Main Text
Pharmaceutical developers are continuously searching for potential drug development targets.1 The time frame to develop and bring a new drug to the market may take 15 years, on average, starting from concept to final marketing, and the cost is likely around a billion dollars. Although technological advances, such as high-throughput identification of agents, have expedited the process of finding new drug targets,2 and a steady increase in new therapy concepts can be noted, many malignancies, for instance, still cannot be efficiently tackled or cured.3 The majority of new therapies continues to be small molecule based; advanced therapy medicinal products (ATMPs), a category that enfolds cell and gene therapies and tissue-engineered products, make up only a minor aspect of this market, although their market share is expected to be increasing. ATMPs have gained much attention because they offer potential treatments for serious conditions where small molecule therapies are inadequate.4 ATMPs are complex products by definition, requiring special research and development plus new regulatory and pricing strategies.5
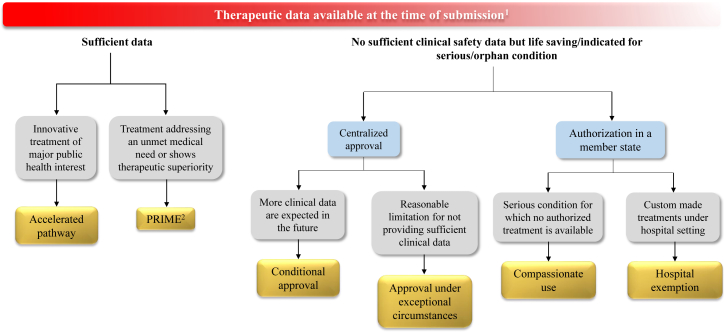
Traditionally, ATMPs have been developed in the academic setting, where a lack of regulatory expertise and marketing knowledge is intrinsic.5 However, fanned by the promising clinical results in the CAR T cell field,6 pharmaceutical industry and venture capital investors have recently become more engaged. At the same time, regulatory frameworks for ATMPs have been evolving, and marketing authorization constraints have been imposed 7, 8 to limit and prevent the spread of unproven cellular interventions.9 Although most critical hurdles faced by ATMP developers today are at the commercialization end,5 the increased European Union (EU) regulatory demands for providing ATMPs have also been viewed as a major burden and have been accused of hampering the entry of new ATMPs into clinical application and the market.10, 11 Here, we highlight that, despite these challenges, ATMPs actually offer a number of regulatory advantages over conventional medicinal products and discuss the early access pathways available in the EU for ATMPs that developers should be aware of and benefit from (Figure 1).
Figure 1.
Expedited and Exceptional Authorization Schemes in the EU
1The amount of therapeutic data available at the time of application submission is decreasing from the left to the right side of the scheme.
2PRIME-designated products are eligible to accelerated assessment.
Orphan diseases, which, by definition, are rare conditions that affect no more than 5 in 10,000 individuals, present no ideal target for traditional drug developers due to low patient numbers and the expected small market size for these products. To stimulate the development of therapeutics for orphan diseases, the European Medicines Agency (EMA) is offering several incentives5, 12 (Table 1). For instance, orphan-designated products are granted 10 years of market exclusivity to protect these therapies from any competition targeting the same orphan indication to assure revenue for rare disease therapy developers. In addition, the EMA offers attractive fee reduction for scientific advice (called protocol assistance) as well as other procedural and administrative assistance. Notably, the fee-reduction scale also depends on the status of a sponsor. ATMP developers should apply first for small- and medium-sized enterprises (SMEs) status whenever applicable before requesting financial or administrative assistance. To be eligible, companies must be established in the EU/European Economic Area (EEA) and meet the EMA’s definition of an SME.13 The extension of market exclusivity for two additional years is also possible for orphan-designated products in which a pediatric investigation plan must be obtained. In addition, both the EMA and FDA offer grants to support the development of orphan products. To date, four out of eight EMA authorized ATMPs have obtained orphan designation and gained benefit from these incentives, namely Glybera, Holoclar, Strimvelis and Zalmoxis (Table 2).
Table 1.
