Abstract
Zolpidem is a short-acting non-benzodiazepine hypnotic that is used to improve sleep architecture in patients with burn injuries. This study evaluated the relationship between zolpidem administration and sleep parameters in a cohort of children with severe burn injuries. Standard age-based zolpidem dosing practices were employed. Polysomnography data were recorded at 30-second intervals throughout the night. Serum concentrations of zolpidem were measured at 0, 1, 2, 4, 5, 6, and 8 hours after administration of the first dose. The relationship between zolpidem concentrations and sleep parameters was evaluated using Markov mixed-effects pharmacodynamic models. Ten children received two doses of zolpidem at 22:00 and 02:00 hours. The median total amount of sleep was 361.0 (interquartile range [IQR]: 299.0–418.5) minutes; approximately 65% of the normal reference value for an 8-hour period. Slow-wave and rapid eye movement (REM) sleep were also dramatically reduced (18–37% of normal). With two doses of zolpidem, stage 2 sleep was 99% of normal levels. Higher peak zolpidem concentrations were associated with increased stage 2 sleep (r2 = .54; P = .04). Despite this, a median of 120.0 (IQR: 99.5–143.5) transitions between nocturnal sleep stages were recorded, with a median of 55.5 (IQR: 36–75) night-time awakenings per patient. In pediatric burn patients, higher zolpidem serum concentrations were associated with restoration of stage 2 sleep to normal levels. Nonetheless, slow-wave and REM sleep were profoundly depressed with frequent transitions between sleep stages, suggesting that alternative hypnotic agents may be required to restore normal sleep architecture in severely burned children.
Severe burn injuries are accompanied by a pronounced catabolic phase that is characterized by an increase in energy expenditure, protein catabolism, and cachexia.1,2 Sleep disturbances have been reported to affect approximately 50% of patients hospitalized with burn injuries and alterations in normal sleep architecture have been associated with impaired ventilation, poor wound healing, immunosuppression, heightened perception of pain, and increased mortality.3–12 These detrimental effects suggest that medications that enhance sleep may reduce morbidity and mortality associated with severe burn injuries.13 However, the use of sedative and hypnotic agents in children remains understudied.14
Zolpidem tartrate is a short-acting, non-benzodiazepine sedative, and hypnotic agent, which has been used for more than two decades to improve sleep duration and quality among insomniacs.15–18 In 2008, we randomized 40 children with severe burn injuries to receive zolpidem or haloperidol with continuous polysomnographic recording to evaluate the effects of these agents on sleep architecture.19 Zolpidem was found to have a small but significant effect in improving the proportion of stage 3/4 and rapid eye movement (REM) sleep (0.8 vs 0.6 hours) but did not improve the total duration of sleep. More recently, we evaluated the pharmacokinetics of zolpidem in a cohort of severely burned children and reported sub-therapeutic effects following a single dose at the onset of night-time and recommended that a second dose be administered 4 hours after the first.20 The objective of the current study was to characterize the effects of this second dose of zolpidem on multiple measures of sleep efficacy for the same cohort of severely burned children.
METHODS
Subjects and Study Design
Children 3 to 18 years of age with severe burn injuries were recruited to participate in this open-label pharmacokinetic and pharmacodynamic study if they were willing to receive zolpidem for use as a sleep-enhancing agent. To be eligible for inclusion in the study, the total burn surface area must have been ≥20% and they must have been admitted to Shriners Hospital for Children within 5 days of the burn injury. Exclusion criteria included: pre-existing neurological, sleep, or psychiatric disorders; brain injury; endocrine disease; a body mass index >97th percentile; questionable 72-hour survival; or planned administration of other sleep-inducing agents within the next 24 hours. Clinical and demographic data were collected for all study subjects.
The University of Cincinnati Institutional Review Board reviewed and approved this study. Parental permission and assent (when applicable) were obtained before conducting any study-related procedures.
Drug Administration
A standard age-based zolpidem dosing regimen was employed, in which children 2 to 4 years of age received 2.5 mg, children 5 to 10 years received 5 mg, and children >10 years received 10 mg of zolpidem. Zolpidem tablets were crushed, dissolved in 5 ml of water, and administered via a nasoduodenal feeding tube, followed by a 5 ml flush, in conjunction with continuous delivery of small amounts of liquid enteral nutrition substrate.
