Abstract
With the use of magnetic resonance imaging (MRI) and brain analysis tools, it has become possible to measure brain volume changes up to around 0.5%. Besides long-term brain changes caused by atrophy in aging or neurodegenerative disease, short-term mechanisms that influence brain volume may exist. When we focus on short-term changes of the brain, changes may be either physiological or pathological. As such determining the cause of volumetric dynamics of the brain is essential. Additionally for an accurate interpretation of longitudinal brain volume measures by means of neurodegeneration, knowledge about the short-term changes is needed. Therefore, in this review, we discuss the possible mechanisms influencing brain volumes on a short-term basis and set-out a framework of MRI techniques to be used for volumetric changes as well as the used analysis tools. 3D T1-weighted images are the images of choice when it comes to MRI of brain volume. These images are excellent to determine brain volume and can be used together with an analysis tool to determine the degree of volume change. Mechanisms that decrease global brain volume are: fluid restriction, evening MRI measurements, corticosteroids, antipsychotics and short-term effects of pathological processes like Alzheimer's disease, hypertension and Diabetes mellitus type II. Mechanisms increasing the brain volume include fluid intake, morning MRI measurements, surgical revascularization and probably medications like anti-inflammatory drugs and anti-hypertensive medication. Exercise was found to have no effect on brain volume on a short-term basis, which may imply that dehydration caused by exercise differs from dehydration by fluid restriction. In the upcoming years, attention should be directed towards studies investigating physiological short-term changes within the light of long-term pathological changes. Ultimately this may lead to a better understanding of the physiological short-term effects of pathological processes and may aid in early detection of these diseases.
Abbreviations: CVR, Cerebrovascular reactivity
Keywords: Brain volume, Magnetic resonance imaging, Dehydration, FSL
Highlights
-
•
Fluid-restriction, evening MRI, corticosteroids, & antipsychotics decrease volume
-
•
Fluid-intake, morning MRI, surgical revascularization & medications increase volume
-
•
Short-term changes within the light of long-term pathological changes should be investigated
-
•
Short-term changes may introduce bias in longitudinal data
1. Introduction
Nowadays, magnetic resonance imaging (MRI) is used to non-invasively investigate tissue loss overtime (Ashburner and Ridgway, 2013, Fox and Freeborough, 1997, Leung et al., 2012, Rudick et al., 1999, Smith et al., 2002), whereas in the early days it was predominantly investigated through post-mortem studies (Alzheimer, 1907, Braak and Braak, 1995, Braak and Braak, 1991, Dawson, 1916). Therefore, brain tissue loss can be assessed prior to death and changes can be monitored during life (Chetelat and Baron, 2003, Fox et al., 2000, Fox et al., 2014, Fox and Freeborough, 1997, Frisoni, 2001). Characterization of chronic brain atrophy by means of MRI is already well established and used widely in longitudinal studies (Fox and Freeborough, 1997, Rudick et al., 1999). The current brain imaging techniques and analysis tools are, however, so sensitive and precise that changes in brain volume can be measure up to around 0.5% (Caramanos et al., 2010, Chard et al., 2002, Rudick et al., 1999, Smith et al., 2001). These changes lie within the range of yearly brain changes in normal aging (Fisher et al., 2008) and may be short-term physiological fluctuations of the brain caused by fluid restriction (Biller et al., 2015, Dickson et al., 2005, Duning et al., 2005, Kempton et al., 2011, Kempton et al., 2009, Meyers et al., 2016, Streitbürger et al., 2012) or use of medication, but could also be caused by pathological processes like diabetes, hypertension and Alzheimer's disease (AD) (Meusel et al., 2014, Thornton, 2014). In addition, it is though that during the night a redistribution of body fluids occurs, therefore brain volume even changes during the day, with a decrease in volume observed by the end of the day (Nakamura et al., 2015). For an accurate interpretation of brain volume measurements it is, therefore, essential to know to what extent the short-term changes – ranging from hours to a couple of days – are caused by physiological fluctuations or due to pathological differences. Furthermore for the interpretation of longitudinal data knowledge of the short-term changes is also crucial since they may introduce bias and additional corrections may be needed. Therefore, in this review, we discuss the possible mechanisms that can influence global brain volumes on a short-time basis. We first focus on the MRI sequences used to image brain changes, then we shift focus to the analysis tools and finally we elaborate on the different mechanisms influencing volumetric brain dynamics.
2. Magnetic resonance imaging and analysis tools
MRI allows for the detection of both structural and functional changes. For the short-term changes the used techniques are: 3D T1- and T2-weighted imaging, 1H-MR spectroscopy, blood‑oxygenated level dependent functional MRI (BOLD-fMRI), and pseudo-continuous arterial spin labeling (pcASL). 3D T1-and T2-weighted imaging is used to measure structural brain changes, like atrophy. 1H-MR Spectroscopy determines the magnetic properties of 1H atoms. BOLD-fMRI enables the indirect measure of differences in neural activity of the resting-state and pcASL provides information about the perfusion status of the brain and permits quantification of cerebral blood flow perfusion, thus measuring blood flow alterations and perfusion deficits in various disorders (Alsop et al., 2015).
Many analysis tools exist to analyze the above-mentioned imaging techniques. Analysis libraries commonly used for this are FMRIB's software library (FSL) (Jenkinson et al., 2012, Smith et al., 2004, Woolrich et al., 2009) and statistical parameter mapping (SPM) (Ashburner, 2012). The libraries contain of different analysis tools, either to analyze functional imaging data obtained from fMRI or to analyze anatomical data from the 3D T1-weighted images. Combining the tools available in the libraries allow for robust measurements. When focusing on normal aging, atrophy rates are shown to lie between 0.1 and 0.3% per year (Fisher et al., 2008, Fotenos et al., 2005, RI et al., 2003). For the neurodegenerative disorders, atrophy rates are higher, with annual rates between 1.0 and 3.0% (Fox and Freeborough, 1997, Jack et al., 2004). As such, these analysis tools have led to increased knowledge about annual atrophy rates in normal aging and neurodegenerative disorders, but can also be used so assess the short-term changes and their role in longitudinal studies.
3. Short-term mechanisms
Now we shift focus towards the short-term mechanisms of brain volume changes. As mentioned, these changes may be physiological fluctuations of the brain, but can also be caused by pathological processes. Furthermore short-term mechanisms may influence different regions of the brain, depending on the mechanism but also on the method used to analyze changes. Regions to be affected are: gray and white matter, total brain, subcortical regions and ventricles. Moreover the overall BOLD response and perfusion may be affected as well. Short-term changes are defined as changes caused within hours to a couple of days, depending on the investigated mechanism. In the sections below we will discuss each of these mechanisms in more detail.
