Abstract
This unit describes protocols for the generation of clinical-grade patient-specific induced pluripotent stem cell (iPSC)-derived retinal cells from patients with inherited retinal degenerative blindness. Specifically, we describe how using xeno-free reagents in an ISO class 5 environment, one can isolate and culture dermal fibroblasts, generate iPSCs and derive autologous retinal cells via 3D differentiation. The universal methods described herein for the isolation of dermal fibroblasts, and generation of iPSCs can be employed regardless of disease, tissue or cell type of interest.
Keywords: fibroblasts, retina, induced pluripotent stem cells, xeno-free, current Good Manufacturing Practice, photoreceptor precursor cells
Introduction
IPSC-derived photoreceptor cells hold great promise as a potential treatment for blinding inherited disorders of the retina such as Leber congenital amaurosis, Stargardt disease and retinitis pigmentosa. These cells have been demonstrated to have the capacity to integrate within and even restore retinal function to diseased host retinas (Zhou et al., 2011; Lamba et al., 2010; Tucker et al., 2013b; Mandai et al., 2017). As the iPSC field moves closer and closer to using these cells therapeutically, there is a growing need for defined protocols describing clinically-feasible cell production. We recently described the isolation of dermal fibroblast and subsequent generation of patient-specific iPSCs and iPSC-derived photoreceptor precursor cells using completely xeno-free methods and current Good Manufacturing Practices (cGMP) (Wiley et al., 2016a). In this unit, we provide a detailed outline of the specific reagents and protocols used for this process.
Briefly, we describe the components of our non-profit clean room facility, which is dedicated to the xeno-free production of autologous patient-derived cells, including the isolation of dermal fibroblasts from 3mm skin biopsies (Protocol 1), generation of patient-specific iPSCs (Protocol 2) and derivation of iPSC-derived retinal organoids (Protocol 3). Although this unit specifically describes the production of iPSCs and iPSC-derived retinal cells for the purpose of treating retinal degenerative diseases, the methods described herein for the generation of iPSCs from dermal fibroblasts can be employed regardless of disease, tissue or cell type of interest.
DESCRIPTION OF NON-PROFIT cGMP FACILITY
The ability to generate patient-specific iPSCs, derive retinal cells, and rescue visual deficits following transplantation into diseased host retinas now exist. With stem cell-derived cellular therapies on the cusp of becoming a reality, protocols for generating and deploying cells clinically are required. Specifically, for autologous iPSC-derived products to be used clinically they will need to be generated, maintained, differentiated and stored in a manner that is compliant with cGMP practices.
As the equipment, expertise and facilities required for production of clinical-grade products are highly specialized, commercial contract research organizations (CROs) with validated cGMP production capabilities are often used. Although this strategy has been largely effective, the resources required to generate and fully validate iPSC-derived cells on a per patient basis is immense. Likewise, when using offsite CROs, it is logistically difficult to devise a strategy that does not include cryopreservation and shipment of the final product from the CRO to the site of the clinical trial. For trials focused on replacement of delicate neurons, avoiding this step is preferable. To address these issues we designed and constructed a non-profit cGMP facility that is dedicated to the development of stem cell-based therapies for the treatment of individuals with inherited retinal degenerative blindness. This facility, which was strategically placed in close proximity to our surgical suites, contains an ISO Class 7 material storage and handling room, two ISO Class 7 gowning areas, and two ISO Class 6 cell processing rooms both of which are equipped with custom closed-system BioSpherix Xvivo cell culture and incubation units (BioSpherix, Ltd.; Parish, NY) (Figure 1A). The BioSpherix Xvivo systems are designed to maintain and monitor atmospheric conditions in real time and exceed ISO Class 5 air quality standards.
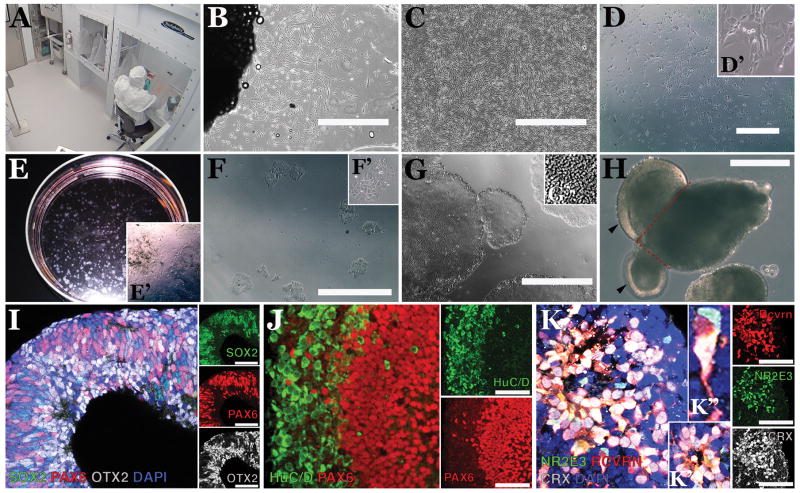
Figure 1. Generation of patient-specific fibroblasts, iPSCs and iPSC-derived retinal cells within an FDA-registered, cGMP-compatible non-profit facility.
A) Image acquired from a secure live-feed camera within the Dezii Translational Vision Research Facility at the Stephen A. Wynn Institute at the University of Iowa showing one of two working suites that include a BioSpherix Xvivo Closed Incubation System and a cellular technician feeding iPSCs through protective arm inserts. B) Light micrograph of dermal fibroblasts migrating and growing from a piece of a skin biopsy. C) Confluent patient-specific dermal fibroblasts. D) Patient fibroblasts 3 days post-Sendai viral transduction. Transduced cells are beginning to abandon the spindle appearance that is typical of fibroblasts (D′: high magnification inset). E) Developing iPSC colonies 25 days post-transduction (i.e., 21 days following passage from the 6-well plate into a 100 mm LN521-coated culture dish). Large distinct colonies can be readily identified at this stage. Note that cultures still contain un-reprogrammed patient-specific dermal fibroblasts (E′: high magnification inset). F) iPSC colonies at 24hrs post-manual isolation and passage from the 100 mm dish into a 12-well LN521-coated culture plate (note absence of un-reprogrammed dermal fibroblasts; F′: high magnification inset). G) Typical patient-specific iPSC colonies at passage 10 post-reprogramming. Cells within densely packed colonies display a classic iPSC morphology, including a high nuclear-to-cytoplasm ratio (G′). H) Example of an early iPSC-derived retinal organoid featuring evaginating loops of neural epithelium (arrowheads) that, following mechanical dissection (along red dotted lines) and further culture in neural retinal medium, will go on to develop cells of the retinal lineage. I) Retinal organoids at 30 days post-differentiation displaying clusters of cells that express the retinal progenitor-cell transcription factors, SOX2 (green), PAX6 (red) and OTX2 (gray). J) At 70 days post-differentiation developing organoids possess independent layers of cells that express the inner retinal-specific marker, HuC/D (green) and photoreceptor precursor cells that robustly express PAX6 (red). K) At 150 days post-differentiation the outer most layer is largely made up of neural rosettes (K′), which contain cells that express the photoreceptor-specific markers NR2E3 (green), Recoverin (RCVRN, red) and CRX (grey). Inset (K′) shows high magnification of a recoverin-positive photoreceptor-like cell with extended neural process. Small adjacent panels (I-K) are of individual fluorophores. Nuclei in I and K were counterstained with DAPI. Scale bars = 1000 μm in B-D and F, 400 μm in G-H and 100 μm in I-K.