Sample Collection
Serial blood samples were obtained at 0, 1, 2, 4, 5, 6, and 8 hours after administration of the first dose of zolpidem. The total volume of blood collected during each sampling interval did not exceed 3.0 ml. Whole-blood samples were centrifuged at 1500g (~3000 rpm) for 10 minutes at 4°C. Centrifuged samples were then stored at −80°C.
Analytical Assay
Serum zolpidem concentrations were established using a validated high-performance liquid chromatography assay with a fluorescence detector.21 The assay was linear over the range from 25 to 1000 ng/ml. Intra- and interday coefficients of variation were <8.2%.
Sleep Measurement
Each study participant went to sleep at their habitual bedtime and underwent polysomnography evaluation for 8 hours. The polysomnography recording began at the time that the subject received their first dose of zolpidem. Polysomnography recordings were conducted using standard methods, which included evaluation of frontal, central, parietal and occipital electroencephalograms, submental electromyogram, electrooculogram, anterior tibialis electromyogram, and electrocardiogram.22
Polysomnography data were digitally recorded and scored for each 30-second interval (epoch). Sleep stages were determined according to previously published criteria, in which the subject was determined to be either awake, in stage 1, stage 2, stage 3, stage 4, or REM sleep for each epoch.23 Stage 3 and stage 4 were collectively aggregated into a single stage called “slow-wave sleep.”24
Thirteen sleep efficacy measurements proposed by Kjellsson et al24 were evaluated to quantify sleep quantity and quality in this study. A list of these measurements is featured in Table 2.
Table 2.
Measures of sleep efficacy among 10 severely burned children who received zolpidem
| Sleep Efficacy Measurements |
Definition | Observed Data Median (Interquartile Range) |
|---|---|---|
| Latency to persistent sleep (min) | Elapsed time since the start of the polysomnography recording to sleep lasting at least 5 minutes | 19.7 (9.0–33.5) |
| Total sleep time (min) | Time from sleep onset to final awakening minus the time spent awake | 361.0 (299.0–418.5) |
| Sleep efficiency (%) | The proportion of sleep, defined as total sleep time divided by the amount of time in which polysomnography data were recorded | 62.3 (58.7–65.6) |
| Number of awakenings | Number of arousals to wakefulness during the night | 55.5 (36.0–75.0) |
| Number of body movements | Number of interruptions in polysomnography recording because of body movements during the night | 99.5 (60.0–139.0) |
| Stage transitions | Number of transitions from one stage of sleep to another during the night (excluding transitions from periods of movement) | 212.0 (146.0–279.0) |
| Sleep onset latency (min) | Elapsed time from the start of the polysomnography recording until stage 1 sleep is recorded | 6.0 (3.7–17.0) |
| REM sleep latency (min) | Elapsed time from the start of the polysomnography recording until REM sleep is recorded | 121.0 (61.7–283.0) |
| Total time in stage 1 (min) | Total time spent in stage 1 during the night | 40.5 (25–53) |
| Total time in stage 2 (min) | Total time spent in stage 2 during the night | 216.3 (177.5–230.0) |
| Total time in slow-wave sleep (min) | Total time spent in stage 3 or stage 4 (slow-wave sleep) during the night | 16.3 (8.0–93.0) |
| Total time in REM sleep (min) | Total time spent in REM sleep during the night | 13.3 (0.5–44.5) |
| Total time in non-REM sleep (min) | Total time spent in stage 1, stage 2, stage 3, or stage 4 sleep during the night | 321.0 (248.0–369.5) |
REM, rapid eye movement.
Sleep efficacy measurements were adapted from Kjellsson et al.24
Dataset Preparation
The dataset was prepared for analysis with sleep stage as the dependent variable. Coded patient identifiers and a date/time stamp were recorded for each 30 second interval throughout the night. Also included in the dataset were measurements of the sleep stage for the previous observation (30 seconds before) and for the subsequent observation (30 seconds later). Additionally, the dataset included measurements of the relative bedtime (defined as the time elapsed since going to bed divided by the total time spent asleep) and a flag to identify transitions between sleep stages. Lastly, we included individually-predicted zolpidem pharmacokinetic parameters for each participant using data from a previously published analysis.25
The objective of this study was to model the probability of transitioning from one sleep stage to another and to determine the effect of zolpidem serum concentrations on the likelihood of entering and exiting each sleep stage. There were five distinct sleep stages that a participant could potentially inhabit over the course of the night: wake, stage 1, stage 2, slow-wave, and REM. On the basis of prior literature describing the effects of zolpidem on different sleep stages and to reduce the computational time required to model each of the 21 possible transitions, we focused our analysis on the probability of transitioning from stage 1 sleep to: wakefulness, stage 2 sleep, slow-wave sleep, and REM sleep.26
Pharmacodynamic Analysis
Traditional mathematical models assume independence between measurements; however, stochastic Markov models make it possible to model processes in which a future state depends entirely on the current state (eg, tomorrow’s weather is likely to depend on today’s weather). For this reason, Markov models are ideally suited to the analysis of longitudinal polysomnography data.