3.1. Hydration status
3.1.1. Fluid restriction
Since the introduction of automated analysis tools many studies have been conducted to investigate annual atrophy rates in several neurodegenerative disorders. Moreover, with the use of these tools evaluation on therapy can be monitored. There are, however, factors that influence brain volume on a physiological level. Therefore corrections are needed to overcome potential overshadowing or overestimation of treatment effect or over- or underestimations of the annual atrophy rates in longitudinal studies by these fluctuations. One mechanism influencing brain volume is the hydration status of the brain. A couple of studies investigated the effect of dehydration on brain volume (Biller et al., 2015, Duning et al., 2005, Meyers et al., 2016, Nakamura et al., 2014, Streitbürger et al., 2012). These studies relatively consistently showed that brain volume is affected by fluid restriction for 9 (Meyers et al., 2016), 12 (Biller et al., 2015), or 16 (Duning et al., 2005) hours or longer (Streitbürger et al., 2012), with a decrease in brain volume ranging from − 0.03% (Meyers et al., 2016) to − 1.7% (Streitbürger et al., 2012) when examining structural 3D T1-weighted MRI scans (Table 1). However, the study from Nakamura et al. (2014) did not find any differences during the dehydration state. But baseline scans, indicating normal hydration, were obtained several days or even weeks before the dehydration scan resulting in a less-controlled research set-up, which may have contributed to these negative results. Moreover, the observed decrease in brain volume nicely corresponded to an increase in cerebrospinal fluid (CSF) volume between 0.22% and 2.6% as was demonstrated by Meyers et al. (2016) and Streitbürger et al. (2012), respectively. Focusing on the effects of rehydration, also consistent results have been shown (Biller et al., 2015, Duning et al., 2005, Meyers et al., 2016, Nakamura et al., 2014, Streitbürger et al., 2012), demonstrating increased brain volumes after rehydration with a range between 0.15% (Meyers et al., 2016) and 3.5% (Streitbürger et al., 2012) with a corresponding decrease in CSF volume (Meyers et al., 2016) (Table 1). Additional, 1H–MR Spectroscopy also demonstrated a decrease and an increase of brain tissue fluid after dehydration and rehydration, respectively (Biller et al., 2015).
Table 1.
Studies investigating mechanisms of brain volume fluctuations.
| Mechanism | Author | MRI sequence | Analysis tool (version) | Study population | Number of patients | Effect on brain volume (numbers in %) |
Effect on ventricular volume |
|---|---|---|---|---|---|---|---|
| Dehydration-Fluid restriction | Duning et al. (2005) | -3D T1-weighted | SIENA (FSL) | Healthy volunteers | 20 | ↓ 0.55⁎⁎–↑ 0.72⁎ | n.a |
| Streitbürger et al. (2012) | -3D T1-weighted | VBM8 (SPM), SIENAr (v5.0) and FreeSurfer (v4.5) | Healthy volunteers | 6 | ↓ 1.7⁎⁎–↑ 3.5⁎ | ↑ 2.6⁎⁎ | |
| Nakamura et al. (2014) | -3D T1-weighted -2D dual echo T2-weighted (for T2-relaxation) |
BET, SIENAX & Jacobian integration method (all FSL v5.0) | Healthy volunteers | 14 | No effect of dehydration -↑ 0.36⁎ | n.a | |
| Biller et al. (2015) | -3D T1-weighted | FreeSurfer (v5.3.0) | Healthy volunteers | 15 | ↓ 0.36⁎⁎–↑ 0.87⁎ | n.a | |
| -FLASH (T1-relaxation) | – | No effect | n.a. | ||||
| -1H-MR Spectroscopy (brain tissue fluid H2O) | LCModel | ↓ 1.63⁎⁎–↑ 0.43⁎ | |||||
| Meyers et al. (2016) | -3D T1-weighted | FLIRT, FAST, BET (all FSL v5.0) and ALVIN (v1.06) | Healthy volunteers | 20 | ↓ 0.03 (ns)⁎⁎–↑ 0.15 (ns)⁎ | ↑ 0.22 (ns)⁎⁎–↓ 0.62 (ns)⁎ | |
| -T2 relaxation | |||||||
| Dehydration-Exercise | Dickson et al. (2005) | -3D T1-weighted | Analyze (v7.0) | Trained healthy volunteers | 6 | No effect | No effect |
| Kempton et al. (2009) | -3D T1-weighted | SIENA (v2.4), MEASURE | Trained healthy volunteers | 7 | No effect | ↑(No percentages mentioned)⁎⁎ | |
| Kempton et al. (2011) | -3D T1-weighted | SPM5 | Healthy volunteers | 10 | No effect | ↑(No percentages mentioned)⁎⁎ | |
| -BOLD-fMRI | ↑ BOLD response | n.a | |||||
| -pcASL | ↑ Blood flow | n.a | |||||
| Diurnal fluctuations | Nakamura et al. (2015) | -3D T1-weighted | BEaST (v1.15) and FAST (FSL v5.0) | MS and AD | 1589 | ↓ 0.180–0.438 | n.a |
n.a. = not applicable.
after rehydration.
after dehydration.
3.1.2. Exercise
Different from the dehydration studies examining the effects of fluid restriction, studies focusing on dehydration as caused by thermal-exercise (Dickson et al., 2005, Kempton et al., 2011, Kempton et al., 2009) did not find any differences in brain volume. Three layers of clothing created the thermal exercise condition. After extensive exercise the brain volumes were comparable to the volumes before exercise. However, two studies have found increased volumes of CSF after a thermal-exercise dehydration challenge (Kempton et al., 2011, Kempton et al., 2009), which is comparable to the effect of fluid restriction (Table 1). One of these studies (Kempton et al., 2011) also investigated the effect of thermal-exercise on the blood oxygenation level dependent (BOLD) response and on cerebral blood flow by means of pcASL. They showed that both the BOLD response and the cerebral blood flow go up after thermal-exercise for 90 min (Table 1). Taken together, it seems that exercise induces a different kind of dehydration status in the brain as compared with fluid restriction, but the exact difference need to be elucidated.
