Abstract
Higher urine albumin-to-creatinine ratio (UACR) has been associated with cardiac dysfunction in the general population. We assessed the association of UACR with cardiac structure and function in the Echocardiographic Study of Latinos (Echo-SOL), an ancillary study of the Hispanic Community Health Study/Study of Latinos (HCHS/SOL) across 4 U.S. sites. Echo-SOL participants underwent standard 2-dimensional echocardiography, including speckle-tracking strain analysis. UACR was categorized as normal and high-normal (based on the midpoint of values below microalbuminuria), microalbuminuria (≥17 mg/g for men; ≥25 mg/g for women), and macroalbuminuria (≥250 mg/g; ≥355 mg/g). Simultaneous assessments were made of left ventricular (LV) mass index and hypertrophy, and measures of LV systolic and diastolic dysfunction. We assessed the association of UACR with subclinical cardiac measures, adjusting for sociodemographic and cardiometabolic factors. Among 1,815 participants (median age 54, female 65%), 42% had normal UACR, 43% high-normal UACR, 13% microalbuminuria, and 2% macroalbuminuria. Prevalence of LV hypertrophy was 13%, LV systolic dysfunction (ejection fraction <50%) 3%, and diastolic dysfunction 53%. After covariate adjustment, both micro- and macroalbuminuria were significantly associated with a two-fold increase in LV hypertrophy. Microalbuminuria but not macroalbuminuria was associated with worse global longitudinal strain. Elevated UACR, even at high-normal levels, was significantly associated with greater diastolic dysfunction. In conclusion, elevated UACR was associated with LV hypertrophy and diastolic dysfunction in the largest known population sample of U.S. Hispanic/Latinos. Screening and detection of even high-normal UACR could be of value to guide CVD prevention efforts among Hispanic/Latino Americans.
Keywords: cardiac dysfunction, echocardiography, kidney, strain
INTRODUCTION
Because U.S. Hispanics/Latinos have a high prevalence of diabetes and obesity,1 as well as poor health care access and cultural and linguistic barriers that may exacerbate health disparities,2 they may be particularly prone to heart failure and its complications. However, only limited data are available regarding heart failure and its precursors, which include structural heart disease in the absence of overt symptoms,3 among Hispanics/Latinos.4,5 In non-Hispanic populations, the urine albumin-to-creatinine ratio (UACR), a measure of proteinuria and marker of kidney damage, has been associated with cardiovascular disease (CVD) mortality and heart failure,6,7 as well as subclinical measures of cardiac structure and function.8 To our knowledge, no studies have examined the association between albuminuria and cardiac dysfunction in U.S. Hispanics/Latinos, despite a disproportionate burden of risk factors leading to chronic kidney disease in some Hispanic groups.9,10 Using data from the Echocardiographic Study of Latinos (Echo-SOL), we examined the association between UACR and measures of cardiac structure and function among 1,815 Hispanic/Latino individuals who underwent a standardized 2-D echocardiography protocol, including speckle-tracking analysis for measurement of longitudinal strain. We hypothesized that higher UACR would be associated with increased left ventricular (LV) mass and greater systolic and diastolic dysfunction, and that this association would be amplified among participants with impaired kidney function, diabetes mellitus, or hypertension. We also examined associations by sex and by Hispanic/Latino background.
METHODS
The Hispanic Community Health Study (HCHS)/Study of Latinos (SOL) is a community-based cohort study of 16,415 self-identified Hispanic/Latino persons from randomly selected households near 4 U.S. field centers (Bronx, Chicago, Miami, San Diego). The baseline examination was conducted from February 2008 to July 2011. Sample design and cohort selection have been previously described.11 Echo-SOL is an ancillary study to the HCHS/SOL designed to characterize cardiac remodeling and systolic and diastolic function in a representative subsample of participants age ≥45 years who were seen within 36 months of their initial visit.12 Echo-SOL used stratified random sampling to assure representation of the overall HCHS/SOL population. Echo-SOL participation rates averaged ~80% among those invited, and enrollment occurred from October 2011 through June 2014.
Echo-SOL participants included in this analysis had an interpretable echocardiogram and data available from the baseline HCHS/SOL visit on UACR and other biomarkers. For assessments of diastolic dysfunction, exclusion criteria included current pregnancy, atrial fibrillation identified through electrocardiography, more than mild valvular disease, LV ejection fraction <50%, and LV end-diastolic volume index >97 mL/m2. Transmitral E/A was not assessed in participants with an absent A wave owing to non-sinus rhythm at echocardiography.
Philips Ultrasound IE-33 or Sonos 5500/7500 was used for echocardiographic measurements at all study sites. This equipment was interfaced with a standard 2.5- to 3.5-MHz phased-array probe, according to American Society of Echocardiography (ASE) recommendations. Standard echocardiographic examination, including M-mode, 2-D, spectral, color-flow and tissue-Doppler study, was performed by experienced sonographers at each center. Speckle-tracking strain (STS) analysis was performed offline using the TomTec Cardiac Performance Analysis package (v1.2.2.7) on acquired 2-D images.13 A single reader technician and over-reader were used for all studies. Additional details are in the Supplemental Material.
