The 26S proteasome catalyzes the great majority (at least 80%) of the protein degradation in growing mammalian cells, including both the rapid degradation of misfolded and regulatory proteins and most of the slower breakdown of the bulk of cellular proteins (Zhao et al., 2015). Consequently, proteasome function is essential for protein homeostasis and influences the regulation of most cellular processes, and inhibitors of the proteasome have proven to be very valuable research tools and therapeutic agents that have prolonged the lives of thousands of patients with multiple myeloma (Goldberg, 2012). Since the discoveries of the critical role of ubiquitin (Ub) in protein turnover (Hershko et al., 1980) and of the 26S complex in digesting ubiquitin conjugates (Hough et al., 1987; Waxman et al., 1987), it has been generally assumed that rates of proteolysis by this pathway are regulated solely through protein ubiquitylation. However, it is now clear that ubiquitylation and even the association of a ubiquitylated protein with the proteasome do not necessarily lead to its degradation (Crosas et al., 2006). Thus, the proteasome is not simply a machine for efficient, automatic destruction of ubiquitin conjugates and ubiquitin recycling, but its properties also determine whether a ubiquitylated protein undergoes degradation or survives intact. In addition, the proteasome's degradative capacity and selectivity are not fixed, but are precisely regulated by multiple post-synthetic mechanisms.
In the proteasome (Fig 1), polypeptides are digested to short peptides, 90% of which range between 2 and 10 residues in length (Kisselev et al., 1999). Nearly all are digested in seconds to amino acids by cytosolic peptidases, but in mammals some serve as precursors for antigenic peptides displayed on MHC-class I molecules. Because proteolysis is irreversible, the consequences for cells can be severe if proteasomes destroy proteins non-selectively or function non-processively and release partially degraded polypeptides. Therefore, proteasomes have evolved intricate mechanisms to avoid such failures and to ensure efficient, selective degradation.
Fig 1.
Genetic and biochemical studies have greatly advanced our understanding of the multiple steps in proteasomal degradation: the binding of ubiquitylated proteins, their deubiquitylation, and their ATP-driven unfolding and translocation into the 20S chamber for proteolysis (Fig 2). Although the roles of many of its 60-odd subunits and associated proteins are still unclear, dramatic progress has been made recently through cryo-EM in capturing the dynamism of the 26S complex. The goal of this article is not to summarize these various advances, as has been done in many valuable reviews (Finley et al., 2016; Inobe and Matouschek, 2014; Lander et al., 2013; Liu and Jacobson, 2013; Livneh et al., 2016; Tomko and Hochstrasser, 2013; Wehmer and Sakata, 2016). Instead, we build on many of these discoveries to try to understand the inherent logic of proteasome function and how its catalytic and regulatory features promote efficient and selective proteolysis. This analysis also highlights important gaps in our understanding that merit further study.
Fig 2.
Recognition of Ubiquitylated Substrates
Although it was long believed that ubiquitylation is sufficient to mark a protein for degradation, Matouschek and colleagues (Lee et al., 2001) provided the fundamental insight that efficient degradation of a protein by the 26S requires not only the attachment of a Ub chain or multiple single Ub molecules, but also a loosely folded region in the substrate. Thus, protein half-lives, which generally range in mammalian cells from 10 minutes to several days, are determined not just by the presence of sequences in proteins recognized by Ub ligases and ligase activities, but also by differences in protein folding, which influence susceptibility to the proteasome. Before the discovery of the Ub proteasome pathway (UPP), it was recognized that such wide variations in half-lives were determined by inherent structural features of the proteins, and that loosely folded and misfolded proteins were degraded especially rapidly in all cells (Goldberg and Dice, 1974). Their selective destruction is thus more ancient than the UPP and evolved in prokaryotes, where degradation is catalyzed by compartmentalized proteases, which like the proteasome, rely on AAA ATPase complexes for substrate recognition. Protein ubiquitylation and a proteasome regulatory complex that recognizes Ub conjugates evolved more recently with the emergence of eukaryotes. This linkage of ubiquitylation to proteolysis enabled protein degradation to be much more selective and precisely regulated.
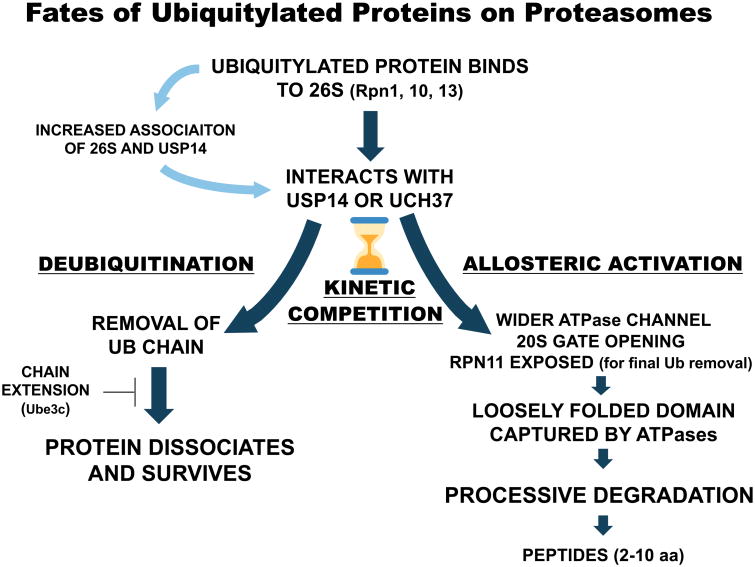
The ability of the proteasome to recognize both a Ub chain and a loosely folded region provides the fundamental basis for how it determines which proteins to degrade and which to spare. This critical life-or-death decision can be explained by the discovery of two types of conjugate binding: 1) an initial, reversible step in which the Ub chain undergoes high affinity binding to receptors on the 26S particle, and 2) a subsequent tighter-binding step that depends on the ubiquitylated protein's structure and requires ATP hydrolysis (Peth et al., 2010). This sequence and the “dwell-time” of the substrate on the 26S provide an opportunity for competing processes to determine the protein's fate. On one hand, the proteasome's multiple de-ubiquitylation enzymes (DUBs) (Fig 3) shorten the substrate's dwell-time and promote the release of some, perhaps many, of the ubiquitylated proteins that initially bind (Lee et al., 2016). However, if the substrate becomes tightly bound through its loosely folded domain, the six proteasome ATPases are activated (Peth et al., 2013a), and the substrate becomes committed to the steps leading to its destruction —further deubiquitylation, unfolding, processive translocation, and hydrolysis to small peptides in the 20S core particle.
Fig 3.
Initial Binding of Ubiquitylated Proteins to the Proteasome
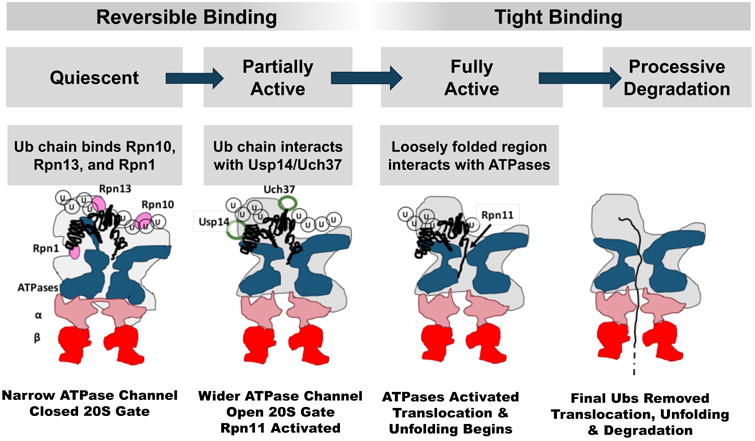
The association of ubiquitylated proteins to the 26S complex, though of high affinity, is readily reversible and easily disrupted by competition with other Ub binding domains or high salt concentrations (Peth et al., 2010). This initial binding depends only on the presence of a Ub chain, is independent of ATP hydrolysis, and even occurs at 4°C. In contrast, the second tight-binding step that commits the substrate to degradation requires ATP hydrolysis and a loosely folded region in the substrate (Fig 2).
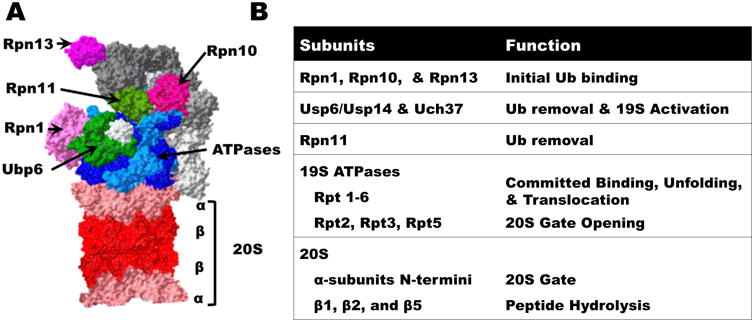
Two 19S subunits, Rpn10 and Rpn13, are particularly important for initial binding of Ub chains (Finley et al., 2016). Rpn10 which binds to Ub chains through its Ub-interacting motif (UIM) was the first “ubiquitin receptor” described (Deveraux et al., 1994). Rpn13 was more recently discovered (Qiu et al., 2006; Yao et al., 2006) and binds Ub chains via its PRU domain (Husnjak et al., 2008). Both Rpn10 and Rpn13 also bind strongly proteins bearing Ub-like (UBL) domains but have only weak affinity for free Ub. They appear to have overlapping yet distinct roles. For example, unanchored Ub chains in cells bind preferentially to Rpn13 and block degradation of certain proteins (Dayal et al., 2009). In vitro yeast 26S lacking either Rpn13 or the UIM domain of Rpn10 show reduced binding of conjugates by ∼50% (Peth et al., 2010). In cells, it is necessary to knockdown Rpn13 and delete Rpn10's UIM domain to cause an accumulation of Ub conjugates and a large decrease in the binding of UBL proteins to the 26S (Hamazaki et al., 2015). Unlike other 26S subunits, Rpn10 also exists in high amounts free in the cytosol and probably has roles outside the 26S (Kim et al., 2009; Rubin et al., 1997). Though often defined as a stoichiometric subunit, Rpn13 is not present on all 19S complexes and may be absent from one regulatory particle in doubly capped 26S (Berko et al., 2014). Curiously the recent cryo-EM studies of human 26S could not visualize Rpn13 (Chen et al., 2016; Huang et al., 2016; Schweitzer et al., 2016).
Although Rpn10 and Rpn13 are the major sites of initial conjugate binding, other 19S subunits also bind Ub-conjugates or UBL proteins. The combined deletion of both Rpn10 and Rpn13 was not lethal in budding and fission yeast, unlike the deletion of most proteasome subunits. Therefore, another Ub binding subunit must be present on the 26S (Husnjak et al., 2008). Accordingly, purified proteasomes lacking both Rpn13 and Rpn10's UIM domain retain about 20% the affinity of normal 26S for Ub conjugates (Peth et al., 2010). Recently, the 19S subunit Rpn1 was identified as a binding site for Ub chains (Shi et al., 2016) as well as UBL domains (Elsasser et al., 2002; Gomez et al., 2011). Many ubiquitylated substrates bind to the 26S indirectly via proteins that contain both a UBL-domain and a Ub-associated (UBA) domain, giving them the ability to bind simultaneously Ub conjugates and the 26S. Therefore, these proteins (in yeast: Ddi1, Dsk2, and Rad23) have been proposed to function as “shuttling factors”. Rpn1 contains two conjugate binding sites: T1, where UBL-UBA proteins and Ub chains bind, and T2, where the UbL domain of the DUB Ubp6 binds (Shi et al., 2016). Another 19S subunit, Dss1, has also been implicated in Ub binding (Paraskevopoulos et al., 2014), but its role in conjugate degradation has not been studied, and its accessibility within the 26S structure has been questioned (Schweitzer et al., 2016). It is bewildering why the proteasome contains so many binding sites for ubiquitylated proteins, and it is not clear that all the sites have been identified. Determining the relative affinities of Ub conjugates for these sites in intact proteasomes and whether they function cooperatively, sequentially, or are specific for certain substrates could fill important gaps in our understanding of the degradative process.