ISOLATION AND PROCESSING OF PATIENT SKIN BIOPSY: ISOLATION, CULTURE AND FREEZING OF PATIENT-SPECIFIC FIBROBLASTS (PROTOCOL 1)
This protocol describes how to obtain a 3 mm skin biopsy and isolate and culture patient-specific human dermal fibroblasts.
Human Subjects
All skin biopsies, fibroblasts and iPSCs used to demonstrate the protocols throughout this article were derived from human patients. All patients provided written, informed consent for this study, which was approved by the Institutional Review Board of the University of Iowa (project approval #199904167) and adhered to the tenets set forth in the Declaration of Helsinki.
Materials and Equipment
Covidien Webcol 70% ethanol swabs (Medline Industries; Northfield, IL; Cat#: KDL5033)
Sterile hypodermic needle (Becton, Dickinson & Co.; Franklin Lakes, NJ; Cat#: 305106)
Sterile 1 mL Syringe (Becton, Dickinson & Co.; Franklin Lakes, NJ; Cat#: 309628)
Lidocaine HCL/Epinephrine mix (anesthetic) (Hospira Inc.; Lake Forest, IL; Cat#: 0409-3182-01)
Sterile 3 mm disposable skin punch (Integra Miltex; York, PA; Cat#: 33-32)
Sterile Forceps (Stephens Instruments; Lexington, KY; Cat#: S5-1570)
Sterile Scalpel (Bard-Parker; Caledonia, MI; Cat#: 371610)
Covidien Dermacea Sterile 2 × 2 gauze (Medline Industries; Northfield, IL; Cat#: 441205)
Tegaderm (3M Healthcare; Oakdale, MN; Cat#: 1624W)
2 mL cell cryo-vials (Simport; Beloeil, QC, Canada; Cat#: T311-2)
-
biopsy collection media
499 mL MEM-alpha (Gibco/Thermo Fisher Scientific; Grand Island, NY; Cat# 12571-063)
1 mL Primocin (InvivoGen; San Diego, CA; Cat# ant-pm-2)
BioSpherix Xvivo closed incubator/cell culture system (BioSpherix, Ltd.; Parish, NY)
EVOS Digital microscope (Thermo Fisher Scientific; Waltham, MA; Cat#: AME 3300)
Sorvall ST 8 tabletop centrifuge (Thermo Fisher Scientific; Waltham, MA; Cat#: 75007205)
−80°C freezer (Sanyo; San Diego, CA; Cat#: MDF-U700VXC)
15 mL and 50 mL polypropylene screw cap conical tubes (CellTreat Scientific Products; Pepperell, MA; Cat#s: 229411, 229418, 229421 and 229428)
Clean room marker (Mirconova, Torrance, CA, Cat#: PEN 40IR)
CoolCell LX cryo-freeze container (BioCision; San Rafael, CA; Cat#: BCS-405)
100 mm tissue culture-treated sterile dishes (Techno Plastic Products; Trasadingen, Switzerland; Cat#: 93040)
Costar 6-well tissue culture-treated sterile plates (Corning Life Sciences; Tewksbury, CA; Cat#: 3516)
Sterile forceps (Fine Science Tools; Foster City, CA; Cat#: 11251-10)
-
Skin biopsy rinsing solution
500 mL 1X HBSS no calcium, no magnesium (Gibco/Thermo Fisher Scientific; Grand Island, NY; Cat#: 14170-112)
1 mL Primocin (InvivoGen; San Diego, CA; Cat#: ant-pm-2)
-
University of Iowa xeno-free biopsy media (UIxMedia)
395 mL MEM-alpha (Gibco/Thermo Fisher Scientific, Grand Island, NY; Cat#: 12571-063)
50 mL CTS KnockOut Serum Replacement XenoFree Medium (Gibco/Thermo Fisher Scientific; Grand Island, NY; Cat#: 12618013)
50 mL Heat-Inactivated Human Serum (Innovative Research, Novi, MI; Cat#: IPLA-SERAB-HI)
5 mL CTS GlutaMAX-I Supplement (Gibco/Thermo Fisher Scientific; Grand Island, NY; Cat#: A1286001)
1 mL Primocin (InvivoGen, San Diego, CA; Cat#: ant-pm-2)
CTS TrypLE Select Enzyme (Gibco/Thermo Fisher Scientific, Grand Island, NY; Cat#: A1285901)
-
Fibroblast freezing media
heat-inactivated human serum (Innovative Research; Novi, MI; Cat#: IPLA-SERAB-HI)
20% DMSO (Sigma-Aldrich; St. Louis, MO; Cat#: D2438-5×10ML)
Scepter 2.0 Cell Counter and 60 μM sensors (EMD Millipore; Billerica, MA; Cat#s: PHCC20060 and PHCC60050)
Isolation of dermal punch biopsy
Dermal skin biopsies are best obtained from a non-sun exposed area of either the upper arm or abdomen.
-
1
Thoroughly cleanse the area to be biopsied with 70% ethanol (alcohol swabs work well).
-
2
Inject Lidocaine HCL/Epinephrine mix beneath the epidermis in the site to be biopsied. The injection should continue until a “bleb” or bubble has formed under the skin greater than 3 mm in diameter. A slight burning sensation, that will quickly subside as the site becomes numb, may be detected.
-
3
Wait 5 minutes for the Lidocaine HCL/Epinephrine mix to take effect and test the area to be biopsied for numbness prior to proceeding. A pressure sensation is normal and expected but there should be no pain. If the area requires more anaesthesia another injection (with a new syringe) can be made until the skin is completely anesthetized.
-
4
Using a sterile 3 mm skin punch the physician or nurse applies pressure while twisting in a “drilling” motion until the blade of the skin punch has pierced the epidermis of the skin. The blade should be about ½ exposed. It is normal for the patient to experience a pressure and twisting sensation, but no pain.
-
5
After the blade has sufficiently “cored” or carved out a 3 mm cylinder of skin, the skin punch is removed using sterile forceps. It is normal for the area to bleed after the punch is removed. Excess blood is wiped off using sterile 2 × 2 gauze to expose the biopsy site.
