Abstract
Background
There is a paucity of normative echocardiographic data in preterm infants. The objectives of this study were: (1) derive left ventricular (LV) M-mode reference values, and (2) compare the performance of alternative methods of indexing LV dimensions and mass (LVM) in preterm infants. We propose that indexing LV measures to weight in preterm infants is a practical approach given the variability associated with tape-measure length measurement in infants.
Methods
In this retrospective study LV M-mode echocardiographic measurements of end diastolic interventricular septal thickness (IVSd), end diastolic LV posterior wall thickness (LVPWd), LV end diastolic and systolic dimensions (LVEDD, LVESD), LVM, and relative wall thickness (RWT) were remeasured in 503 hospitalized preterm infants ≤ 2 Kg (372 from a retrospective sample and 131 prospectively enrolled). Measures for all variables did not differ between retrospective and prospective samples so results were pooled. LV dimensions and LVM indexed for weight, length, and body surface area (BSA) sex-specific centile curves and corresponding Z scores were generated using Cole’s lambda-mu-sigma method. Threshold limits (10th, and 80th percentile; P10, P80) were used to generate RWT normative range.
Results
Sex-specific centile curves using LVM, IVSd, LVPWd, LVEDD, and LVESD indexed to weight were similar to the curves generated using length and BSA. The mean [P10, P80] normal range for RWT was 0.33 (0.26, 0.38).
Conclusions
From this large cohort of preterm infants, we developed LV M-mode dimension and LVM centile curves indexed to weight as a practical method to assess LV morphology in preterm infants.
Keywords: Echocardiography, Reference values, M-mode, Preterm, Left Ventricle
INTRODUCTION
Two dimensionally guided M-mode echocardiography is commonly used to obtain measures of left ventricular (LV) chamber size and wall thickness as well as derived values of left ventricular mass (LVM) and relative wall thickness (RWT) in children and adults. Because left ventricular hypertrophy (LVH) is known to be associated with adverse cardiac events in adults, increased attention is being paid to the identification of early stages of LVH during childhood.1–5 Elevated LVM, derived from M-mode echocardiography, is of particular interest because it is often used to define LVH.6 M-mode echocardiography offers the advantage of quick acquisition in irritable preterm infants with sedation risks. Appropriate normalization of LV measures is especially critical in young infants because of the enormous variability in body size and altered body proportions with variable gestational age and growth.7 Numerous methods have been proposed to normalize cardiac dimensions to body size, including simple division by height, weight, or body surface area (BSA) or more complex allometric relationships of these body measures.7–13 LVM indexed to height (g/m2.7 or g/m2.16) has gained wide acceptance, but may not be an ideal method for standardizing LVM for body size in infants.4–7, 14 Furthermore indexing to length or body surface may not be optimal in preterm infants because of the inaccuracy of the commonly used tape-measure technique for length measurement in neonates.15, 16 More recently centile curves used for pediatric growth charts by the National Center for Health Statistics, have been demonstrated to be useful for evaluating LVM in children.7, 17–20
Improved survival of extremely premature babies has further led to the recognition of LVH in preterm infants.21 Studies of former preterm infants at 5 and 7 years of age found decreased LV chamber size and increased ventricular septal thickness but did not track cardiac abnormalities from the nursery.22, 23 The paucity of normative echocardiographic data in preterm infants limits the identification of patients that might be at risk for persistent cardiac abnormalities. Biased or imprecise cardiac growth curves can lead to inappropriate clinical or management decisions.8, 24–30 In this study we sought to derive LV M-mode reference values with centile curves as well as compare the performance of alternative methods of indexing LV measures in preterm infants (length, weight, and BSA).
METHODS
Study Design
For the purpose of this retrospective study, LV M-mode echocardiographic remeasurements were made in two cohorts of preterm infants: (1) a prospective cohort of 131 preterm infants (born less than 29 weeks gestational age) was recruited between August 2011 and November 2013, and (2) a retrospective database generated cohort of 372 preterm infants from January 1, 2005 through December 31, 2014. The institutional review board of Washington University School of Medicine approved the study. All subject guardians in the prospective sample provided written informed consent.
Retrospective study population
Last ten year echocardiographic and clinical databases for St. Louis Children’s Hospital were retrospectively reviewed. All preterm infants ≤ 2 Kg born from 2005 to 2014, with a technically adequate echocardiographic evaluation (defined as an echocardiogram with measurable M mode) performed at St. Louis Children’s Hospital were eligible for inclusion. Exclusion criteria were: 1) congenital heart disease including moderate or large atrial level shunt; 2) moderate or large patent ductus arteriosus; 3) known genetic cardiomyopathy including hypertrophic cardiomyopathy, genetic syndromes (such as Noonan, Pompe’s disease), neuromuscular disease, chromosomal abnormalities, diagnosis of pulmonary hypertension (diagnosed based on clinical chart review or echocardiographic interpretation), connective tissue disease, and clinical or radiologic diagnosis of kidney disease 4) patients with incomplete medical records; 5) enrollment in the prospective sample (described below). Patients with moderate or large shunts on a prior echocardiogram were eligible if at least one month elapsed until the time of the study echocardiogram.
Prospective study population
Additionally, 131 preterm infants were prospectively enrolled from among infants participating in the Prematurity and Respiratory Outcomes Program (PROP), a 7-center initiative sponsored by the National Heart, Lung and Blood Institute (Clinical Trials number: NCT01435187).31 All infants in the prospective sample were enrolled at St. Louis Children’s Hospital neonatal intensive care unit between August 2011 and November 2013. All prospective subjects had structurally normal hearts; none had a family history of genetic cardiomyopathy, genetic syndromes or known chromosomal abnormality. All prospectively enrolled subjects were reevaluated 1 year later to validate that they remained free of any recognizable systemic disorder, including hypertension. All patients enrolled in the prospective study routinely had echocardiograms performed per the Prematurity and Respiratory Outcomes Program study protocol.31 All subjects with initial echocardiographic readings of moderate or large shunts were excluded without review. If the initial reading of shunt size was small to moderate, a senior echocardiographer (MCJ) reviewed the studies to exclude any with moderate or larger shunts. PDA was graded as small if the ratio of the smallest ductal diameter to ostium of the left pulmonary artery was < 0.5.32 Atrial shunts were qualitatively graded as small if there was no right ventricular or right atrial enlargement and the color flow Doppler diameter of the shunt was less than 20% of the length of the atrial septum.