Early Access Routes in the EU
| Early Access Option | Description/Eligibility Criteria |
|---|---|
| Accelerated assessment | Offers reduced evaluation time by the CHMP/CAT for innovative medicinal products expected to be of major public health interest. The evaluation time is reduced from 210 days to 150 days. |
| PRIME | Medicines entitled to treat conditions that have no available treatment options or medicines showing therapeutic superiority over the current treatments for a specific indication are designated as PRIority MEdicines (PRIME) and benefit from early scientific advice and eligibility to accelerated assessment, aiming to reach patients earlier. |
| Compassionate use | Member states can allow the use of unauthorized medicines to treat patients with life-threatening, long-lasting, or seriously debilitating illnesses for which no currently authorized medicine is available for treatment. It is not a centralized endorsement, but, also, the EMA may provide recommendations for the national authorities about the compassionate use of certain therapies when requested. |
| Orphan designation | A denomination granted to medicines intended to be used as a treatment for rare conditions where the EU prevalence is not more than 5 in 10,000 or it is unlikely that marketing of the product would generate sufficient returns to justify the investment needed for its development. An Orphan designated product benefits from scientific advice, procedural assistance, 10-year market exclusivity for each particular indication, which can be extended by 2 additional years upon obtaining a pediatric investigation plan (PIP), and potential administrative fee reduction. |
| Hospital exemption | A permission that can be granted by EU member states for unauthorized ATMPs to be used on a named-patient basis in a hospital setting within the same member state only and under the exclusive responsibility of the treating physician. |
| Conditional approval | Marketing authorization of medicine that fulfills unmet medical needs based on less comprehensive safety and efficacy data than normally required. The authorization is valid for 1 year and can be renewed based on new clinical data provided. |
| Exceptional circumstances | Marketing authorization of medicine in absence of comprehensive safety and efficacy data based on unmanageable circumstances, e.g., rarity of patients. This authorization is annually reviewed for risk-benefit balance. |
Table 2.
ATMP’s Special Approval Considerations
| ATMP | Glybera | MACI | Provenge | Holoclar | Imlygic | Strimvelis | Zalmoxis |
|---|---|---|---|---|---|---|---|
| Special approval considerations | Orphan designation, additional monitoring,a and exceptional circumstances | Additional monitoringa | Additional monitoringa | Orphan designation, conditional approval and additional monitoringa | Additional monitoringa | Orphan designation and additional monitoringa | Orphan designation, conditional approval, and additional monitoringa |
A medicinal product is usually subject to additional monitoring when there is less information available on it than other medicines, for example, because it is new to the market or there is limited data on its long-term use. It does not mean that the medicine is unsafe.
Most regulatory authorities around the world, especially in the EU, US, Japan and South Korea have been realizing the need for accelerated novel drug evaluation and approval schemes to ensure early access to innovative therapies that may improve patients’ quality of lives or even cure life-threatening conditions.14 As consequence, several early access pathways and expedited evaluation programs have been launched, aiming at providing patients with innovative therapies for unmet medical needs as early as possible, without compromising the benefits - risks balance (Table 1).
The nature of ATMPs makes them especially eligible for early access options: Most ATMPs are intended for a one-time treatment and may provide a lifetime cure. In example, Imlygic, a gene therapy product approved in 2015 by EMA and US Food and Drug Administration (FDA) is intended to treat patients with surgically unresectable or distantly metastatic melanoma. Strimvelis, a recently EMA-approved gene therapy, is a one-time treatment for rare patients with severe combined immunodeficiency due to adenosine deaminase deficiency (ADA-SCID), for whom no suitable human leukocyte antigen (HLA)-matched allogeneic bone marrow stem cell donor is available.
A low number of target patients is not only a market limiting factor, it also limits the preceding evaluation of safety and efficacy, as most of clinical trials require a statistically significant number of patients. Regular clinical trial design and the number of subjects per study phase are, therefore, often not suitable for orphan products. Unfortunately, the scarcity of the patient populations may not be the only hurdle. Low batch numbers coming along with high therapeutics manufacturing costs per batch15 and complicated administration procedures at bedside may limit the utility of the product.5 In favor of orphan medications, including ATMPs, the EMA developed a conditional marketing authorization pathway. As such, products are expected to provide more clinical data over the long run. For products incapable of providing sufficient clinical data at all due to reasonable limitations, such as the rarity of subjects, authorization under exceptional circumstances can be granted. Glybera was approved under exceptional circumstances, and Zalmoxis and Holoclar were granted conditional authorizations (Table 2).