-
6
When the skin has been cored and cleared of excess blood, the next step is to remove the biopsy from the rest of the skin. Great care should be taken not to damage the epidermis by crushing it with forceps or by cutting it with a scalpel unnecessarily. The physician or nurse uses the sterile forceps to take hold of the dermis of the cored skin, pulls up the core to reveal excess dermis and subdermal fat, and uses the sterile scalpel in one or two cutting motions to cut the cored skin free.
-
7
Once the biopsy has been removed from the skin there will usually be some degree of bleeding which should be absorbed with sterile 2 × 2 gauze. The biopsy site is then covered with sterile Tegaderm and possibly fortified with sterile gauze if the bleeding threatens to soak the Tegaderm and/or the patient’s clothing. This “hole” in the skin will continue to bleed for the rest of the day and may or may not form a scab in a few days’ time.
-
8
The patient should be informed that the biopsy site should be kept clean. The biopsy site should not be submerged in water (i.e. no swimming, hot tubs, baths, etc.) for 5–7 days. The bandage should be changed at least once a day and should be changed if it should become wet or damp. Once a substantial scab has formed, or new skin begins to grow and bleeding has stopped, the bandage can be removed. In the long term, minimal scaring may occur. In most cases the biopsy site is indistinguishable within a few months. In a few instances the biopsy site may form a protrusion or bump but will continue to heal normally.
-
9
Clearly label the biopsy tube containing biopsy medium with the patient’s name, date of birth, and date of collection.
-
10
Place biopsy in sterile biohazard bag and place in a lockable transport case. Lock case and transport directly to a cGMP facility.
Processing of skin biopsy
-
11
Following transport, remove the biohazard bag from transport case and pass into the cGMP facility and, in turn, the BioSpherix unit according to facility SOPs.
-
12
Once the tube containing the skin biopsy is placed inside of the BioSpherix unit, transfer the contents of the vial onto a sterile 100 mm petri dish. Pick the sample up using sterile forceps and transfer to a 50 ml tube containing 25 ml of skin biopsy rinsing solution. Invert the tube 10 times. Allow the biopsy to settle, aspirate the rinse solution and repeat with a fresh 25 ml of skin biopsy rinsing solution.
-
13
Following the second rinse, transfer the skin biopsy to a 6-well tissue culture-treated plate. Mince the biopsy into fine pieces using a sterile scalpel blade.
-
14
Allow skin biopsy pieces to air dry for 5 minutes. Carefully add 3 mL of UIxMedia to each well.
-
15
Label the plate with correct patient biopsy information, date and scientist’s initials.
-
16
Place plate in incubator with the following settings: 5% CO2, 20% O2, 37°C.
-
17
Feed cells with 3 mL of fresh UIxMedia every other day. Typically, you will begin to see significant dermal fibroblast growth at 3–5 days post-plating (Figure 1B).
Passaging and freezing of patient-derived dermal fibroblasts
-
18
Once cells are 100% confluent (Figure 1C), cultures can be passaged and either re-plated for iPSC generation or cryopreserved for future use (i.e., iPSC generation or long-term storage).
-
19
To passage fibroblasts add 1 mL of CTS TrypLE Select Enzyme to each well and incubate for 5 minutes at 37°C. After incubation, agitate the cells and transfer them into a 15 mL conical tube with an equal amount of UIxMedia.
-
20
Centrifuge the cell mixture for 3 minutes at room temperature at 1000 rpm.
-
21
Carefully aspirate the supernatant leaving only the cell pellet behind.
-
22
To continue culturing, re-suspend the pellet in UIxMedia at a density of 100,000 cells/mL (Cell concentration determined using the Scepter 2.0 Cell Counter). Plate in a 6-well tissue culture-treated plate at a density of 250,000 cells/well, and add media until there is a total of 3 mL of fresh UIxMedia.
-
23
To freeze, re-suspend the pellet in 0.5 mL of UIxMedia per 500,000 cells and add 0.5 mL of cell suspension to each cryo-vial. Next add 0.5 mL of fibroblast freezing media to each cryo-vial. Transfer vials into cell cryo-freeze container and place in −80°C freezer overnight. 16–24 hours later, move cells into liquid nitrogen for long-term storage.
GENERATION OF PATIENT-SPECIFIC INDUCED PLURIPOTENT STEM CELLS: REPROGRAMMING, MAINTENANCE AND FREEZING OF IPSCS (PROTOCOL 2)
This protocol describes the xeno-free production of patient-specific dermal fibroblast-derived induced pluripotent stem cells (iPSCs).
Materials and Equipment
BioSpherix Xvivo closed incubator/cell culture system (BioSpherix, Ltd.; Parish, NY)
Sorvall ST 8 tabletop centrifuge (Thermo Fisher Scientific; Waltham, MA; Cat#: 75007205)
EVOS Digital microscope (Thermo Fisher Scientific; Waltham, MA; Cat#: AME 3300)
Recombinant human laminin-521 (Corning Life Sciences; Tewksbury, CA; Cat#: 354222)
Costar 6-well tissue culture-treated sterile plates (Corning Life Sciences; Tewksbury, CA; Cat#: 3516)
Costar 12-well tissue culture-treated sterile plates (Corning Life Sciences; Tewksbury, CA; Cat#: 3513)
100 mm tissue culture-treated sterile dishes (Techno Plastic Products; Trasadingen, Switzerland; Cat#: 93040)
CTS TrypLE Select Enzyme (Gibco/Thermo Fisher Scientific, Grand Island, NY; Cat#: A1285901)
Y-27632 ROCK inhibitor (EMD Millipore; Billerica, MA; Cat#: 688000)
Hank’s Balanced Salt Solution plus magnesium, plus calcium (HBSS++; Gibco/Thermo Fisher Scientific; Grand Island, NY; Cat#: 14025092)
-
Serum-free Iowa xeno-free biopsy media (serum-free UIxMedia)
445 mL MEM-alpha (Gibco/Thermo Fisher Scientific, Grand Island, NY; Cat#: 12571-063)
50 mL CTS KnockOut Serum Replacement XenoFree Medium (Gibco/Thermo Fisher Scientific; Grand Island, NY; Cat#: 12618013)
5 mL CTS GlutaMAX-I Supplement (Gibco/Thermo Fisher Scientific; Grand Island, NY; Cat#: A1286001)
1 mL Primocin (InvivoGen, San Diego, CA; Cat#: ant-pm-2)
CytoTune-iPS 2.0 Sendai Reprogramming Kit driving expression of human OCT4, SOX2, KLF4 and c-MYC (Invitrogen/Thermo Fisher Scientific; Waltham, MA; Cat#: A16517)
Essential 8 Medium (Gibco/Thermo Fisher Scientific; Grand Island, NY; Cat#: A1517001)
rhFGF2 cGMP Grade (Waisman Biomanufacturing, Madison, WI; Cat#: rhFGF)
RevitaCell (Gibco/Thermo Fisher Scientific; Grand Island, NY; Cat#: A2644501)
Versene (Gibco/Thermo Fisher Scientific; Grand Island, NY; Cat#: 15040-066)
Viral transduction of patient fibroblasts and iPSC generation
-
24
The day prior to viral transduction and initiation of iPSC generation, plate 250,000 patient-specific fibroblasts into 1 well of a 6-well tissue culture-treated plate.