In this study, we developed Markov models that allow estimation of the probability of observing a transition between sleep stages. For example, it is possible to estimate the probability of measuring a specific sleep stage (eg, stage 2 sleep) as a function of the fact that the subject was in a different stage in the preceding 30 second period (eg, stage 1). This probability is represented as P (Stage 2|Stage 1).
Using techniques developed by Karlsson et al27 and Kjellsson et al,24 we developed Markov mixed-effects models to analyze the relationship between zolpidem concentrations and sleep quantity and quality. Sleep transitions were modelled separately as a function of zolpidem exposure, which was based on the individual pharmacokinetic parameters estimated previously.25 To calculate the average probability that stage 2 sleep will occur directly after stage 1 sleep [P (Stage 2|Stage 1)], the complete dataset may be reduced to only those observations of stage 1 sleep that are immediately preceded by stage 2 sleep. This transition probability can then be estimated independently of all other sleep stage transitions, which do not have to be included in the P (Stage 2|Stage 1) dataset.
To simultaneously estimate the transition probability between any two sleep stages for all 10 participants, we developed a mixed-effects model that was implemented in NONMEM 7.2 (nonlinear mixed effects modelling; ICON Development Solutions, Ellicott City, MD).
RESULTS
Participant Characteristics
Data were collected from 10 children with acute burn injuries, seven of whom were male and three were female (Table 1). The median age of the subjects included in this study was 8.0 (interquartile range [IQR]: 7.0–12.0) years. Additional demographic characteristics of the study cohort are featured in Table 1. The extent of the median TBSA burn was 52.5% (IQR: 35.3–82.5%). All 10 children had thermal burn injuries and 2 (20%) patients also suffered an inhalation injury.
Table 1.
Demographic and clinical characteristics of 10 severely burned children who received zolpidem
| Participant Characteristics |
|
|---|---|
| Gender | |
| Male | 7 (70%) |
| Female | 3 (30%) |
| Age (yrs) | |
| Median | 8.0 |
| Interquartile range | 7.0–12.0 |
| Range | 4.0–17.0 |
| Weight (kg) | |
| Median | 30.75 |
| Interquartile range | 23.0–45.0 |
| Range | 17.0–59.8 |
| Height (cm) | |
| Median | 131.5 |
| Interquartile range | 119.0–153.0 |
| Range | 102.0–170.0 |
| Body mass index (kg/m2) | |
| Median | 17.0 |
| Interquartile range | 16.3–19.2 |
| Range | 14.9–23.1 |
| TBSA* (%) | |
| Median | 52.5 |
| Interquartile range | 35.3–82.5 |
| Range | 28.0–84.9 |
| Third-degree burns (%) | |
| Median | 37.6 |
| Interquartile range | 23.0–74.3 |
| Range | 19.0–83.2 |
| Days after burn | |
| Median | 15.5 |
| Interquartile range | 11.0–19.0 |
| Range | 8.0–44.0 |
TBSA reflects the cumulative percentage of the burned area for each major section of the body.