Another fundamental unanswered question concerns the precise roles in conjugate degradation of the “shuttling factors.” Although genetic studies clearly support an important role in degradation of some substrates, there seems to be no a priori need to have shuttling factors, since Ub-conjugates associate with the 26S with similar affinities as the UBL-UBA proteins and bind to the same sites on the 26S as ubiquitylated proteins (Shi et al., 2016). Nevertheless, Rad23 is found on most Ub conjugates binding to the proteasome after processing by the p97/VCP/Cdc48 ATPase complex (Tsuchiya et al., 2017) and the loss of these shuttling factors, like mutations in proteasome subunits, results in increased sensitivity to conditions causing protein misfolding (Wilkinson et al., 2001). Thus, these proteins must serve important functions in vivo, but efforts to reconstitute in vitro the stimulation of proteolysis by shuttling factors have failed so far. Although there is appreciable functional redundancy of these UBL-UBA proteins (Wilkinson et al., 2001), mutations in certain shuttling factors produce specific phenotypes. For example, mutations in UBQLN2 (a Dsk2 homologue) cause Amyotrophic Lateral Sclerosis (ALS) (Deng et al., 2011), and thus it must serve some distinct role, probably in ubiquitylation and shuttling of client proteins to the 26S (Itakura et al., 2016). Also, human Rad23s show a strong preference for Ub chains containing Ub molecules linked through their lysine 48 residues (K48) over chains formed by lysine 63 linkages (K63). Thus, these shuttling factors seem to help direct K48 Ub conjugates to the proteasome (Nathan et al., 2013).
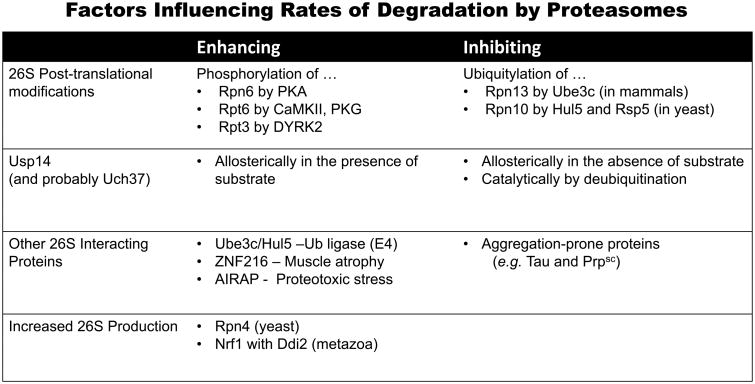
The binding of Ub conjugates to proteasomes can be regulated by post-synthetic modifications. In response to proteasome inhibitors or conditions that impair 26S function (e.g. heat shock or expression of aggregation-prone proteins), Rpn13 becomes ubiquitylated by the 26S-associated Ub ligase Ube3c (Besche et al., 2014; Jacobson et al., 2014). This modification prevents further binding of Ub conjugates inactivating the 26S (despite the presence of other Ub-binding sites) (Besche et al., 2014). This inactivation seems to occur when proteasomes encounter difficulties in degrading substrates (e.g. aggregated proteins) and presumably redirects Ub conjugates to functioning proteasomes (Besche et al., 2014). It seems likely that other regulatory factors will also affect the initial binding step. In mammalian cells, Rpn13 ubiquitylation is reversed by cytosolic DUBs However, in plants, where proteasome inhibition also causes Rpn13 ubiquitylation, this modification targets the 26S to autophagy (Marshall et al., 2015). In S. cerevisiae, the Ube3c homolog, Hul5, and Rsp5 ubiquitylate a different Ub binding protein, Rpn10 (Isasa et al., 2010), which may cause Rpn10 dissociation from the 26S (Zuin et al., 2015) or trigger autophagy of proteasomes (Marshall et al., 2016).
Proteasomes Are Not Fastidious About Ubiquitin Chain Preference
Proteasomes face the seemingly difficult challenge of binding and disassembling many different types of Ub chains that vary both in length and linkage specificity. Despite widespread impressions to the contrary, proteasomes are not fastidious regarding the type or length of Ub chains on substrates. Isolated 26S can degrade substrates containing multiple short Ub chains (Dimova et al., 2012) or even a single Ub (Braten et al., 2016), although these different Ub conjugates may differ widely in their affinities for the proteasome and once bound in their likelihood of being degraded. The importance of chain-length on substrate binding was first revealed by Thrower et al (Thrower et al., 2000). While often cited as indicating a requirement for only K48 tetra-Ub chains, these investigators actually found that longer chains containing 5-9 ubiquitins on dihydrofolate reductase (DHFR) provided greater affinity for proteasomes, but did not change its degradation rate (Thrower et al., 2000). Thus, for this protein, binding to the 26S is not the rate-limiting step in proteasomal degradation. Additionally, longer chains are probably important in vivo in preventing substrate escape from degradation by slowing deubiquitylation both before 26S binding and on the 26S, prior to the transition to tight, Ub-independent binding.
The importance of the Ub chain in influencing dwell-time was nicely demonstrated by Lu et al using fluorescent Ub conjugates and single-molecule imaging to measure both the degradation rate and the dwell-time of conjugates on the 26S before they are either released or degraded (Lu et al., 2015). Degradation rates increased with the number of Ub molecules attached up to eight (the longest chain measured). Multi-ubiquitylated substrates had longer dwell times than ones with the same number of Ub molecules in a single chain (Lu et al., 2015). However, a single Ub on the substrate contributed little to 26S binding. Because a single Ub is particularly likely to be removed by cytosolic or 26S-associated DUBs, mono-ubiquitylation of substrates is probably not important in driving proteolysis in vivo, unless another Ub ligase rapidly catalyzes chain extension as reported by several groups (Crosas et al., 2006; Koegl et al., 1999; Scott et al., 2016).
Although much attention has been paid to the nature of the Ub linkages formed by different ligases, chains containing nearly all linkages can support proteasome dependent proteolysis. Upon proteasome inhibition in cells, ubiquitylated proteins accumulate that contain all seven different types of Ub linkages except K63 (Xu et al., 2009), strongly suggesting that K63 chains do not primarily target proteins to proteasomes. Nevertheless, proteins bearing K63 and K48 Ub chains bind to purified proteasomes with similar high affinities (Peth et al., 2010) and are degraded rapidly (Hofmann and Pickart, 2001). However, in the cytosol, K63 chains do not typically reach the 26S because they bind preferentially to ESCRT proteins and are thereby shunted to lysosomes (Nathan et al., 2013). Because ESCRT proteins are not in the nucleus, the fates of nuclear K63-linked proteins remain unknown, although proteasomal degradation seems likely considering the high concentrations of 26S in nuclei (Wójcik and DeMartino, 2003).
Despite this lack of strong specificity, certain types of Ub chains are not efficiently degraded. If two Ub chains are formed on adjacent lysines of the donor Ub, creating a forked chain, they are not degraded (Kim et al., 2009). Although purified RING E3s tend to form such non-degradable forked chains, cytosolic UIM proteins (e.g. Rpn10) have the surprising capacity to prevent this apparent dead-end ubiquitylation (Kim et al., 2009). By contrast, proteasomes efficiently degrade substrates bearing branched chains, where the modified lysines are more distantly separated on the donor Ub (Meyer and Rape, 2014). The major cell cycle regulatory E3, APC (the Anaphase Promoting Complex), forms such conjugates on several proteins that are degraded particularly rapidly in mitosis (Meyer and Rape, 2014), and recent findings indicate that branched chains are frequently found on short-lived proteins in vivo (Liu et al., 2017).
The Step Committing the Substrate for Degradation
In contrast to the initial binding through the Ub chain, the tighter association that enables the 26S to processively degrade the substrate resists disruption by competing Ub-binding domains or salt. This transition to tighter binding requires ATP hydrolysis and a loosely folded region in the protein (Peth et al., 2010), and thus makes the degradative process dependent on both ubiquitylation and protein conformation (Lee et al., 2001; Prakash et al., 2004). For example, ubiquitylated DHFR is rapidly degraded in cells and in vitro; however, in the presence of its inhibitor, the widely-used drug methotrexate, DHFR assumes a tightly folded structure that prevents DHFR degradation (Johnston et al., 1995; Thrower et al., 2000). Methotrexate does not affect the initial Ub-dependent binding to the 26S, but does block the transition to the tightly bound, committed state (Peth et al., 2010).
Such a stabilizing effect of ligands on conformation and proteasome susceptibility is probably a general determinant of protein half-lives. Interestingly, initial examples of regulated degradation of proteins were instances where the binding of substrates (e.g. tryptophan for tryptophan pyrrolase) or cofactors (e.g. heme for globin) that stabilize a protein's conformation were shown to raise its cellular content by slowing its degradation (Goldberg and Dice, 1974). Similarly, the tendency of proteins to become more stable upon forming multimeric complexes is probably through elimination of loosely folded or structureless domains that would otherwise favor ubiquitylation and proteasomal hydrolysis (McShane et al., 2016).
Despite their importance, the commitment step and its requirement for a loosely folded region remain vague concepts. The nature of the protein sequences promoting proteasomal degradation has been examined by Matouschek and coworkers who concluded that to promote degradation, the unstructured region should contain at least 30 amino acids (van der Lee et al., 2014), have little flexibility and be composed of varied amino acids, but biased towards hydrophobic residues (Yu et al., 2016). Highly repetitive amino acid sequences, such as polyglutamines (as cause several neurodegenerative diseases) or polyalanines (as cause certain muscular dystrophies) or dipeptide repeats (as can cause ALS) probably impair translocation (Kraut et al., 2012) and degradation. These effects and the resistance of repetitive sequences to hydrolysis by the 20S (Venkatraman et al., 2004) can explain the inability of the UPP to efficiently degrade such proteins and their accumulation in some proteotoxic diseases.
Such loosely-folded domains are present at some time in most proteins through continual protein “breathing,” but can become more frequent with mutations, covalent modifications, post-synthetic damage, or actions of unfoldases (e.g. p97/VCP/Cdc48). The influence of such a loosely folded domain also probably depends on its position relative to the Ub chain and the location on the 26S when the Ub chain binds. The key components that bind these loosely folded regions almost certainly are the tyrosine pore loops inside the 19S ATPases. These loops are hydrophobic projections into the substrate channel through the ATPase ring, whose role in substrate translocation is discussed below. The difficulties in degrading highly repetitive sequences presumably is due to their resistance to translocation by these pore loops (Coffino et al., 2014; Kraut, 2013).
Deubiquitylation of Potential Substrates on the 26S
After binding a ubiquitylated protein, proteasomes disassemble and release the Ub chain using several associated DUBs. In most eukaryotes, there are three major DUBs associated with the proteasome: the metalloprotease Rpn11 (Verma et al., 2002; Yao and Cohen, 2002) and the cysteine proteases, Usp14 (Ubp6 in S. cerevisiae) (Borodovsky et al., 2001), and Uch37/UchL5 (Stone et al., 2004). Although proteasomes are generally very similar in mammals and S. cerevisiae, major insights about the UPP have been made through genetic studies in this yeast, which lacks Uch37. The functional consequences of having only two DUBs on the proteasome remain a mystery, especially because Ubp6/Usp14 is present on only a small fraction of the 26S complexes in yeast and mammalian cells. Lower amounts of other DUBs (Usp5 (Isopeptidase T), Usp7, Usp9x, Usp13, Usp15, Usp25, and Usp38) have also been reported to associate with the 26S (Besche et al., 2014; Scanlon et al., 2009), although little is known about their possible contributions to proteasome function. Among them, Usp5 selectively hydrolyzes unattached Ub chains and thus appears to be important in promoting rapid elimination of such chains, whose accumulation can interfere with 26S activity and impede the degradation of certain substrates (Dayal et al., 2009).
One fundamental function of these 26S DUBs is to release the Ub molecules so that they can be reutilized in degradation of other proteins. Cells maintain high concentrations (∼20μM) of free Ub (Kaiser et al., 2011), but deleting Ubp6/Usp14 prevents efficient Ub recycling leading to proteasomal destruction of Ub. The resulting decrease in Ub levels limits cellular proteolysis and hinders growth (Chernova et al., 2003; Hanna et al., 2003). In yeast lacking Ubp6, proteolysis is reduced, unless Ub is also overexpressed (Hanna et al., 2003). Similarly, mice lacking Usp14 in neurons develop Ub deficiency leading to synaptic dysfunction and ataxia (Wilson et al., 2002), which is also reversed by Ub overexpression (Chen et al., 2011). Such effects on the Ub pool are surprising because Usp14/Ubp6 is present on only a small fraction of the proteasomes (Aufderheide et al., 2015), which suggests that this fraction is most active in conjugate degradation.