-
25
The following day (1-day post-plating) replace media with serum-free UIxMedia.
-
26
On day 2 post-plating, add viral transduction media and incubate overnight at 5% CO2, 20% O2, 37°C. Viral Transduction Media is comprised of 1 ml serum-free UIxMedia, 10 μM Y-27632 ROCK inhibitor and CytoTune-iPS 2.0 Sendai Reprogramming Kit at an MOI of 3–5.
-
27
12–24 hours post-transduction wash out viral transduction media with serum-free UIxMedia. Culture transduced cells for an additional 5 days, feeding cells with fresh serum-free UIxMedia media every other day. At 2–3 days post-transduction a change in dermal fibroblast morphology can begin to be seen (Figure 1D and 1D′).
-
28
On day 4 post-transduction, prepare recombinant human laminin-521 (10 μg/mL recombinant human laminin-521 in HBSS++)-coated 100 mm tissue culture dishes.
-
29
On day 5, use CTS TrypLE Select Enzyme to passage 200,000 transduced cells onto recombinant human laminin-521-coated 100 mm dishes with serum-free UIxMedia and 10 μM Y-27632 ROCK inhibitor (ROCKi can be substituted with RevitaCell if so desired).
-
30
On day 6, feed cells with equal parts serum-free UIxMedia and Essential 8 media supplemented with rhFGF2 (10 ng/ml).
-
31
From day 7 to day 30, feed cells every day with Essential 8 media supplemented with rhFGF2 (10 ng/ml).
-
32
Within 1–4 weeks post-transduction, distinct iPSC colonies will appear. Once colonies reach 1–2 mm in diameter (Figure 1E and 1E′), manually passage colonies onto 12-well recombinant human laminin-521-coated tissue culture plates (Figure 1F and 1F′, 24 hours post-manual passage, note lack of contaminating dermal fibroblasts) for clonal expansion in Essential 8 media plus rhFGF2 (10 ng/ml).
-
33
Feed iPSCs daily with fresh Essential 8 media supplemented with rhFGF2 (10 ng/ml).
-
34
Expand iPSCs using Versene as per the manufacturers recommendations for a minimum of 10 passages (Figure 1G and 1G′) prior to post-generation analysis (i.e., karyotyping (Songstad et al., 2015; Wiley et al., 2016a), TaqMan Scorecard analysis (Wiley et al., 2016a), whole genome sequencing (Wiley et al., 2016a) and rt-PCR (Tucker et al., 2011b; 2013b; Wiley et al., 2016b)) to demonstrate endogenous expression of pluripotency factors, loss of expression of Sendai reprogramming vectors and lack of genetic anomalies.
PATIENT-SPECIFIC RETINAL CELL GENERATION VIA 3-DIMENSIONAL DIFFERENTIATION (PROTOCOL 3)
This protocol describes how to generate retinal cells from patient-specific iPSCs using xeno-free, cGMP-validated reagents via a 3-dimensional (3D) suspension culture approach.
Materials and Equipment
Patient-specific iPSCs (from Protocol 2 above)
BioSpherix Xvivo closed incubator/cell culture system (BioSpherix, Ltd.; Parish, NY)
EVOS Digital microscope (Thermo Fisher Scientific; Waltham, MA; Cat#: AME 3300)
Sorvall ST 8 Tabletop Centrifuge (Thermo Fisher Scientific; Waltham, MA; Cat#: 75007205)
Scepter 2.0 Cell Counter and 40 μM sensors (EMD Millipore; Billerica, MA; Cat#s: PHCC20040 and PHCC40050)
CTS TrypLE Select Enzyme (Gibco/Thermo Fisher Scientific, Grand Island, NY; Cat#: A1285901)
Corning spheroid 96-well microplate, ultra-low attachment (Corning Life Sciences; Tewksbury, CA; Cat#: #4520 or CLS7007)
ClipTip 30–300 μl multi-channel pipettor (Thermo Fisher Scientific; Grand Island, NY; Cat#: 4661140)
100 mm ultra-low attachment culture dishes (Corning Life Sciences; Tewksbury, CA; Cat#: 3262)
-
3D differentiation media
395 mL CTS KnockOut DMEM (Gibco/Thermo Fisher Scientific; Grand Island, NY; Cat#: A1286101)
50 mL heat-inactivated human serum (Innovative Research; Novi, MI; Cat#: IPLA-SERAB-HI)
100 mL CTS KnockOut Serum Replacement XenoFree medium (Gibco/Thermo Fisher Scientific; Grand Island, NY; Cat#: 12618013)
0.1 mM MEM non-essential amino acids (Gibco/Thermo Fisher Scientific; Grand Island, NY; Cat#: 11140-050)
1 mL Primocin (InvivoGen; San Diego, CA; Cat#: ant-pm-2)
1 mM Sodium Pyruvate (Gibco/Thermo Fisher Scientific; Grand Island, NY; Cat#: 11360-070)
-
0.1 mM 2-Mercaptoethanol (Gibco/Thermo Fisher Scientific; Grand Island, NY; Cat#: 21985-023)
The following are added as supplements to the working aliquot of 3D Differentiation Media:
-
1% ECM (1% ECM mixture)
human type 1 collagen (Advanced BioMatrix, Carlsbad, CA; Cat#: 5007-20ML)
human type 3 collagen (Advanced BioMatrix, Carlsbad, CA; Cat#: 5021-10MG)
human vitronectin (Advanced BioMatrix, Carlsbad, CA; Cat#: 5051-0.1MG)
human fibronectin (Advanced BioMatrix, Carlsbad, CA; Cat#: 5050-1MG)
10 μM Y-27632 ROCK inhibitor (EMD Millipore; Billerica, MA; Cat#: 688000)
3 μM IWR1e (Cayman Chemical; Ann Arbor, MI; Cat#: 13659)
40 nM StemMACS CHIR99021 (Miltenyi Biotec Inc.; San Diego, CA; Cat#: 130-103-926)
100 nM SAG (Enzo Life Sciences; Farmingdale, NY, Cat#: ALX-270-426)
-
Neural Retina culture medium (NR media)
489 mL CTS KnockOut DMEM/F12 (Gibco/Thermo Fisher Scientific; Grand Island, NY; Cat#: A1370801)
2 mM CTS GlutaMAX-I Supplement (Gibco/Thermo Fisher Scientific; Grand Island, NY; Cat#: A1286001)
1 mL Primocin (InvivoGen; San Diego, CA; Cat#: ant-pm-2)
-
5 mL CTS N-2 supplement (Gibco/Thermo Fisher Scientific; Grand Island, NY; Cat#: A1370701)
The following is added to the working aliquot of NR media:
10 μM DAPT (EMD Millipore; Billerica, MA; Cat#: 565770).