3.2. Diurnal fluctuations
A recent study has demonstrated that brain volume also fluctuates during the day (Nakamura et al., 2015). Investigating both AD (ADNI trial) and MS (RESTORE (Fox et al., 2014) and DEFINE (Gold et al., 2012) trials) patients they have showed that brain volumes are larger in the morning, and decrease during the day (Nakamura et al., 2015). They demonstrated a reduction of − 0.180% in the MS group and a reduction of even − 0.442% in the AD group when comparing morning brain volumes with evening brain volumes (Table 1). Since annual atrophy rates lie between − 0.253% in MS and − 0.913% in elderly people (Nakamura et al., 2015), it is not assumed to be an irreversible effect and this difference must be accompanied with a similar increase during the night. However, this feels somewhat counterintuitive since brain volume tends to decrease after dehydration and the fluid intake is often smaller during nighttime. The authors hypothesized that redistribution of body fluid may be the possible mechanism involved in the increase in brain volume after sleep. Other mechanisms may be the effect of daily medication intake, which most often takes place during the morning and after lunch and may be diurnal. Also, cortisol levels fluctuate during the day and are higher in the morning (Geerlings et al., 2015), possible affecting brain volumes during the day.
3.3. Medication, surgery, and pathological processes
As where the percentages change in brain volume are given for dehydration and day-to-night fluctuation studies, the percentages are relatively sparse for the effect of medication, surgery and pathological processes on global brain volume. Therefore, in this section, we will speculate on the potential mechanisms that may influence brain volume on a short-term (hours to days), but sometimes also on a long-term (approximately one year) basis, where we focus on anti-inflammatory drugs, antipsychotics, surgical revascularization, hypertension, diabetes mellitus and AD. Moreover, here we will also address the regional brain volume changes as caused by the above-mentioned mechanisms.
3.3.1. Medication – anti-inflammatory drugs
Water-related brain volume fluctuations are also believed to occur in inflammatory brain edema (Zivadinov et al., 2008). It was shown that in MS patients, atrophy rates were accelerated during the first years of treatment with anti-inflammatory drugs like Natulizumab (Miller et al., 2007, Rudick et al., 1999), but disappeared during the second year. As such it is suggested that a so-called “pseudoatrophy” phenomenon exists where the observed reduction in brain volume is not the consequence of atrophy and cellular loss itself but rather caused by water shifts. A study (Rudick et al., 1999) investigating this phenomenon in more detail in MS patients demonstrated that the volumetric brain changes were mainly driven by white matter (Fisher et al., 2016), indicating that the inflammatory white matter lesions may be related to these changes. As a result pseudoatrophy may lead to an overshadowing of the real treatment effect and makes it complicated to assess the effect of true brain atrophy if not taken into account.
3.3.2. Medication – corticosteroids
Corticosteroids are drugs given for a variety of diseases, and have an anti-inflammatory and immunosuppressive action. Therefore, many side effects are observed for this type of medication. Focusing on brain volumetric effects two different regions are found to be affected: the hippocampus and the amygdala, both playing a role in short-term memory processing. The first study investigating the effect of chronic corticosteroid exposure (year) on hippocampal volume demonstrated a volumetric decrease of 8% on the left and 9% on the right as compared to healthy volunteers (Brown et al., 2004). This study was however retrospective. Later, prospective, studies demonstrated volume differences ranging from no effect at all (Coluccia et al., 2008, Hájek et al., 2006) to inconsistent results (11% decrease on the left, and 4% increase on the right) (Brown et al., 2007) to a mean decrease of 1.69% (Brown et al., 2014). Besides hippocampal volume differences, differences in amygdala volume are observed (Brown et al., 2008), with a decrease of 20% on the left side and 11% on the right. Although most studies investigated chronic corticosteroid exposure, brain volume changes have been observe and should be accounted for in the interpretation of longitudinal studies.
3.3.3. Medication – antipsychotics
Antipsychotic drugs can be classified into typical (first generation antipsychotics) and atypical (second generation antipsychotics) antipsychotics. A recent meta-analysis comparing the typical to the atypical revealed differences in gray matter volume loss between the two groups (Vita et al., 2015). The latter group was associated with less progressive gray matter loss with higher mean daily medication intake as compared to the first group (Vita et al., 2015). Therefore, this part will be subdivided into the effects of typical and the atypical classes on brain volumes. Since there are many studies that have investigated this issue and a thorough review on this topic is not the focus of this review, we will only focus on the most recent studies. First, for the typical drugs an increase in basal ganglia volume (Ebdrup et al., 2013, Jørgensen et al., 2015, Scherk and Falkai, 2006) and lateral ventricles (Jørgensen et al., 2015) has been observed. Smaller total brain volumes and hippocampal volumes accompany the increase in ventricle volume (Jørgensen et al., 2015). Second, the atypical drugs are associated with both reductions (Ahmed et al., 2015, Jørgensen et al., 2015, Scheepers et al., 2001, van Haren et al., 2007) in volume as with increases in volume (Guo et al., 2015, Jørgensen et al., 2015, Yue et al., 2016) and thus very inconclusive. Some studies combine both typical and atypical antipsychotics (Andreasen et al., 2013, Fusar-Poli et al., 2013, Ho et al., 2011, Nørbak-Emig et al., 2016) and effects are again inconsistent. For more details and the chronic impact of antipsychotic drugs in general read the recent review of Amato et al. (2016). Most of the studies discussed are longitudinal studies, they do, however, show that the progression of brain volume loss already starts relatively early after the start with antipsychotics. As such, antipsychotic medication intake should be collected in longitudinal studies.
3.3.4. Surgical revascularization
Chronic regional blood flow impairments can lead to thinning of the cortex, without global tissue loss (Fierstra et al., 2010). Brain perfusion may be sufficient enough to prevent from ischemia in these cases, but when neuronal activity increases it fails (Fierstra et al., 2011). The cerebrovascular reactivity (CVR) – the cerebral blood flow in response to a vasodilatory or vasoconstrictive stimulus –, can be used to assess the integrity of blood flow and CVR reductions are found to range between blunted increases in blood flow to a phenomenon called steal where blood flow is taken from the region with reduced cerebral blood flow in patients with severe steno-occlusive disease (Fierstra et al., 2011). Hence, it was hypothesized that the reduction in vascular reserve is associated with cortical thinning (Fierstra et al., 2011). Fierstra et al. (2011) studied patients presented with steno-occlusive disease and the steal phenomenon. They demonstrated that restoring the CVR by means of surgical revascularization, resulted in a 5.1% increase in mean cortical thickness in the revascularized hemisphere.