Sleep Patterns and Efficacy Parameters
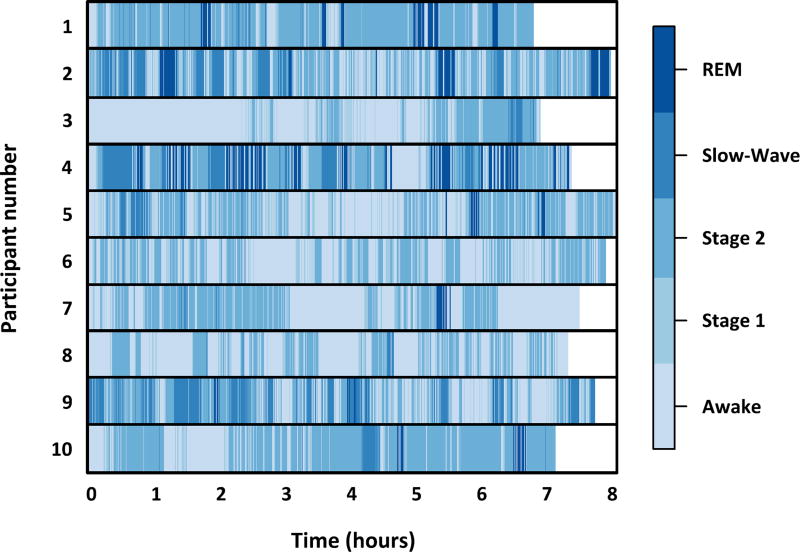
The median amount of time from the onset of sleep to final awakening, minus the time spent awake, was 361.0 (IQR: 299.0–418.5) minutes. A minimum of 218.5 minutes of total sleep time was recorded in one patient and a maximum of 427.0 minutes was recorded in another patient. The distribution of sleep stages is presented graphically for each of the study participants in Figure 1. Overall, 40.8% of the night was spent in stage 2, 8.2% in stage 1, 7.8% in slow-wave, and 4.9% in REM sleep. In this population, 29.1% of the night was spent awake and a further 9.2% of the time the patient was moving and a reliable polysomnographic measurement could not be established.
Figure 1.
Distribution of sleep stages for 10 severely burned children who received zolpidem. Each participant had polysomnographic measurements obtained every 30 seconds for the duration of the night. The total duration of sleep was approximately 65% of normal age- and gender-matched reference values. This was primarily because of markedly lower amounts of slow-wave and rapid eye movement (REM) sleep than would be expected for healthy children. Two doses of zolpidem effectively restored the amount of stage 2 sleep to normal levels. The sleep patterns observed in these children exhibit signs of sleep fragmentation, including more than 50 nocturnal awakenings, which has been previously reported among children with severe burn injuries.20
Additional sleep efficacy measures are featured in Table 2, including the relative sleep efficiency, the number of body movements recorded, the number of transitions between sleep stages, and the number of minutes spent in each sleep stage. Characteristic of this study population,22 the median number of night-time awakenings was 55.5 (IQR: 36–75).
Sleep Stage Transitions
Transitions between sleep stages occurred often in this population, with a median of 212.0 (IQR: 60.0–139.0) transitions recorded per patient. The distribution of the number of transitions, divided into hourly intervals, is presented in Table 3. The most common transition was to stage 2 sleep (34.2%). Transitions into slow-wave and REM sleep were infrequently recorded (12.5 and 6.1%, respectively).
Table 3.
Distribution of the number of sleep stage transitions among 10 severely burned children who received the sleep-enhancing agent zolpidem
| Sleep Stage in the Next 30 Seconds | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| Hourly Interval |
Current Sleep Stage |
Awake | Stage 1 | Stage 2 | Slow-Wave | REM |
| 0 | Awake | 32 | 3 | |||
| Stage 1 | 6 | 27 | ||||
| Stage 2 | 14 | 3 | 33 | 2 | ||
| Slow-wave | 2 | 29 | ||||
| REM | 2 | |||||
| 1 | Awake | 32 | 2 | |||
| Stage 1 | 5 | 25 | 1 | 1 | ||
| Stage 2 | 13 | 2 | 22 | 7 | ||
| Slow-wave | 1 | 18 | ||||
| REM | 3 | 4 | ||||
| 2 | Awake | 23 | 5 | 1 | ||
| Stage 1 | 5 | 27 | ||||
| Stage 2 | 9 | 7 | 32 | 9 | ||
| Slow-wave | 2 | 1 | 28 | |||
| REM | 4 | 1 | 4 | |||
| 3 | Awake | 44 | 5 | 1 | ||
| Stage 1 | 16 | 28 | ||||
| Stage 2 | 19 | 3 | 16 | 6 | ||
| Slow-wave | 4 | 11 | ||||
| REM | 1 | 4 | ||||
| 4 | Awake | 46 | 6 | |||
| Stage 1 | 13 | 26 | ||||
| Stage 2 | 12 | 6 | 10 | 9 | ||
| Slow-wave | 1 | 1 | 9 | 3 | ||
| REM | 3 | 3 | 3 | |||
| 5 | Awake | 42 | 3 | |||
| Stage 1 | 6 | 31 | 3 | |||
| Stage 2 | 16 | 6 | 9 | 7 | ||
| Slow-wave | 5 | |||||
| REM | 2 | 1 | 4 | |||
| 6 | Awake | 39 | 3 | |||
| Stage 1 | 7 | 34 | 3 | |||
| Stage 2 | 18 | 7 | 12 | 10 | ||
| Slow-wave | 2 | 12 | ||||
| REM | 3 | 1 | 4 | 1 | ||
| 7 | Awake | 36 | 8 | |||
| Stage 1 | 18 | 28 | ||||
| Stage 2 | 16 | 7 | 7 | 12 | ||
| Slow-wave | 4 | |||||
| REM | 2 | 1 | 4 | |||
| 8 | Awake | 10 | 2 | |||
| Stage 1 | 1 | 11 | ||||
| Stage 2 | 12 | 3 | 10 | 4 | ||
| Slow-wave | 1 | 8 | ||||
| REM | 2 | 3 | ||||
REM, rapid eye movement.