3D differentiation of patient-derived iPSCs into retinal lineages
-
35
Aspirate cell culture media from iPSCs and add CTS TrypLE Select Enzyme and incubate for 3–5 minutes at 37°C.
-
36
After incubation, vigorously tap the sides of the culture plate to release cells. Use a 1 mL pipettor to gently flush cells from the plate. Pipette cells into a 50 mL sterile conical centrifuge tube. Rinse remaining adherent cells with 2 mL of 3D differentiation media/well and add to the cell suspension in the centrifuge tube.
-
37
Centrifuge cell suspension at 200 × g for 3 minutes, aspirate the media and resuspend cell pellet in 1mL of 3D differentiation media. Count cells using the Scepter 2.0 Cell Counter and sensors.
-
38
For seeding of each 96-well plate, add 1×106 cells to 10 mL of 3D differentiation media supplemented with 10 μM Y-27632 ROCK inhibitor and 3 μM IWR1e.
-
39
Using a multichannel pipettor, add 1×104 cells/100 μL 3D differentiation media supplemented with 10 μM Y-27632 ROCK inhibitor and 3 μM IWR1e. Incubate at 5% CO2, 20% O2, 37°C.
-
40
Two days later, add 100 μL 3D differentiation media supplemented with 10 μM Y-27632 ROCK inhibitor, 3 μM IWR1e and 1% ECM.
-
41
On days 6 and 10, use the multichannel pipettor to remove 100 μL of media and carefully add 100 μL fresh 3D differentiation media supplemented with 10 μM Y-27632 ROCK inhibitor, 3 μM IWR1e and 1% ECM (same formula as day 2).
-
42
On day 12, use a 1 mL pipettor (using a sterile scalpel inside the Biospherix unit cut off tip of pipette to avoid breaking spheres) to transfer spheres to 100 mm ultra-low attachment culture dishes at a density of one 96-well plate per dish. Remove media and replace with fresh 3D differentiation media supplemented with 1% ECM only. Feed cells with fresh media on Monday, Wednesday and Friday from this point forward.
-
43
On days 14–17, feed spheres with 3D differentiation media supplemented with 1% ECM, 40 nM CHIR and 100 nM SAG.
-
44
On day 18, remove 3D differentiation media from spheres and switch to NR media. Continue feeding every Monday, Wednesday and Friday.
-
45
On days 30–40, supplement NR media with 10 μM DAPT to encourage photoreceptor cell production.
-
46
Neural epithelium isolation: Between days 30 and 4o neural epithelium, which is destined to become neural retina, can clearly be identified as distinct evaginations from the surface of the larger sphere (Figure 1H, arrowheads). These neural epithelium protrusions can be manually dissected and cultured free from the remaining tissue. This is typically performed under 4–10X magnification using an 18-gauge needle to pin the main sphere in place and a micro dissection scalpel to cut the neural epithelium free (Figure 1H, dotted lines). Following dissection neural epithelium are moved to a new 100 mm culture dish using a wide bore 1 mL pipette tip (i.e. as above cut the tip to allow adequate space for the retinal cup to be aspirated without damaging).
-
47
Continue feeding organoids with NR Media for as long as differentiation is desired. Although there is some inter-patient/line variability, in general mature photoreceptor precursor cells expressing the transcription factors CRX and NR2E3 and the photoreceptor cell marker recoverin can be detected between 120–150 days post-differentiation (Figure 1K).
COMMENTARY
Background Information
Since the seminal discovery by Shinya Yamanaka (Takahashi et al., 2007) that four independent transcription factors, OCT4, SOX2, KLF4 and c-MYC, were sufficient to reprogram dermal fibroblasts into induced pluripotent stem cells (iPSCs), many groups in the field of eye research have been utilizing this technology to investigate the pathophysiological mechanisms of inherited retinal disease and develop autologous cell replacement-based treatment approaches (Tucker et al., 2011b; 2013a; 2013b; Burnight et al., 2014; Zhong et al., 2014; Meyer et al., 2011; Gamm and Meyer, 2010; Jin et al., 2011; Takahashi et al., 2007). While early studies utilized a two-dimensional differentiation protocol (Tucker et al., 2011a; 2013a; Lamba et al., 2006; Jin et al., 2012; Osakada et al., 2009; Buchholz et al., 2013; 2009; Klassen et al., 2004; MacLaren et al., 2006; Carr et al., 2009), recently many groups have adopted a three-dimensional approach that more closely mimics the spatio-temporal aspects of retinal development in vivo (Small et al., 2015; Phillips et al., 2014; Meyer et al., 2009; Nakano et al., 2012; Eiraku et al., 2011; Zhong et al., 2014).
Phase I/II clinical safety trials using embryonic stem cell (ESC)-derived retinal pigmented epithelial (RPE) cells to treat patients with advanced dry (i.e., geographic atrophy) age-related macular degeneration (AMD) (NCT01344993) and Stargardt macular dystrophy (NCT01345006) were recently completed. ESC-derived RPE cells were reported to be well-tolerated following subretinal transplantation, and there was not evidence of adverse cellular proliferation or tumor formation (Schwartz et al., 2015). Although these findings were promising, as ESC cell-based transplants are by definition allogenic (i.e., donor cells are genetically and immunologically different than the transplant recipient) long-term success will likely require life-long immunosuppression. Although efforts are underway to make ESCs more universal, that is, immunologically-tolerable through human leukocyte antigen-matching (Riolobos et al., 2013; Gourraud et al., 2012), iPSCs represent a source of patient-specific cells that are immunologically matched and can be generated in large numbers from the individuals for whom they are intended.
While patient-specific iPSCs offer an advantage over ESCs with respect to immunologic matching, as indicated above, iPSC generation, differentiation and end product validation is cost-prohibitive when using a standard commercial CRO pipeline on a population-wide scale. In this unit we describe the infrastructure (i.e., small dedicated cGMP-compatible facility), detailed protocols and reagents suitable for the xeno-free culture of human skin fibroblasts, generation of patient-specific iPSCs and derivation of retinal cells suitable for autologous iPSC-based phase 1 clinical safety trials.