3.3.5. Pathological conditions
In a recent review the authors discussed the potential bias of atrophy rates caused by type 2 diabetes mellitus or hypertension (Meusel et al., 2014). Diabetes can alter brain function and structure probably on two levels. First, insulin transport across the blood brain barrier is reduced in patients with type 2 diabetes (Heni et al., 2014). Consequently, the resulting low levels of brain insulin directly affect the cognitive state of the patient, especially in the medial temporal lobe where insulin receptors are abundant (Convit, 2005, Craft, 2006). Moreover, an indirect pathway leads to increased levels in amyloid beta and tau caused by down-regulation of insulin degrading enzyme (Luchsinger, 2008). This enzyme plays a role in the degradation of amyloid and as a result aggregation of amyloid takes place (Carlsson, 2010), which is one of the hallmarks of AD and essential in neurodegeneration (Braak and Braak, 1991). Second, hyperglycemia can cause macro-and microvascular damage. Additional, type 2 diabetes may decrease blood flow velocities, increase cerebrovascular resistance and impair vasoreactivity (Novak et al., 2006). These changes may ultimately lead to brain volume changes and altered function (Meusel et al., 2014). This is in line with studies demonstrating modest brain volume loss in diabetes (Biessels, 2015, Wisse et al., 2014). As for hypertension, cerebral circulation is stressed by the increase in blood pressure (Pires et al., 2013). As a consequence there will be thickening of the vessel wall and an increased vascular resistance, mainly in the smaller blood vessels (Meusel et al., 2014). This increase in vascular resistance leads to blood flow reduction and has been linked to cognitive impairment (Meusel et al., 2014) and brain volume decreases (Beason-Held et al., 2007, Nagai et al., 2009). Since both diabetes and hypertension, but also AD, affect brain volume, it is tempting to suggest that a common pathological process exists between these disease entities. Thornton (2014) suggested hypohydration to be a mechanism possibly involved in the development of these diseases. Linking this hypothesis to the effects of hydration status on brain volume, there might indeed be a potential role of dehydration as a mechanism for the diseases. In that case AD, diabetes and hypertension may reflect a chronically dehydrated status, resulting in decreased brain volumes (Thornton, 2014).
4. Discussion and future directions
Many short-term mechanisms do have an effect on brain volume. For the interpretation of longitudinal data the effect of short-term mechanisms should not be underestimated. The percentage brain volume change during the day is in the same order of magnitude as yearly atrophy rates in normal aging (Fisher et al., 2008). As such longitudinal brain volume measurements may include effects from short-term physiological mechanisms, confounding the measurements and interpretation of longitudinal studies. Especially studies that assess therapy response or deterioration in brain volume may be impacted and biased by the effect of short-term physiological changes. One example of bias created by short-term mechanisms is the diurnal fluctuations in brain volume, creating bias in longitudinal data when scanning is not aligned. Thus the moment of scanning for every longitudinal point should be approximately the same for a single volunteer. The other mechanisms, medication, hypertension, diabetes, and (de)hydration, should be collected and adjusted for in longitudinal studies to overcome bias created by these mechanisms.
Taking the effects of (de)hydration, day-to-night fluctuations, medication and pathological processes like hypertension and diabetes together, it would be interested to investigate the role of these different mechanisms on the healthy aging brain and the brain of demented patients. In that way we might be able to assess whether different mechanisms may influence brain volume in different ways when comparing healthy elderly people to demented patients. For the hydration status it may be hypothesized that rehydration may be more affective in healthy aging, because when AD is already present the chronic dehydration status may overrule the effect of rehydration on brain volume, resulting in a decrease in volume increase after rehydration. When comparing diurnal fluctuations, it might be that different volume effects exist. The healthy brain may possibly have a better ability to regulate its volume during the day, therefore it may be hypothesized that brain volume changes over the day may be smaller (Thornton, 2014). Third, as we know hypertension to decrease brain volume, it would be interesting to what other effects hypertension has on brain and CSF volume, like diurnal fluctuations or medication effects. It may be suggested that similar to AD and MS, diurnal fluctuations exist. Hence it can be hypothesized that in de morning brain and CSF volume is greater as compared to the evening. The decrease by the end of the day in CSF volume may be a result of glymphatic activity during the night, where the interstitial fluid increased by approximately 60% to clear the brain from toxins and metabolites (Xie et al., 2013). Similar to the medication studies, the other factors influencing brain volume should also be investigated on a regional level. This will uncover differences in tissue type (white matter, gray matter, basal ganglia) and may show differences in specific areas rather than whole brain alone. Fourth, age-related differences may exist with regard to brain volume and the effect of short-term mechanisms. Since younger, developing, brains are more prone to disturbances it may be that for instance medication has a greater effect on brain volume in children and adolescents as it has in adults. Yet, to our knowledge, these studies have not been conducted but would be of great value to understand the role of short-term changes in the developing brain and whether these changes may reversible or not.
5. Conclusion
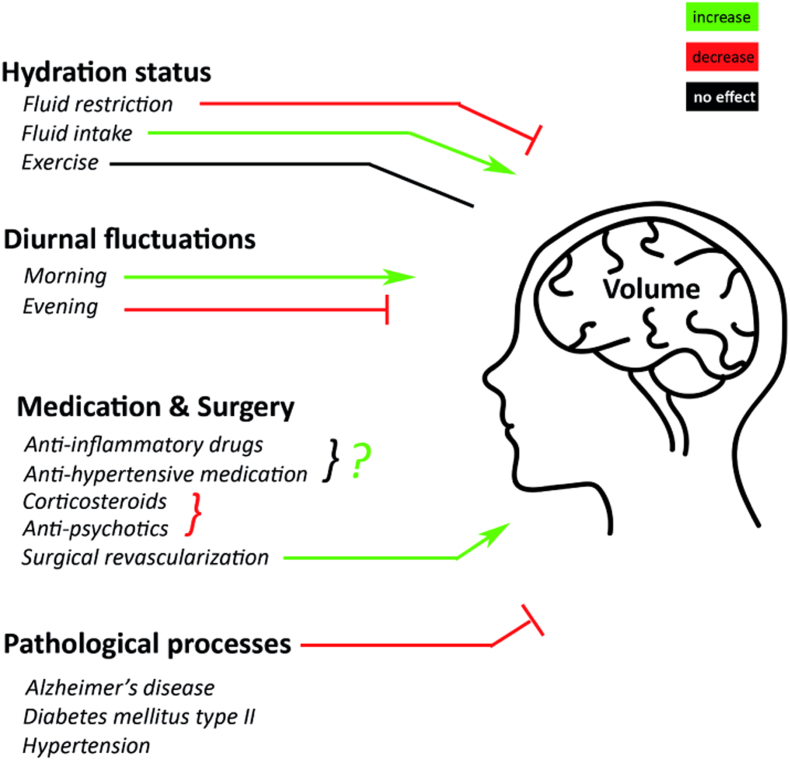
Taken together, with the use of MRI and different analysis tools it is shown that multiple mechanisms influence volumetric brain dynamics. These mechanisms may increase, decrease or have no effect on brain volume (Fig. 1). But all may influence an accurate interpretation of longitudinal brain volume measurements. In the upcoming years, attention should be directed towards studies investigating physiological short-term changes within the light of long-term chronic pathological changes. Ultimately this may lead to a better understanding of the physiological short-term effects of pathological processes and may aid in early detection of brain vulnerability or resilience to atrophy.