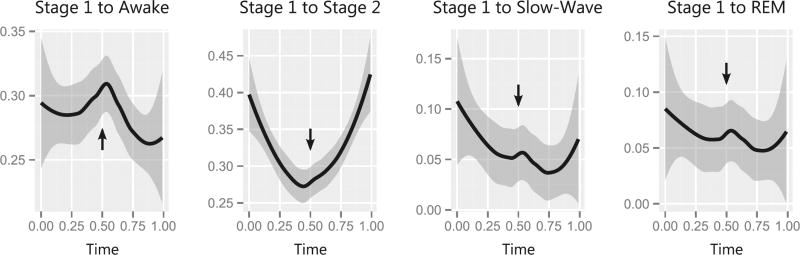
Consistent with earlier studies involving zolpidem use among insomniacs,26 the probability of transitioning from stage 1 into stage 2 sleep increased following administration of the second dose of zolpidem. This is depicted graphically in Figure 2, in which the probability of transitioning from stage 1 sleep is modelled as a function of the night-time (scaled from 0 to 1 to represent the proportion of the night). Critically, administration of the second dose of zolpidem half-way through the night (denoted by the black arrows at 0.5) also decreased the probability of waking from stage 1 sleep. However, zolpidem did not increase the probability of transitioning to slow-wave or REM sleep.
Figure 2.
The probability of transiting between sleep stages changes following zolpidem administration. The x-axis represents the total duration of the night-time and is scaled from 0 (initial sleeplessness) to 1 (final awakening), as a proportion of the 8-hour study period. Solid black lines depict the predicted transition probabilities within the next 30 seconds for severely burned children who received zolpidem (shaded grey areas represent the 95% confidence intervals). Black arrows indicate the timing of the second administration of zolpidem. Administration of the second dose of zolpidem was associated with an increased likelihood of transitioning from stage 1 to stage 2 sleep. The second dose of zolpidem was also associated with a decrease in the likelihood of waking from stage 1 sleep. In contrast, the second dose of zolpidem was associated with a reduced likelihood of transitioning from stage 1 sleep to slow-wave and rapid eye movement (REM) sleep.
Associations Between Zolpidem Concentrations and Sleep Parameters
Zolpidem serum concentrations were significantly higher 1 hour after the administration of the second dose as compared to 1 hour after the first dose (mean = 407.7 ± 72 vs 269 ± 34 ng/ml; P < .01). The area under the concentration time curve was also significantly higher in the second half of the night as compared to the first (mean = 1193 ± 248 vs 809 ± 138 ng × hour/ml; P < .01). Compared to previously published polysomnography data for children with severe burn injuries, the administration of zolpidem was not associated with statistically significant changes in the total amount of time spent asleep or the amount of time spent in REM sleep (P > .05 for both). However, the amount of time spent in stage 2 sleep was highly correlated with zolpidem exposure, with higher peak concentrations associated with increased stage 2 sleep (r2 = .54; P = .04). Additionally, administration of a second dose of zolpidem was associated with an increase in the total amount of sleep when compared with burned children who received only a single dose (two dose mean = 344.8 ± 79 vs one dose mean = 288 ± 18 minutes; P < .01).