These findings imply that the presence of a single Ub or short chains on the substrate do not prevent its translocation and destruction (Singh et al., 2016). However, removing large Ub chains from the substrate must be important for substrate translocation because a long Ub chain should prevent efficient passage through the ATPase ring into the 20S. By removing the Ub chain close to the substrate backbone, Rpn11 plays an essential role. Inactivating Rpn11 traps ubiquitylated proteins on the 26S (Matyskiela et al., 2013). Rpn11 is positioned just above the substrate entry channel (Beck et al., 2012; Lander et al., 2012), and thus can scan substrates as they enter the ATPase ring. Its function is therefore linked to ATP hydrolysis and substrate translocation (see below). To what extent, Usp14, Uch37, or Rpn11 contribute to the removal of Ub molecules from different substrates presumably depends on the length, size, position, and number of chains attached. However, the actions of these three DUBs are not independent, and the timing of their respective functions is critical. Usp14 and Uch37 appear to act before the commitment to proteolysis. The subsequent exposure and activation of Rpn11 presumably avoids premature deubiquitylation and dissociation of potential substrates.
A Life or Death Kinetic Competition
In deubiquitinating substrates, Usp14 and Uch37 also serve to limit the substrate's dwell-time on the 26S (Crosas et al., 2006). These DUB activities thus constitute a timing mechanism determining whether a conjugate becomes committed to degradation or released from the 26S and escapes destruction (Fig 3). This kinetic competition mechanism was first recognized by Finley and coworkers (Crosas et al., 2006) and provided the rationale for their development of small molecule Usp14 inhibitors to enhance substrate degradation (Lee et al., 2010). By slowing conjugate deubiquitylation, these inhibitors should prolong the substrate's dwell-time on the proteasome and enhance the probability of degradation. Usp14 appears to specifically deubiquitylate proteins with multiple short Ub chains (Lee et al., 2016), which presumably are frequently generated in vivo. Most likely, Usp14 inhibitors promote the selective destruction of such proteins, which were reported to have longer dwell times on the proteasome than proteins with a single Ub chain (Lu et al., 2015). Thus, the rate of Ub chain removal is a primary determinant of dwell-time and therefore the likelihood of degradation. Moreover, this step can be altered pharmacologically with Usp14 inhibitors and perhaps regulated physiologically by varying the Usp14 content on. In fact, Usp14 continually binds and dissociates from the proteasome, and increasing the levels of Ub conjugates on the particles stimulates Usp14 binding (Kuo and Goldberg, 2017).
In addition to the DUBs that determine substrate dwell-time, several Ub ligases have been found associated with mammalian proteasomes: Ube3a, Ube3c, Rnf181, Huwe1, and Ubr4 (Besche et al., 2014). In yeast the Ube3c homolog, Hul5, is the most abundant ligase on the 26S and has important effects on proteolysis. Because deleting Hul5 suppresses the phenotypic effects of Ubp6 deletion, Hul5 was proposed to counter Ubp6's actions in deubiquitinating conjugates on the 26S (Leggett et al., 2002), by functioning as an “E4”, which elongates Ub chains on the substrate and thereby prolongs its association with the proteasome (Crosas et al., 2006). Hul5 has been shown to prevent the release of partially degraded proteins (Aviram and Kornitzer, 2010). Incomplete degradation and release of hard-to-unfold globular domains (e.g. GFP) often occur with purified 26S, which lack ubiquitylating enzymes (Berko et al., 2012). Because protein fragments can be toxic, preventing non-processive degradation is likely to be especially important in eukaryotes, where large multi-domain proteins are more abundant than in prokaryotes. In addition, if proteasomes are inhibited or stalled, Ube3c serves a very different regulatory function by ubiquitylating Rpn13 and inactivating the 26S, which presumably prevents conjugate binding to nonfunctional proteasomes (Besche et al., 2014).
The roles of other E3 ligases associated with the mammalian proteasome —whether they regulate its activity, modify substrates, or serve some distinct regulatory function —remain unclear. The functions of 26S-associated proteins are difficult to study because they are present on a low fraction of the particles, are easily removed during purification, and may only function under special conditions. It is noteworthy that although purified proteasomes effectively digest some structureless Ub conjugates, they fail to digest many ubiquitylated proteins that are degraded rapidly in cells, perhaps because these associated ligases or key cofactors are removed during proteasome isolation.
Allosteric Activation of Proteolysis by the DUBs and Ub conjugates
In this kinetic competition, Usp14 and Uch37 play another important regulatory role by mediating the activation of proteasomes by Ub conjugates (Fig 3) (Peth et al., 2009; Peth et al., 2013a). Substrate binding to Usp14 triggers major alterations in 19S structure that result in gate opening and allow ATPase activation (see below). The DUB's interaction with a substrate thus appears to function as a sentinel that signals the presence of a potential substrate. This activating role of Usp14 is surprising, because it simultaneously inhibits proteolysis by catalyzing deubiquitylation (Leggett et al., 2002), and in the absence of a Ub conjugate allosterically reduces protein degradation (Hanna et al., 2006). Thus, during the substrate's dwell-time, the DUBs, while catalyzing removal of ubiquitin and promoting the protein's survival, are also allosterically enhancing several proteasomal processes that favor substrate destruction.
The two key roles of the proteasome, substrate deubiquitylation and ATP-driven proteolysis are linked functionally through Usp14 and Uch37 (Peth et al., 2013a). The binding of Ub conjugates to the active site of Usp14 allosterically stimulates substrate entry into the 20S which involves opening its gated central pore (Peth et al., 2009; Peth et al., 2013a) and enlargement of the channel through the ATPase ring (Matyskiela et al., 2013). This activation of the entry step by substrate-bound Usp14/Ubp6 is blocked by mutations in any ATPase subunit that prevent nucleotide binding or disrupts its gate-opening motifs (Peth et al., 2009). Although substrate binding to Usp14 triggers major alterations in 19S structure (see below), exactly how this binding induces these changes is still unclear. An important recent clue is that Usp14's UBL domain by itself can recapitulate the activation by Ub conjugates, and thus this domain seems to mediate the activation upon conjugate binding (Kim and Goldberg, unpublished). Interestingly, the association of a Ub conjugate with Uch37 can also allosterically activate peptide entry. Because Uch37 lacks a UBL domain and is bound to the 26S through Rpn13 rather than Rpn1 (Qiu et al., 2006; Yao et al., 2006), substrate binding to Uch37 must enhance 26S activity by a very different mechanism.
The exact order of events after substrate binding to the 26S is still only superficially understood (Fig 2), and many basic questions about this kinetic competition between deubiquitylation and destruction remain unanswered. Do these DUBs digest all types of conjugates or do they serve distinct roles (as suggested by Usp14's preference for proteins with multiple chains) (Lee et al., 2016)? How important is this allosteric activation for efficient proteolysis? It cannot be essential for proteolysis since S. cerevisiae lack Uch37 and are viable without Ubp6 (Leggett et al., 2002). If a conjugate associates directly with a Ub- binding subunit, or if it binds via a shuttling factor, do Usp14 and Uch37 trim the Ub chains in a similar manner or are they processed by distinct mechanisms?
This activation by substrates is noteworthy since in the absence of a substrate, Usp14 plays an important function in allosterically suppressing basal proteasome activity (Hanna et al., 2006). Proteasomes from cells lacking Usp14 show higher ATPase, peptidase, and Rpn11 activity than those in WT, and can even degrade some non-ubiquitylated proteins (Kim and Goldberg, unpublished). Thus, the presence of Usp14 on the 26S helps reduce wasteful ATP consumption and nonselective proteolysis.
Structural Changes Facilitating Substrate Entry into the 20S Particle
The binding of a ubiquitylated substrate triggers many structural changes in the 19S complex (Matyskiela et al., 2013) that presumably account for its enhanced catalytic activity. The most prominent changes observed by cryo-EM are the widening of the central substrate transit channel through the ATPase ring and its alignment with the gated entry into the 20S. Similar structural changes are also observed upon binding of the non-hydrolyzed nucleotide ATPγS which freezes the enzyme in a state that presumably occurs transiently upon ATP binding (Śledź et al., 2013).
Non-selective entry and destruction of cell proteins in the 20S particle are prevented by N-termini of the 20S α-subunits, which protrude into this central channel to form a gate (Groll et al., 2000). The importance of this gate has been demonstrated by deletion of one or two of these N-terminal extensions, which increases the hydrolysis of peptides and proteins even in vivo (Bajorek et al., 2003; Choi et al., 2016). Biochemical and structural studies established that gate opening occurs when the C-terminal HbYX (Hydrophobic-Tyrosine-X) motifs on three of the 19S ATPases (Rpt2, Rpt3, and Rpt5) bind to lysines in the inter-subunit pockets of the 20S's outer ring (Rabl et al., 2008; Smith et al., 2007; Yu et al., 2010). This interaction occurs upon nucleotide binding to the ATPase subunits and triggers movement of the α-subunits' N-termini out of the 20S opening, which permits substrate entry. The remaining three non-HbYX termini seem to maintain the association between the regulatory and core particles.
Physiologically, this activation when a substrate binds must be important in preventing non-specific, Ub-independent proteolysis. Tight regulation of gate-opening ensures that free 20S particles, which are present in many cells in comparable amounts to the 26S complex, are maintained in a latent state and do not cause unregulated proteolysis. Thus, proteasome activation occurs upon binding of Ub conjugates and ATPγS, which trigger reversible 19S structural changes (Matyskiela et al., 2013; Śledź et al., 2013) and 20S gate-opening (Peth et al., 2009; Smith et al., 2005). This activated state is then maintained until the substrate either is deubiquitylated and escapes or undergoes degradation. Substrate entry into the 20S or singly capped 19S-20S complexes can also be enhanced by the binding of the 11S complexes (PA28αβ, and PA28γ (Ma et al., 1992)) or Blm10/PA200 (Ortega et al., 2005), which markedly stimulate hydrolysis of peptides and certain non-ubiquitylated proteins (e.g. histones; (Qian et al., 2013)).
For ubiquitylated proteins to be translocated into the 20S, other structural changes are required beyond gate-opening. The substrate entry channel extends from the 20S gate through the ATPases, which resemble two stacked hexameric rings. The ring proximal to the 20S contains the ATPase domains and HbYX motifs, while the distal ring is formed by the N-terminal domains of the ATPases. In the basal state, the ATPases form a narrow, disjointed channel, which enlarges and aligns to facilitate protein entry upon activation (Matyskiela et al., 2013; Śledź et al., 2013).
Another important structural change upon binding a Ub conjugate or ATPγS is the repositioning and activation of the DUB, Rpn11. In the basal state, Rpn11's active site faces away from the ATPases' substrate entry channel and is blocked by other subunits, but upon activation, it is repositioned so that its active site lies just above the opening of this channel (Matyskiela et al., 2013; Śledź et al., 2013). In addition to its other allosteric consequences, the binding of Ub conjugates to Usp14 exposes Rpn11's active site (Bashore et al., 2015) and enhances its catalytic activity. Thus, the activities of these two DUBs are linked. This previously unappreciated coordination probably both prevents premature substrate deubiquitylation by Rpn11 also ensures efficient removal of Ub chains that might block translocation.
Substrate Activation of ATP Hydrolysis
The 26S proteasome belongs to a set of compartmentalized proteolytic complexes whose activity is regulated by the hexameric AAA ATPases. This group includes the archaeal 20S proteasome with its PAN regulatory ATPase, the ClpP proteases regulated by ClpA, ClpC, and ClpX ATPases, the HslV complex regulated by HslU ATPase, and the single-subunit proteases, Lon and FtsH (Striebel et al., 2009). Unlike the eukaryotic 19S complex, which contains six distinct but homologous ATPases (Rpt1-6), these substrate-recognizing ATPases are simple hexameric homopolymers, whose functions are not linked to Ub. Nevertheless, their mechanisms for substrate binding, unfolding, and translocation seem to be largely conserved. Therefore, insights gained into how these prokaryotic and mitochondrial ATPase subunits coordinate nucleotide hydrolysis and link it to the mechanical forces that drive substrate translocation and unfolding should be applicable to the 19S complex. An important gap in our understanding concerns the functional significance of the sequence divergences between the six 19S ATPase subunits. Each contains a distinct N-terminal extension, which pair up forming three coiled coils that interact with other 19S subunits. No similar extension is found in the bacterial AAA ATPases. Presumably these extensions help couple structural changes in the ATPases upon nucleotide binding and hydrolysis to the functions of other 19S components.