Fig. 1.
Mechanisms influencing volumetric brain dynamics. Several mechanisms may influence brain volume on a short-term, or long-term basis. Mechanisms with a negative influence on global brain volume, thus decreasing it, are: fluid restriction, evening MRI measurements, corticosteroids, antipsychotics and pathological processes such as Alzheimer's disease, hypertension and diabetes mellitus type 2. Mechanisms with a positive influence, thereby increasing brain volume are: fluid intake, morning MRI measurements, surgical revascularization and probably medications like anti-inflammatory drugs and anti-hypertensive medication. Exercise does not seem to have any effect on brain volume.
6. Search strategy and selection criteria
References for this Review were identified through a search of PubMed between March 1968 and June 2016, and references from relevant articles. A snowball search using Nakamura et al., 2015 as reference paper was used to select these relevant papers. In addition, the search terms “brain volume MRI”, “brain volume dehydration”, “brain volume anti-inflammatory”, and “magnetic resonance imaging” were used. Again, a snowball search was used to identify additional papers relevant to this topic. Only papers in English were reviewed and had to be restricted to the human brain. The final reference list was generated on the basis of relevance to the topics covered in this Review.
References
- Ahmed M., Cannon D.M., Scanlon C., Holleran L., Schmidt H., McFarland J., Langan C., McCarthy P., Barker G.J., Hallahan B., McDonald C. Progressive brain atrophy and cortical thinning in schizophrenia after commencing clozapine treatment. Neuropsychopharmacology. 2015;40:1–30. doi: 10.1038/npp.2015.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsop D.C., Detre J.A., Golay X., Günther M., Hendrikse J., Hernandez-Garcia L., Lu H., Macintosh B.J., Parkes L.M., Smits M., Van Osch M.J.P., Wang D.J.J., Wong E.C., Zaharchuk G. Recommended implementation of arterial spin-labeled perfusion mri for clinical applications: a consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magn. Reson. Med. 2015;73:102–116. doi: 10.1002/mrm.25197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alzheimer A. Uber eine eigenartige Erkrankung der Hirnrinde. Allg Zeits Psychiatry Psych. Y Gerichtl. Med. 1907;64:146–148. [Google Scholar]
- Amato D., Beasley C.L., Hahn M.K., Vernon A.C. Neuroadaptations to antipsychotic drugs: insights from pre-clinical and human post-mortem studies. Neurosci. Biobehav. Rev. 2016 doi: 10.1016/j.neubiorev.2016.10.004. [DOI] [PubMed] [Google Scholar]
- Andreasen N.C., Liu D., Ziebell S., Vora A., Ho B.C. Relapse duration, treatment intensity, and brain tissue loss in schizophrenia: a prospective longitudinal MRI study. Am. J. Psychiatry. 2013;170:609–615. doi: 10.1176/appi.ajp.2013.12050674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J. SPM: a history. NeuroImage. 2012;62:791–800. doi: 10.1016/j.neuroimage.2011.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J., Ridgway G.R. Symmetric diffeomorphic modeling of longitudinal structural MRI. Front. Neurosci. 2013;6:197. doi: 10.3389/fnins.2012.00197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beason-Held L.L., Moghekar A., Zonderman A.B., Kraut M.A., Resnick S.M. Longitudinal changes in cerebral blood flow in the older hypertensive brain. Stroke. 2007;38:1766–1773. doi: 10.1161/STROKEAHA.106.477109. [DOI] [PubMed] [Google Scholar]
- Biessels G.J. Diabetes: brain changes in T1DM—a microvascular complication? Nat. Rev. Endocrinol. 2015;11:447–448. doi: 10.1038/nrendo.2015.93. [DOI] [PubMed] [Google Scholar]
- Biller A., Reuter M., Paternaude B., Homola G., Breuer F., Bendszus M., Bartsch A. Responses of the human brain to mild dehydration and rehydration explored in vivo by 1H-MR imaging and spectroscopy. AJNR Am. J. Neuroradiol. 2015;36:2277–2284. doi: 10.3174/ajnr.A4508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H., Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Braak H., Braak E. Staging of Alzheimer's disease-related neurofibrillary changes. Neurobiol. Aging. 1995;16:271–284. doi: 10.1016/0197-4580(95)00021-6. [DOI] [PubMed] [Google Scholar]
- Brown E.S., Woolston D.J., Frol A., Bobadilla L., Khan D.A., Hanczyc M., Rush A.J., Fleckenstein J., Babcock E., Cullum C.M. Hippocampal volume, spectroscopy, cognition, and mood in patients receiving corticosteroid therapy. Biol. Psychiatry. 2004;55:538–545. doi: 10.1016/j.biopsych.2003.09.010. [DOI] [PubMed] [Google Scholar]
- Brown E.S., Vera E., Frol A.B., Woolston D.J., Johnson B. Effects of chronic prednisone therapy on mood and memory. J. Affect. Disord. 2007;99:279–283. doi: 10.1016/j.jad.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown E.S., Woolston D.J., Frol A.B. Amygdala volume in patients receiving chronic corticosteroid therapy. Biol. Psychiatry. 2008;63:705–709. doi: 10.1016/j.biopsych.2007.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown E.S., Jeon-Slaughter H., Lu H., Jamadar R., Issac S., Shad M., Denniston D., Tamminga C., Nakamura A., Thomas B.P. Hippocampal volume in healthy controls given 3-day stress doses of hydrocortisone. Neuropsychopharmacology. 2014;40:1216–1221. doi: 10.1038/npp.2014.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caramanos Z., Fonov V.S., Francis S.J., Narayanan S., Pike G.B., Collins D.L., Arnold D.L. Gradient distortions in MRI: characterizing and correcting for their effects on SIENA-generated measures of brain volume change. NeuroImage. 2010;49:1601–1611. doi: 10.1016/j.neuroimage.2009.08.008. [DOI] [PubMed] [Google Scholar]