Although this approach does reduce the cost to the point where early phase clinical trials become feasible, the fact remains that it is difficult for a single technician in a small cGMP facility to produce more than a handful of patient-specific iPSC-derived retinal cell lines per year. Although one could increase throughput by increasing the size of the workforce, this approach introduces the possibility of inter-technician variability and is accompanied by an increased risk for human error. An alternative approach would be to automate, via introduction of robotic technologies (Paull et al., 2015), primary cell culture, iPSC generation and tissue-specific differentiation. Robotics have the potential to significantly increase the number of cell lines that can be produced while simultaneously decreasing the risk of human-mediated error. Such an approach would increase reproducibility between cells lines while also reducing costs.
Critical Parameters and Troubleshooting
The protocols described in this unit have been used for the successful generation of patient-specific iPSCs and iPSC-derived retinal cells (Wiley et al., 2016a). That said, there are several critical parameters that are worthy of discussion prior to establishing a stem cell program intended for downstream therapeutic application. For instance, in 2016 iPSCs were first used in human clinical trial at the RIKEN Institute in Japan for the replacement of RPE cells in patients with AMD. This trial was interrupted when one of the cGMP-produced patient-specific iPSC lines was found to have a genomic mutation in an oncogene which was introduced during the process of reprogramming (Chakradhar, 2016). In addition to cellular reprogramming, genetic mutations have been shown to arise with time and passage in culture (Liu et al., 2014), regardless of the cellular origin of the stem cells. As such, to identify potentially harmful variants induced via reprogramming or cell culture, it is essential that newly-established iPSC lines be genetically characterized. Two well developed approaches suitable for this purpose are karyotyping, which can be used to identify gross chromosomal abnormalities, and whole genome sequencing, which can be used to find defects in a single base pair (Wiley et al., 2016a; Bhutani et al., 2016).
Unfortunately, not all reagents required for xeno-free production of patient-specific iPSCs and iPSC-derived retinal tissue are produced under cGMP conditions and/or come with sufficient certificates of analysis to be used for clinical application. For this reason, some of the reagents used may need to be validated prior to release and subsequent use in a cGMP facility. For example, the CytoTune-iPS 2.0 Sendai Reprogramming Kit (Invitrogen/Thermo Fisher Scientific) is sterile and xeno-free, but is provided as a non-commercial research-grade kit only. Confirmation that this kit meets cGMP standards must be established prior to release to the cGMP facility and use for production of clinical-grade iPSCs (Wiley et al., 2016a).
The ability to isolate and expand dermal fibroblasts and in turn generate patient-specific iPSCs is highly influenced by patient age. It is sometimes difficult to obtain dermal fibroblasts from elderly individuals in sufficient numbers for successful generation of high quality iPSCs. This is especially true when using xeno-free media, which by definition does not contain FBS, a major component of standard fibroblast growth medias. For elderly patients that we have had difficulty growing a sufficient number of dermal fibroblasts for iPSC generation, we have found that addition of cGMP-grade bFGF (10 ng/mL, Waisman Biomanufacturing; Madison, WI; Cat#: rhFGF) and a 1% extracellular matrix protein mixture to the UIxMedia [Advanced BioMatrix; Carlsbad, CA; collagen type 1 (Cat#: 5007-20ML), collagen type 3 (Cat#: 5021-MG), vitronectin (Cat#: 5051-0.1MG) and fibronectin (Cat#: 5050-1MG)] is sufficient to overcome age-dependent fibroblast senescence. Typically, addition of the 1% ECM mixture is only required for the first 48 hours post-plating.
As previously published (Wiley et al., 2016a), the efficiency of eye cup formation can be quite variable, i.e., the number of spheres generated with well-formed neural epithelia can be different between experiments and patient-specific iPSC lines. One of the key steps in the differentiation process is determining the appropriate plating cell density used for sphere formation (Step 39). If the cell density is too low there is typically a significant degree of cell death and spheres are often loosely packed and poorly organized. If cell density is too high the spheres tend to grow very rapidly, the core of the sphere rapidly becomes necrotic and it is difficult to identify and isolate the neural epithelium for dissection and subsequent retinal cup formation (Step 46). Using the above described protocol, we have found the optimal plating density can vary between 5,000 and 15,000 cells per well of a 96-well sphere forming plate. The number used is typically determined empirically and depends largely upon the growth characteristics and propensity of the iPSC line in question to differentiate. As indicated above (Step 39) we typically start differentiating a new line using a plating density of 10,000 cells per well. If excessive cell death is detected and spheres remain small or spheres rapidly become too large and neural epithelial protrusions are difficult to identify and/or isolate, a second dose escalation experiment should be performed, in which 10,000 cells are used as either the lower limit, if the original spheres were small and diffuse, or the upper limit, if spheres were too large.
Anticipated Results
We are confident that adherence to the protocols and reagents described within this unit will yield successful and efficient generation of patient-specific dermal fibroblasts, iPSCs and iPSC-derived retinal cells. Protocols 1 and 2 that detail the isolation and culture of human fibroblasts from a patient dermal biopsy and the reprogramming of patient fibroblasts are a reusable approach that can be applied universally for the generation of human iPSCs, regardless of patient age and tissue type of interest. Protocol 3 that describes the derivation of iPSC-derived retinal cells will result in robust generation of retinal-specific cells and, in turn, photoreceptor precursor cells suitable for retinal transplantation.
Time Considerations
The most necessary and key component for implementation of a strategy for the generation of xeno-free, cGMP-compatible patient-specific iPSCs is the design and construction of an on-site cGMP facility. The facility shown in Figure 1 took approximately 1 year to design and construct, and it took approximately 1 additional year for facility validation, SOP and process development. The isolation, expansion and cryopreservation of fibroblasts from patient skin biopsy takes ~20 days (Wiley et al., 2016a). Reprogramming of fibroblasts, clonal selection and expansion of patient-specific iPSCs and genomic and pluripotency analysis of iPSCs typically takes ~70 days, but can vary depending on how smoothly analysis goes (Wiley et al., 2016a). The initial stages of three-dimensional differentiation of iPSCs, including early organoid development to manual dissection/isolation of organoids with good neural epithelium takes ~30 days. Subsequent neural retinal development and maturation can take an additional ~40–100 days (Figure 1I–K) depending on the stage of retinal lineage you are interested in (i.e., retinal precursor cells versus photoreceptor precursor cells) (Wiley et al., 2016a).
Significance Statement.
Although there has been much discussion surrounding the potential of patient-specific induced pluripotent stem cells (iPSCs) for autologous cell replacement, clinical translation requires that cells are generated in a clinically-compatible manner. Thus, there is a need for detailed protocols, which adhere to current Good Manufacturing Practices (cGMP), that describe how to generate iPSCs and in turn differentiate them into the cell types of interest. In this unit we describe how to culture and reprogram patient-specific dermal fibroblasts into iPSCs, using xeno-free, cGMP-compatible methods, and in turn how to differentiate these cells using a three-dimensional retinal organoid system.