DISCUSSION
In this study, the total amount of sleep recorded among children with burn injuries who received two doses of zolpidem was approximately 65% of the normal reference value for age-matched healthy children.28 This aligns well with our earlier study in which 40 children with severe burn injuries were randomized to receive a single dose of zolpidem and had a mean recorded duration of sleep that was 49% of normal.19,28 The current study employed a two dose regimen, which was associated with a slightly longer total sleep time.
Slow-wave and REM sleep were similarly disrupted, with values ranging from 18 to 37% of normal age and gender matched reference values.28 In contrast, the amount of time spent in stage 2 sleep was restored to 99% of normal.28 In studies conducted among adults with insomnia, zolpidem has been widely reported to improve the duration of stage 2 sleep.26,29,30 Unlike benzodiazepine hypnotics, these adult studies demonstrated that zolpidem had no effect on slow-wave sleep or REM sleep. Normal pediatric reference ranges suggest that healthy children spend 50% of their total sleep time in slow-wave or REM sleep.28 However, in this study, slow-wave and REM sleep accounted for 20% of the total sleep time, demonstrating the profound alterations to normal sleep architecture that are observed among children with severe burn injuries.
Further exacerbating normal sleep patterns, the children in this study experienced more than double the number of night-time awakenings reported among control subjects.19 This suggests that two doses of zolpidem are insufficient to sustain sleep throughout the night for children with severe burn injuries and that alternative hypnotic agents may be required to achieve long-lasting improvements in sleep quality and duration. However, until more effective agents are discovered, a second dose of zolpidem halfway through the night appears to improve the duration of stage 2 sleep and achieves a slightly longer duration of sleep than has been reported following administration of a single dose.
In 2013, the United States Food and Drug Administration recommended that the initial dose of immediate- and extended-release formulations of zolpidem be lowered to reduce the risk of driving impairment the following morning.31,32 Although the updated product labelling did not provide any recommendations on the dosage of zolpidem to be administered to children, our data suggest that lowering the dose of zolpidem is not likely to be appropriate for children with severe burn injuries.
Interpretation of these findings should be considered in light of several limitations. First, only 10 subjects were evaluated in this study. Nevertheless, this is the first study to conduct an integrated assessment of the pharmacokinetics and pharmacodynamics of zolpidem in pediatric burn patients. Although approximately 1000 polysomnographic measurements were obtained for each participant over the course of the night (typically an 8 to 10-hour period), these results cannot be interpreted to reflect the total amount of time slept over a 24-hour period. Second, because of computational constraints, our Markov model analyses were limited to evaluating transitions from stage 1 sleep. Additionally, the children enrolled in this study underwent polysomnographic evaluation at a median of 18 days after the burn injury. Therefore, the findings described here may not be generalizable to children during the shock phase (lasting 3–5 days postinjury), peak hypermetabolic stage (approximately 5–12 days postburn), nor after substantive wound healing and convalescence has occurred. It should also be noted that zolpidem was administered in conjunction with small amounts of liquid enteral nutrition, which has been reported to decrease zolpidem exposure.33 Lastly, limited demographic and clinical data were collected after informed consent was obtained and we did not have access to information regarding other medical and environmental factors that may have influenced our patients’ sleep patterns.
This study reinforces the fact that alterations in normal sleep patterns are common among children with severe burn injuries. Use of the sleep-enhancing agent zolpidem resulted in a modest improvement in sleep efficacy parameters, including a notable increase in stage 2 sleep. However, these children had profoundly fragmented sleep patterns, with more than 50 awakenings per night. Additionally, large aberrations in slow-wave and REM sleep that are characteristic of severely burned patients were not ameliorated despite the administration of two doses of zolpidem. This finding suggests that zolpidem improves cycling to non-slow-wave sleep and that alternative hypnotic agents are needed to restore deep sleep architecture in children with severe burn injuries.
Acknowledgments
We thank the contributions of Chris Allgeier, DTR, and the departments of clinical research, pharmacy, lab medicine, nursing, and medical staff for their invaluable assistance.
This work was sponsored by a grant from Shriners Hospitals for Children® to M.M.G. (grants 70097 and 71005).