We focused on several echocardiographic parameters of cardiac structure and function. LV mass index (LVMI) was estimated using 2-D guided M-mode measurements and indexed by sex to body surface area.14 LV hypertrophy was defined by dichotomizing LVMI as >115 g/m2 for men and >95 g/m2 for women.14 Two measures of global LV systolic dysfunction were defined: first, based on an LV ejection fraction <50%, obtained using the bi-plane Simpson method of disks, and second, based on global longitudinal strain (GLS) obtained from STS analysis. GLS is a more sensitive measure of systolic performance than LV ejection fraction, particularly among those with relatively preserved ejection fraction.15 As previously described,16 diastolic dysfunction was graded as 0, I, II, or III following an algorithm (Supplemental Figure) that combined ASE guidelines and Redfield criteria using 3 echocardiographic parameters: E/A ratio, E/e’ ratio and left atrial volume index (LAVI).17,18 We dichotomized diastolic dysfunction as grades I–III compared with grade 0, and separately examined LAVI; e’, defined as the average of early diastolic annular velocities of the septal and lateral mitral annulus; and E/e’ ratio.
UACR was measured in mg/g from spot urine samples at the baseline HCHS/SOL visit. Creatinine was assessed on a Roche Modular P Chemistry Analyzer using a creatinase enzymatic method. Urine albumin was assessed using an immunoturbidimetric method on the ProSpec nephelometric analyzer (Dade Behring, Marburg, Germany). We grouped UACR into 4 categories. More severe categories of kidney dysfunction were based on established sex-specific thresholds for microalbuminuria (≥17 and ≥25 mg/g for men and women, respectively) and macroalbuminuria (≥250 and ≥355 mg/g), whereas for the remaining UACR levels we used the midpoint of the distribution below microalbuminuria to create “normal” and “high-normal” levels.19 We also performed analyses grouping UACR into quartiles after stratification by sex.
Covariates included age, sex, Hispanic/Latino background (Cuban, Dominican, Mexican, Puerto Rican, other), acculturation (U.S.-born, lived in U.S. ≥10 years, lived in U.S. <10 years), study center, and cardiometabolic traits: body mass index (BMI), systolic blood pressure (SBP), use of antihypertensive medications, diabetes mellitus (including both self-reported physician diagnosis and undiagnosed diabetes mellitus identified through serum glucose ≥126 mg/dL if fasting >8 hours or ≥200 mg/dL if fasting ≤8 hours; post-OGTT glucose ≥200 mg/dL; or HgA1C ≥6.5%), smoking, HDL- and LDL-cholesterol levels, use of lipid-lowering medications, and estimated glomerular filtration rate (eGFR). eGFR was considered as a measure of impaired kidney function and determined based on serum cystatin C and creatinine, age, sex, and race.20
We examined bivariate relationships between UACR and cardiac outcomes using both categories defined by established cutpoints and sex-specific quartiles. To determine associations with UACR, we developed linear regression models for continuous outcomes and Poisson regression models for dichotomous outcomes to generate prevalence ratios. We adjusted for confounders through serial adjustment, starting first with a model with only UACR as the independent variable; then adding confounders related to demographic characteristics, including age, sex, and Hispanic/Latino background; and finally cardiometabolic risk factors.
We assessed effect modification by sex, eGFR (<60 versus ≥60 mL/min/1.73 m2 of body-surface area), diabetes, hypertension status (taking into account self-reported physician diagnosis, measured SBP and DBP, and use of anti-hypertensive medications). On the basis that ancestral or lifestyle differences may modify the role of albuminuria on cardiovascular health,21 we also examined groups defined by Hispanic/Latino background and level of acculturation. We tested statistical significance of effect modification through use of interaction terms in fully adjusted models, and conducted stratified analyses for interactions significant at the p<0.05 level. No differences were found in sensitivity analyses that excluded participants with pre-existing CVD (i.e., self-reported coronary heart disease, stroke, and heart failure [1% of the study population]), and therefore we report results in the entire group.
Analyses were conducted using R 3.2.0. To address missing data (<3% of values), we implemented multiple imputation using chained equations with the R package mice. All reported effect estimates were weighted to account for the disproportionate selection of the sample and to at least partially adjust for any bias effects due to differential nonresponse in the selected sample at the household and person levels. All participants provided informed consent, and the study was approved by each center’s institutional review board.
RESULTS
Demographic and clinical characteristics of the 1,815 study participants are shown in Table 1. Of note, nearly two-thirds of the mostly middle-aged cohort were women. Diagnosed diabetes mellitus was reported by nearly one-fifth, with another 9% having diabetes mellitus identified only through study visit measurements. Almost half had a history of hypertension.
Table 1.
Demographic and clinical characteristics of the Echo-SOL population (N=1,815).
| N | % | |
|---|---|---|
| Age (median, IQR) | 54 | 49–60 |
| Sex | ||
| Female | 1184 | 65% |
| Male | 631 | 35% |
| Field Center | ||
| Bronx | 549 | 30% |
| Miami | 500 | 28% |
| San Diego | 349 | 19% |
| Chicago | 417 | 23% |
| Ethnicity | ||
| Dominican | 326 | 18% |
| Central American | 176 | 10% |
| Cuban | 356 | 20% |
| Mexican | 456 | 25% |
| Puerto Rican | 347 | 19% |
| South American | 150 | 8% |
| Multiple or other | 4 | <1% |
| Acculturation | ||
| U.S.-born | 162 | 9% |
| Lived in U.S. for ≥10 years | 1291 | 71% |
| Lived in U.S. for <10 years | 359 | 20% |
| Body mass index (kg/m2) (median, IQR) | 29.4 | 26.3–33.5 |
| Systolic blood pressure (mm Hg) (median, IQR) | 135 | 123–148 |
| Diastolic blood pressure (mm Hg) (median, IQR) | 78 | 71–86 |
| Use of antihypertensive medications | 480 | 26% |
| History of hypertension | 860 | 47% |
| Diabetes diagnosis | ||
| Diagnosed diabetic before baseline | 336 | 19% |
| Undiagnosed diabetic | 161 | 9% |
| Non-diabetic | 1317 | 73% |
| Use of anti-diabetes medications | 290 | 16% |
| Smoking status | ||
| Never | 1068 | 59% |
| Former | 441 | 24% |
| Current | 304 | 17% |
| HDL-cholesterol (mg/dL) (median, IQR) | 49 | 42–58 |
| LDL-cholesterol (mg/dL) (median, IQR) | 127 | 104–151 |
| Use of lipid-lowering medications | 356 | 20% |
| Estimated glomerular filtration rate (mL/min/1.73 m2 of body-surface area) (median, IQR) | 96.8 | 84.5–107.2 |
| <60 | 69 | 4% |
| ≥60 | 1726 | 95% |
| History of coronary heart disease | 5 | <1% |
| History of stroke/transient ischemic attack | 9 | <1% |
| History of heart failure | 21 | 1% |
N and % presented unless otherwise indicated. IQR = interquartile range.