- Carlsson C.M. Type 2 diabetes mellitus, dyslipidemia, and Alzheimer's disease. J. Alzheimers Dis. 2010;20:711–722. doi: 10.3233/JAD-2010-100012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chard D.T., Parker G.J.M., Griffin C.M.B., Thompson A.J., Miller D.H. The reproducibility and sensitivity of brain tissue volume measurements derived from an SPM-based segmentation methodology. J. Magn. Reson. Imaging. 2002;15:259–267. doi: 10.1002/jmri.10064. [DOI] [PubMed] [Google Scholar]
- Chetelat G., Baron J. Early diagnosis of Alzheimer's disease: contribution of structural neuroimaging. NeuroImage. 2003;18:525–541. doi: 10.1016/s1053-8119(02)00026-5. [DOI] [PubMed] [Google Scholar]
- Coluccia D., Wolf O.T., Kollias S., Roozendaal B., Forster A., de Quervain D.J.F. Glucocorticoid therapy-induced memory deficits: acute versus chronic effects. J. Neurosci. 2008;28:3474–3478. doi: 10.1523/JNEUROSCI.4893-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Convit A. Neurobiology of Aging. 2005. Links between Cognitive Impairment in Insulin Resistance: An Explanatory Model. [DOI] [PubMed] [Google Scholar]
- Craft S. Insulin resistance syndrome and Alzheimer disease: pathophysiologic mechanisms and therapeutic implications. Alzheimer Dis. Assoc. Disord. 2006;20:298–301. doi: 10.1097/01.wad.0000213866.86934.7e. [DOI] [PubMed] [Google Scholar]
- Dawson J.W. XVIII the histology of disseminated sclerosis. Earth Environ. Sci. Trans. R. Soc. Edinb. 1916;50:517–740. [Google Scholar]
- Dickson J.M., Weavers H.M., Mitchell N., Winter E.M., Wilkinson I.D., Van Beek E.J.R., Wild J.M., Griffiths P.D. The effects of dehydration on brain volume - preliminary results. Int. J. Sports Med. 2005;26:481–485. doi: 10.1055/s-2004-821318. [DOI] [PubMed] [Google Scholar]
- Duning T., Kloska S., Steinsträter O., Kugel H., Heindel W., Knecht S. Dehydration confounds the assessment of brain atrophy. Neurology. 2005;64:548–550. doi: 10.1212/01.WNL.0000150542.16969.CC. [DOI] [PubMed] [Google Scholar]
- Ebdrup B.H., Nørbak H., Borgwardt S., Glenthøj B. Volumetric changes in the basal ganglia after antipsychotic monotherapy: a systematic review. Curr. Med. Chem. 2013;20:438–447. doi: 10.2174/0929867311320030015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fierstra J., Poublanc J., Han J.S., Silver F., Tymianski M., Crawley A.P., Fisher J.A., Mikulis D.J. Steal physiology is spatially associated with cortical thinning. J. Neurol. Neurosurg. Psychiatry. 2010;81:290–293. doi: 10.1136/jnnp.2009.188078. [DOI] [PubMed] [Google Scholar]
- Fierstra J., MacLean D.B., Fisher J.A., Han J.S., Mandell D.M., Conklin J., Poublanc J., Crawley A.P., Regli L., Mikulis D.J., Tymianski M. Surgical revascularization reverses cerebral cortical thinning in patients with severe cerebrovascular steno-occlusive disease. Stroke. 2011;42:1631–1637. doi: 10.1161/STROKEAHA.110.608521. [DOI] [PubMed] [Google Scholar]
- Fisher E., Lee J.C., Nakamura K., Rudick R.A. Gray matter atrophy in multiple sclerosis: a longitudinal study. Ann. Neurol. 2008;64:255–265. doi: 10.1002/ana.21436. [DOI] [PubMed] [Google Scholar]
- Fisher E., Nakamura K., Lee J.-C., You X., Sperling B., Rudick R.A. Effect of intramuscular interferon beta-1a on gray matter atrophy in relapsing-remitting multiple sclerosis: a retrospective analysis. Mult. Scler. 2016;22:668–676. doi: 10.1177/1352458515599072. [DOI] [PubMed] [Google Scholar]
- Fotenos A.F., Snyder A.Z., Girton L.E., Morris J.C., Buckner R.L. Normative estimates of cross-sectional and longitudinal brain volume decline in aging and AD. Neurology. 2005;64:1032–1039. doi: 10.1212/01.WNL.0000154530.72969.11. [DOI] [PubMed] [Google Scholar]
- Fox N.C., Freeborough P.A. Brain atrophy progression measured from registered serial MRI: validation and application to Alzheimer's disease. J. Magn. Reson. Imaging. 1997;7:1069–1075. doi: 10.1002/jmri.1880070620. [DOI] [PubMed] [Google Scholar]
- Fox N.C., Cousens S., Scahill R., Harvey R.J., Rossor M.N. Using serial registered brain magnetic resonance imaging to measure disease progression in Alzheimer disease. Arch. Neurol. 2000;57:339. doi: 10.1001/archneur.57.3.339. [DOI] [PubMed] [Google Scholar]
- Fox R.J., Cree B.A.C., De Séze J., Gold R., Hartung H.P., Jeffery D., Kappos L., Kaufman M., Montalbán X., Weinstock-Guttman B., Anderson B., Natarajan A., Ticho B., Duda P. MS disease activity in RESTORE: a randomized 24-week natalizumab treatment interruption study. Neurology. 2014;82:1491–1498. doi: 10.1212/WNL.0000000000000355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisoni G.B. Structural imaging in the clinical diagnosis of Alzheimer's disease: problems and tools. J. Neurol. Neurosurg. Psychiatry. 2001;70:711–718. doi: 10.1136/jnnp.70.6.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusar-Poli P., Smieskova R., Kempton M.J., Ho B.C., Andreasen N.C., Borgwardt S. Progressive brain changes in schizophrenia related to antipsychotic treatment? A meta-analysis of longitudinal MRI studies. Neurosci. Biobehav. Rev. 2013;37:1680–1691. doi: 10.1016/j.neubiorev.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geerlings M.I., Sigurdsson S., Eiriksdottir G., Garcia M.E., Harris T.B., Gudnason V., Launer L.J. Salivary cortisol, brain volumes, and cognition in community-dwelling elderly without dementia. Neurology. 2015;85:976–983. doi: 10.1212/WNL.0000000000001931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold R., Kappos L., Arnold D.L., Bar-Or A., Giovannoni G., Selmaj K., Tornatore C., Sweetser M.T., Yang M., Sheikh S.I., Dawson K.T. Placebo-controlled phase 3 study of oral BG-12 for relapsing multiple sclerosis. N. Engl. J. Med. 2012;367:1098–1107. doi: 10.1056/NEJMoa1114287. [DOI] [PubMed] [Google Scholar]