References
- 1.Herndon DN, Tompkins RG. Support of the metabolic response to burn injury. Lancet. 2004;363:1895–902. doi: 10.1016/S0140-6736(04)16360-5. [DOI] [PubMed] [Google Scholar]
- 2.Wilmore DW, Aulick LH. Metabolic changes in burned patients. Surg Clin North Am. 1978;58:1173–87. doi: 10.1016/s0039-6109(16)41685-3. [DOI] [PubMed] [Google Scholar]
- 3.Lawrence JW, Fauerbach J, Eudell E, Ware L, Munster A. The 1998 Clinical Research Award. Sleep disturbance after burn injury: a frequent yet understudied complication. J Burn Care Rehabil. 1998;19:480–6. doi: 10.1097/00004630-199811000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Raymond I, Nielsen TA, Lavigne G, Manzini C, Choinière M. Quality of sleep and its daily relationship to pain intensity in hospitalized adult burn patients. Pain. 2001;92:381–8. doi: 10.1016/S0304-3959(01)00282-2. [DOI] [PubMed] [Google Scholar]
- 5.White DP, Douglas NJ, Pickett CK, Zwillich CW, Weil JV. Sleep deprivation and the control of ventilation. Am Rev Respir Dis. 1983;128:984–6. doi: 10.1164/arrd.1983.128.6.984. [DOI] [PubMed] [Google Scholar]
- 6.Plyley MJ, Shephard RJ, Davis GM, Goode RC. Sleep deprivation and cardiorespiratory function. Influence of intermittent submaximal exercise. Eur J Appl Physiol Occup Physiol. 1987;56:338–44. doi: 10.1007/BF00690902. [DOI] [PubMed] [Google Scholar]
- 7.Bonnet MH, Berry RB, Arand DL. Metabolism during normal, fragmented, and recovery sleep. J Appl Physiol (1985) 1991;71:1112–8. doi: 10.1152/jappl.1991.71.3.1112. [DOI] [PubMed] [Google Scholar]
- 8.Rechtschaffen A, Gilliland MA, Bergmann BM, Winter JB. Physiological correlates of prolonged sleep deprivation in rats. Science. 1983;221:182–4. doi: 10.1126/science.6857280. [DOI] [PubMed] [Google Scholar]
- 9.Everson CA, Bergmann BM, Rechtschaffen A. Sleep deprivation in the rat: III. Total sleep deprivation. Sleep. 1989;12:13–21. doi: 10.1093/sleep/12.1.13. [DOI] [PubMed] [Google Scholar]
- 10.Kushida CA, Bergmann BM, Rechtschaffen A. Sleep deprivation in the rat: IV. Paradoxical sleep deprivation. Sleep. 1989;12:22–30. doi: 10.1093/sleep/12.1.22. [DOI] [PubMed] [Google Scholar]
- 11.Palmblad J, Petrini B, Wasserman J, Akerstedt T. Lymphocyte and granulocyte reactions during sleep deprivation. Psychosom Med. 1979;41:273–8. doi: 10.1097/00006842-197906000-00001. [DOI] [PubMed] [Google Scholar]
- 12.Benca RM, Kushida CA, Everson CA, Kalski R, Bergmann BM, Rechtschaffen A. Sleep deprivation in the rat: VII. Immune function. Sleep. 1989;12:47–52. doi: 10.1093/sleep/12.1.47. [DOI] [PubMed] [Google Scholar]
- 13.Jaffe SE, Patterson DR. Treating sleep problems in patients with burn injuries: practical considerations. J Burn Care Rehabil. 2004;25:294–305. doi: 10.1097/01.bcr.0000124793.99886.6a. [DOI] [PubMed] [Google Scholar]
- 14.Owens JA, Babcock D, Blumer J, et al. The use of pharmacotherapy in the treatment of pediatric insomnia in primary care: rational approaches. A consensus meeting summary. J Clin Sleep Med. 2005;1:49–59. [PubMed] [Google Scholar]
- 15.Holm KJ, Goa KL. Zolpidem: an update of its pharmacology, therapeutic efficacy and tolerability in the treatment of insomnia. Drugs. 2000;59:865–89. doi: 10.2165/00003495-200059040-00014. [DOI] [PubMed] [Google Scholar]
- 16.Roth T, Roehrs T, Vogel G. Zolpidem in the treatment of transient insomnia: a double-blind, randomized comparison with placebo. Sleep. 1995;18:246–51. doi: 10.1093/sleep/18.4.246. [DOI] [PubMed] [Google Scholar]