Body Size Parameters
Measurements for weight and length were based on neonatal intensive care clinical records with daily weight and weekly tape measured length while the infant was supine with stretched legs. The most recent length, and weight measurement on the day echocardiogram performed was collected. We used the Haycock formula for calculation of body surface area: weight0.5378 × height0.3964 × 0.024265.33
Echocardiography
All echocardiographic studies were performed on commercially available cardiac ultrasound scanners according to the guidelines of the American Society of Echocardiography.28 All of the 503 echocardiograms were re-measured offline for the purposes of the present study by SC. MCJ remeasured 100 studies in a blinded fashion and was allowed to choose the M-mode image for measurement for interobserver variability determination. Measurements were made by 2-dimensional guided M-mode echocardiography using the parasternal short-axis view at the level of the papillary muscles. End diastole was defined as the time of maximum LV dimension. Electronic calipers were used to measure end diastole interventricular septal thickness (IVSd), left ventricular (LV) posterior wall thickness (LVPWd), and LV dimension at end diastole (LVEDD) and end systole (LVESD). Measurements were repeated over 3 consecutive cardiac cycles and averaged. LVM was estimated by the Devereux equation, LVM (grams) = 0.8{1.04 [(LVEDD + LVPWd + IVSd)3 − (LVEDD)3 + 0.6.34 Relative wall-thickness (RWT) was calculated using 2 formulas: (1) RWT P equals twice the posterior wall thickness (LVPWd) over LVEDD; (2) RWT SP equals the ratio of the sum of LVPWd and IVSd over LVEDD.35, 36
Statistical analysis
Descriptive statistics were used to summarize demographic and echocardiographic measures. Continuous variables were summarized as mean ± SD, or mean (10th, and 80thpercentile [P10, P80]), as appropriate. Categorical variables were presented by the absolute and relative frequencies or as numbers and percentages. Comparisons between the retrospective and prospective samples were performed using independent-samples t test or Mann-Whitney U test for continuous variables and chi square or Fisher’s exact test for categorical variables. Intraobserver and interobserver variability of IVSd, LVPWd, LVEDD, LVESD and LVM measurements were determined in 100 randomly selected patients using intraclass correlation coefficient (ICC). A 2-sided p value < 0.05 was considered statistically significant and performed by using SAS 9.3 version (SAS Institute, Inc, Cary, NC).
Centile curves
The lambda-mu-sigma (LMS) method was used to construct smoothed reference centile curves for LV dimensions (IVSd, LVPWd, LVEDD, and LVESD) and LVM indexed for three body size parameters (weight, length and body surface area).37 The LMS method fits 3 curves, lambda (L), mu (M) and sigma (S) which represent the Box-Cox power transformation of skewness, the mean and the coefficient of variation, respectively. The data was assessed for influential outliers using LOESS regression, a robust regression technique, in SAS. Observations with residuals outside of the 2nd and 98th percentiles were removed. Separate sex-specific curves were constructed for each of the above 5 M-mode echocardiographic measures and the 3 body size parameters. Using the LMS function in the Generalized Additive Models for Location, Scale and Shape (GAMLSS) R package, the effective degrees of freedom parameters (for lambda, mu, and sigma) with the lowest generalized Akaike information criterion (gAIC) was identified by an automated algorithm. The reference centile curves were generated to reflect the 5th, 10th, 25th, 50th, 75th, 80th, 90th, and 95th centiles. Z scores were computed using the following formula: Z score = [(LVM/M(weight))L(weight) — 1]/(L(weight)xS(weight)). Comparison of Z scores derived from weight were compared to the indexes of length and Haycock BSA using Bland-Altman plots and the ICC.38
Confounding effects
The effect of the following potential confounding factors on LVM indexed to weight were evaluated using quantile regression: 1) postmenstrual age (defined as the time elapsed between the first day of the last menstrual period and birth [gestational age] plus the time elapsed after birth [chronological age],39 2) chronological age, 3) sex, and 4) PDA(yes/no). Quantile regression is a robust regression model which does not make any assumption regarding normality and allows for estimation of the quantiles of the distribution of the outcome variable. While it cannot be used to compute Z scores, it can estimate the effect of covariates for different quantiles of LVM. Weight was modeled using a spline effect and covariates considered were postmenstrual age, sex, and PDA(yes/no). An additional model was constructed to consider the effect of chronological age instead of postmenstrual age. Covariate effects were tested using a likelihood ratio test and a significance level of 0.05 was used.
RESULTS
Demographics
We reviewed 692 preterm infant (who were 2 Kilogram or less) charts and their echocardiograms. Of those, 503 patients met inclusion/exclusion criteria and were included in the final analysis. Of the 189 excluded patients 107 had moderate/large PDA and/or atrial level shunt and 82 patients had congenital heart disease. About a third of the patients (n = 39) that were excluded due to moderate/large PDA and or atrial level shunt had underlying pulmonary hypertension. The baseline characteristics of all 503 preterm infants who participated in the study are presented in Table 1. The study population had equal sex distribution (249/503, 49.5% males). A small atrial level shunt was seen in the majority of the patients (90%). Small ductal shunts were noted in about 40% of patients. Of note, 189 patients (37.5%) were extremely low birth weight (weight < 1 Kg on day of scan).
Table 1.
Baseline patient characteristics.
| Retrospective (n = 372) | Prospective (n = 131) | P value | |
|---|---|---|---|
| Males | 186 (50%) | 63 (48%) | 0.7 |
| Weight (kilograms) | 1.2 ± 0.47 | 1.3 ± 0.35 | 0.01 |
| Gestational age | 27.05 ± 3.24 | 26.27 ± 1.46 | 0.6 |
| PMA, weeks | 30.09 ± 4.29 | 30.89 ± 2.59 | 0.004 |
| Chronological age | 21.29 ± 22.56 | 32.4 ± 19.1 | <0.0001 |
| Length, cm | 36.58 ± 4.95 | 37.04 ± 2.94 | 0.2 |
| BSA, Kg/m2 | 0.11 ± 0.03 | 0.12 ± 0.02 | 0.01 |
| PDA | 177 (47.6%) | 52 (40%) | 0.3 |
| Atrial shunt | 332 (89%) | 121 (92%) | 0.7 |
Note: Values expressed as mean ± SD or number (percentage). Postmenstrual age (PMA, weeks); Weight, in kilograms on the day of scan; Body surface area (BSA, Kg/m2); Patent ductus arteriosus (PDA).
Echocardiographic values
Results were pooled because there were no differences in echocardiographic (mean ± SD) measures when comparing retrospective versus (vs.) prospective groups, respectively: IVSd (2.7 ± 0.6 vs. 2.7 ± 0.4 mm, P = 0.8), LVPWd (2.4 ± 0.5 vs. 2.5 ± 0.4 mm, P = 0.1), LVEDD (13.8 ± 2.9 vs. 14.1 ± 2.2 mm, P = 0.2), LVESD (8.9 ± 2.2 vs. 9.1 ± 1.8 mm, P = 0.3), and LVM (4.27 ± 2.04 vs. 4.38 ± 1.49 g, P = 0.5). LV dimensions (IVSd, LVPWd, LVEDD, and LVESD) and LVM indexed for weight sex-specific centile curves were generated (Figure 1, 2). The L, M and S measures to compute Z scores for weight is provided in Tables 2, and 3 (for males and females respectively). Bland-Altman plots with corresponding ICCs comparing weight versus length or weight versus BSA for all measured indexed LV dimensions including LVM are shown in Supplementary appendix, figure S1–S10. The ICCs demonstrate the strong agreement of weight with both length and BSA. The mean [P10, P80] normal range for RWT was 0.33 (0.26, 0.38).