Missing data: acculturation, N=3; body mass index and diabetes, N=1; systolic blood pressure, N=21; diastolic blood pressure, N=22; use of medications, N=36; smoking and HDL cholesterol, N=2; LDL-cholesterol, N=26; eGFR, N=20.
Median UACR was 6.3 mg/g (IQR 4.3, 13.4) among men and 7.7 mg/g (IQR 5.3, 14.3) among women. Using established thresholds, 15% of men and 11% of women met criteria for microalbuminuria, while 3% of men and 1% of women met criteria for macroalbuminuria. Distributions of UACR among participants are shown by clinically defined categories of albuminuria in Table 2 and by quartiles of UACR in Supplemental Table 1.
Table 2.
Distribution of urine albumin-to-creatinine ratio (UACR) categories across measures of cardiac structure and function.
| Normal (N=727, 42%) | High-normal (N=733, 43%) | Microalbuminuria (N=222, 13%) | Macroalbuminuria (N=37, 2%) | |||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| N or mean | % or SD | N or mean | % or SD | N or mean | % or SD | N or mean | % or SD | |
| Cardiac structure | ||||||||
| Left ventricular mass index (mean g/m2, SD) | 77.9 | 17.5 | 81.7 | 19.0 | 90.3 | 24.6 | 104.4 | 31.5 |
| Left ventricular hypertrophy (N, %) | 56 | 8% | 97 | 13% | 62 | 28% | 13 | 35% |
| Systolic function | ||||||||
| Left ventricular ejection fraction (mean %, SD) | 60.1% | 5.7% | 60.4% | 5.3% | 58.9% | 6.0% | 60.4% | 5.5% |
| Left ventricular systolic dysfunction (ejection fraction <50%) (N, %) | 22 | 3% | 21 | 3% | 12 | 5% | 3 | 8% |
| Global longitudinal strain (mean %, SD) | −17.9% | 2.7% | −17.6% | 2.9% | −16.4% | 3.1% | −15.4% | 3.7% |
| Diastolic function | ||||||||
| Diastolic dysfunction* (N, %) | ||||||||
| Normal | 364 | 56% | 270 | 41% | 57 | 31% | 3 | 10% |
| Grade I | 63 | 10% | 99 | 15% | 40 | 22% | 12 | 41% |
| Grade II | 203 | 31% | 273 | 42% | 84 | 46% | 14 | 48% |
| Grade III | 20 | 3% | 14 | 2% | 3 | 2% | 0 | 0% |
| Grade I–III | 286 | 44% | 386 | 59% | 127 | 69% | 26 | 90% |
| Left atrial volume index (mean mL/m2, SD) | 22.6 | 6.5 | 23.5 | 7.5 | 24.4 | 9.0 | 25.9 | 7.5 |
| Early diastolic annular velocity (e’) (mean cm/sec, SD) | 8.56 | 1.99 | 7.97 | 2.06 | 7.42 | 1.99 | 6.66 | 1.82 |
| E/e’ ratio (mean, SD) | 9.43 | 2.61 | 10.19 | 3.04 | 10.95 | 3.55 | 12.80 | 4.57 |
SD = standard deviation.
Excluding current pregnancy, atrial fibrillation, more than mild valvular disease, left ventricular ejection fraction <50%, or left ventricular end-diastolic volume index >97 mL/m2.
For men, normal UACR range 1.36–<5.42, high normal range 5.42–<17, microalbuminuria range 17–<250, and macroalbuminuria range 250–≤3,429. For women, normal UACR range 2.33–<6.98, high normal range 6.98–<25, microalbuminuria range 25–<355, and macroalbuminuria range 355–≤11,470. Missing data: Left ventricular mass index and hypertrophy, N=41; left ventricular ejection fraction, N=89; global longitudinal strain and diastolic dysfunction, N=32; left atrial volume index, N=37; e’, N=10; E/e’ ratio, N=21.
Median LVMI among men was 87.3 g/m2 (IQR 74.6, 101.6) and among women 74.4 g/m2 (64.1, 86.2). Compared with normal UACR levels, macroalbuminuria was associated with a statistically significant 19.2 g/m2 increase in LVMI in fully adjusted analyses, while microalbuminuria was associated with a significant 5.6 g/m2 increase (Table 3). High-normal UACR levels did not show a significant association with LVMI as compared with normal levels.
Table 3.