- Guo J.Y., Huhtaniska S., Miettunen J., Jääskeläinen E., Kiviniemi V., Nikkinen J., Moilanen J., Haapea M., Mäki P., Jones P.B., Veijola J., Isohanni M., Murray G.K. Longitudinal regional brain volume loss in schizophrenia: relationship to antipsychotic medication and change in social function. Schizophr. Res. 2015;168:297–304. doi: 10.1016/j.schres.2015.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hájek T., Kopeček M., Preiss M., Alda M., Höschl C. Prospective study of hippocampal volume and function in human subjects treated with corticosteroids. Eur. Psychiatry. 2006;21:123–128. doi: 10.1016/j.eurpsy.2005.01.005. [DOI] [PubMed] [Google Scholar]
- van Haren N.E., Hulshoff Pol H.E., Schnack H.G., Cahn W., Mandl R.C., Collins D.L., Evans A.C., Kahn R.S. Focal gray matter changes in schizophrenia across the course of the illness: a 5-year follow-up study. Neuropsychopharmacology. 2007;32:2057–2066. doi: 10.1038/sj.npp.1301347. [DOI] [PubMed] [Google Scholar]
- Heni M., Schöpfer P., Peter A., Sartorius T., Fritsche A., Synofzik M., Häring H.U., Maetzler W., Hennige A.M. Evidence for altered transport of insulin across the blood-brain barrier in insulin-resistant humans. Acta Diabetol. 2014;51:679–681. doi: 10.1007/s00592-013-0546-y. [DOI] [PubMed] [Google Scholar]
- Ho B.-C., Andreasen N.C., Ziebell S., Peirson R., Magnotta V. Long-term antipsychotic treatment and brain volumes. Arch. Gen. Psychiatry. 2011;68:128–137. doi: 10.1001/archgenpsychiatry.2010.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack C.R., Shiung M.M., Gunter J.L., O'Brien P.C., Weigand S.D., Knopman D.S., Boeve B.F., Ivnik R.J., Smith G.E., Cha R.H., Tangalos E.G., Petersen R.C. Comparison of different MRI brain atrophy rate measures with clinical disease progression in AD. Neurology. 2004;62:591–600. doi: 10.1212/01.wnl.0000110315.26026.ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M., Beckmann C.F., Behrens T.E.J., Woolrich M.W., Smith S.M. Neuroimage. 2012. FSL. [Google Scholar]
- Jørgensen K.N., Nesvåg R., Gunleiksrud S., Raballo A., Jönsson E.G., Agartz I. First- and second-generation antipsychotic drug treatment and subcortical brain morphology in schizophrenia. Eur. Arch. Psychiatry Clin. Neurosci. 2015;266:451–460. doi: 10.1007/s00406-015-0650-9. [DOI] [PubMed] [Google Scholar]
- Kempton M.J., Ettinger U., Schmechtlg A., Winter E.M., Smith L., McMorris T., Wilkinson I.D., Williams S.C.R., Smith M.S. Effects of acute dehydration on brain morphology in healthy humans. Hum. Brain Mapp. 2009;30:291–298. doi: 10.1002/hbm.20500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempton M.J., Ettinger U., Foster R., Williams S.C.R., Calvert G.A., Hampshire A., Zelaya F.O., O'Gorman R.L., McMorris T., Owen A.M., Smith M.S. Dehydration affects brain structure and function in healthy adolescents. Hum. Brain Mapp. 2011;32:71–79. doi: 10.1002/hbm.20999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung K.K., Ridgway G.R., Ourselin S., Fox N.C. Consistent multi-time-point brain atrophy estimation from the boundary shift integral. NeuroImage. 2012;59:3995–4005. doi: 10.1016/j.neuroimage.2011.10.068. [DOI] [PubMed] [Google Scholar]
- Luchsinger J.A. Adiposity, hyperinsulinemia, diabetes and Alzheimer's disease. An epidemiological perspective. Eur. J. Pharmacol. 2008;585(1):119–129. doi: 10.1016/j.ejphar.2008.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meusel L.A.C., Kansal N., Tchistiakova E., Yuen W., MacIntosh B.J., Greenwood C.E., Anderson N.D. A systematic review of type 2 diabetes mellitus and hypertension in imaging studies of cognitive aging: time to establish new norms. Front. Aging Neurosci. 2014;6:1–17. doi: 10.3389/fnagi.2014.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers S.M., Tam R., Lee J.S., Kolind S.H., Vavasour I.M., Mackie E., Zhao Y., Laule C., Mädler B., Li D.K.B., MacKay A.L., Traboulsee A.L. Does hydration status affect MRI measures of brain volume or water content? J. Magn. Reson. Imaging. 2016;44:296–304. doi: 10.1002/jmri.25168. http://dx.doi.org/10.1002/jmri.25168 [DOI] [PubMed] [Google Scholar]
- Miller D.H., Soon D., Fernando K.T., MacManus D.G., Barker G.J., Yousry T.A., Fisher E., O'Connor P.W., Phillips J.T., Polman C.H., Kappos L., Hutchinson M., Havrdova E., Lublin F.D., Giovannoni G., Wajgt A., Rudick R., Lynn F., Panzara M.A., Sandrock A.W. MRI outcomes in a placebo-controlled trial of natalizumab in relapsing MS. Neurology. 2007;68:1390–1401. doi: 10.1212/01.wnl.0000260064.77700.fd. [DOI] [PubMed] [Google Scholar]
- Nagai M., Hoshide S., Ishikawa J., Shimada K., Kario K. Insular cortex atrophy as an independent determinant of disrupted diurnal rhythm of ambulatory blood pressure in elderly hypertension. Am. J. Hypertens. 2009;22:723–729. doi: 10.1038/ajh.2009.71. [DOI] [PubMed] [Google Scholar]
- Nakamura K., Brown R.A., Araujo D., Narayanan S., Arnold D.L. Correlation between brain volume change and T2 relaxation time induced by dehydration and rehydration: implications for monitoring atrophy in clinical studies. NeuroImage Clin. 2014;6:166–170. doi: 10.1016/j.nicl.2014.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K., Brown R.A., Narayanan S., Collins D.L., Arnold D.L. Diurnal fluctuations in brain volume: statistical analyses of MRI from large populations. NeuroImage. 2015;118:126–132. doi: 10.1016/j.neuroimage.2015.05.077. [DOI] [PubMed] [Google Scholar]