Acknowledgments
We would like to achknowledge our funding sources: The Stephen A. Wynn Foundation, The Elmer and Sylvia Sramek Charitable Foundation, National Institutes of Health Grants (NIH; Bethesda, MD, USA) EY-024605.
Literature Cited
- Chakradhar S. An eye to the future: Researchers debate best path for stem cell-derived therapies. Nature Medicine. 2016;22(2):116–9. doi: 10.1038/nm0216-116. [DOI] [PubMed] [Google Scholar]
- Bhutani K, Nazor KL, Williams R, Tran H, Dai H, Džakula Ž, Cho EH, Pang AW, Rao M, Cao H, Schork NJ, Loring JF. Whole-genome mutational burden analysis of three pluripotency induction methods. Nature Communications. 2016;7:10536. doi: 10.1038/ncomms10536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchholz DE, Pennington BO, Croze RH, Hinman CR, Coffey PJ, Clegg DO. Rapid and efficient directed differentiation of human pluripotent stem cells into retinal pigmented epithelium. Stem Cells Translational Medicine. 2013;2(5):384–93. doi: 10.5966/sctm.2012-0163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchholz DE, Hikita ST, Rowland TJ, Friedrich AM, Hinman CR, Johnson LV, Clegg DO. Derivation of functional retinal pigmented epithelium from induced pluripotent stem cells. Stem Cells. 2009;27(10):2427–34. doi: 10.1002/stem.189. [DOI] [PubMed] [Google Scholar]
- Burnight ER, Wiley LA, Drack AV, Braun TA, Anfinson KR, Kaalberg EE, Halder JA, Affatigato LM, Mullins RF, Stone EM, Tucker BA. CEP290 gene transfer rescues Leber congenital amaurosis cellular phenotype. Gene Therapy. 2014;21(7):662–72. doi: 10.1038/gt.2014.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr AJ, Vugler AA, Hikita ST, Lawrence JM, Gias C, Chen LL, Buchholz DE, Ahmado A, Semo M, Smart MJ, Hasan S, da Cruz L, Johnson LV, Clegg DO, Coffey PJ. Protective effects of human iPS-derived retinal pigment epithelium cell transplantation in the retinal dystrophic rat. PloS One. 2009;4(12):e8152. doi: 10.1371/journal.pone.0008152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiraku M, Takata N, Ishibashi H, Kawada M, Sakakura E, Okuda S, Sekiguchi K, Adachi T, Sasai Y. Self-organizing optic-cup morphogenesis in three-dimensional culture. Nature. 2011;472(7341):51–6. doi: 10.1038/nature09941. [DOI] [PubMed] [Google Scholar]
- Gamm DM, Meyer JS. Directed differentiation of human induced pluripotent stem cells: a retina perspective. Regenerative Medicine. 2010;5(3):315–7. doi: 10.2217/rme.10.28. [DOI] [PubMed] [Google Scholar]
- Gourraud PA, Gilson L, Girard M, Peschanski M. The role of human leukocyte antigen matching in the development of multiethnic “haplobank” of induced pluripotent stem cell lines. Stem Cells. 2012;30(2):180–6. doi: 10.1002/stem.772. [DOI] [PubMed] [Google Scholar]
- Mandai M, Fujii M, Hashiguchi T, Sunagawa GA, Ito S, Sun J, Kaneko J, Sho J, Yamada C, Takahashi M. iPSC-Derived Retina Transplants Improve Vision in rd1 End-Stage Retinal-Degeneration Mice. Stem Cell Reports. 2017;8(1):69–83. doi: 10.1016/j.stemcr.2016.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin ZB, Okamoto S, Osakada F, Homma K, Assawachananont J, Hirami Y, Iwata T, Takahashi M. Modeling retinal degeneration using patient-specific induced pluripotent stem cells. PloS One. 2011;6(2):e17084. doi: 10.1371/journal.pone.0017084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin ZB, Okamoto S, Xiang P, Takahashi M. Integration-free induced pluripotent stem cells derived from retinitis pigmentosa patient for disease modeling. Stem Cells Translational Medicine. 2012;1(6):503–9. doi: 10.5966/sctm.2012-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klassen HJ, Ng TF, Kurimoto Y, Kirov I, Shatos M, Coffey P, Young MJ. Multipotent retinal progenitors express developmental markers, differentiate into retinal neurons, and preserve light-mediated behavior. Investigative Ophthalmology & Visual Science. 2004;45:4167–4173. doi: 10.1167/iovs.04-0511. [DOI] [PubMed] [Google Scholar]
- Lamba DA, Karl MO, Ware CB, Reh TA. Efficient generation of retinal progenitor cells from human embryonic stem cells. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(34):12769–74. doi: 10.1073/pnas.0601990103. Epub 2006 Aug 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamba DA, McUsic A, Hirata RK, Wang PR, Russell D, Reh TA. Generation, purification and transplantation of photoreceptors derived from human induced pluripotent stem cells. PloS One. 2010;5(1):e8763. doi: 10.1371/journal.pone.0008763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P, Kaplan A, Yuan B, Hanna JH, Lupski JR, Reiner O. Passage number is a major contributor to genomic structural variations in mouse iPSCs. Stem Cells. 2014;32(10):2657–67. doi: 10.1002/stem.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLaren RE, Pearson RA, MacNeil A, Douglas RH, Salt TE, Akimoto M, Swaroop A, Sowden JC, Ali RR. Retinal repair by transplantation of photoreceptor precursors. Nature. 2006;444(7116):203–7. doi: 10.1038/nature05161. [DOI] [PubMed] [Google Scholar]
- Meyer JS, Howden SE, Wallace KA, Verhoeven AD, Wright LS, Capowski EE, Pinilla I, Martin JM, Tian S, Stewart R, Pattnaik B, Thomson JA, Gamm DM. Optic vesicle-like structures derived from human pluripotent stem cells facilitate a customized approach to retinal disease treatment. Stem Cells. 2011;29(8):1206–18. doi: 10.1002/stem.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano T, Ando S, Takata N, Kawada M, Muguruma K, Sekiguchi K, Saito K, Yonemura S, Eiraku M, Sasai Y. Self-formation of optic cups and storable stratified neural retina from human ESCs. Cell Stem Cell. 2012;10(6):771–85. doi: 10.1016/j.stem.2012.05.009. [DOI] [PubMed] [Google Scholar]
- Osakada F, Jin ZB, Hirami Y, Ikeda H, Danjyo T, Watanabe K, Sasai Y, Takahashi M. In vitro differentiation of retinal cells from human pluripotent stem cells by small-molecule induction. Journal of Cell Science. 2009;122(Pt 17):3169–79. doi: 10.1242/jcs.050393. [DOI] [PubMed] [Google Scholar]