Association of urine albumin-to-creatinine ratio (UACR) with measures of cardiac structure and function (N=1,815).
| Outcome and level of albuminuria | Model 1 | Model 2 | Model 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Left ventricular mass index | β, g/m2 | 95% CI | P-value | β, g/m2 | 95% CI | P-value | β, g/m2 | 95% CI | P-value |
| Normal UACR | 0.00 | Ref. | 0.00 | Ref. | 0.00 | Ref. | |||
| High-normal UACR | 2.27 | −0.60, 5.15 | 0.12 | 2.31 | −0.31, 4.93 | 0.08 | 1.03 | −1.48, 3.54 | 0.42 |
| Microalbuminuria | 10.92 | 6.85, 14.99 | <0.0001 | 8.85 | 5.03, 12.66 | <0.0001 | 5.57 | 1.97, 9.18 | 0.002 |
| Macroalbuminuria | 29.10 | 14.42, 43.78 | 0.0001 | 24.42 | 8.44, 40.40 | 0.003 | 19.24 | 3.57, 34.91 | 0.02 |
| Left ventricular hypertrophy | PrevR | 95% CI | P-value | PrevR | 95% CI | P-value | PrevR | 95% CI | P-value |
| Normal UACR | 1.00 | Ref. | 1.00 | Ref. | 1.00 | Ref. | |||
| High-normal UACR | 1.17 | 0.75, 1.82 | 0.49 | 1.11 | 0.70, 1.77 | 0.66 | 0.95 | 0.61, 1.49 | 0.83 |
| Microalbuminuria | 2.82 | 1.84, 4.31 | <0.0001 | 2.60 | 1.69, 3.98 | <0.0001 | 1.77 | 1.18, 2.66 | 0.01 |
| Macroalbuminuria | 3.37 | 1.68, 6.77 | 0.0006 | 3.14 | 1.55, 6.36 | 0.001 | 2.18 | 1.20, 3.97 | 0.01 |
| Left ventricular systolic dysfunction (ejection fraction <50%) | PrevR | 95% CI | P-value | PrevR | 95% CI | P-value | PrevR | 95% CI | P-value |
| Normal UACR | 1.00 | Ref. | 1.00 | Ref. | 1.00 | Ref. | |||
| High-normal UACR | 0.73 | 0.35, 1.53 | 0.41 | 0.76 | 0.37, 1.58 | 0.47 | 0.67 | 0.34, 1.32 | 0.25 |
| Microalbuminuria | 1.69 | 0.83, 3.45 | 0.15 | 1.58 | 0.77, 3.24 | 0.22 | 1.20 | 0.57, 2.51 | 0.64 |
| Macroalbuminuria | 2.39 | 0.54, 10.51 | 0.25 | 1.69 | 0.39, 7.26 | 0.48 | 1.71 | 0.43, 6.86 | 0.45 |
| Global longitudinal strain | β, % | 95% CI | P-value | β, % | 95% CI | P-value | β, % | 95% CI | P-value |
| Normal UACR | 0.00 | Ref. | 0.00 | Ref. | 0.00 | Ref. | |||
| High-normal UACR | 0.21 | −0.28, 0.69 | 0.40 | 0.22 | −0.19, 0.63 | 0.30 | 0.12 | −0.27, 0.51 | 0.55 |
| Microalbuminuria | 1.49 | 0.92, 2.05 | <0.0001 | 1.15 | 0.64, 1.66 | <0.0001 | 0.57 | 0.07, 1.07 | 0.02 |
| Macroalbuminuria | 1.91 | 0.56, 3.25 | 0.01 | 1.40 | −0.14, 2.94 | 0.07 | 0.81 | −0.63, 2.25 | 0.27 |
| Diastolic dysfunction (Grade I or higher) (N=1,628) | PrevR | 95% CI | P-value | PrevR | 95% CI | P-value | PrevR | 95% CI | P-value |
| Normal UACR | 1.00 | Ref. | 1.00 | Ref. | 1.00 | Ref. | |||
| High-normal UACR | 1.43 | 1.20, 1.70 | 0.0001 | 1.29 | 1.11, 1.50 | 0.0007 | 1.21 | 1.05, 1.39 | 0.01 |
| Microalbuminuria | 1.65 | 1.38, 1.98 | <0.0001 | 1.50 | 1.26, 1.78 | <0.0001 | 1.31 | 1.11, 1.54 | 0.001 |
| Macroalbuminuria | 2.29 | 1.97, 2.67 | <0.0001 | 1.98 | 1.63, 2.39 | <0.0001 | 1.61 | 1.27, 2.05 | 0.0001 |
| Left atrial volume index | β, mL/m2 | 95% CI | P-value | β, mL/m2 | 95% CI | P-value | β, mL/m2 | 95% CI | P-value |
| Normal UACR | 0.00 | Ref. | 0.00 | Ref. | 0.00 | Ref. | |||
| High-normal UACR | 0.73 | −0.22, 1.67 | 0.13 | 0.60 | −0.26, 1.45 | 0.17 | 0.55 | −0.34, 1.44 | 0.23 |
| Microalbuminuria | 1.48 | −0.12, 3.07 | 0.07 | 1.46 | −0.0009, 2.92 | 0.05 | 1.48 | 0.002, 2.96 | 0.05 |
| Macroalbuminuria | 4.36 | 0.92, 7.79 | 0.01 | 4.10 | 0.57, 7.63 | 0.02 | 3.15 | −0.003, 6.30 | 0.05 |
CI = confidence interval, PrevR = prevalence ratio, UACR = urine albumin-to-creatinine ratio.
Model 1 is unadjusted. Model 2 is adjusted for demographic characteristics (sex, age, Hispanic background, field center). Model 3 is adjusted for demographic characteristics and cardiometabolic risk factors (body mass index, systolic blood pressure, use of antihypertensive medications, diabetes, smoking status, HDL- and LDL-cholesterol, use of lipid-lowering medications, and estimated glomerular filtration rate).