Figure 1.
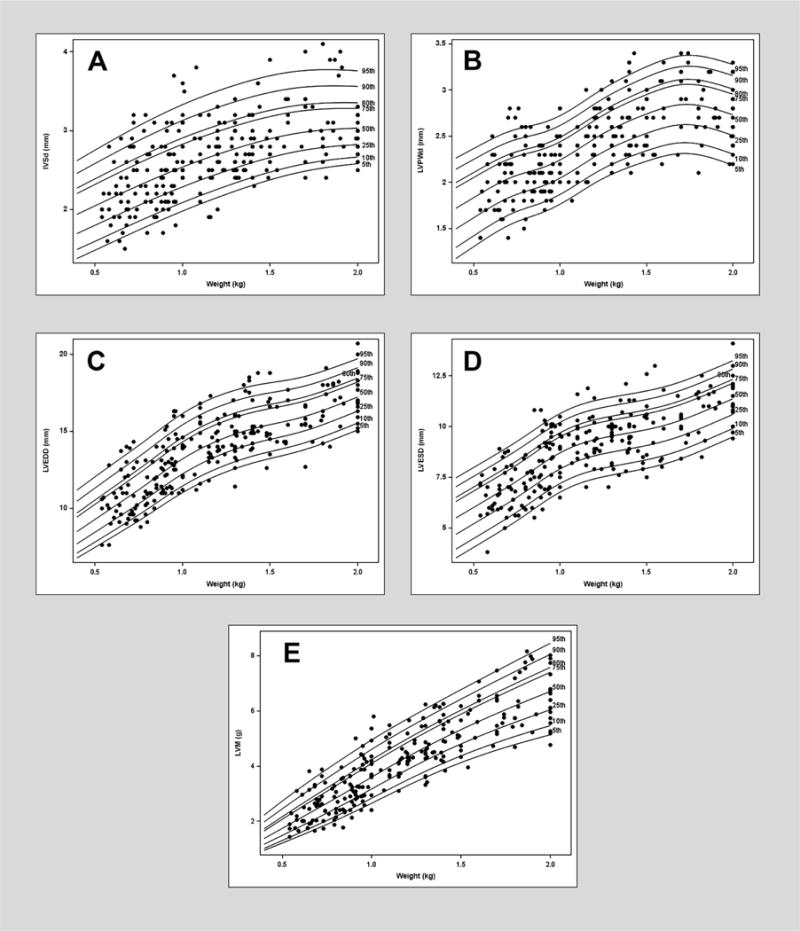
Reference centile curve for males generated using lambda-mu-sigma (LMS) method for A) end diastole interventricular septal thickness (IVSd) normalized by weight on the day of scan B) left ventricular posterior wall thickness (LVPWd) normalized by weight on the day of scan C) left ventricular dimension end diastole (LVEDD) normalized by weight on the day of scan D) left ventricular dimension end systole (LVESD) normalized by weight on the day of scan E) left ventricular mass (LVM) normalized by weight on the day of scan.
Figure 2.
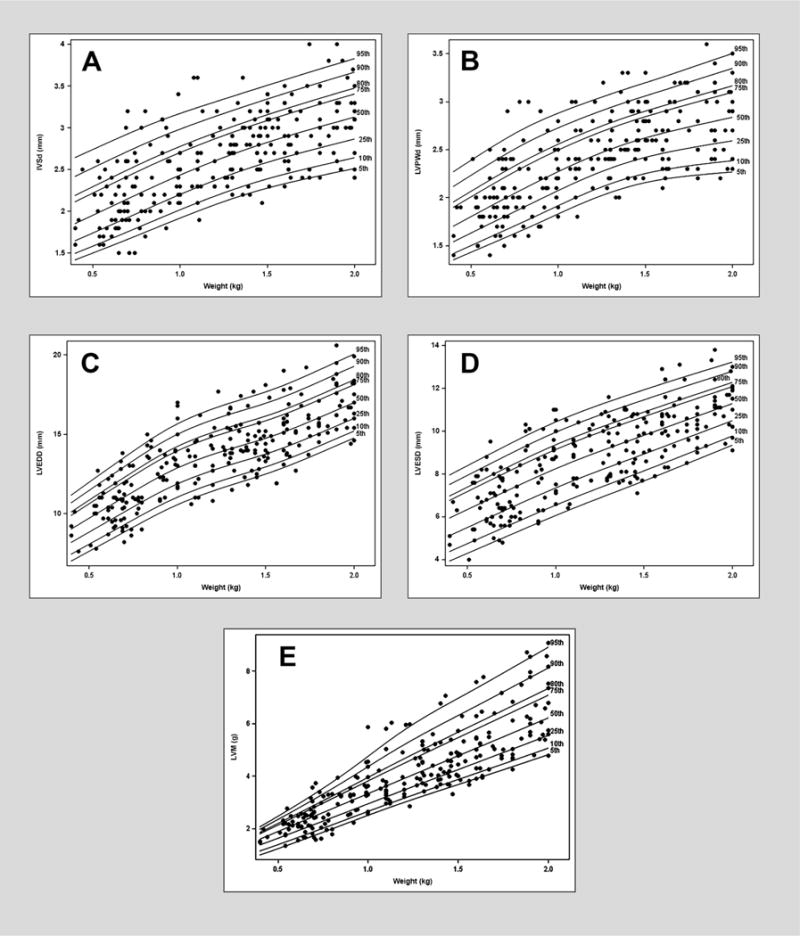
Reference centile curve for females generated using lambda-mu-sigma (LMS) method for A) end diastole interventricular septal thickness (IVSd) normalized by weight on the day of scan B) left ventricular posterior wall thickness (LVPWd) normalized by weight on the day of scan C) left ventricular dimension end diastole (LVEDD) normalized by weight on the day of scan D) left ventricular dimension end systole (LVESD) normalized by weight on the day of scan E) left ventricular mass (LVM) normalized by weight on the day of scan.
Table 2.