Unlike most eubacteria, archaea contain a four-ring 20S proteasome organized similarly to the eukaryotic complex as well as the simple homohexameric, ATPase, PAN, which appears to be the direct evolutionary antecedent of the 19S ATPases (Zwickl et al., 1999). During the evolution of eukaryotes, this ATPase complex became functionally linked to substrate ubiquitylation to provide exquisite selectivity to the proteolytic process. The resulting addition of many 19S subunits to an ATPase ring resulted in a single complex capable of substrate recognition, Ub-recycling, and protein translocation.
Our understanding of the cycle of ATP binding, hydrolysis, and ADP release in the 26S has benefited from studies of PAN (Kim et al., 2015; Smith et al., 2011). Though all six subunits are identical, PAN normally binds two ATPs plus two ADPs, leaving two sites unoccupied (Smith et al., 2011). Although it initially seemed most likely that the two ATPs and ADPs become bound to the most distant (para) subunits (Smith et al., 2011), recent, elegant FRET analysis indicated that the two ATPs bind to adjacent subunits (Kim et al., 2015). A critical feature for their integrated function is that ATP binding and hydrolysis allosterically alter the binding of nucleotides to the neighboring subunits. The structural basis for the resulting cooperativity is that an arginine residue from one subunit, “the arginine finger”, contributes to the nucleotide-binding site on the adjacent subunit (Kim et al., 2015).
This cross talk between subunits triggers a hydrolytic cycle in which the loss of any one nucleotide-binding site markedly reduces the ATPase activity of the particle (Peth et al., 2013b). This requirement for all six Rpt subunits implies that they function in an ordered cycle (Smith et al., 2011). After two new ATPs bind to the unoccupied subunits, they are rapidly hydrolyzed, and their phosphates released. The rate-limiting step in this cycle appears to be the release of the two ADPs (Rodriguez-Aliaga et al., 2016; Smith et al., 2011), which is triggered by ATP hydrolysis in the preceding subunit. Further analysis of this ATPase cycle is important, because it drives and integrates multiple steps in conjugate degradation, and ATP hydrolysis increases during proteolysis and can be stimulated by 26S phosphorylation (see below).
The rate of ATP hydrolysis by the 26S complex increases 2-3 fold upon binding a Ub conjugate (Peth et al., 2013b). A similar activation of ATP hydrolysis by loosely folded substrates is a general feature of the homologous ATP-dependent proteases in E. coli, archaea, and mitochondria, but in the 19S complex ATPase activation also requires the binding of Ub chains. Thus, this activation has two requirements: 1) an interaction of the Ub chain with Usp14 or Uch37, and 2) the loosely folded polypeptide with one of the ATPases. These two signals do not have to be covalently linked; the 19S ATPases can be activated by addition of Ub aldehyde or a Ub chain (but not free Ub) together with casein (Peth et al., 2013a). Thus, this activation of the ATPases, which is probably the critical step that commits substrates to degradation, is more restrictive than the signal to open the substrate entry channel, which only requires a Ub chain. The structural basis of this regulation by Ubp6 is still not very clear despite recent cryo-EMs studies (Aufderheide et al., 2015; Bashore et al., 2015), because without a substrate present, Ubp6's location in the 26S cannot be resolved. However, upon binding the substrate analog, Ub aldehyde, Ubp6 assumes a more definite structure that interacts with the ATPases. To stimulate ATP hydrolysis, the loosely folded region most likely interacts with the ATPases, specifically with their pore loops. Mutations in these loops, like ones affecting nucleotide binding, not only have a major impact on substrate translocation and degradation rates (Beckwith et al., 2013), but also can increase the basal rate of ATP hydrolysis (Erales et al., 2012).
Translocation of the Polypeptide through the ATPase ring
Although substrate translocation and unfolding by the 19S have been difficult to study, valuable insights have been gained from the homologous bacterial ATPases, ClpX and HslU. Unfolding occurs because the ATP-powered translocation of the polypeptide through the ATPases' central channel causes upstream folded domains to linearize (Kenniston et al., 2003). Translocation seems to be driven by the tugging on the substrate by the six central tyrosine pore loops (Hinnerwisch et al., 2005). Martin and colleagues recently used single-molecule analysis to analyze the unfolding of a GFP-fusion protein by ClpXP and showed that unfolding depends of the power generated by the pore loops and thus requires frequent and forceful pulling on the substrate (Rodriguez-Aliaga et al., 2016). However, Smith an coworkers showed that translocation by the 26S or PAN is more processive than by ClpXP and depends predominantly on the force generated by the pore loops rather than the frequency with which these loops pull on the substrate (Snoberger et al., 2017).
The ease of unfolding any region must depend on the thermodynamic stability of that domain and its ability to withstand the force on the upstream sequence generated by these tyrosine loops (Lee et al., 2001). Whether translocation begins from the protein's N or C terminus, or even starts from an internal loop (Piwko and Jentsch, 2006) depends on the relative ease of unfolding of these regions (Berko et al., 2012). Thus, the initial association of the pore loops with a loosely folded domain is critical not only for the commitment to degradation, but also determines the direction of translocation. Since the start site depends on which loosely folded domain initially interacts with the ATPases, it probably also depends on what part of the substrate is ubiquitylated, and where on the 26S, it binds. Because the direction of translocation determines how the polypeptide is delivered to and cleaved by the 20S's peptidase sites, the direction of translocation even influences the nature of the peptides generated for antigen presentation on MHC class I molecules (Berko et al., 2012).
The Energy Costs of Proteasome Function
The discovery that ATP was required for intracellular proteolysis in both animal and bacterial cells raised two obvious questions. 1) Why do cells devote valuable energy to a hydrolytic reaction that is thermodynamically favored? It is now clear that ATP is consumed in the UPP to make the degradation process highly selective through ubiquitylation and at the proteasome, where ATP consumption enables substrate unfolding and translocation into an isolated chamber within which proteolysis is efficient and processive. 2) How much metabolic energy do cells devote to achieve selective protein breakdown? Even though ATP binding and hydrolysis are necessary for multiple steps in conjugate degradation, the rate of hydrolysis of ubiquitylated proteins by the 26S is proportional to its rate of ATP hydrolysis (Peth et al., 2013b). The total ATP consumed in degrading Ub conjugates is surprisingly large. For example, at Vmax, it takes roughly 50–80 ATP molecules and about 23 seconds for a purified 26S to degrade a ubiquitylated DHFR molecule. If, however, DHFR binds the substrate folate and thus assumes a more tightly-folded conformation, the number of ATP molecules consumed and the time necessary to degrade DHFR doubles (Peth et al., 2013b). The greater ATP consumption with the more tightly-folded substrate occurs not because the proteasome hydrolyzes ATP at a higher rate, but because degradation takes longer (i.e. the activated state must be maintained longer to complete the unfolding of the DHFR).
Substrate size also determines the time for proteolysis and thus the amount of ATP consumed. Degradation of ubiquitylated Sic1, a loosely folded protein that is twice the size of DHFR, requires about twice the time and ATP. The breakdown of large multi-domain proteins must consume much greater time and ATP for the 26S to unwind and degrade each successive domain (Rodriguez-Aliaga et al., 2016). With such large proteins, it is unclear if the rate-limiting step for proteasomal digestion is the time until a loose domain is captured by the ATPases or the conformation-dependent unfolding and translocation, as no multi-domain ubiquitylated substrates have been studied. These measurements of the energy costs for 26S function indicate that conjugate degradation consumes about a third of the ATP required for ribosomal synthesis of polypeptides, but proteolysis in vivo must consume even more ATP to support substrate ubiquitylation and perhaps repeated rounds of proteasomal binding and deubiquitylation before degradation occurs.
The Structural Changes Underlying 26S Function
The recent advances in single particle cryo-EM technology have made possible the near atomic resolution of the structures of yeast and human proteasomes, which certainly represents a major achievement of the Baumeister (Beck et al., 2012; Wehmer et al., 2017) and Martin (Dambacher et al., 2016; Lander et al., 2012) laboratories and more recently from Shi's (Huang et al., 2016), Mao's (Chen et al., 2016), and Cong's (Ding et al., 2017) labs. The structure of the 19S particle has been a major challenge because of its inherent complexity, and because it is highly dynamic and heterogeneous. Based upon hierarchical classifications of images, several proposals for a sequence of structural changes have been made. Initially two states of the 26S were recognized (Matyskiela et al., 2013), but further studies led to the recognition of four different forms (Chen et al., 2016; Schweitzer et al., 2016). These structures were solved with and without Usp14, ATPγS, ATP, or ADP-AlFx present or with Rpn11 inactivated to capture a substrate during translocation. The clustering of the multiple forms has been interpreted as a series of snapshots of the 26S at different steps in the degradative process, although strong evidence that these different states function sequentially during degradation is lacking in most studies. Consequently, considerable uncertainty remains concerning the sequence of structural changes leading to proteasomal activation and the commitment to proteolysis (Fig 2).
These studies have illuminated the structural basis for the enzymatic activation upon Ub conjugate binding, although some clear discrepancies exist. Rpn11 activation correlates with the repositioning of Rpn11 and the exposure of its active site, while the activation of peptide hydrolysis by ATPγS or a Ub chain correlates with the realignment of the ATPase ring and widening of its substrate entry channel. Surprisingly, recent cryo-EM analysis suggests that the enhanced peptide entry occurs without the anticipated opening of the 20S gate entry channel, as occurs with addition of HbYX peptides or PA28 complexes. The most recent models suggest that the opening of the 20S gate, which is evident in only a small subset of the particles, occurs as the final step (Chen et al., 2016; Ding et al., 2017; Huang et al., 2016; Schweitzer et al., 2016). Further time-dependent analysis of the events after substrate addition should resolve these important issues.
Moreover, as several of these studies point out, additional transitional structures are likely. In fact, the biochemical investigations already indicate multiple degrees of activation; e.g. although the structural changes with ATPγS and Ub aldehyde seem similar, biochemically they are distinct, and the maximal activation of peptide hydrolysis by ATPγS can be further enhanced by several factors including subunit phosphorylation (see below). The structural basis for these different degrees of activation, and especially their effects on 20S gating, are important to delineate. Among the unresolved fundamental issues are 1) the precise relationship between these various structural classes and the particle's enzymatic activities, 2) the order of conformational changes between conjugate binding and degradation, 3) whether other structural intermediate states exist that correspond to the commitment step and activation of the ATPases, and 4) whether there may be alternative steps for activation and degradation depending on whether the conjugate binds directly to the 26S and interacts with Usp14 or Uch37 or via a shuttling factor. While the key role of the ATPases' pore loops is evident, it is unclear how their movements which drive substrate translocation are coupled to the nucleotide binding-hydrolysis cycle. These major mechanistic questions are critical for understanding the overall regulation of proteasomal degradation.
The Multiple Forms of the 26S in Cells
Because the substrate-activated form of the 26S complex is different in quaternary structure from its basal state, Baumeister and colleagues could recognize the active proteasomes when they developed methods for high resolution cryo-EM of fixed cells. By computationally fitting individual particles to previous cryo-EM images, they found that about 80% of the 26S in cultured hypothalamic neurons were in the inactive or substrate-free state (Asano et al., 2015). This conclusion fits with the observations that Usp14 and Ube3c are present on only a small fraction of 26S, especially those containing Ub conjugates (Kuo and Goldberg, 2017). Thus, in these and presumably most cells, there is a large excess of unengaged proteasomes that can be mobilized in stressful conditions when ubiquitylation rates increase, e.g. upon nutrient deprivation (Zhao et al., 2015) or disposed of in prolonged starvation (Cohen-Kaplan et al., 2016).