LV hypertrophy was present in 11% of men and 14% of women. Associations of UACR with LV hypertrophy were similar in directionality to associations with LVMI (Table 3). In fully adjusted analyses, macroalbuminuria was significantly associated with a 2.2-fold increase in the prevalence of LV hypertrophy, compared with normal UACR, and microalbuminuria was significantly associated with an almost 2-fold increase. High-normal levels of UACR were associated with LV hypertrophy, but this association did not reach statistical significance.
Regarding LV systolic function, the median LV ejection fraction (LVEF) was 59.3% (IQR 54.9, 62.9) among men and 61.3% (IQR 57.9, 64.2) among women, with LV systolic dysfunction (defined as LVEF <50%) present among 3% of participants. Among men, median GLS was −16.6% (IQR −18.4, −14.8), and among women, it was −18.3% (IQR −20.1, −16.5), with less negative (i.e., more positive) values denoting worse LV function during systole.
In adjusted analyses, both macroalbuminuria and microalbuminuria showed greater systolic dysfunction (LVEF <50%), but these findings were not statistically significant (Table 3). In contrast, there was a significant association between microalbuminuria and less negative GLS (i.e., worse LV systolic function). A similar relationship was seen for macroalbuminuria but was not statistically significant. High-normal UACR was not associated with GLS.
There were 1,628 individuals eligible for analyses of diastolic dysfunction. Some degree of diastolic dysfunction was present in 53%, with grade I dysfunction occurring in 14%, grade II in 37%, and grade III in 2%. We found a graded association between UACR levels and presence of any diastolic dysfunction after adjusting for demographic and cardiometabolic risk factors (Table 3). Those with macroalbuminuria had the highest prevalence of diastolic dysfunction (61% higher than the normal UACR group), followed by those with microalbuminuria (31% higher). Notably, even participants with high-normal UACR had significantly higher prevalence of diastolic dysfunction compared with participants with normal UACR (by 21%). In analyses evaluating diastolic dysfunction grade as an ordinal variable, there was evidence of a dose-response relationship between levels of albuminuria and diastolic dysfunction (Supplemental Materials).
Analyses of LAVI were conducted in the entire study population. Median LAVI was 22.5 mL/m2 (IQR 18.3, 27.8) among men and 22.0 mL/m2 (IQR 18.1, 26.7) among women. Those with microalbuminuria, but not those with milder UACR elevations, had significantly elevated LAVI in fully adjusted analyses. There was suggestion of a stronger association for macroalbuminuria, but this did not attain statistical significance (Table 3).
Analyses of the associations of UACR with e’ and E/e’ showed similar findings as with diastolic dysfunction defined by grade (Supplemental Table 2) Both e’ and E/e’ exhibited a significant dose response with albuminuria, even at high-normal UACR levels.
We conducted secondary analyses that categorized UACR into quartiles instead of clinical categories of albuminuria (Supplemental Table 3). In general, we found that measures of diastolic dysfunction were more likely than other study outcomes to have a gradient across the UACR distribution. For example, each higher quartile of UACR was associated with a 12% increase in diastolic dysfunction after full adjustment (ptrend<0.0001). In contrast, LVMI and LV hypertrophy were significantly associated only with the highest quartile, and GLS and LAVI showed marginally significant associations for the highest quartile but not the other quartiles.
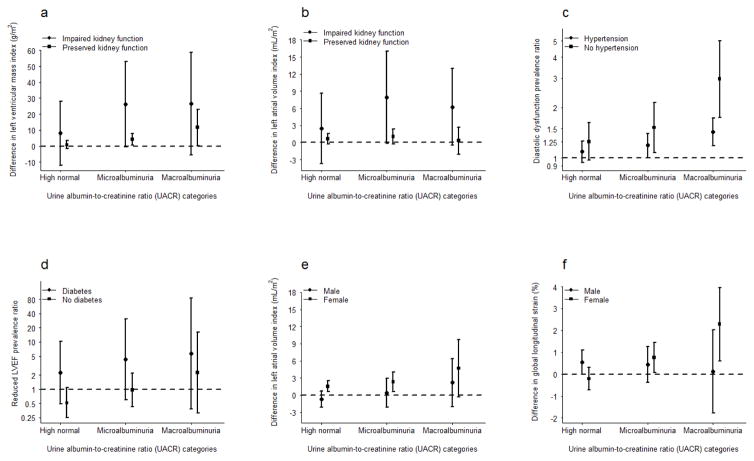
We explored whether associations of albuminuria with cardiac features were different in those with high-risk disorders. Figure 1 shows stratified associations of measures for which analyses yielded significant (p<0.05) interactions. Associations of UACR with LVMI and LAVI were more pronounced among those with low versus preserved eGFR. The association of UACR with reduced LVEF was stronger among diabetic than non-diabetic participants. By contrast, hypertensive participants showed a diminished association of albuminuria with diastolic dysfunction as compared with normotensive participants.
Figure 1. Association of albuminuria with (1a) left ventricular mass index and (1b) left atrial volume index, by eGFR; (1c) diastolic dysfunction, by hypertension status; (1d) reduced left ventricular ejection fraction, by diabetes status; and (1e) left atrial volume index and (1f) global longitudinal strain, by sex.
Reference group is normal UACR. Bars represent 95% confidence intervals. For men, high-normal range 5.42–<17 mg/g, microalbuminuria range 17–<250 mg/g, and macroalbuminuria range 250–≤3,429 mg/g. For women, high-normal range 6.98–<25 mg/g, microalbuminuria range 25–<355 mg/g, and macroalbuminuria range 355–≤11,470 mg/g. Impaired kidney function defined as eGFR <60 mL/min/1.73 m2 of body surface area.