L, M and S values for males for each left ventricular M mode echocardiographic measurement indexed using weight.
| Weight (Kg) | LVM | IVSd | LVPWd | LVEDD | LVESD | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M | S | L | M | S | L | M | S | L | M | S | L | M | S | L | |
| 0.4 | 1.366 | 0.257 | −0.563 | 0.194 | 0.195 | 0.389 | 0.173 | 0.207 | −0.737 | 0.847 | 0.151 | −0.807 | 0.548 | 0.120 | 1.000 |
| 0.5 | 1.729 | 0.246 | −0.492 | 0.204 | 0.188 | 0.268 | 0.182 | 0.190 | −0.608 | 0.924 | 0.144 | −0.764 | 0.599 | 0.120 | 1.000 |
| 0.6 | 2.092 | 0.236 | −0.420 | 0.214 | 0.182 | 0.146 | 0.190 | 0.173 | −0.479 | 1.001 | 0.138 | −0.721 | 0.649 | 0.119 | 1.000 |
| 0.7 | 2.456 | 0.225 | −0.348 | 0.225 | 0.175 | 0.025 | 0.199 | 0.157 | −0.350 | 1.078 | 0.132 | −0.677 | 0.701 | 0.119 | 1.000 |
| 0.8 | 2.823 | 0.214 | −0.277 | 0.235 | 0.169 | −0.097 | 0.207 | 0.143 | −0.221 | 1.157 | 0.127 | −0.634 | 0.757 | 0.119 | 1.000 |
| 0.9 | 3.200 | 0.202 | −0.205 | 0.244 | 0.164 | −0.218 | 0.215 | 0.131 | −0.092 | 1.240 | 0.121 | −0.591 | 0.816 | 0.118 | 1.000 |
| 1 | 3.583 | 0.190 | −0.134 | 0.253 | 0.158 | −0.340 | 0.224 | 0.122 | 0.037 | 1.317 | 0.116 | −0.548 | 0.868 | 0.118 | 1.000 |
| 1.1 | 3.958 | 0.178 | −0.062 | 0.262 | 0.153 | −0.461 | 0.235 | 0.118 | 0.167 | 1.382 | 0.112 | −0.504 | 0.907 | 0.118 | 1.000 |
| 1.2 | 4.318 | 0.168 | 0.009 | 0.269 | 0.147 | −0.582 | 0.246 | 0.116 | 0.296 | 1.435 | 0.107 | −0.461 | 0.933 | 0.117 | 1.000 |
| 1.3 | 4.663 | 0.160 | 0.081 | 0.277 | 0.142 | −0.704 | 0.258 | 0.118 | 0.425 | 1.479 | 0.103 | −0.418 | 0.951 | 0.117 | 1.000 |
| 1.4 | 4.992 | 0.155 | 0.152 | 0.283 | 0.137 | −0.825 | 0.268 | 0.121 | 0.554 | 1.514 | 0.100 | −0.374 | 0.967 | 0.116 | 1.000 |
| 1.5 | 5.305 | 0.151 | 0.224 | 0.289 | 0.133 | −0.947 | 0.274 | 0.124 | 0.683 | 1.541 | 0.096 | −0.331 | 0.983 | 0.116 | 1.000 |
| 1.6 | 5.603 | 0.149 | 0.295 | 0.294 | 0.128 | −1.068 | 0.278 | 0.126 | 0.812 | 1.566 | 0.093 | −0.288 | 1.003 | 0.116 | 1.000 |
| 1.7 | 5.889 | 0.148 | 0.367 | 0.298 | 0.124 | −1.190 | 0.279 | 0.127 | 0.941 | 1.595 | 0.090 | −0.244 | 1.030 | 0.115 | 1.000 |
| 1.8 | 6.164 | 0.148 | 0.438 | 0.300 | 0.120 | −1.311 | 0.278 | 0.125 | 1.070 | 1.632 | 0.087 | −0.201 | 1.062 | 0.115 | 1.000 |
| 1.9 | 6.430 | 0.149 | 0.510 | 0.302 | 0.115 | −1.432 | 0.277 | 0.122 | 1.199 | 1.675 | 0.084 | −0.158 | 1.098 | 0.115 | 1.000 |
| 2 | 6.690 | 0.151 | 0.581 | 0.303 | 0.111 | −1.554 | 0.275 | 0.118 | 1.328 | 1.720 | 0.081 | −0.115 | 1.135 | 0.114 | 1.000 |
| gAIC | 548.110 | −908.968 | −1018.491 | −228.268 | −346.066 | ||||||||||
Note: Left ventricular mass (LVM), interventricular septum thickness at end diastole (IVSd), thickness of posterior wall of the left ventricle at end diastole (LVPWd), left ventricular dimension at end diastole (LVEDD) and end systole (LVESD). For calculation of the Z score please use the formula, Weight, Z score = [(LVM/M(weight))L(weight) ‒ 1]/(L(weight)xS(weight)). LMS corresponds to lambda (L), mu (M), and sigma (S) values respectively.
Table 3.
L, M and S values for females for each left ventricular M mode echocardiographic measurement indexed using weight.
| Weight (Kg) | LVM | IVSd | LVPWd | LVEDD | LVESD | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M | S | L | M | S | L | M | S | L | M | S | L | M | S | L | |
| 0.4 | 1.586 | 0.200 | 1.741 | 0.185 | 0.184 | −0.924 | 0.170 | 0.155 | −0.850 | 0.900 | 0.140 | 0.752 | 0.577 | 0.206 | 0.720 |
| 0.5 | 1.875 | 0.199 | 1.349 | 0.195 | 0.179 | −0.842 | 0.180 | 0.154 | −0.779 | 0.968 | 0.137 | 0.639 | 0.618 | 0.197 | 0.649 |
| 0.6 | 2.164 | 0.197 | 0.954 | 0.205 | 0.175 | −0.760 | 0.190 | 0.153 | −0.708 | 1.035 | 0.133 | 0.525 | 0.661 | 0.189 | 0.577 |
| 0.7 | 2.453 | 0.196 | 0.554 | 0.214 | 0.170 | −0.677 | 0.199 | 0.151 | −0.637 | 1.101 | 0.130 | 0.412 | 0.706 | 0.181 | 0.506 |
| 0.8 | 2.742 | 0.195 | 0.140 | 0.224 | 0.164 | −0.595 | 0.209 | 0.148 | −0.566 | 1.167 | 0.126 | 0.299 | 0.751 | 0.174 | 0.435 |
| 0.9 | 3.031 | 0.194 | −0.289 | 0.234 | 0.158 | −0.513 | 0.218 | 0.143 | −0.495 | 1.230 | 0.123 | 0.185 | 0.794 | 0.167 | 0.363 |
| 1 | 3.319 | 0.192 | −0.687 | 0.243 | 0.152 | −0.430 | 0.227 | 0.138 | −0.424 | 1.285 | 0.120 | 0.072 | 0.830 | 0.160 | 0.292 |
| 1.1 | 3.608 | 0.191 | −0.989 | 0.252 | 0.147 | −0.348 | 0.235 | 0.132 | −0.353 | 1.331 | 0.117 | −0.042 | 0.859 | 0.153 | 0.220 |
| 1.2 | 3.897 | 0.190 | −1.157 | 0.260 | 0.141 | −0.266 | 0.243 | 0.127 | −0.282 | 1.371 | 0.114 | −0.155 | 0.884 | 0.147 | 0.149 |
| 1.3 | 4.186 | 0.189 | −1.215 | 0.268 | 0.137 | −0.184 | 0.250 | 0.123 | −0.211 | 1.406 | 0.111 | −0.268 | 0.905 | 0.141 | 0.078 |
| 1.4 | 4.475 | 0.187 | −1.206 | 0.275 | 0.134 | −0.101 | 0.256 | 0.121 | −0.140 | 1.439 | 0.108 | −0.382 | 0.927 | 0.135 | 0.006 |
| 1.5 | 4.764 | 0.186 | −1.167 | 0.282 | 0.131 | −0.019 | 0.262 | 0.120 | −0.068 | 1.473 | 0.105 | −0.495 | 0.955 | 0.130 | −0.065 |
| 1.6 | 5.053 | 0.185 | −1.132 | 0.289 | 0.130 | 0.063 | 0.267 | 0.121 | 0.003 | 1.511 | 0.103 | −0.609 | 0.989 | 0.125 | −0.136 |
| 1.7 | 5.341 | 0.184 | −1.118 | 0.295 | 0.129 | 0.146 | 0.271 | 0.123 | 0.074 | 1.555 | 0.100 | −0.722 | 1.025 | 0.119 | −0.208 |
| 1.8 | 5.630 | 0.183 | −1.117 | 0.301 | 0.129 | 0.228 | 0.275 | 0.126 | 0.145 | 1.601 | 0.097 | −0.835 | 1.059 | 0.115 | −0.279 |
| 1.9 | 5.919 | 0.181 | −1.121 | 0.307 | 0.128 | 0.310 | 0.279 | 0.129 | 0.216 | 1.649 | 0.095 | −0.949 | 1.091 | 0.110 | −0.351 |
| 2 | 6.208 | 0.180 | −1.122 | 0.312 | 0.128 | 0.393 | 0.283 | 0.132 | 0.287 | 1.697 | 0.092 | −1.062 | 1.121 | 0.105 | −0.422 |
| gAIC | 546.185 | −914.746 | −1003.141 | −218.723 | −324.249 | ||||||||||
Note: Left ventricular mass (LVM), interventricular septum thickness at end diastole (IVSd), thickness of posterior wall of the left ventricle at end diastole (LVPWd), left ventricular dimension at end diastole (LVEDD) and end systole (LVESD). For calculation of the Z score please use the formula, Weight, Z score = [(LVM/M(weight))L(weight) − 1]/(L(weight)×S(weight)). LMS corresponds to lambda (L), mu (M), and sigma (S) values respectively.