- 17.Maarek L, Cramer P, Attali P, Coquelin JP, Morselli PL. The safety and efficacy of zolpidem in insomniac patients: a long-term open study in general practice. J Int Med Res. 1992;20:162–70. doi: 10.1177/030006059202000208. [DOI] [PubMed] [Google Scholar]
- 18.Hajak G, Bandelow B. Safety and tolerance of zolpidem in the treatment of disturbed sleep: a post-marketing surveillance of 16944 cases. Int Clin Psychopharmacol. 1998;13:157–67. doi: 10.1097/00004850-199807000-00002. [DOI] [PubMed] [Google Scholar]
- 19.Armour A, Gottschlich MM, Khoury J, Warden GD, Kagan RJ. A randomized, controlled prospective trial of zolpidem and haloperidol for use as sleeping agents in pediatric burn patients. J Burn Care Res. 2008;29:238–47. doi: 10.1097/BCR.0b013e31815f384e. [DOI] [PubMed] [Google Scholar]
- 20.Stockmann C, Sherwin CM, Buterbaugh W, et al. Preliminary assessment of zolpidem pharmacokinetics in pediatric burn patients. Ther Drug Monit. 2014;36:295–301. doi: 10.1097/FTD.0000000000000017. [DOI] [PubMed] [Google Scholar]
- 21.Ring PR, Bostick JM. Validation of a method for the determination of zolpidem in human plasma using LC with fluorescence detection. J Pharm Biomed Anal. 2000;22:495–504. doi: 10.1016/s0731-7085(99)00311-8. [DOI] [PubMed] [Google Scholar]
- 22.Gottschlich MM, Jenkins ME, Mayes T, et al. The 1994 clinical research award. A prospective clinical study of the polysomnographic stages of sleep after burn injury. J Burn Care Rehabil. 1994;15:486–92. [PubMed] [Google Scholar]
- 23.Rechtschaffen A, Kales A. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. Los Angeles: UBISBR Institute; 1968. [DOI] [PubMed] [Google Scholar]
- 24.Kjellsson MC, Ouellet D, Corrigan B, Karlsson MO. Modeling sleep data for a new drug in development using markov mixed-effects models. Pharm Res. 2011;28:2610–27. doi: 10.1007/s11095-011-0490-x. [DOI] [PubMed] [Google Scholar]
- 25.Stockmann C, Sherwin CM, Buterbaugh W, et al. Preliminary assessment of zolpidem pharmacokinetics in pediatric burn patients. Ther Drug Monit. 2014;36:295–301. doi: 10.1097/FTD.0000000000000017. [DOI] [PubMed] [Google Scholar]
- 26.Monti JM. Effect of zolpidem on sleep in insomniac patients. Eur J Clin Pharmacol. 1989;36:461–6. doi: 10.1007/BF00558070. [DOI] [PubMed] [Google Scholar]
- 27.Karlsson MO, Schoemaker RC, Kemp B, et al. A pharmacodynamic Markov mixed-effects model for the effect of temazepam on sleep. Clin Pharmacol Ther. 2000;68:175–88. doi: 10.1067/mcp.2000.108669. [DOI] [PubMed] [Google Scholar]
- 28.Ohayon MM, Carskadon MA, Guilleminault C, Vitiello MV. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep. 2004;27:1255–73. doi: 10.1093/sleep/27.7.1255. [DOI] [PubMed] [Google Scholar]
- 29.Roth T, Zorick F, Wittig R, Roehrs T. Pharmacological and medical considerations in hypnotic use. Sleep. 1982;5(Suppl 1):S46–52. doi: 10.1093/sleep/5.suppl_1.s46. [DOI] [PubMed] [Google Scholar]
- 30.Monti JM, Alterwain P, Debellis J, Altier H, Pellejero T, Monti D. Short-term sleep laboratory evaluation of midazolam in chronic insomniacs. Preliminary results. Arzneimittelforschung. 1987;37:54–7. [PubMed] [Google Scholar]
- 31.U.S. Food and Drug Administration. FDA Drug Safety Communication: FDA approves new label changes and dosing for zolpidem products and a recommendation to avoid driving the day after using Ambien CR. Rockville, MD: MedWatch; 2013. [Google Scholar]
- 32.Kuehn BM. FDA warning: driving may be impaired the morning following sleeping pill use. JAMA. 2013;309:645–6. doi: 10.1001/jama.2013.323. [DOI] [PubMed] [Google Scholar]
- 33.Ambien (zolpidem tartrate) [package insert] Bridgewater, NJ: Sanofi-Aventis; 2013. [Google Scholar]