We also explored potential effect modification of the association between UACR and cardiac dysfunction by sex and by Hispanic background and acculturation. There was evidence of more pronounced associations of UACR with GLS and LAVI among women (p<0.05, Figure 1). While reduced LVEF, GLS, and LV diastolic dysfunction showed significant effect modification by Hispanic background (p<0.05), no one group showed consistently increased strength of associations. We found no evidence of effect modification by time in the U.S.
DISCUSSION
In a broad Hispanic/Latino population largely free of clinical CVD, we found significant associations between albuminuria and several measures of subclinical cardiac structure and function. Our finding with respect to LV mass index is consistent with those from studies in other populations. High-normal UACR was significantly associated with greater LV mass in the MESA cohort,19 and LV mass increased with increasing quartiles of UACR in the HyperGEN study.22 Our results are similar, with the exception that LV mass index was not significantly elevated among those with high-normal as compared with normal UACR levels. This discrepancy may be due to differences in sample size or the younger age of our participants.
For diastolic function measures, even high-normal UACR was associated with greater impairment, suggesting that elevated levels below clinical thresholds for albuminuria may signal cardiac dysfunction and potential future heart failure risk. These findings are consistent with a previously reported dose-response relationship of albuminuria with clinical CVD events.23 In the present study, the relationship of albuminuria with diastolic dysfunction was of much greater magnitude than associations with LV structure or deformation measures. This observation supports the well-documented clinical usefulness of diastolic function parameters as measures of CVD risk.24 Indeed, diastolic dysfunction tends to precede the development of LV hypertrophy, and thus represents one of the earliest changes in hypertensive heart disease.25 A simple measure like UACR, when in the high-normal range, may indicate the presence of cardiac microvascular or endothelial dysfunction. This microvascular dysfunction has been proposed to be central to the development of diastolic dysfunction by leading to diminished NO bioavailability, reduced cGMP/PKG signaling, titin hypophosphorylation, increased stiffness of cardiomyocytes, and decreased LV compliance.26 Further, endothelial dysfunction may produce microvascular ischemia, resulting in impaired relaxation.
We did not find significant associations of UACR categories with systolic dysfunction defined by reduced LVEF, but participants with microalbuminuria exhibited significantly impaired strain as compared with those having normal UACR levels. There are conflicting findings in the literature with respect to the association between UACR and LV systolic function,8 likely in part due to the lack of sensitivity of LVEF for mild systolic dysfunction. Our inclusion of strain allowed us to better characterize systolic dysfunction as compared with most existing studies, and we found that microalbuminuria was significantly associated with impaired strain. The only other large study to use this measure to assess the relationship between UACR and systolic dysfunction, of which we are aware, found progressively impaired strain with increasing quartiles of UACR.27 We found a similar gradient, but it was not as steep and only marginally significant, possibly due a modest number of participants with macroalbuminuria in our study.
There was some evidence of more pronounced associations within higher-risk groups as we hypothesized; the associations of UACR with LV mass index and LAVI were accentuated in subgroups defined by low eGFR, and the association between UACR and reduced LVEF was stronger in diabetic participants. This latter finding could be due in part to relatively low treatment levels for diabetes. In contrast, the relationship of UACR with diastolic dysfunction appeared stronger in normotensive participants, which ran counter to our expectation. Because the observed effect modification was largely driven by the association in the macroalbuminuria subgroup, which had few participants, this finding will require replication in larger studies.
Because Echo-SOL comprised almost two-thirds women, we were able to assess interactions by sex. We detected stronger associations of UACR with strain and LAVI in women. Prior studies have found sex differences in the association of kidney disease with clinical CVD events,28 but not with LV dysfunction,15 and additional research is warranted to more fully understand these differences.
A limitation of our study is its cross-sectional design. Also, the number of participants with reduced LVEF was modest, and such reductions mostly fell in the category of mid-range (40%≤LVEF<50%),29 precluding separate assessment of more severe LV systolic dysfunction. Despite these limitations, our study is to our knowledge the largest and most comprehensive dataset of echocardiographic parameters focused solely on U.S. Hispanics/Latinos. We used state-of-the-art echocardiographic techniques to gain a more detailed understanding of cardiac function in the context of albuminuria, including GLS, which is a powerful technique for the assessment of early LV systolic dysfunction with distinct advantages over LVEF, including identification of more subtle degrees of dysfunction. Use of a single technician minimized inter-observer variability associated with strain measurements. Finally, we undertook detailed assessment of diastolic dysfunction incorporating transmitral Doppler, tissue Doppler, and LA volume measures, an approach that to our knowledge has been applied in one Chinese study of albuminuria.30 In summary, our findings support greater attention by providers to albuminuria, even at high-normal levels, as a risk factor for CVD. Interventions to screen for, prevent, and treat albuminuria may be important in Hispanics/Latinos, even in the absence of overt diabetes or hypertension, but this will require further evaluation.
Supplementary Material
Acknowledgments
Funding: The Hispanic Community Health Study/Study of Latinos was carried out as a collaborative study supported by contracts from the National Heart, Lung, and Blood Institute (NHLBI) to the University of North Carolina [grant number N01-HC65233], University of Miami [grant number N01-HC65234], Albert Einstein College of Medicine [grant number N01-HC65235], Northwestern University [grant number N01-HC65236], and San Diego State University [grant number N01-HC65237]. The following Institutes/Centers/Offices contribute to the HCHS/SOL through a transfer of funds to the NHLBI: National Institute on Minority Health and Health Disparities, National Institute on Deafness and Other Communication Disorders, National Institute of Dental and Craniofacial Research, National Institute of Diabetes and Digestive and Kidney Diseases, National Institute of Neurological Disorders and Stroke, NIH Institution-Office of Dietary Supplements. Echo-SOL was supported by a grant from NHLBI [grant number R01-HL104199, PI: Rodriguez]. D.B.H. was partially supported by an American Heart Association Mentored Clinical & Population Research Award [grant number 17MCPRP33410166]. The funding sources had no role in study design; collection, analysis and interpretation of data; writing of the report; or decision to submit the article for publication.