If the ATMP is regarded as a major interest for public health and therapeutic innovation, it can also benefit from accelerated assessment. This procedure is intended to speed up the review time, shortening the “time to market” period. Whereas the regular evaluation procedure of a marketing authorization application is designed for 210 days (excluding clock stops where applicants have to provide clarification on identified deficiencies), the time frame is reduced to 150 days.
Criticisms arise from the fact that marketing authorizations granted via conditional or accelerated pathways are based on less data than normally required, injecting a higher grade of uncertainty in the system and downgrading the trust in regulatory decisions. One might also argue that early market access may put more risk on reimbursement and taxpayer money. Therefore, flanking concomitant analysis and compensatory pharmacovigilance measures are deemed essential to allow re-evaluation and ongoing monitoring of the medicines’ benefit-risk profile.
The EMA has also launched the adaptive pathways approach, a conceptual effort seeking to improve timely access for patients to new medicines in areas of high medical need. The idea is to provide a prospective progressive approval scenario that allows a stepwise convergence of benefit-risk balance exploration of a product, e.g., starting with a restricted but well-defined patient population and/or based on early data, to be iteratively expanded in subsequent stages. The concept uses the already existing compassionate use and conditional approval mechanisms together with post-marketing pharmacovigilance tools, such as patient registries, to generate supporting real-life data. Most interestingly, with EMA and Health Technology Assessment (HTA) parallel scientific advice being integrated into this approach, the uncertainties and financial risks imposed on the developers might be reduced. A pilot exploratory project was launched to test the adaptive pathways implications and has successfully identified 10 eligible products out of 64 applications, of which 6 products were selected for detailed EMA and HTA scientific discussion, reflecting the approach selectivity.
Recently, the Committee for Medicinal Products for Human Use (CHMP) together with the EMA developed a stand-alone assessment pathway tool called priority medicines (PRIME), which aims to accelerate the development of medicines targeting unmet medical needs to reach patients within a shortened time. In early March of 2016, the PRIME scheme became effective, offering several advantages to applicants when accepted for the scheme. For example, the PRIME fosters the early dialogue, coordination, and advice from regulatory authorities on the developmental program and may initiate the accelerated assessment procedure at the time of the marketing authorization application.
By July 2017, twenty five medicinal products were granted eligibility for the PRIME scheme, of which eleven products are ATMPs, which are comprised of five oncology products, one product for neurology indication, and five products targeting severe blood illnesses (Table 3). Thus, based on their innovative mechanisms to target severe/life-threatening illnesses, ATMPs appear to have greater acceptability for entering the PRIME scheme compared to other drugs. Therefore, ATMP developers should always check the availability of this special evaluation scheme for their products and pursue an early dialog with regulators.
Table 3.