Confounders
The quantile regression model with postmenstrual age found that weight was independently associated with LVM between the 5th and 95th percentiles (P < 0.0001 for all percentiles). Sex was independently associated with LVM between the 5th and 75th percentiles where females had lower LVM compared to males in these percentiles (5th, P = 0.04; 10th, P < 0.0001; 25th, P = 0.0002, 50th, P = 0.02, 75th P = 0.04). PDA and postmenstrual age were not found to be independently associated with LVM. The quantile regression model using chronological age as a covariate found that weight, sex, and PDA had associations with LVM similar to the model using postmenstrual age. However, chronological age was independently associated with LVM for the 10th (P = 0.03), 25th (P = 0.006), 75th (P = 0.01) and 80th (P = 0.03) percentiles. For every one-day increase in days of life, the LVM increases by 0.008 (95% confidence interval: 0.0015, 0.0141) to 0.011 grams (95% confidence interval: 0.0023, 0.0190) for the significant quantiles. The impact of chronological age on the model for LVM indexed to weight in males is shown with a graph of the smoothed fitted parameter in Figure 3.
Figure 3.
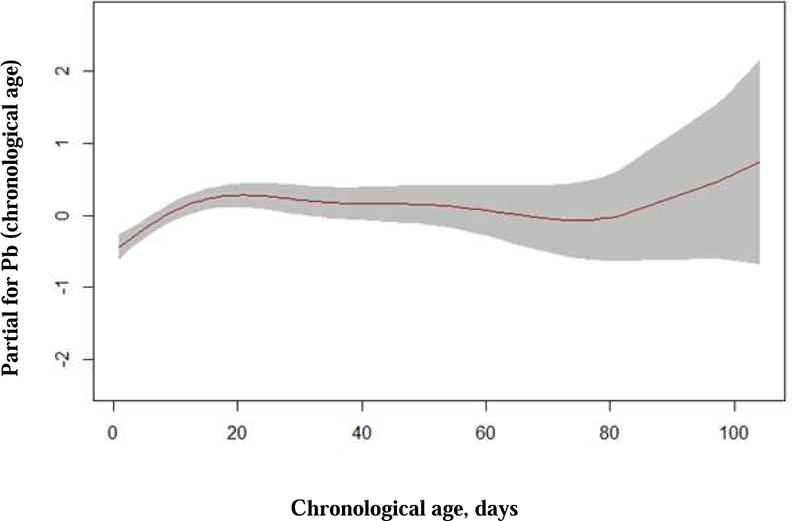
Fitted smoothing parameter (spline) for the additive predictor chronological age for the model of LVM indexed to weight in males.
Interobserver and Intraobserver variability
The reproducibility analysis is summarized in Table 3. No significant differences were observed within observers (P > 0.05). For all interobserver measurements, there was an interclass correlation coefficient ≥ 0.96 with P < 0.01.
DISCUSSION
This study provides normative M-mode reference values with sex-speific centile curves from a large population of preterm infants up to 2 kilograms. Allometric analysis with LMS centile curves affirmed that indexing these measures to weight is appropriate. Because preterm infants are a heterogeneous group of hospitalized patients, and it is difficult to define a ‘healthy preterm’ population it may be appropriate to use tighter normal ranges than the standard P5 and P95. The smoothed centiles curves along with LMS parameters generated allow for the calculation of the percentiles and Z scores facilitating the interpretation of LV M-mode measures in preterm infants. Additionally, these data allow for graphical or calculated Z score threshold limits to vary depending on the clinical question. We included determination of normal values for RWT because this measure does not require indexing to body size and has been widely used in adults to classify patterns of remodeling.40 Upper limits of RWT in this preterm population are increased compared to pediatric values possibly in part secondary to morbidity inherent in hospitalized preterm infants or normal age related changes.41, 42
Because our retrospective cohort was a 10-year echocardiographic database generated group and did not represent a consecutive birth cohort we validated these results with a smaller prospectively enrolled cohort. The absence of differences between these groups offers some evidence against a bias based on test indication. Our large dataset allowed for a robust comparison of body size indexing methods in preterm infants ≤ 2 Kg. Our results demonstrated that LV dimensions indexed to weight were similar to the curves generated using BSA, and length. Smaller studies of preterm infants by Skelton et al (n = 79), Zecaca et al (n = 35), and Abushaban et al (n = 268) did not explore allometric relationships and did not calculate LVM.43–45 A study of 40 preterm infants comparing small versus appropriate for gestational age groups included LVM and found that indexing to length as compared with weight removed differences between groups at the same time acknowledging the practical difficulties of length measurement.16 Nagasawa et al. concluded length was an appropriate index for LVEDD in patients under age 1 year with only 32 patients less than 32 weeks gestation.46 These prior studies did not utilize centile curves for LV dimensions.