- Paull D, Sevilla A, Zhou H, Hahn AK, Kim H, Napolitano C, Tsankov A, Shang L, Krumholz K, Jagadessan P, Woodard CM, Sun B, Vilboux T, Zimmer M, Forero E, Moroziewicz DN, Martinez H, Malicdan MC, Weiss KA, Vensand LB, Dusenberry CR, Polus H, Sy KT, Kahler DJ, Gahl WA, Solomon SL, Chang S, Meissner A, Eggan K, Noggle SA. Automated, high-throughput derivation, characterization and differentiation of induced pluripotent stem cells. Nature Methods. 2015;12(9):885–92. doi: 10.1038/nmeth.3507. [DOI] [PubMed] [Google Scholar]
- Phillips MJ, Perez ET, Martin JM, Reshel ST, Wallace KA, Capowski EE, Singh R, Wright LS, Clark EM, Barney PM, Stewart R, Dickerson SJ, Miller MJ, Percin EF, Thomson JA, Gamm DM. Modeling human retinal development with patient-specific induced pluripotent stem cells reveals multiple roles for visual system homeobox 2. Stem Cells. 2014;32(6):1480–92. doi: 10.1002/stem.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riolobos L, Hirata RK, Turtle CJ, Wang PR, Gornalusse GG, Zavajlevski M, Riddell SR, Russell DW. HLA engineering of human pluripotent stem cells. Molecular Therapy. 2013;21(6):1232–41. doi: 10.1038/mt.2013.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz SD, Regillo CD, Lam BL, Eliott D, Rosenfeld PJ, Gregori NZ, Hubschman JP, Davis JL, Heilwell G, Spirn M, Maguire J, Gay R, Bateman J, Ostrick RM, Morris D, Vincent M, Anglade E, Del Priore LV, Lanza R. Human embryonic stem cell-derived retinal pigment epithelium in patients with age-related macular degeneration and Stargardt’s macular dystrophy: follow-up of two open-label phase 1/2 studies. Lancet. 2015;385(9967):509–16. doi: 10.1016/S0140-6736(14)61376-3. [DOI] [PubMed] [Google Scholar]
- Small KW, DeLuca AP, Whitmore SS, Rosenberg T, Silva-Garcia R, Udar N, Puech B, Garcia CA, Rice TA, Fishman GA, Heon E, Folk JC, Streb LM, Haas CM, Wiley LA, Scheetz TE, Fingert JH, Mullins RF, Tucker BA, Stone EM. North Carolina Macular Dystrophy Is Caused by Dysregulation of the Retinal Transcription Factor PRDM13. Ophthalmology. 2016;123(1):9–18. doi: 10.1016/j.ophtha.2015.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Songstad AE, Wiley LA, Duong K, Kaalberg E, Flamme-Wiese MJ, Cranston CM, Riker MJ, Levasseur D, Stone EM, Mullins RF, Tucker BA. Generating iPSC-Derived Choroidal Endothelial Cells to Study Age-Related Macular Degeneration. Investigative Ophthalmology & Visual Science. 2015;56(13):8258–67. doi: 10.1167/iovs.15-17073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861–72. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Tucker BA, Anfinson KR, Mullins RF, Stone EM, Young MJ. Use of a synthetic xeno-free culture substrate for induced pluripotent stem cell induction and retinal differentiation. Stem Cells Translational Medicine. 2013a;2(1):16–24. doi: 10.5966/sctm.2012-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker BA, Mullins RF, Streb LM, Anfinson K, Eyestone ME, Kaalberg E, Riker MJ, Drack AV, Braun TA, Stone EM. Patient-specific iPSC-derived photoreceptor precursor cells as a means to investigate retinitis pigmentosa. Elife. 2013b;2:e00824. doi: 10.7554/eLife.00824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker BA, Park IH, Qi SD, Klassen HJ, Jiang C, Yao J, Redenti S, Daley GQ, Young MJ. Transplantation of adult mouse iPS cell-derived photoreceptor precursors restores retinal structure and function in degenerative mice. PloS One. 2011a;6(4):e18992. doi: 10.1371/journal.pone.0018992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker BA, Scheetz TE, Mullins RF, DeLuca AP, Hoffmann JM, Johnston RM, Jacobson SG, Sheffield VC, Stone EM. Exome sequencing and analysis of induced pluripotent stem cells identify the cilia-related gene male germ cell-associated kinase (MAK) as a cause of retinitis pigmentosa. Proceedings of the National Academy of Sciences of the United States of America. 2011b;108(34):E569–76. doi: 10.1073/pnas.1108918108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker BA, Cranston CM, Anfinson KA, Shrestha S, Streb LM, Leon A, Mullins RF, Stone EM. Using patient specific iPSCs to interrogate the pathogenicity of a novel RPE65 cryptic splice site mutation and confirm eligibility for enrollment into a clinical gene augmentation trial. Translational Research. 2015;166(6):740–749. e1. doi: 10.1016/j.trsl.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer JS, Shearer RL, Capowski EE, Wright LS, Wallace KA, McMillan EL, Zhang SC, Gamm DM. Modeling early retinal development with human embryonic and induced pluripotent stem cells. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(39):16698–703. doi: 10.1073/pnas.0905245106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley LA, Burnight ER, DeLuca AP, Anfinson KR, Cranston CM, Kaalberg EE, Penticoff JA, Affatigato LM, Mullins RF, Stone EM, Tucker BA. cGMP production of patient-specific iPSCs and photoreceptor precursor cells to treat retinal degenerative blindness. Scientific Reports. 2016a;6:30742. doi: 10.1038/srep30742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley LA, Burnight ER, Drack AV, Banach BB, Ochoa D, Cranston CM, Madumba RA, East JS, Mullins RF, Stone EM, Tucker BA. Using Patient-Specific Induced Pluripotent Stem Cells and Wild-Type Mice to Develop a Gene Augmentation-Based Strategy to Treat CLN3-Associated Retinal Degeneration. Human Gene Therapy. 2016b doi: 10.1089/hum.2016.049. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong X, Gutierrez C, Xue T, Hampton C, Vergara MN, Cao LH, Peters A, Park TS, Zambidis ET, Meyer JS, Gamm DM, Yau KW, Canto-Soler MV. Generation of three-dimensional retinal tissue with functional photoreceptors from human iPSCs. Nature Communications. 2014;5:4047. doi: 10.1038/ncomms5047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Wang W, Liu Y, Fernandez de Castro J, Ezashi T, Telugu BP, Roberts RM, Kaplan HJ, Dean DC. Differentiation of induced pluripotent stem cells of swine into rod photoreceptors and their integration into the retina. Stem Cells. 2011;29(6):972–80. doi: 10.1002/stem.637. [DOI] [PMC free article] [PubMed] [Google Scholar]