Although widely assumed to be a single entity, 26S proteasomes in cells are clearly quite heterogeneous. They vary in content of Ub conjugates, Usp14, Ube3c, other associated proteins, activator complexes, and subunit modifications. Under specific conditions, certain proteasome-activating proteins are induced and associate with the 26S when proteolysis rises, e.g. in proteotoxic stress, AIRAP and AIRAPL (Stanhill et al., 2006) and in muscle atrophy, ZNF216 (Hishiya et al., 2006). However, the biological significance of most of these differences in 26S composition are still unclear or controversial, including the association of proteasomes with the activators PA28αβ, PA28γ, or PA200/Blm10 or the inhibitor PI31, and the physiological functions, if any, of the free 20S particles that are present in large amounts in many cells but appear to have little or no role in normal protein turnover.
Alterations of Proteasome Activity Upon Phosphorylation or Proteotoxic Disease
Most likely, each of the key steps in 26S function from initial conjugate binding to ATPase activation and proteolysis is regulated physiologically or can become impaired in disease. There is growing evidence that the proteasome's capacity to degrade ubiquitylated proteins is defective in major proteotoxic diseases. Decreased 26S activity has often been suggested in models of neurodegenerative disease (Dantuma and Bott, 2014) and even cardiac disease (Ranek et al., 2013). Although most early studies only assayed 20S peptidase activity, whose biological significance remains doubtful, clear loss of 26S activity has now been demonstrated in neurons infected by the prion protein, PrPsc, the cause of Bovine Spongiform Encephalitis (Mad Cow Disease) (Deriziotis et al., 2011), and in the brains of mice overproducing mutant tau in a model of Frontotemporal Dementia (Myeku et al., 2016). Overexpression of the disease-associated, aggregation-prone tau led to a decreased capacity of brain 26S to degrade Ub conjugates, ATP, and peptides (Myeku et al., 2016). As proteasome function decreased ubiquitylated proteins and model UPP substrates accumulated in neurons along with phospho-tau. Impaired 26S function by aggregation-prone proteins leading to inadequate protein homeostasis may represent a common feature of many proteotoxic diseases.
The recognition that proteasomes do not function at a maximal level and that their degradative capacity can be activated by post-synthetic modifications —especially phosphorylation —suggested rational new therapies to enhance the clearance of such toxic proteins. Proteomic studies have noted phosphorylation of many 26S subunits, and several protein kinases have been reported to stimulate proteasomal activity (Guo et al., 2017). For example, raising cAMP and activation of protein kinase A (PKA) causes phosphorylation of the 19S subunit Rpn6 (Lokireddy et al., 2015), which increases the rates of hydrolysis of peptides, ATP, and Ub conjugates (Lokireddy et al., 2015; Myeku et al., 2016). Rpn6 is a lid subunit that interacts with both the ATPases and the α-ring, which probably accounts for its ability to influence multiple 26S activities. This activation of proteasomes leads to enhanced breakdown only of short-lived cell proteins and thus a greater capacity to degrade misfolded proteins including several aggregation-prone mutant proteins known to cause neurodegenerative disease (Lokireddy et al., 2015; Myeku et al., 2016). Surprisingly, this phosphorylation does not promote degradation of long-lived proteins, which comprise the bulk of cell constituents. Although the mechanism by which the PKA-phosphorylated 26S can degrade misfolded proteins more rapidly is unclear, this capacity is of major pharmacological interest because of its potential to treat neurodegenerative diseases. In fact, Duff and coworkers showed that treatment of a mouse model of Frontotemporal Dementia with an agent that raised cAMP and activated brain proteasomes reversed the accumulation of phospho-tau and the failure of proteostasis (Myeku et al., 2016).
Because signal transduction by cAMP and PKA mediate the actions of many hormones and neurotransmitters and is critical in fasting and exercise, this type of proteasomal activation probably occurs frequently in vivo. In fact, Rpn6 phosphorylation and proteasome activation were recently observed upon treatment of hepatocytes with glucagon or epinephrine and heart with epinephrine, as well as in muscle upon intense exercise or after a brief fast (Lokireddy et al, submitted). These intriguing findings imply an enhanced cellular capacity to degrade short-lived misfolded and regulatory proteins. Perhaps proteasome activation, by enhancing the degradation of preexistent regulatory proteins, facilitates cellular adaptation to new conditions or after exercise the enhanced clearance of misfolded proteins may help remove proteins damaged by oxygen radicals or repeated contractions.
There is compelling evidence that other kinases activate proteasome function by subunit phosphorylation, including PKG in the mammalian heart (Ranek et al., 2013) and CamKII in neurons, which promotes proteasomal movement into dendrites (Bingol et al., 2010). These kinases were reported to modify the ATPase subunit Rpt6, but their effects on 26S activities and on protein turnover are still unclear. Further studies of these actions are needed not only for their mechanistic interest but because many pharmacological and physiological stimuli activate these ubiquitous kinases. In addition, proteasome activation by phosphorylation appears to regulate progression through the cell cycle. Many steps in the cell cycle are triggered by phosphorylation of cyclins or CDK inhibitors leading to their ubiquitylation and degradation. Guo et al recently demonstrated that phosphorylation of the ATPase subunit Rpt3 by DYRK2 also increases 26S activity and promotes progression through S-phase and cell proliferation (Guo et al., 2016).
The ability of PKA and DYRK2 to enhance the proteasome's capacity to degrade Ub conjugates implies either that a greater fraction of the ubiquitylated proteins that bind to the 26S are hydrolyzed, or that after tight binding, the rate-limiting step in substrate degradation is accelerated. It is unclear whether these different kinases activate the same proteasomal processes, since they appear to phosphorylate different 19S subunits. Quite different proteins are likely targeted for degradation by the growth-enhancing phosphorylation of Rpt3 by DYRK2 during S-phase and the exercise-induced phosphorylation of Rpn6 by PKA, which eliminates misfolded proteins in non-dividing tissues.
These findings with cAMP also indicate that even in non-proliferating cells, the capacity to eliminate misfolded, potentially toxic proteins does not normally operate at its maximal level, as had been generally assumed. Since the rapid degradation of such proteins is of clear selective advantage and is important for longevity (Hartl et al., 2011), there must be negative consequences if proteasomes always functioned with maximal efficiency, perhaps by causing excessive degradation of key proteins.
In addition to post-synthetic mechanisms to activate proteasomes rapidly, there are slower adaptive mechanisms that increase proteasome abundance to meet physiological demand. In yeast, a simple feedback system exists in which the expression of 26S genes is activated by the short-lived transcription factor, Rpn4 (Xie and Varshavsky, 2001). If proteasome function is impaired, Rpn4 accumulates and stimulates proteasome production. A more complex feedback mechanism functions in higher eukaryotes where proteasome inhibitors stimulate production of new 26S and the p97/VCP complex by activating the transcription factor, Nrf1 (Radhakrishnan et al., 2010; Sha and Goldberg, 2014; Steffen et al., 2010). This short-lived protein is expressed as a larger precursor fixed to the ER. When proteasomes are inhibited, Nrf1 is cleaved by the endoprotease Ddi2 (Koizumi et al., 2016; Lehrbach and Ruvkun, 2016; Sha and Goldberg, 2016). The released fragment enters the nucleus and induces expression of all 26S subunits, p97, and its cofactors.
This mechanism is of medical interest, because expression of new proteasomes may limit the efficacy of proteasome inhibitors in multiple myeloma patients. However, such regulation of proteasome content clearly did not evolve to allow cells to escape the effects of bortezomib or MG132. Most likely, the production of new 26S represents a mechanism to withstand disease processes where aggregation-prone proteins impair proteasome activity. For example, in mouse models of β-thalassemia caused by an imbalance of hemoglobin subunits (Khandros et al., 2012) or Charcot Marie Tooth disease due to myelin mutations (VerPlank, 2017), 26S function is impaired, but the cells partially compensate by increasing Nrf1 processing and proteasome production. Despite its importance, it is still not understood how inadequate proteasome function is detected and how Ddi2-dependent processing of Nrf1 is activated.
Concluding Thoughts
Among the fundamental insights emerging from these recent developments are that some, perhaps many, of the ubiquitylated proteins that bind to the proteasome are deubiquitylated and escape proteolysis, and that proteasomal degradation, like ubiquitylation, depends on structural features of the substrate that determine whether the 26S degrades or only deubiquitylates and releases the protein. Thus, the simple view of ubiquitylation as a death sentence for proteins is obsolete. A more appropriate analogy is that the cell's ubiquitylation machinery functions as a policing system that arrests proteins, while the proteasome serves as a combination of discerning judge and efficient executioner.
The control of degradative rates through changes in proteasome activity and not just through control of substrate ubiquitylation indicates a new and generally unappreciated mode of cell regulation. The various studies discussed here emphasize that proteasome function is regulated at multiple levels. This recent work has illuminated several inherent mechanisms that coordinate deubiquitylation, ATP hydrolysis, and proteolysis. In the absence of a substrate, Usp14 helps to maintain the 26S in a latent state, minimizing wasteful hydrolysis of ATP and non-selective protein degradation until the 26S binds a ubiquitylated protein. Then multiple changes occur: this basal inhibition is reversed, more cytosolic Usp14 and Ube3c associate with the 26S particle, and the interaction of the Ub chain with Usp14 (or Uch37) activates the particle's ATPase and proteolytic capacity, all of which favor the selective and efficient degradation of ubiquitylated proteins (Fig 3).
In addition to these inherent forms of regulation, cells contain many mechanisms that modulate 26S activity (Fig 4). Several protein kinases can rapidly enhance 26S activities and stimulate the breakdown of ubiquitylated proteins in vivo. Moreover, the association of activating proteins and complexes seem to enable the proteasome to serve specialized roles e.g. when proteolysis increases in proteotoxic stress (Stanhill et al., 2006) or muscle atrophy (Hishiya et al., 2006).
Fig 4.