- Nørbak-Emig H., Pinborg L.H., Raghava J.M., Svarer C., Baaré W.F.C., Allerup P., Friberg L., Rostrup E., Glenthøj B., Ebdrup B.H. Extrastriatal dopamine D2/3 receptors and cortical grey matter volumes in antipsychotic-naïve schizophrenia patients before and after initial antipsychotic treatment. World J. Biol. Psychiatry. 2016;0:1–11. doi: 10.1080/15622975.2016.1237042. [DOI] [PubMed] [Google Scholar]
- Novak V., Last D., Alsop D.C., Abduljalil A.M., Hu K., Lepicovsky L., Cavallerano J., Lipsitz L.A. Cerebral blood flow velocity and periventricular white matter hyperintensities in type 2 diabetes. Diabetes Care. 2006;29:1529–1534. doi: 10.2337/dc06-0261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pires P.W., Dams Ramos C.M., Matin N., Dorrance A.M. The effects of hypertension on the cerebral circulation. Am. J. Physiol. Heart Circ. Physiol. 2013;304:1598–1614. doi: 10.1152/ajpheart.00490.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RI S., Frost C., Jenkins R., JL W., MN R., NC F. A longitudinal study of brain volume changes in normal aging using serial registered magnetic resonance imaging. Arch. Neurol. 2003;60:989–994. doi: 10.1001/archneur.60.7.989. [DOI] [PubMed] [Google Scholar]
- Rudick R.A., Fisher E., Lee J.C., Simon J., Jacobs L. Use of the brain parenchymal fraction to measure whole brain atrophy in relapsing-remitting MS. multiple sclerosis collaborative research group. Neurology. 1999;53:1698–1704. doi: 10.1212/wnl.53.8.1698. [DOI] [PubMed] [Google Scholar]
- Scheepers F.E., De Wied C.C.G., Pol H.E.H., Van De Flier W., Van Der Linden J.A., Kahn R.S. The effect of clozapine on caudate nucleus volume in schizophrenic patients previously treated with typical antipsychotics. Neuropsychopharmacology. 2001;24:47–54. doi: 10.1016/S0893-133X(00)00172-X. [DOI] [PubMed] [Google Scholar]
- Scherk H., Falkai P. Effects of antipsychotics on brain structure. Curr. Opin. Psychiatry. 2006;19:145–150. doi: 10.1097/01.yco.0000214339.06507.d8. [DOI] [PubMed] [Google Scholar]
- Smith S.M., De Stefano N., Jenkinson M., Matthews P.M. Normalized accurate measurement of longitudinal brain change. J. Comput. Assist. Tomogr. 2001;25:466–475. doi: 10.1097/00004728-200105000-00022. [DOI] [PubMed] [Google Scholar]
- Smith S.M., Zhang Y., Jenkinson M., Chen J., Matthews P.M., Federico A., De Stefano N. Accurate, robust, and automated longitudinal and cross-sectional brain change analysis. NeuroImage. 2002;17:479–489. doi: 10.1006/nimg.2002.1040. [DOI] [PubMed] [Google Scholar]
- Smith S.M., Jenkinson M., Woolrich M.W., Beckmann C.F., Behrens T.E., Johansen-Berg H., Bannister P.R., De Luca M., Drobnjak I., Flitney D.E., Niazy R.K., Saunders J., Vickers J., Zhang Y., De Stefano N., Brady J.M., Matthews P.M. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage. 2004;23(Suppl. 1):S208–19. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Streitbürger D.P., Möller H.E., Tittgemeyer M., Hund-Georgiadis M., Schroeter M.L., Mueller K. Investigating structural brain changes of dehydration using voxel-based morphometry. PLoS One. 2012:7. doi: 10.1371/journal.pone.0044195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton S.N. Diabetes and hypertension, as well as obesity and Alzheimer's disease, are linked to hypohydration-induced lower brain volume. Front. Aging Neurosci. 2014 doi: 10.3389/fnagi.2014.00279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vita A., De Peri L., Deste G., Barlati S., Sacchetti E. The effect of antipsychotic treatment on cortical gray matter changes in schizophrenia: does the class matter? A meta-analysis and meta-regression of longitudinal magnetic resonance imaging studies. Biol. Psychiatry. 2015;78:403–412. doi: 10.1016/j.biopsych.2015.02.008. [DOI] [PubMed] [Google Scholar]
- Wisse L.E.M., De Bresser J., Geerlings M.I., Reijmer Y.D., Portegies M.L.P., Brundel M., Kappelle L.J., Van Der Graaf Y., Biessels G.J. Global brain atrophy but not hippocampal atrophy is related to type 2 diabetes. J. Neurol. Sci. 2014;344:32–36. doi: 10.1016/j.jns.2014.06.008. [DOI] [PubMed] [Google Scholar]
- Woolrich M.W., Jbabdi S., Patenaude B., Chappell M., Makni S., Behrens T., Beckmann C., Jenkinson M., Smith S.M. Bayesian analysis of neuroimaging data in FSL. NeuroImage. 2009;45 doi: 10.1016/j.neuroimage.2008.10.055. [DOI] [PubMed] [Google Scholar]
- Xie L., Kang H., Xu Q., Chen M.J., Liao Y., Thiyagarajan M., O'Donnell J., Christensen D.J., Nicholson C., Iliff J.J., Takano T., Deane R., Nedergaard M. Sleep drives metabolite clearance from the adult brain. Science. 2013;342(80):373–377. doi: 10.1126/science.1241224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue Y., Kong L., Wang J., Li C., Tan L., Su H., Xu Y. Regional abnormality of grey matter in schizophrenia: effect from the illness or treatment? PLoS One. 2016;11:1–12. doi: 10.1371/journal.pone.0147204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zivadinov R., Reder A.T., Filippi M., Minagar A., Stüve O., Lassmann H., Racke M.K., Dwyer M.G., Frohman E.M., Khan O. Mechanisms of action of disease-modifying agents and brain volume changes in multiple sclerosis. Neurology. 2008;71:136–144. doi: 10.1212/01.wnl.0000316810.01120.05. [DOI] [PubMed] [Google Scholar]