PRIME Products
| Proprietary Name | Description | Therapeutic Area | Therapeutic Indication | Developer |
|---|---|---|---|---|
| SPK-9001 | adeno-associated viral vector containing factor IX gene variant | hematology | treatment of haemophilia B | Spark Therapeutics |
| BMN 270 | adeno-associated viral vector serotype 5 containing a B-domain deleted variant of human coagulation factor VIII gene | hematology | treatment of haemophilia A | BioMarin |
| AMT-060 | adeno-associated viral vector serotype 5 containing human factor IX gene | hematology | treatment of haemophilia B | uniQure |
| AVXS-101 | Adeno-associated viral vector serotype 9 containing the human SMN gene | neurology | treatment of pediatric patients diagnosed with spinal muscular atrophy type 1 | AveXis |
| DNX-2401 | adenovirus serotype 5 containing partial E1A deletion and an integrin-binding domain | oncology | treatment of recurrent glioblastoma in patients for which a gross total resection is not possible or advisable or who refuse further surgery | DNAtrix Therapeutics |
| ATA129 | allogeneic Epstein-Barr virus-specific cytotoxic T lymphocytes | hematology | treatment of patients with Epstein-Barr virus-associated post-transplant lymphoproliferative disorder in the allogeneic hematopoietic cell transplant setting refractory to rituximab | Atara Biotherapeutics |
| Lentiglobin | autologous CD34+ hematopoietic stem cells transduced with lentiviral vector encoding the human βA-T87Q-globin gene | hematology | treatment of beta-thalassaemia major | Bluebird Bio |
| NY-ESO-1c259T | autologous CD4 and CD8 T cells transduced with lentiviral vector containing an affinity-enhanced T cell receptor to target the cancer-testis tumor antigen | oncology | treatment of HLA-A*0201, HLA-A*0205, or HLA-A*0206 allele-positive patients with inoperable or metastatic synovial sarcoma who have received prior chemotherapy and whose tumor expresses the NY-ESO-1 tumor antigen | Adaptimmune |
| JCAR017 | autologous CD4+ and CD8+ T cells expressing a CD19-specific chimeric antigen receptor | oncology | treatment of relapsed/refractory diffuse large B cell lymphoma (DLBCL) | Juno Therapeutics |
| CTL019 | autologous T cells transduced with lentiviral vector containing a chimeric antigen receptor directed against CD19 | oncology | treatment of pediatric patients with relapsed or refractory B cell acute lymphoblastic leukemia | Novartis |
| KTE-C19 | autologous T cells transduced with retroviral vector encoding an anti-CD19 CD28/CD3-zeta chimeric antigen receptor |
oncology | treatment of adult patients with DLBCL who have not responded to their prior therapy or have had disease progression after autologous stem cell transplant (ASCT) | Kite Pharma |
Date of search: May 24, 2017. Source of information: http://www.ema.europa.eu/ema/index.jsp?curl=pages/regulation/general/general_content_000660.jsp.
With the introduction of the ATMP regulation that aims for a mandatory EU-centralized marketing authorization, the regulatory expectations at the time of marketing approval are set rather high, and suitability for a EU-wide supply to serve patients is expected, upholding a regulatory burden on ATMP developers. Exemptions and alternative access options, therefore, become strongly needed for patients to benefit from ATMPs that are not suitable to be authorized under the centralized scheme, especially those intended to treat life-threatening conditions. The compassionate use program, for instance, allows EU member states to permit the use of investigational ATMPs that are not yet centrally authorized in case of a life-threatening unmet medical need. Article 3 No. 7 of Directive 2001/83/EC, the so-called hospital exemption, allows member states to authorize the application of so–called non-routine ATMPs in a national setting under defined restrictions, such as direct physician responsibility.16 Hospital-exempt products often are prepared and/or used in special hospital settings, fitting many ATMPs very well, as they often feature a short shelf-life or a short time interval between administrations as well as in depth clinical expertise.5, 17
Germany is one of the main markets for ATMPs, currently, with two somatic cell therapies and seven tissue-engineered products carrying a temporary hospital exemption authorization (Table 4). What should be strongly emphasized, however, is the mandate that compassionate use programs and hospital exemptions cannot be used for prospective product development, circumventing high-evidence clinical trials, or even misused for treatments prone to medical tourism, such as unproven stem cell therapies.
Table 4.