The two levels for regulating degradation by the UPP, substrate-specific ubiquitylation and global control at the proteasome, seem analogous to the two levels for controlling protein expression —the transcription of specific genes and the more global control of translation. These multiple levels of regulation provide the organism with alternative ways to tailor the proteome to different conditions. Accelerated breakdown of preexistent proteins allows for more rapid adaptations than just changing synthesis, but it is not yet clear what is the relative importance of ubiquitylation or proteasome function in the degradation of specific proteins under different conditions. It is also uncertain how these different kinases and activating factors alter protein turnover rates. Perhaps the phosphorylation of different 19S subunits or cofactors alters the particle's recognition and processing of certain ubiquitylated proteins. Alternatively, when proteasomes are activated by these kinases, selective degradation of some proteins may result from the simultaneous stimulation of their ubiquitylation. Clearly, the recognition of the cellular importance of proteasome regulation raises many fundamental mechanistic questions, and the recent advances in our understanding of proteasome mechanisms provide a strong basis for resolving these outstanding issues.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- Asano S, Fukuda Y, Beck F, Aufderheide A, Förster F, Danev R, Baumeister W. Proteasomes. A molecular census of 26S proteasomes in intact neurons. Science. 2015;347:439–442. doi: 10.1126/science.1261197. [DOI] [PubMed] [Google Scholar]
- Aufderheide A, Beck F, Stengel F, Hartwig M, Schweitzer A, Pfeifer G, Goldberg AL, Sakata E, Baumeister W, Förster F. Structural characterization of the interaction of Ubp6 with the 26S proteasome. Proc Natl Acad Sci U S A. 2015;112:8626–8631. doi: 10.1073/pnas.1510449112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviram S, Kornitzer D. The ubiquitin ligase Hul5 promotes proteasomal processivity. Mol Cell Biol. 2010;30:985–994. doi: 10.1128/MCB.00909-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajorek M, Finley D, Glickman MH. Proteasome disassembly and downregulation is correlated with viability during stationary phase. Curr Biol. 2003;13:1140–1144. doi: 10.1016/s0960-9822(03)00417-2. [DOI] [PubMed] [Google Scholar]
- Bashore C, Dambacher CM, Goodall EA, Matyskiela ME, Lander GC, Martin A. Ubp6 deubiquitinase controls conformational dynamics and substrate degradation of the 26S proteasome. Nat Struct Mol Biol. 2015;22:712–719. doi: 10.1038/nsmb.3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck F, Unverdorben P, Bohn S, Schweitzer A, Pfeifer G, Sakata E, Nickell S, Plitzko JM, Villa E, Baumeister W, et al. Near-atomic resolution structural model of the yeast 26S proteasome. Proc Natl Acad Sci U S A. 2012;109:14870–14875. doi: 10.1073/pnas.1213333109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckwith R, Estrin E, Worden EJ, Martin A. Reconstitution of the 26S proteasome reveals functional asymmetries in its AAA+ unfoldase. Nat Struct Mol Biol. 2013;20:1164–1172. doi: 10.1038/nsmb.2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berko D, Herkon O, Braunstein I, Isakov E, David Y, Ziv T, Navon A, Stanhill A. Inherent asymmetry in the 26S proteasome is defined by the ubiquitin receptor RPN13. J Biol Chem. 2014;289:5609–5618. doi: 10.1074/jbc.M113.509380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berko D, Tabachnick-Cherny S, Shental-Bechor D, Cascio P, Mioletti S, Levy Y, Admon A, Ziv T, Tirosh B, Goldberg AL, et al. The direction of protein entry into the proteasome determines the variety of products and depends on the force needed to unfold its two termini. Mol Cell. 2012;48:601–611. doi: 10.1016/j.molcel.2012.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besche HC, Sha Z, Kukushkin NV, Peth A, Hock EM, Kim W, Gygi S, Gutierrez JA, Liao H, Dick L, et al. Autoubiquitination of the 26S proteasome on Rpn13 regulates breakdown of ubiquitin conjugates. EMBO J. 2014;33:1159–1176. doi: 10.1002/embj.201386906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingol B, Wang CF, Arnott D, Cheng D, Peng J, Sheng M. Autophosphorylated CaMKIIalpha acts as a scaffold to recruit proteasomes to dendritic spines. Cell. 2010;140:567–578. doi: 10.1016/j.cell.2010.01.024. [DOI] [PubMed] [Google Scholar]
- Borodovsky A, Kessler BM, Casagrande R, Overkleeft HS, Wilkinson KD, Ploegh HL. A novel active site-directed probe specific for deubiquitylating enzymes reveals proteasome association of USP14. EMBO J. 2001;20:5187–5196. doi: 10.1093/emboj/20.18.5187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braten O, Livneh I, Ziv T, Admon A, Kehat I, Caspi LH, Gonen H, Bercovich B, Godzik A, Jahandideh S, et al. Numerous proteins with unique characteristics are degraded by the 26S proteasome following monoubiquitination. Proc Natl Acad Sci U S A. 2016;113:E4639–4647. doi: 10.1073/pnas.1608644113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen PC, Bhattacharyya BJ, Hanna J, Minkel H, Wilson JA, Finley D, Miller RJ, Wilson SM. Ubiquitin homeostasis is critical for synaptic development and function. J Neurosci. 2011;31:17505–17513. doi: 10.1523/JNEUROSCI.2922-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Wu J, Lu Y, Ma YB, Lee BH, Yu Z, Ouyang Q, Finley DJ, Kirschner MW, Mao Y. Structural basis for dynamic regulation of the human 26S proteasome. Proc Natl Acad Sci U S A. 2016;113:12991–12996. doi: 10.1073/pnas.1614614113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernova TA, Allen KD, Wesoloski LM, Shanks JR, Chernoff YO, Wilkinson KD. Pleiotropic effects of Ubp6 loss on drug sensitivities and yeast prion are due to depletion of the free ubiquitin pool. J Biol Chem. 2003;278:52102–52115. doi: 10.1074/jbc.M310283200. [DOI] [PubMed] [Google Scholar]
- Choi WH, de Poot SA, Lee JH, Kim JH, Han DH, Kim YK, Finley D, Lee MJ. Open-gate mutants of the mammalian proteasome show enhanced ubiquitin-conjugate degradation. Nat Commun. 2016;7:10963. doi: 10.1038/ncomms10963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffino P, Too PH, Erales J. Slippery substrates impair ATP-dependent protease function by slowing unfolding. J Biol Chem. 2014;289:3826. doi: 10.1074/jbc.L113.532622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen-Kaplan V, Livneh I, Avni N, Fabre B, Ziv T, Kwon YT, Ciechanover A. p62- and ubiquitin-dependent stress-induced autophagy of the mammalian 26S proteasome. Proc Natl Acad Sci U S A. 2016;113:E7490–E7499. doi: 10.1073/pnas.1615455113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosas B, Hanna J, Kirkpatrick DS, Zhang DP, Tone Y, Hathaway NA, Buecker C, Leggett DS, Schmidt M, King RW, et al. Ubiquitin chains are remodeled at the proteasome by opposing ubiquitin ligase and deubiquitinating activities. Cell. 2006;127:1401–1413. doi: 10.1016/j.cell.2006.09.051. [DOI] [PubMed] [Google Scholar]
- Dambacher CM, Worden EJ, Herzik MA, Martin A, Lander GC. Atomic structure of the 26S proteasome lid reveals the mechanism of deubiquitinase inhibition. Elife. 2016;5:e13027. doi: 10.7554/eLife.13027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantuma NP, Bott LC. The ubiquitin-proteasome system in neurodegenerative diseases: precipitating factor, yet part of the solution. Front Mol Neurosci. 2014;7:70. doi: 10.3389/fnmol.2014.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayal S, Sparks A, Jacob J, Allende-Vega N, Lane DP, Saville MK. Suppression of the deubiquitinating enzyme USP5 causes the accumulation of unanchored polyubiquitin and the activation of p53. J Biol Chem. 2009;284:5030–5041. doi: 10.1074/jbc.M805871200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng HX, Chen W, Hong ST, Boycott KM, Gorrie GH, Siddique N, Yang Y, Fecto F, Shi Y, Zhai H, et al. Mutations in UBQLN2 cause dominant X-linked juvenile and adult-onset ALS and ALS/dementia. Nature. 2011;477:211–215. doi: 10.1038/nature10353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deriziotis P, André R, Smith DM, Goold R, Kinghorn KJ, Kristiansen M, Nathan JA, Rosenzweig R, Krutauz D, Glickman MH, et al. Misfolded PrP impairs the UPS by interaction with the 20S proteasome and inhibition of substrate entry. EMBO J. 2011;30:3065–3077. doi: 10.1038/emboj.2011.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deveraux Q, Ustrell V, Pickart C, Rechsteiner M. A 26 S protease subunit that binds ubiquitin conjugates. J Biol Chem. 1994;269:7059–7061. [PubMed] [Google Scholar]
- Dimova NV, Hathaway NA, Lee BH, Kirkpatrick DS, Berkowitz ML, Gygi SP, Finley D, King RW. APC/C-mediated multiple monoubiquitylation provides an alternative degradation signal for cyclin B1. Nat Cell Biol. 2012;14:168–176. doi: 10.1038/ncb2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Z, Fu Z, Xu C, Wang Y, Li J, Kong L, Chen J, Li N, Zhang R, Cong Y. High-resolution cryo-EM structure of the proteasome in complex with ADP-AlFx. Cell Res. 2017 doi: 10.1038/cr.2017.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsasser S, Gali RR, Schwickart M, Larsen CN, Leggett DS, Müller B, Feng MT, Tübing F, Dittmar GA, Finley D. Proteasome subunit Rpn1 binds ubiquitin-like protein domains. Nat Cell Biol. 2002;4:725–730. doi: 10.1038/ncb845. [DOI] [PubMed] [Google Scholar]
- Erales J, Hoyt MA, Troll F, Coffino P. Functional asymmetries of proteasome translocase pore. J Biol Chem. 2012;287:18535–18543. doi: 10.1074/jbc.M112.357327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finley D, Chen X, Walters KJ. Gates, Channels, and Switches: Elements of the Proteasome Machine. Trends Biochem Sci. 2016;41:77–93. doi: 10.1016/j.tibs.2015.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg AL. Development of proteasome inhibitors as research tools and cancer drugs. J Cell Biol. 2012;199:583–588. doi: 10.1083/jcb.201210077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg AL, Dice JF. Intracellular protein degradation in mammalian and bacterial cells. Annu Rev Biochem. 1974;43:835–869. doi: 10.1146/annurev.bi.43.070174.004155. [DOI] [PubMed] [Google Scholar]
- Gomez TA, Kolawa N, Gee M, Sweredoski MJ, Deshaies RJ. Identification of a functional docking site in the Rpn1 LRR domain for the UBA-UBL domain protein Ddi1. BMC Biol. 2011;9:33. doi: 10.1186/1741-7007-9-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groll M, Bajorek M, Köhler A, Moroder L, Rubin DM, Huber R, Glickman MH, Finley D. A gated channel into the proteasome core particle. Nat Struct Biol. 2000;7:1062–1067. doi: 10.1038/80992. [DOI] [PubMed] [Google Scholar]
- Guo X, Huang X, Chen MJ. Reversible phosphorylation of the 26S proteasome. Protein Cell. 2017;8:255–272. doi: 10.1007/s13238-017-0382-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X, Wang X, Wang Z, Banerjee S, Yang J, Huang L, Dixon JE. Site-specific proteasome phosphorylation controls cell proliferation and tumorigenesis. Nat Cell Biol. 2016;18:202–212. doi: 10.1038/ncb3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamazaki J, Hirayama S, Murata S. Redundant Roles of Rpn10 and Rpn13 in Recognition of Ubiquitinated Proteins and Cellular Homeostasis. PLoS Genet. 2015;11:e1005401. doi: 10.1371/journal.pgen.1005401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna J, Hathaway NA, Tone Y, Crosas B, Elsasser S, Kirkpatrick DS, Leggett DS, Gygi SP, King RW, Finley D. Deubiquitinating enzyme Ubp6 functions noncatalytically to delay proteasomal degradation. Cell. 2006;127:99–111. doi: 10.1016/j.cell.2006.07.038. [DOI] [PubMed] [Google Scholar]
- Hanna J, Leggett DS, Finley D. Ubiquitin depletion as a key mediator of toxicity by translational inhibitors. Mol Cell Biol. 2003;23:9251–9261. doi: 10.1128/MCB.23.24.9251-9261.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartl FU, Bracher A, Hayer-Hartl M. Molecular chaperones in protein folding and proteostasis. Nature. 2011;475:324–332. doi: 10.1038/nature10317. [DOI] [PubMed] [Google Scholar]