The authors acknowledge the investigators, staff, and participants of HCHS-SOL and Echo-SOL for their dedication and commitment to the success of this study. Investigator website: http://www.cscc.unc.edu/hchs/
Footnotes
Disclosures: J.R.K. has served as a consultant for ClearView Health Partners and reports stock ownership in Pfizer, Inc. and Gilead Sciences, Inc. All other authors report no potential conflicts.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Daviglus ML, Talavera GA, Aviles-Santa ML, Allison M, Cai J, Criqui MH, Gellman M, Giachello AL, Gouskova N, Kaplan RC, LaVange L, Penedo F, Perreira K, Pirzada A, Schneiderman N, Wassertheil-Smoller S, Sorlie PD, Stamler J. Prevalence of major cardiovascular risk factors and cardiovascular diseases among Hispanic/Latino individuals of diverse backgrounds in the United States. Jama. 2012;308:1775–1784. doi: 10.1001/jama.2012.14517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rodriguez CJ, Allison M, Daviglus ML, Isasi CR, Keller C, Leira EC, Palaniappan L, Pina IL, Ramirez SM, Rodriguez B, Sims M. Status of cardiovascular disease and stroke in Hispanics/Latinos in the United States: a science advisory from the American Heart Association. Circulation. 2014;130:593–625. doi: 10.1161/CIR.0000000000000071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL. 2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:1810–1852. doi: 10.1161/CIR.0b013e31829e8807. [DOI] [PubMed] [Google Scholar]
- 4.Bahrami H, Kronmal R, Bluemke DA, Olson J, Shea S, Liu K, Burke GL, Lima JA. Differences in the incidence of congestive heart failure by ethnicity: the multi-ethnic study of atherosclerosis. Arch Intern Med. 2008;168:2138–2145. doi: 10.1001/archinte.168.19.2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fernandes VR, Cheng S, Cheng YJ, Rosen B, Agarwal S, McClelland RL, Bluemke DA, Lima JA. Racial and ethnic differences in subclinical myocardial function: the Multi-Ethnic Study of Atherosclerosis. Heart. 2011;97:405–410. doi: 10.1136/hrt.2010.209452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS, de Jong PE, Coresh J, Gansevoort RT. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375:2073–2081. doi: 10.1016/S0140-6736(10)60674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matsushita K, Coresh J, Sang Y, Chalmers J, Fox C, Guallar E, Jafar T, Jassal SK, Landman GW, Muntner P, Roderick P, Sairenchi T, Schottker B, Shankar A, Shlipak M, Tonelli M, Townend J, van Zuilen A, Yamagishi K, Yamashita K, Gansevoort R, Sarnak M, Warnock DG, Woodward M, Arnlov J. Estimated glomerular filtration rate and albuminuria for prediction of cardiovascular outcomes: a collaborative meta-analysis of individual participant data. Lancet Diabetes Endocrinol. 2015;3:514–525. doi: 10.1016/S2213-8587(15)00040-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matsushita K, Ballew SH, Coresh J. Influence of chronic kidney disease on cardiac structure and function. Curr Hypertens Rep. 2015;17:581. doi: 10.1007/s11906-015-0581-x. [DOI] [PubMed] [Google Scholar]
- 9.Fischer MJ, Go AS, Lora CM, Ackerson L, Cohan J, Kusek JW, Mercado A, Ojo A, Ricardo AC, Rosen LK, Tao K, Xie D, Feldman HI, Lash JP. CKD in Hispanics: Baseline characteristics from the CRIC (Chronic Renal Insufficiency Cohort) and Hispanic-CRIC Studies. Am J Kidney Dis. 2011;58:214–227. doi: 10.1053/j.ajkd.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ricardo AC, Flessner MF, Eckfeldt JH, Eggers PW, Franceschini N, Go AS, Gotman NM, Kramer HJ, Kusek JW, Loehr LR, Melamed ML, Peralta CA, Raij L, Rosas SE, Talavera GA, Lash JP. Prevalence and correlates of CKD in Hispanics/Latinos in the United States. Clin J Am Soc Nephrol. 2015;10:1757–1766. doi: 10.2215/CJN.02020215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lavange LM, Kalsbeek WD, Sorlie PD, Aviles-Santa LM, Kaplan RC, Barnhart J, Liu K, Giachello A, Lee DJ, Ryan J, Criqui MH, Elder JP. Sample design and cohort selection in the Hispanic Community Health Study/Study of Latinos. Ann Epidemiol. 2010;20:642–649. doi: 10.1016/j.annepidem.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rodriguez CJ, Dharod A, Allison MA, Shah SJ, Hurwitz B, Bangdiwala SI, Gonzalez F, Kitzman D, Gillam L, Spevack D, Dadhania R, Langdon S, Kaplan R. Rationale and Design of the Echocardiographic Study of Hispanics/Latinos (ECHO-SOL) Ethn Dis. 2015;25:180–186. [PMC free article] [PubMed] [Google Scholar]