LVH is recognized as an independent predictor of cardiovascular morbidity and mortality in adults.1, 2 Accurate diagnosis of LVH depends on normalization of LVM for body size. There has been debate regarding the best method of standardizing LVM.7, 17, 47 Numerous authors have described both lean body mass (LBM) and fat free mass (FFM) as ideal methods for indexing LVM, however; these measures have practical barriers.17, 47 Therefore, surrogates of LBM such as weight, height, and BSA, have been used to index LVM in various studies.7, 14, 48, 49 Although LVM/height2.7 is a widely accepted method of normalizing LVM, it has limitations in young children.7, 17 LVM index is known to increase with decreasing height, particularly for height < 140 cm.7, 14 Furthermore indexing to length or body surface may not be optimal in preterm infants because of the inaccuracy of the commonly used tape-measure technique.15, 16 In addition, length is more prone to error because it is obtained less frequently than weight in neonatal intensive care units. In small studies of term and preterm neonates body weight had the best correlation with fat free mass measured with dual energy x-ray absorptiometry (DEXA).50, 51
Based on these considerations for LVM we tested weight as a method of indexing for all LV measures and generated sex-specific weight centile curves for these preterm infants ≤ 2Kg. Because weight is a practical measurement in preterm infants and our analysis found that length or BSA were not superior indexes, we propose weight should be used as the index for LV dimensions in small premature infants. Foster et al recently published LBM predictive equations for children and adolescents that have not been validated for young infants.17 Future work could include deriving similar predictive equation for young infants and validating it with LBM or FFM.
Limitations
This study has some notable limitations. First, the retrospective study population is likely biased because patients underwent echocardiography for clinical reasons and did not represent a consecutive birth cohort. Moreover, all of these infants were cared for at a tertiary care referral center suggesting bias towards worse disease. Next, our cohort comprised heterogeneous ethnic preterm infants. We could not evaluate the effect of ethnicity on LV dimensions. We limited the analysis of confounding factors to LVM secondary to the complex nature of quantile regression and to reduce the potential for multiple comparison errors. The high incidence of PDA in the preterm population makes it difficult to eliminate PDA as a confounding factor. These infants may have had persistent altered LV dimensions from a moderate to large PDA present at some time prior to the study echocardiogram. We did not measure LBM, which is considered the optimal method for standardizing LVM. Although 2-dimensional measurements have been recommended in the pediatric population28 we found that M-mode had practical advantages in these preterm infants. Methods that use 2 dimensional echocardiography such as the area length algorithm52 are difficult to utilize in agitated preterm infants with risk factors that prevent sedation. MRI imaging would be an ideal validation tool but also has practical challenges in this population. Lastly, our analysis of confounding factors for LVM suggests that at ages far from the mean chronological age (80 days and beyond), these centile curves are less accurate. Clinical outcome studies are needed to validate the use of LV dimensions indexed to weight with LMS centile curves.
Conclusions
Data from this large study in preterm infants provide reference values for LV dimensions and LVM with sex-specific weight centile curves as a practical method to assess LV morphology in preterm infants up to 2 kilograms. These findings may influence risk assessment and impact decision making of clinicians caring for these high risk infants. Further long term clinical follow-up of these subjects will enable validation of these reference values and uncover additional links between preterm morbidities and cardiac abnormalities.
Table 4.
Comparison of the interobserver variability of the two observers for measuring the left ventricular dimensions.
| Variable | N | ICC (95% CI) | P value |
|---|---|---|---|
| LVM (g) | 100 | 0.99 (0.985, 0.993) | < 0.01 |
| IVSd (mm) | 100 | 0.96 (0.941, 0.973) | < 0.01 |
| LVPWd (mm) | 100 | 0.96 (0.941, 0.973) | < 0.01 |
| LVEDD (mm) | 100 | 0.99 (0.985, 0.993) | < 0.01 |
| LVESD (mm) | 100 | 0.99 (0.985, 0.993) | < 0.01 |
Note: Interclass Correlation Coefficient (ICC), Left ventricular mass (LVM), interventricular septum thickness at end diastole (IVSd), thickness of posterior wall of the left ventricle at end diastole (LVPWd), left ventricular dimension at end diastole (LVEDD) and end systole (LVESD).
Acknowledgments
Funding Sources: This work was supported in part by a grant from the Premature and Respiratory Outcomes Program (PROP) National Institutes of Health [NIH] (grant U01 HL101794 and HL1014650).
Abbreviations
- BSA
Body surface area
- DEXA
Dual energy x-ray absorptiometry
- EDF
effective degrees of freedom
- FFM
Fat free mass
- GAMLSS
Generalized Additive Models for Location, Scale and Shape
- ICC
intraclass correlation coefficient
- gAIC
generalized Akaike information criterion (gAIC)
- IVSd
Interventricular septal thickness diastole
- LBM
Lean body mass
- LV
Left ventricular
- LVEDD
Left ventricular end diastolic dimension
- LVESD
Left ventricular end systolic dimensions
- LVH
Left ventricular hypertrophy
- LVM
Left ventricular mass
- LVPWd
Left ventricular posterior wall thickness diastole
- L
Lamda
- M
Mu
- PDA
Patent ductus arteriosus
- PROP
Prematurity and Respiratory Outcomes Program (PROP)
- RWT
relative wall thickness
- S
Sigma
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: The authors have reported that they no relationships relevant to the contents of this paper to disclose
This study was presented at the 27th American society of Echocardiography conference, Seattle, U.S.A in June 2016.