- Hershko A, Ciechanover A, Heller H, Haas AL, Rose IA. Proposed role of ATP in protein breakdown: conjugation of protein with multiple chains of the polypeptide of ATP-dependent proteolysis. Proc Natl Acad Sci U S A. 1980;77:1783–1786. doi: 10.1073/pnas.77.4.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinnerwisch J, Fenton WA, Furtak KJ, Farr GW, Horwich AL. Loops in the central channel of ClpA chaperone mediate protein binding, unfolding, and translocation. Cell. 2005;121:1029–1041. doi: 10.1016/j.cell.2005.04.012. [DOI] [PubMed] [Google Scholar]
- Hishiya A, Iemura S, Natsume T, Takayama S, Ikeda K, Watanabe K. A novel ubiquitin-binding protein ZNF216 functioning in muscle atrophy. EMBO J. 2006;25:554–564. doi: 10.1038/sj.emboj.7600945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann RM, Pickart CM. In vitro assembly and recognition of Lys-63 polyubiquitin chains. J Biol Chem. 2001;276:27936–27943. doi: 10.1074/jbc.M103378200. [DOI] [PubMed] [Google Scholar]
- Hough R, Pratt G, Rechsteiner M. Purification of two high molecular weight proteases from rabbit reticulocyte lysate. J Biol Chem. 1987;262:8303–8313. [PubMed] [Google Scholar]
- Huang X, Luan B, Wu J, Shi Y. An atomic structure of the human 26S proteasome. Nat Struct Mol Biol. 2016;23:778–785. doi: 10.1038/nsmb.3273. [DOI] [PubMed] [Google Scholar]
- Husnjak K, Elsasser S, Zhang N, Chen X, Randles L, Shi Y, Hofmann K, Walters KJ, Finley D, Dikic I. Proteasome subunit Rpn13 is a novel ubiquitin receptor. Nature. 2008;453:481–488. doi: 10.1038/nature06926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inobe T, Matouschek A. Paradigms of protein degradation by the proteasome. Curr Opin Struct Biol. 2014;24:156–164. doi: 10.1016/j.sbi.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isasa M, Katz EJ, Kim W, Yugo V, González S, Kirkpatrick DS, Thomson TM, Finley D, Gygi SP, Crosas B. Monoubiquitination of RPN10 regulates substrate recruitment to the proteasome. Mol Cell. 2010;38:733–745. doi: 10.1016/j.molcel.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itakura E, Zavodszky E, Shao S, Wohlever ML, Keenan RJ, Hegde RS. Ubiquilins Chaperone and Triage Mitochondrial Membrane Proteins for Degradation. Mol Cell. 2016;63:21–33. doi: 10.1016/j.molcel.2016.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson AD, MacFadden A, Wu Z, Peng J, Liu CW. Autoregulation of the 26S proteasome by in situ ubiquitination. Mol Biol Cell. 2014;25:1824–1835. doi: 10.1091/mbc.E13-10-0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston JA, Johnson ES, Waller PR, Varshavsky A. Methotrexate inhibits proteolysis of dihydrofolate reductase by the N-end rule pathway. J Biol Chem. 1995;270:8172–8178. doi: 10.1074/jbc.270.14.8172. [DOI] [PubMed] [Google Scholar]
- Kaiser SE, Riley BE, Shaler TA, Trevino RS, Becker CH, Schulman H, Kopito RR. Protein standard absolute quantification (PSAQ) method for the measurement of cellular ubiquitin pools. Nat Methods. 2011;8:691–696. doi: 10.1038/nmeth.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenniston JA, Baker TA, Fernandez JM, Sauer RT. Linkage between ATP consumption and mechanical unfolding during the protein processing reactions of an AAA+ degradation machine. Cell. 2003;114:511–520. doi: 10.1016/s0092-8674(03)00612-3. [DOI] [PubMed] [Google Scholar]
- Khandros E, Thom CS, D'Souza J, Weiss MJ. Integrated protein quality-control pathways regulate free α-globin in murine β-thalassemia. Blood. 2012;119:5265–5275. doi: 10.1182/blood-2011-12-397729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HT, Kim KP, Uchiki T, Gygi SP, Goldberg AL. S5a promotes protein degradation by blocking synthesis of nondegradable forked ubiquitin chains. EMBO J. 2009;28:1867–1877. doi: 10.1038/emboj.2009.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YC, Snoberger A, Schupp J, Smith DM. ATP binding to neighbouring subunits and intersubunit allosteric coupling underlie proteasomal ATPase function. Nat Commun. 2015;6:8520. doi: 10.1038/ncomms9520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisselev AF, Akopian TN, Woo KM, Goldberg AL. The sizes of peptides generated from protein by mammalian 26 and 20 S proteasomes. Implications for understanding the degradative mechanism and antigen presentation. J Biol Chem. 1999;274:3363–3371. doi: 10.1074/jbc.274.6.3363. [DOI] [PubMed] [Google Scholar]
- Koegl M, Hoppe T, Schlenker S, Ulrich HD, Mayer TU, Jentsch S. A novel ubiquitination factor, E4, is involved in multiubiquitin chain assembly. Cell. 1999;96:635–644. doi: 10.1016/s0092-8674(00)80574-7. [DOI] [PubMed] [Google Scholar]
- Koizumi S, Irie T, Hirayama S, Sakurai Y, Yashiroda H, Naguro I, Ichijo H, Hamazaki J, Murata S. The aspartyl protease DDI2 activates Nrf1 to compensate for proteasome dysfunction. Elife. 2016;5 doi: 10.7554/eLife.18357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraut DA. Slippery substrates impair ATP-dependent protease function by slowing unfolding. J Biol Chem. 2013;288:34729–34735. doi: 10.1074/jbc.M113.512533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraut DA, Israeli E, Schrader EK, Patil A, Nakai K, Nanavati D, Inobe T, Matouschek A. Sequence- and species-dependence of proteasomal processivity. ACS Chem Biol. 2012;7:1444–1453. doi: 10.1021/cb3001155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo CL, Goldberg AL. Proc Natl Acad Sci U S A. 2017. Ubiquitinated proteins promote the association of proteasomes with the deubiquitinating enzyme Usp14 and the ubiquitin ligase Ube3c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lander GC, Estrin E, Matyskiela ME, Bashore C, Nogales E, Martin A. Complete subunit architecture of the proteasome regulatory particle. Nature. 2012;482:186–191. doi: 10.1038/nature10774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lander GC, Martin A, Nogales E. The proteasome under the microscope: the regulatory particle in focus. Curr Opin Struct Biol. 2013;23:243–251. doi: 10.1016/j.sbi.2013.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BH, Lee MJ, Park S, Oh DC, Elsasser S, Chen PC, Gartner C, Dimova N, Hanna J, Gygi SP, et al. Enhancement of proteasome activity by a small-molecule inhibitor of USP14. Nature. 2010;467:179–184. doi: 10.1038/nature09299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BH, Lu Y, Prado MA, Shi Y, Tian G, Sun S, Elsasser S, Gygi SP, King RW, Finley D. USP14 deubiquitinates proteasome-bound substrates that are ubiquitinated at multiple sites. Nature. 2016;532:398–401. doi: 10.1038/nature17433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C, Schwartz MP, Prakash S, Iwakura M, Matouschek A. ATP-dependent proteases degrade their substrates by processively unraveling them from the degradation signal. Mol Cell. 2001;7:627–637. doi: 10.1016/s1097-2765(01)00209-x. [DOI] [PubMed] [Google Scholar]
- Leggett DS, Hanna J, Borodovsky A, Crosas B, Schmidt M, Baker RT, Walz T, Ploegh H, Finley D. Multiple associated proteins regulate proteasome structure and function. Mol Cell. 2002;10:495–507. doi: 10.1016/s1097-2765(02)00638-x. [DOI] [PubMed] [Google Scholar]
- Lehrbach NJ, Ruvkun G. Proteasome dysfunction triggers activation of SKN-1A/Nrf1 by the aspartic protease DDI-1. Elife. 2016;5 doi: 10.7554/eLife.17721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Liu W, Ye Y, Li W. Ufd2p synthesizes branched ubiquitin chains to promote the degradation of substrates modified with atypical chains. Nat Commun. 2017;8:14274. doi: 10.1038/ncomms14274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CW, Jacobson AD. Functions of the 19S complex in proteasomal degradation. Trends Biochem Sci. 2013;38:103–110. doi: 10.1016/j.tibs.2012.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livneh I, Cohen-Kaplan V, Cohen-Rosenzweig C, Avni N, Ciechanover A. The life cycle of the 26S proteasome: from birth, through regulation and function, and onto its death. Cell Res. 2016;26:869–885. doi: 10.1038/cr.2016.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lokireddy S, Kukushkin NV, Goldberg AL. cAMP-induced phosphorylation of 26S proteasomes on Rpn6/PSMD11 enhances their activity and the degradation of misfolded proteins. Proc Natl Acad Sci U S A. 2015;112:E7176–7185. doi: 10.1073/pnas.1522332112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Lee BH, King RW, Finley D, Kirschner MW. Substrate degradation by the proteasome: a single-molecule kinetic analysis. Science. 2015;348:1250834. doi: 10.1126/science.1250834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma CP, Slaughter CA, DeMartino GN. Identification, purification, and characterization of a protein activator (PA28) of the 20 S proteasome (macropain) J Biol Chem. 1992;267:10515–10523. [PubMed] [Google Scholar]
- Marshall RS, Li F, Gemperline DC, Book AJ, Vierstra RD. Autophagic Degradation of the 26S Proteasome Is Mediated by the Dual ATG8/Ubiquitin Receptor RPN10 in Arabidopsis. Mol Cell. 2015;58:1053–1066. doi: 10.1016/j.molcel.2015.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall RS, McLoughlin F, Vierstra RD. Autophagic Turnover of Inactive 26S Proteasomes in Yeast Is Directed by the Ubiquitin Receptor Cue5 and the Hsp42 Chaperone. Cell Rep. 2016;16:1717–1732. doi: 10.1016/j.celrep.2016.07.015. [DOI] [PubMed] [Google Scholar]
- Matyskiela ME, Lander GC, Martin A. Conformational switching of the 26S proteasome enables substrate degradation. Nat Struct Mol Biol. 2013;20:781–788. doi: 10.1038/nsmb.2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McShane E, Sin C, Zauber H, Wells JN, Donnelly N, Wang X, Hou J, Chen W, Storchova Z, Marsh JA, et al. Kinetic Analysis of Protein Stability Reveals Age-Dependent Degradation. Cell. 2016;167:803–815.e821. doi: 10.1016/j.cell.2016.09.015. [DOI] [PubMed] [Google Scholar]
- Meyer HJ, Rape M. Enhanced protein degradation by branched ubiquitin chains. Cell. 2014;157:910–921. doi: 10.1016/j.cell.2014.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myeku N, Clelland CL, Emrani S, Kukushkin NV, Yu WH, Goldberg AL, Duff KE. Tau-driven 26S proteasome impairment and cognitive dysfunction can be prevented early in disease by activating cAMP-PKA signaling. Nat Med. 2016;22:46–53. doi: 10.1038/nm.4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan JA, Kim HT, Ting L, Gygi SP, Goldberg AL. Why do cellular proteins linked to K63-polyubiquitin chains not associate with proteasomes? EMBO J. 2013;32:552–565. doi: 10.1038/emboj.2012.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega J, Heymann JB, Kajava AV, Ustrell V, Rechsteiner M, Steven AC. The axial channel of the 20S proteasome opens upon binding of the PA200 activator. J Mol Biol. 2005;346:1221–1227. doi: 10.1016/j.jmb.2004.12.049. [DOI] [PubMed] [Google Scholar]
- Paraskevopoulos K, Kriegenburg F, Tatham MH, Rösner HI, Medina B, Larsen IB, Brandstrup R, Hardwick KG, Hay RT, Kragelund BB, et al. Dss1 is a 26S proteasome ubiquitin receptor. Mol Cell. 2014;56:453–461. doi: 10.1016/j.molcel.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peth A, Besche HC, Goldberg AL. Ubiquitinated proteins activate the proteasome by binding to Usp14/Ubp6, which causes 20S gate opening. Mol Cell. 2009;36:794–804. doi: 10.1016/j.molcel.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peth A, Kukushkin N, Bossé M, Goldberg AL. Ubiquitinated proteins activate the proteasomal ATPases by binding to Usp14 or Uch37 homologs. J Biol Chem. 2013a;288:7781–7790. doi: 10.1074/jbc.M112.441907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peth A, Nathan JA, Goldberg AL. The ATP costs and time required to degrade ubiquitinated proteins by the 26 S proteasome. J Biol Chem. 2013b;288:29215–29222. doi: 10.1074/jbc.M113.482570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peth A, Uchiki T, Goldberg AL. ATP-dependent steps in the binding of ubiquitin conjugates to the 26S proteasome that commit to degradation. Mol Cell. 2010;40:671–681. doi: 10.1016/j.molcel.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piwko W, Jentsch S. Proteasome-mediated protein processing by bidirectional degradation initiated from an internal site. Nat Struct Mol Biol. 2006;13:691–697. doi: 10.1038/nsmb1122. [DOI] [PubMed] [Google Scholar]