- 13.Pirat B, Khoury DS, Hartley CJ, Tiller L, Rao L, Schulz DG, Nagueh SF, Zoghbi WA. A novel feature-tracking echocardiographic method for the quantitation of regional myocardial function: validation in an animal model of ischemia-reperfusion. J Am Coll Cardiol. 2008;51:651–659. doi: 10.1016/j.jacc.2007.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39. e14. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 15.Kalam K, Otahal P, Marwick TH. Prognostic implications of global LV dysfunction: a systematic review and meta-analysis of global longitudinal strain and ejection fraction. Heart. 2014;100:1673–1680. doi: 10.1136/heartjnl-2014-305538. [DOI] [PubMed] [Google Scholar]
- 16.Mehta H, Armstrong A, Swett K, Shah SJ, Allison MA, Hurwitz B, Bangdiwala S, Dadhania R, Kitzman DW, Arguelles W, Lima JA, Youngblood M, Schneiderman N, Daviglus ML, Spevack D, Talavera GA, Raisinghani A, Kaplan R, Rodriguez CJ. Burden of systolic and diastolic left ventricular dysfunction among Hispanics in the United States: Insights from the Echocardiographic Study of Latinos. Circ Heart Failure. 2016;9:e002733. doi: 10.1161/CIRCHEARTFAILURE.115.002733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, Waggoner AD, Flachskampf FA, Pellikka PA, Evangelista A. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr. 2009;22:107–133. doi: 10.1016/j.echo.2008.11.023. [DOI] [PubMed] [Google Scholar]
- 18.Ommen SR, Nishimura RA, Appleton CP, Miller FA, Oh JK, Redfield MM, Tajik AJ. Clinical utility of Doppler echocardiography and tissue Doppler imaging in the estimation of left ventricular filling pressures: A comparative simultaneous Doppler-catheterization study. Circulation. 2000;102:1788–1794. doi: 10.1161/01.cir.102.15.1788. [DOI] [PubMed] [Google Scholar]
- 19.Kramer H, Jacobs DR, Jr, Bild D, Post W, Saad MF, Detrano R, Tracy R, Cooper R, Liu K. Urine albumin excretion and subclinical cardiovascular disease. The Multi-Ethnic Study of Atherosclerosis. Hypertension. 2005;46:38–43. doi: 10.1161/01.HYP.0000171189.48911.18. [DOI] [PubMed] [Google Scholar]
- 20.Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, Kusek JW, Manzi J, Van Lente F, Zhang YL, Coresh J, Levey AS. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367:20–29. doi: 10.1056/NEJMoa1114248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Effoe VS, Chen H, Moran A, Bertoni AG, Bluemke DA, Seeman T, Darwin C, Watson KE, Rodriguez CJ. Acculturation is associated with left ventricular mass in a multiethnic sample: the Multi-Ethnic Study of Atherosclerosis. BMC Cardiovasc Disord. 2015;15:161. doi: 10.1186/s12872-015-0157-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Djousse L, Kochar J, Hunt SC, North KE, Gu CC, Tang W, Arnett DK, Devereux RB. Relation of albuminuria to left ventricular mass (from the HyperGEN Study) Am J Cardiol. 2008;101:212–216. doi: 10.1016/j.amjcard.2007.07.065. [DOI] [PubMed] [Google Scholar]
- 23.Gansevoort RT, Correa-Rotter R, Hemmelgarn BR, Jafar TH, Heerspink HJ, Mann JF, Matsushita K, Wen CP. Chronic kidney disease and cardiovascular risk: epidemiology, mechanisms, and prevention. Lancet. 2013;382:339–352. doi: 10.1016/S0140-6736(13)60595-4. [DOI] [PubMed] [Google Scholar]
- 24.Kane GC, Karon BL, Mahoney DW, Redfield MM, Roger VL, Burnett JC, Jr, Jacobsen SJ, Rodeheffer RJ. Progression of left ventricular diastolic dysfunction and risk of heart failure. Jama. 2011;306:856–863. doi: 10.1001/jama.2011.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Graettinger WF, Longfellow JV, Klein RC, Weber MA. Diastolic blood pressure and left ventricular filling. Am J Hypertens. 1988;1:100s–102s. doi: 10.1093/ajh/1.3.100s. [DOI] [PubMed] [Google Scholar]
- 26.Paulus WJ, Tschope C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62:263–271. doi: 10.1016/j.jacc.2013.02.092. [DOI] [PubMed] [Google Scholar]
- 27.Katz DH, Selvaraj S, Aguilar FG, Martinez EE, Beussink L, Kim KY, Peng J, Sha J, Irvin MR, Eckfeldt JH, Turner ST, Freedman BI, Arnett DK, Shah SJ. Association of low-grade albuminuria with adverse cardiac mechanics: findings from the hypertension genetic epidemiology network (HyperGEN) study. Circulation. 2014;129:42–50. doi: 10.1161/CIRCULATIONAHA.113.003429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hui X, Matsushita K, Sang Y, Ballew SH, Fulop T, Coresh J. CKD and cardiovascular disease in the Atherosclerosis Risk in Communities (ARIC) study: interactions with age, sex, and race. Am J Kidney Dis. 2013;62:691–702. doi: 10.1053/j.ajkd.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lam CS, Teng TH. Understanding Heart Failure With Mid-Range Ejection Fraction. JACC Heart Fail. 2016;4:473–476. doi: 10.1016/j.jchf.2016.03.025. [DOI] [PubMed] [Google Scholar]
- 30.Zhou J, Cui X, Jin X, Zhou J, Zhang H, Tang B, Fu M, Herlitz H, Cui J, Zhu H, Sun A, Hu K, Ge J. Association of renal biochemical parameters with left ventricular diastolic dysfunction in a community-based elderly population in China: a cross-sectional study. PLoS One. 2014;9:e88638. doi: 10.1371/journal.pone.0088638. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.