Hospital Exemption Products in Germany
| Proprietary Name | Description | Therapeutic Area | Therapeutic Indication | Authorization Holder |
|---|---|---|---|---|
| DCVax-L | autologous antigen-presenting dendritic cells | oncology | various types of solid tumors | Northwest Biotherapeutics |
| CIK-Cells | allogenic human cytokine-activated natural killer like T cells | oncology | various types of solid tumors | German Red Cross, blood donation service in Baden-Wuerttemberg and Hessen |
| BioSeed-C | autologous 3D chondrocytes transplant | tissue repair/regenerative medicine | repair of knee cartilage defects | BioTissue Technologies |
| co.don chondrosphere | autologous, matrix-associated chondrocytes for implantation | tissue repair/regenerative medicine | repair of knee cartilage defects | co.don AG, Teltow |
| Human allogeneic mesenchymal Stromal cells | allogeneic mesenchymal stromal cells | tissue repair/regenerative medicine | repair of various tissue types | German Red Cross, blood donation service in Baden-Wuerttemberg and Hessen |
| MukoCell | autologous oral mucosa cells for surgical repair of urethral strictures | urology | surgical treatment for patients with urethral narrowing | UroTiss Europe |
| NOVOCART 3D | autologous 3D matrix-associated chondrocytes for transplantation | tissue repair/regenerative medicine | repair of articular cartilage defects | TETEC |
| NOVOCART Inject | autologous chondrocytes and hydrogel for in situ polymerization. | tissue repair/regenerative medicine | repair of articular cartilage defects | TETEC |
| t2c001 | autologous bone marrow-derived progenitor cells | tissue repair/regenerative medicine | repair of damaged cardiac tissue in patients with ischemic heart disease or myocardial infarction and tissue repair in patients with peripheral vascular disease | t2cure, Frankfurt |
Date of search: March 7, 2017. Source of information: http://www.pei.de/DE/infos/pu/genehmigungen/atmp-4b-amg/antraege-genehmigung-4b-amg-atmp-node.html.
In conclusion, ATMPs are expected to provide a major contribution to the field of next generation medicines. Acknowledging the innovative nature of these products, EU lawmakers and regulatory bodies have not insisted on conventional rigid regulatory expectations, but have installed a number of flexible tools to facilitate marketing and patient access (Figure 1). Although only the hospital exemption pathway is exclusively designed for ATMPs, many characteristics, such as innovative mechanisms of action, therapies for extremely low number of patients or diseases for which no treatment is currently available, and being indicated for life-long and/or life-threatening conditions, qualify ATMPs for several early access and exemption options, such as conditional approval, approval under exceptional circumstance, or compassionate use. In addition, scientific advice and procedural and administrative assistance in favor of ATMP development are offered by national regulatory agencies like the German Paul-Ehrlich-Institut or the EMA.
While the available regulatory pathways offer additional flexibility and the possibility for accelerated commercialization, it is also criticized as too elaborate, complex, or even obscure. The latter is especially conveyed by academics and SME-based ATMP developers with limited regulatory oversight. However, selection of the optimal pathway(s) helps to optimize the allocation of financial and other resources in order to streamline development and shorten the time to approval. ATMP developers may not be fully aware of these opportunities. To the authors’ view, eligibility of most ATMPs to early access options and exemptions should be seen as encouraging, since this illustrates the high interest of the public health sector and regulatory bodies for ATMP development. ATMP guidance should not be perceived as insurmountable, but rather as an opportunity to interact with regulatory bodies for finding pathways tailored for an individual ATMP to serve the common goal of providing safe and effective ATMPs to patients.
Acknowledgments
The views expressed in this article are the personal views of the authors and may not be understood or quoted as being made on behalf of or reflecting the position of the Paul-Ehrlich-Institut (PEI), the European Medicines Agency (EMA), or one of its working parties.