- Prakash S, Tian L, Ratliff KS, Lehotzky RE, Matouschek A. An unstructured initiation site is required for efficient proteasome-mediated degradation. Nat Struct Mol Biol. 2004;11:830–837. doi: 10.1038/nsmb814. [DOI] [PubMed] [Google Scholar]
- Qian MX, Pang Y, Liu CH, Haratake K, Du BY, Ji DY, Wang GF, Zhu QQ, Song W, Yu Y, et al. Acetylation-mediated proteasomal degradation of core histones during DNA repair and spermatogenesis. Cell. 2013;153:1012–1024. doi: 10.1016/j.cell.2013.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu XB, Ouyang SY, Li CJ, Miao S, Wang L, Goldberg AL. hRpn13/ADRM1/GP110 is a novel proteasome subunit that binds the deubiquitinating enzyme, UCH37. EMBO J. 2006;25:5742–5753. doi: 10.1038/sj.emboj.7601450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabl J, Smith DM, Yu Y, Chang SC, Goldberg AL, Cheng Y. Mechanism of gate opening in the 20S proteasome by the proteasomal ATPases. Mol Cell. 2008;30:360–368. doi: 10.1016/j.molcel.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radhakrishnan SK, Lee CS, Young P, Beskow A, Chan JY, Deshaies RJ. Transcription factor Nrf1 mediates the proteasome recovery pathway after proteasome inhibition in mammalian cells. Mol Cell. 2010;38:17–28. doi: 10.1016/j.molcel.2010.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranek MJ, Terpstra EJ, Li J, Kass DA, Wang X. Protein kinase g positively regulates proteasome-mediated degradation of misfolded proteins. Circulation. 2013;128:365–376. doi: 10.1161/CIRCULATIONAHA.113.001971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Aliaga P, Ramirez L, Kim F, Bustamante C, Martin A. Substrate-translocating loops regulate mechanochemical coupling and power production in AAA+ protease ClpXP. Nat Struct Mol Biol. 2016;23:974–981. doi: 10.1038/nsmb.3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin DM, van Nocker S, Glickman M, Coux O, Wefes I, Sadis S, Fu H, Goldberg A, Vierstra R, Finley D. ATPase and ubiquitin-binding proteins of the yeast proteasome. Mol Biol Rep. 1997;24:17–26. doi: 10.1023/a:1006844305067. [DOI] [PubMed] [Google Scholar]
- Scanlon TC, Gottlieb B, Durcan TM, Fon EA, Beitel LK, Trifiro MA. Isolation of human proteasomes and putative proteasome-interacting proteins using a novel affinity chromatography method. Exp Cell Res. 2009;315:176–189. doi: 10.1016/j.yexcr.2008.10.027. [DOI] [PubMed] [Google Scholar]
- Schweitzer A, Aufderheide A, Rudack T, Beck F, Pfeifer G, Plitzko JM, Sakata E, Schulten K, Förster F, Baumeister W. Structure of the human 26S proteasome at a resolution of 3.9 Å. Proc Natl Acad Sci U S A. 2016;113:7816–7821. doi: 10.1073/pnas.1608050113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott DC, Rhee DY, Duda DM, Kelsall IR, Olszewski JL, Paulo JA, de Jong A, Ovaa H, Alpi AF, Harper JW, et al. Two Distinct Types of E3 Ligases Work in Unison to Regulate Substrate Ubiquitylation. Cell. 2016;166:1198–1214.e1124. doi: 10.1016/j.cell.2016.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sha Z, Goldberg AL. Proteasome-mediated processing of Nrf1 is essential for coordinate induction of all proteasome subunits and p97. Curr Biol. 2014;24:1573–1583. doi: 10.1016/j.cub.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sha Z, Goldberg AL. Reply to Vangala et al.: Complete inhibition of the proteasome reduces new proteasome production by causing Nrf1 aggregation. Curr Biol. 2016;26:R836–837. doi: 10.1016/j.cub.2016.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Chen X, Elsasser S, Stocks BB, Tian G, Lee BH, Zhang N, de Poot SA, Tuebing F, Sun S, et al. Rpn1 provides adjacent receptor sites for substrate binding and deubiquitination by the proteasome. Science. 2016;351 doi: 10.1126/science.aad9421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh SK, Sahu I, Mali SM, Hemantha HP, Kleifeld O, Glickman MH, Brik A. Synthetic Uncleavable Ubiquitinated Proteins Dissect Proteasome Deubiquitination and Degradation, and Highlight Distinctive Fate of Tetraubiquitin. J Am Chem Soc. 2016;138:16004–16015. doi: 10.1021/jacs.6b09611. [DOI] [PubMed] [Google Scholar]
- Śledź P, Unverdorben P, Beck F, Pfeifer G, Schweitzer A, Förster F, Baumeister W. Structure of the 26S proteasome with ATP-γS bound provides insights into the mechanism of nucleotide-dependent substrate translocation. Proc Natl Acad Sci U S A. 2013;110:7264–7269. doi: 10.1073/pnas.1305782110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DM, Chang SC, Park S, Finley D, Cheng Y, Goldberg AL. Docking of the proteasomal ATPases' carboxyl termini in the 20S proteasome's alpha ring opens the gate for substrate entry. Mol Cell. 2007;27:731–744. doi: 10.1016/j.molcel.2007.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DM, Fraga H, Reis C, Kafri G, Goldberg AL. ATP binds to proteasomal ATPases in pairs with distinct functional effects, implying an ordered reaction cycle. Cell. 2011;144:526–538. doi: 10.1016/j.cell.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DM, Kafri G, Cheng Y, Ng D, Walz T, Goldberg AL. ATP binding to PAN or the 26S ATPases causes association with the 20S proteasome, gate opening, and translocation of unfolded proteins. Mol Cell. 2005;20:687–698. doi: 10.1016/j.molcel.2005.10.019. [DOI] [PubMed] [Google Scholar]
- Snoberger A, Anderson RT, Smith DM. The Proteasomal ATPases use a slow moving but high power strategy to unfold proteins. Frontiers in Molecular Biosciences. 2017 doi: 10.3389/fmolb.2017.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanhill A, Haynes CM, Zhang Y, Min G, Steele MC, Kalinina J, Martinez E, Pickart CM, Kong XP, Ron D. An arsenite-inducible 19S regulatory particle-associated protein adapts proteasomes to proteotoxicity. Mol Cell. 2006;23:875–885. doi: 10.1016/j.molcel.2006.07.023. [DOI] [PubMed] [Google Scholar]
- Steffen J, Seeger M, Koch A, Krüger E. Proteasomal degradation is transcriptionally controlled by TCF11 via an ERAD-dependent feedback loop. Mol Cell. 2010;40:147–158. doi: 10.1016/j.molcel.2010.09.012. [DOI] [PubMed] [Google Scholar]
- Stone M, Hartmann-Petersen R, Seeger M, Bech-Otschir D, Wallace M, Gordon C. Uch2/Uch37 is the major deubiquitinating enzyme associated with the 26S proteasome in fission yeast. J Mol Biol. 2004;344:697–706. doi: 10.1016/j.jmb.2004.09.057. [DOI] [PubMed] [Google Scholar]
- Striebel F, Kress W, Weber-Ban E. Controlled destruction: AAA+ ATPases in protein degradation from bacteria to eukaryotes. Curr Opin Struct Biol. 2009;19:209–217. doi: 10.1016/j.sbi.2009.02.006. [DOI] [PubMed] [Google Scholar]
- Thrower JS, Hoffman L, Rechsteiner M, Pickart CM. Recognition of the polyubiquitin proteolytic signal. EMBO J. 2000;19:94–102. doi: 10.1093/emboj/19.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomko RJ, Hochstrasser M. Molecular architecture and assembly of the eukaryotic proteasome. Annu Rev Biochem. 2013;82:415–445. doi: 10.1146/annurev-biochem-060410-150257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchiya H, Arai N, Kaiho A, Ohtake F, Tanaka K, Saeki Y. A comprehensive analysis of ubiquitin-binding proteins reveals that Cdc48 is the Lys48 linkage specific gatekeeper for proteasomal degradation. Mol Cell 2017 [Google Scholar]
- van der Lee R, Lang B, Kruse K, Gsponer J, Sánchez de Groot N, Huynen MA, Matouschek A, Fuxreiter M, Babu MM. Intrinsically disordered segments affect protein half-life in the cell and during evolution. Cell Rep. 2014;8:1832–1844. doi: 10.1016/j.celrep.2014.07.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatraman P, Wetzel R, Tanaka M, Nukina N, Goldberg AL. Eukaryotic proteasomes cannot digest polyglutamine sequences and release them during degradation of polyglutamine-containing proteins. Mol Cell. 2004;14:95–104. doi: 10.1016/s1097-2765(04)00151-0. [DOI] [PubMed] [Google Scholar]
- Verma R, Aravind L, Oania R, McDonald WH, Yates JR, Koonin EV, Deshaies RJ. Role of Rpn11 metalloprotease in deubiquitination and degradation by the 26S proteasome. Science. 2002;298:611–615. doi: 10.1126/science.1075898. [DOI] [PubMed] [Google Scholar]
- VerPlank J. Impairment in the ubiquitin proteasome system in hereditary peripheral neuropathies. State University of New York; Buffalo: 2017. In Press. [Google Scholar]
- Waxman L, Fagan JM, Goldberg AL. Demonstration of two distinct high molecular weight proteases in rabbit reticulocytes, one of which degrades ubiquitin conjugates. J Biol Chem. 1987;262:2451–2457. [PubMed] [Google Scholar]
- Wehmer M, Rudack T, Beck F, Aufderheide A, Pfeifer G, Plitzko JM, Förster F, Schulten K, Baumeister W, Sakata E. Structural insights into the functional cycle of the ATPase module of the 26S proteasome. Proc Natl Acad Sci U S A. 2017;114:1305–1310. doi: 10.1073/pnas.1621129114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehmer M, Sakata E. Recent advances in the structural biology of the 26S proteasome. Int J Biochem Cell Biol. 2016;79:437–442. doi: 10.1016/j.biocel.2016.08.008. [DOI] [PubMed] [Google Scholar]
- Wilkinson CR, Seeger M, Hartmann-Petersen R, Stone M, Wallace M, Semple C, Gordon C. Proteins containing the UBA domain are able to bind to multi-ubiquitin chains. Nat Cell Biol. 2001;3:939–943. doi: 10.1038/ncb1001-939. [DOI] [PubMed] [Google Scholar]
- Wilson SM, Bhattacharyya B, Rachel RA, Coppola V, Tessarollo L, Householder DB, Fletcher CF, Miller RJ, Copeland NG, Jenkins NA. Synaptic defects in ataxia mice result from a mutation in Usp14, encoding a ubiquitin-specific protease. Nat Genet. 2002;32:420–425. doi: 10.1038/ng1006. [DOI] [PubMed] [Google Scholar]
- Wójcik C, DeMartino GN. Intracellular localization of proteasomes. Int J Biochem Cell Biol. 2003;35:579–589. doi: 10.1016/s1357-2725(02)00380-1. [DOI] [PubMed] [Google Scholar]
- Xie Y, Varshavsky A. RPN4 is a ligand, substrate, and transcriptional regulator of the 26S proteasome: a negative feedback circuit. Proc Natl Acad Sci U S A. 2001;98:3056–3061. doi: 10.1073/pnas.071022298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu P, Duong DM, Seyfried NT, Cheng D, Xie Y, Robert J, Rush J, Hochstrasser M, Finley D, Peng J. Quantitative proteomics reveals the function of unconventional ubiquitin chains in proteasomal degradation. Cell. 2009;137:133–145. doi: 10.1016/j.cell.2009.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao T, Cohen RE. A cryptic protease couples deubiquitination and degradation by the proteasome. Nature. 2002;419:403–407. doi: 10.1038/nature01071. [DOI] [PubMed] [Google Scholar]
- Yao T, Song L, Xu W, DeMartino GN, Florens L, Swanson SK, Washburn MP, Conaway RC, Conaway JW, Cohen RE. Proteasome recruitment and activation of the Uch37 deubiquitinating enzyme by Adrm1. Nat Cell Biol. 2006;8:994–1002. doi: 10.1038/ncb1460. [DOI] [PubMed] [Google Scholar]
- Yu H, Singh Gautam AK, Wilmington SR, Wylie D, Martinez-Fonts K, Kago G, Warburton M, Chavali S, Inobe T, Finkelstein IJ, et al. Conserved Sequence Preferences Contribute to Substrate Recognition by the Proteasome. J Biol Chem. 2016;291:14526–14539. doi: 10.1074/jbc.M116.727578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Smith DM, Kim HM, Rodriguez V, Goldberg AL, Cheng Y. Interactions of PAN's C-termini with archaeal 20S proteasome and implications for the eukaryotic proteasome-ATPase interactions. EMBO J. 2010;29:692–702. doi: 10.1038/emboj.2009.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Zhai B, Gygi SP, Goldberg AL. mTOR inhibition activates overall protein degradation by the ubiquitin proteasome system as well as by autophagy. Proc Natl Acad Sci U S A. 2015;112:15790–15797. doi: 10.1073/pnas.1521919112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuin A, Bichmann A, Isasa M, Puig-Sàrries P, Díaz LM, Crosas B. Rpn10 monoubiquitination orchestrates the association of the ubiquilin-type DSK2 receptor with the proteasome. Biochem J. 2015;472:353–365. doi: 10.1042/BJ20150609. [DOI] [PubMed] [Google Scholar]
- Zwickl P, Ng D, Woo KM, Klenk HP, Goldberg AL. An archaebacterial ATPase, homologous to ATPases in the eukaryotic 26 S proteasome, activates protein breakdown by 20 S proteasomes. J Biol Chem. 1999;274:26008–26014. doi: 10.1074/jbc.274.37.26008. [DOI] [PubMed] [Google Scholar]