References
- 1.Levy D, Anderson KM, Savage DD, Kannel WB, Christiansen JC, Castelli WP. Echocardiographically detected left ventricular hypertrophy: Prevalence and risk factors. The Framingham Heart Study. Ann Int Med. 1988;108:7–13. doi: 10.7326/0003-4819-108-1-7. [DOI] [PubMed] [Google Scholar]
- 2.Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med. 1990;322:1561–1566. doi: 10.1056/NEJM199005313222203. [DOI] [PubMed] [Google Scholar]
- 3.Urbina EM, Gidding SS, Bao W, Pickoff AS, Berdusis K, Berenson GS. Effect of body size, ponderosity, and blood pressure on left ventricular growth in children and young adults in the Bogalusa Heart Study. Circulation. 1995;91:2400–6. doi: 10.1161/01.cir.91.9.2400. [DOI] [PubMed] [Google Scholar]
- 4.de Simone G, Daniels SR, Devereux RB, Meyer RA, Roman MJ, Divitiis OD, et al. Left ventricular mass and body size in normotensive children and adults: Assessment of allometric relations and impact of overweight. J Am Coll Cardiol. 1992;20:1251–60. doi: 10.1016/0735-1097(92)90385-z. [DOI] [PubMed] [Google Scholar]
- 5.Chinali M, Emma F, Esposito C, Rinelli G, Franceschini A, Doyon A, et al. Left Ventricular Mass Indexing in Infants, Children, and Adolescents: A Simplified Approach for the Identification of Left Ventricular Hypertrophy in Clinical Practice. J Pediatr. 2016;170:193–8. doi: 10.1016/j.jpeds.2015.10.085. [DOI] [PubMed] [Google Scholar]
- 6.Foster BJ, Gao T, Mackie AS, Zemel BS, Ali H, Platt RW, et al. Limitations of Expressing Left Ventricular Mass Relative to Height and to Body Surface Area in Children. J Am Soc Echocardiogr. 2013;26:410–8. doi: 10.1016/j.echo.2012.11.018. [DOI] [PubMed] [Google Scholar]
- 7.Foster BJ, Mackie AS, Mitsnefes M, Ali H, Mamber S, Colan SD. A novel method of expressing left ventricular mass relative to body size in children. Circulation. 2008;117:2769–2775. doi: 10.1161/CIRCULATIONAHA.107.741157. [DOI] [PubMed] [Google Scholar]
- 8.Colan Sd. The why and how of Z scores. J Am Soc Echocardiogr. 2013;26:38–40. doi: 10.1016/j.echo.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 9.Mawad W, Drolet C, Dahdah N, Dallaire F. A review and critique of the statistical methods used to generate reference values in pediatric echocardiography. J Am Soc Echocardiogr. 2013;26:29–37. doi: 10.1016/j.echo.2012.09.021. [DOI] [PubMed] [Google Scholar]
- 10.Lauer MS, Anderson KM, Levy D. Influence of contemporary versus 30-year blood pressure levels on left ventricular mass and geometry: the Framingham Heart Study. J Am Coll Cardiol. 1991;18:1287–1294. doi: 10.1016/0735-1097(91)90549-o. [DOI] [PubMed] [Google Scholar]
- 11.Devereux RB, Lutas EM, Casale PN, Kligfield P, Eisenberg RR, Hammond IW, et al. Standardization of M-mode echocardiographic left ventricular anatomic measurements. J Am Coll Cardiol. 1984;4:1222–1230. doi: 10.1016/s0735-1097(84)80141-2. [DOI] [PubMed] [Google Scholar]
- 12.McMahon LP, Roger SD, Levin A. Development, prevention, and potential reversal of left ventricular hypertrophy in chronic kidney disease. J Am Soc Nephrol. 2004;15:1640–1647. doi: 10.1097/01.asn.0000130566.69170.5e. [DOI] [PubMed] [Google Scholar]
- 13.Rigatto C, Foley RN, Kent GM, Guttmann R, Parfrey PS. Long-term changes in left ventricular hypertrophy after renal transplantation. Transplantation. 2000;70:570–575. doi: 10.1097/00007890-200008270-00006. [DOI] [PubMed] [Google Scholar]
- 14.de Simone G, Devereux RB, Daniels SR, Koren MJ, Meyer RA, Laragh JH. Effect of growth on variability of left ventricular mass: assessment of allometric signals in adults and children and their capacity to predict cardiovascular risk. J Am Coll Cardiol. 1995;25:1056–1062. doi: 10.1016/0735-1097(94)00540-7. [DOI] [PubMed] [Google Scholar]
- 15.Corkins MR, Lewis P, Cruse W, Gupta S, Fitzgerald J. Accuracy of Infant Admission Lengths. Pediatrics. 2002;109:1108–1111. doi: 10.1542/peds.109.6.1108. [DOI] [PubMed] [Google Scholar]
- 16.Czernik C, Rhode S, Metze B. Comparison of left ventricular cardiac dimensions between small and appropriate for gestational age preterm infants below 30 weeks of gestation. J Perinat Med. 2013;41:219–226. doi: 10.1515/jpm-2012-0032. [DOI] [PubMed] [Google Scholar]
- 17.Foster BJ, Khoury PR, Kimball TR, Mackie AS, Mitsnefes M. New Reference Centiles for Left Ventricular Mass Relative to Lean Body Mass in Children. J Am Soc Echocardiogr. 2016;29:441–7. doi: 10.1016/j.echo.2015.12.011. [DOI] [PubMed] [Google Scholar]
- 18.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, Flegal KM, Guo SS, Wei R, et al. CDC growth charts: United States. Adv Data. 2000;314:1–27. [PubMed] [Google Scholar]
- 19.Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Flegal KM, Mei Z, et al. 2000 CDC growth charts for the United States: methods and development. Vital Health Stat. 2002;246:1–190. [PubMed] [Google Scholar]
- 20.Cole TJ. The LMS method for constructing normalized growth standards. Eur J Clin Nutr. 1990;44:45–60. [PubMed] [Google Scholar]
- 21.Skelton R, Gill AB, Parsons JM. Cardiac effects of short course dexamethasone in preterm infants. Arch Dis Child Fetal Neonatal Ed. 1998;78:F133–F137. doi: 10.1136/fn.78.2.f133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leipala JA, Boldt T, Turpeinen U, Vuolteenaho O, Fellman V. Cardiac Hypertrophy and Altered Hemodynamic Adaptation in Growth-Restricted Preterm Infants. Pediatric Research. 2003;53:989–993. doi: 10.1203/01.PDR.0000061564.86797.78. [DOI] [PubMed] [Google Scholar]
- 23.Mikkola K, Leipälä J, Boldt T, Fellman V. Fetal growth restriction in preterm infants and cardiovascular function at five years of age. J Pediatr. 2007;151:494–499. doi: 10.1016/j.jpeds.2007.04.030. [DOI] [PubMed] [Google Scholar]
- 24.Cantinotti M, Scalese M, Molinaro S, Murzi B, Passino C. Limitations of current echocardiographic nomograms for left ventricular, valvular and arterial dimensions in children: A critical review. J Am Soc Echocardiogr. 2012;25:142–82. doi: 10.1016/j.echo.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 25.Cantinotti M. Current pediatric nomograms are only one source of error for quantification in pediatric echocardiography: what to expect from future research. J Am Soc Echocardiogr. 2013;26:919. doi: 10.1016/j.echo.2013.04.013. [DOI] [PubMed] [Google Scholar]