References
- 1.Santos R., Ursu O., Gaulton A., Bento A.P., Donadi R.S., Bologa C.G., Karlsson A., Al-Lazikani B., Hersey A., Oprea T.I., Overington J.P. A comprehensive map of molecular drug targets. Nat Rev Drug Discov. 2016;16:19–34. doi: 10.1038/nrd.2016.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sergeeva A., Kolonin M.G., Molldrem J.J., Pasqualini R., Arap W. Display technologies: application for the discovery of drug and gene delivery agents. Adv. Drug Deliv. Rev. 2006;58:1622–1654. doi: 10.1016/j.addr.2006.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Loda M. Challenging roadblocks to cancer cure. Cancer Res. 2016;76:4924–4930. doi: 10.1158/0008-5472.CAN-16-1443. [DOI] [PubMed] [Google Scholar]
- 4.Abou-El-Enein M., Bauer G., Reinke P., Renner M., Schneider C.K. A roadmap toward clinical translation of genetically-modified stem cells for treatment of HIV. Trends Mol. Med. 2014;20:632–642. doi: 10.1016/j.molmed.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 5.Abou-El-Enein M., Elsanhoury A., Reinke P. Overcoming Challenges Facing Advanced Therapies in the EU Market. Cell Stem Cell. 2016;19:293–297. doi: 10.1016/j.stem.2016.08.012. [DOI] [PubMed] [Google Scholar]
- 6.Fesnak A.D., June C.H., Levine B.L. Engineered T cells: the promise and challenges of cancer immunotherapy. Nat. Rev. Cancer. 2016;16:566–581. doi: 10.1038/nrc.2016.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sanzenbacher R., Dwenger A., Schuessler-Lenz M., Cichutek K., Flory E. European regulation tackles tissue engineering. Nat Botechnol. 2007;25:1089–1091. doi: 10.1038/nbt1007-1089. [DOI] [PubMed] [Google Scholar]
- 8.Renner M., Anliker B., Sanzenbacher R., Schuele S. Regulation of clinical trials with advanced therapy medicinal products in Germany. Adv. Exp. Med. Biol. 2015;871:87–101. doi: 10.1007/978-3-319-18618-4_5. [DOI] [PubMed] [Google Scholar]
- 9.Dominici M., Nichols K., Srivastava A., Weiss D.J., Eldridge P., Cuende N., Deans R.J., Rasko J.E.J., Levine A.D., Turner L., 2013–2015 ISCT Presidential Task Force on Unproven Cellular Therapy Positioning a Scientific Community on Unproven Cellular Therapies: The 2015 International Society for Cellular Therapy Perspective. Cytotherapy. 2015;17:1663–1666. doi: 10.1016/j.jcyt.2015.10.007. [DOI] [PubMed] [Google Scholar]
- 10.Ylä-Herttuala S. The need for increased clarity and transparency in the regulatory pathway for gene medicines in the European Union. Mol. Ther. 2012;20:471–472. doi: 10.1038/mt.2012.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schneider C.K., Salmikangas P., Jilma B., Flamion B., Todorova L.R., Paphitou A., Haunerova I., Maimets T., Trouvin J.-H., Flory E., Committee for Advanced Therapies (CAT) CAT Scientific Secretariat Challenges with advanced therapy medicinal products and how to meet them. Nat. Rev. Drug Discov. 2010;9:195–201. doi: 10.1038/nrd3052. [DOI] [PubMed] [Google Scholar]
- 12.Gammie T., Lu C.Y., Babar Z.U. Access to orphan drugs: A comprehensive review of legislations, regulations and policies in 35 countries. PLoS ONE. 2015;10:e0140002. doi: 10.1371/journal.pone.0140002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.The European Commission. (2015). The revised user guide to the SME definition. http://ec.europa.eu/growth/tools-databases/newsroom/cf/itemdetail.cfm?item_id=8274&lang=en&title=The%2Drevised%2Duser%2Dguide%2Dto%2Dthe%2DSME%2Ddefinition.
- 14.Feigal E.G., Tsokas K., Viswanathan S., Zhang J., Priest C., Pearce J., Mount N. Proceedings: international regulatory considerations on development pathways for cell therapies. Stem Cells Transl. Med. 2014;3:879–887. doi: 10.5966/sctm.2014-0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abou-El-Enein M., Bauer G., Medcalf N., Volk H.-D., Reinke P. Putting a price tag on novel autologous cellular therapies. Cytotherapy. 2016;18:1056–1061. doi: 10.1016/j.jcyt.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 16.Buchholz C.J., Sanzenbacher R., Schüle S. The European hospital exemption clause-new option for gene therapy? Hum. Gene Ther. 2012;23:7–12. doi: 10.1089/hum.2011.2529. [DOI] [PubMed] [Google Scholar]
- 17.Cuende N., Boniface C., Bravery C., Forte M., Giordano R., Hildebrandt M., Izeta A., Dominici M., Legal and Regulatory Affairs Committee—Europe, International Society for Cellular Therapy The puzzling situation of hospital exemption for advanced therapy medicinal products in Europe and stakeholders’ concerns. Cytotherapy. 2014;16:1597–1600. doi: 10.1016/j.jcyt.2014.08.007. [DOI] [PubMed] [Google Scholar]