- 26.Cantinotti M, Assanta N, Crocetti M, Marotta M, Murzi B, Iervasi G. Limitations of current nomograms in pediatric echocardiography: just the tip of the iceberg—a call for standardization. J Am Soc Echocardiogr. 2014;27:339. doi: 10.1016/j.echo.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 27.Cantinotti M, Scalese M, Murzi B, Assanta N, Spadoni I, Festa P, et al. Echocardiographic nomograms for ventricular, valvular and arterial dimensions in Caucasian children with a special focus on neonates, infants and toddlers. J Am Soc Echocardiogr. 2014;27:179–191. doi: 10.1016/j.echo.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 28.Lopez L, Colan SD, Frommelt PC, Ending GJ, Kendall K, Younoszai AK, et al. Recommendations for quantification methods during the performance of a pediatric echocardiogram: a report from the Pediatric Measurements Writing Group of the American Society of Echocardiography Pediatric and Congenital Heart Disease Council. J Am Soc Echocardiogr. 2010;23:465–95. doi: 10.1016/j.echo.2010.03.019. [DOI] [PubMed] [Google Scholar]
- 29.Cantinotti M, Kutty S, Franchi E, Paterni M, Scalese M, Iervasi G, et al. Pediatric echocardiographic nomograms: What has been done and what still needs to be done. Trends Cardiovasc Med. 2017 Jan 19; doi: 10.1016/j.tcm.2017.01.006. pii: S1050-1738(17)30009-9. [DOI] [PubMed] [Google Scholar]
- 30.Dani C, Bertini G, Simone P, Rubaltelli FF. Hypertrophic cardiomyopathy in preterm infants treated with methylprednisolone for bronchopulmonary dysplasia. Pediatrics. 2006;117:1866–1867. doi: 10.1542/peds.2006-0055. [DOI] [PubMed] [Google Scholar]
- 31.Levy PT, Dioneda B, Holland MR, Sekarski TJ, Lee CK, Mathur A, et al. Right ventricular function in preterm and term neonates: reference values for right ventricle areas and fractional area of change. J Am Soc Echocardiogr. 2015;28:559–69. doi: 10.1016/j.echo.2015.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ramos FG, Rosenfeld CR, Roy L, Koch J, Ramaciotti C. Echocardiographic predictors of symptomatic patent ductus arteriosus in extremely-low-birth-weight preterm neonates. J Perinatol. 2010;30:535–9. doi: 10.1038/jp.2010.14. [DOI] [PubMed] [Google Scholar]
- 33.Haycock GB, Schwartz GJ, Wisotsky DH. Geometric method for measuring body surface area: a height-weight formula validated in infants, children, and adults. J Pediatr. 1978;93:62–6. doi: 10.1016/s0022-3476(78)80601-5. [DOI] [PubMed] [Google Scholar]
- 34.Devereux RB, Reichek N. Echocardiographic determination of left ventricular mass in man. Anatomic validation of the method. Circulation. 1977;55:613–618. doi: 10.1161/01.cir.55.4.613. [DOI] [PubMed] [Google Scholar]
- 35.Biton Y, Goldenberg I, Kutyifa V, Baman JR, Solomon S, Moss AJ, et al. Relative wall thickness and the risk for ventricular tachyarrhythmias in patients with left ventricular dysfunction. Am Coll Cardiol. 2016;67:303–12. doi: 10.1016/j.jacc.2015.10.076. [DOI] [PubMed] [Google Scholar]
- 36.Lieb W, Gona P, Larson MG, Aragam J, Zile MR, Cheng S, et al. The Natural History of Left Ventricular Geometry in the Community: Clinical Correlates and Prognostic Significance of Change in LV Geometric Pattern. JACC Cardiovasc Imaging. 2014;7:870–878. doi: 10.1016/j.jcmg.2014.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cole TJ, Green PJ. Smoothing reference centile curves: the LMS method and penalized likelihood. Stat Med. 1992;11:1305–19. doi: 10.1002/sim.4780111005. [DOI] [PubMed] [Google Scholar]
- 38.Morgan CJ, Aban I. Methods for evaluating the agreement between diagnostic tests. J Nucl Cardiol. 2016;23:511–3. doi: 10.1007/s12350-015-0175-7. [DOI] [PubMed] [Google Scholar]
- 39.Engle WA, American Academy of Pediatrics Committee on Fetus and Newborn Age terminology during the perinatal period. Pediatrics. 2004;114:1362–4. doi: 10.1542/peds.2004-1915. [DOI] [PubMed] [Google Scholar]
- 40.Gaasch WH, Zile MR. Left Ventricular Structural Remodeling in Health and Disease: With Special Emphasis on Volume, Mass, and Geometry. J Am Coll Cardiol. 2011;58:1733–40. doi: 10.1016/j.jacc.2011.07.022. [DOI] [PubMed] [Google Scholar]
- 41.de Simone G, Daniels SR, Kimball TR, Roman MJ, Romano C, Chinali M, et al. Evaluation of Concentric Left Ventricular Geometry in Humans Evidence for Age-Related Systematic Underestimation. Hypertension. 2005;45:64–68. doi: 10.1161/01.HYP.0000150108.37527.57. [DOI] [PubMed] [Google Scholar]
- 42.Jurko A., Jr Echocardiographic evaluation of left ventricle postnatal growth in newborns and infants. Bratisl Lek Listy. 2004;105:78–85. [PubMed] [Google Scholar]
- 43.Skelton R, Gill AB, Parsons JM. Reference ranges for cardiac dimensions and blood flow velocity in preterm infants. Heart. 1998;80:281–285. doi: 10.1136/hrt.80.3.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zecca E, Romagnoli C, Vento G, De Carolis MP, De Rosa G, Tortorolo G. Left ventricle dimensions in preterm infants during the first month of life. Eur J Pediatr. 2001;160:227–30. doi: 10.1007/s004310000702. [DOI] [PubMed] [Google Scholar]
- 45.Abushaban L, Vel MT, Rathinasamy J, Sharma PN. Normal reference ranges for left ventricular dimensions in preterm infants. Ann Pediatr Cardiol. 2014;7:180–186. doi: 10.4103/0974-2069.140832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nagasawa H. Novel regression equations of left ventricular dimensions in infants less than 1 year of age and premature neonates obtained from echocardiographic examination. Cardiol Young. 2010;20:526–31. doi: 10.1017/S1047951110000661. [DOI] [PubMed] [Google Scholar]
- 47.Daniels SR, Kimball TR, Morrison JA, Khoury P, Meyer RA. Indexing Left Ventricular Mass to Account for Differences in Body size in Children and Adolescents Without Cardiovascular Disease. Am J Cardiol. 1995;76:699–701. doi: 10.1016/s0002-9149(99)80200-8. [DOI] [PubMed] [Google Scholar]
- 48.Khoury PR, Mitsnefes M, Daniels SR, Kimball TR. Age-Specific Reference Intervals for Indexed Left Ventricular Mass in Children. J Am Soc Echocardiogr. 2009;22:709–714. doi: 10.1016/j.echo.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 49.Kuch B, Hense HW, Gneiting B, Doring A, Muscholl M, Brockel U, et al. Body composition and prevalence of left ventricular hypertrophy. Circulation. 2000;102:405–10. doi: 10.1161/01.cir.102.4.405. [DOI] [PubMed] [Google Scholar]
- 50.Quang Dung N, Fusch G, Armbrust S, Jochum F, Fusch C. Body composition of preterm infants measured during the first months of life: bioelectrical impedance provides insignificant additional information compared to anthropometry alone. Eur J Pediatr. 2007;166:215–222. doi: 10.1007/s00431-006-0232-y. [DOI] [PubMed] [Google Scholar]
- 51.Hammami M, Koo WW, Hockman EM. Body composition of neonates from fan beam dual energy X-ray absorptiometry measurement. JPEN J Parenter Enteral Nutr. 2003;27:423–426. doi: 10.1177/0148607103027006423. [DOI] [PubMed] [Google Scholar]
- 52.Margossian R, Chen S, Sleeper LA, Tani LY, Shirali G, Golding F, et al. The reproducibility and absolute values of echocardiographic measurements of left ventricular size and function in children are algorithm dependent. J Am Soc Echocardiogr. 2015;28:549–558. doi: 10.1016/j.echo.2015.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]


