Abstract
Ultrasound reports of 102 children with microbiologically confirmed or clinically diagnosed pulmonary tuberculosis (TB) showed that 23/37 (64%) and 23/65 (36%) had TB suggestive abdominal lymphadenopathy and 16/37 (44%) and 8/65(13%) had splenic micro-abscesses, respectively. Splenic micro-abscesses were associated with HIV-infection (p=0.041). These data suggest that pulmonary TB is often complicated by abdominal TB in children.
Keywords: ultrasound, tuberculosis, HIV, children, extra-pulmonary tuberculosis
Introduction
Childhood tuberculosis (TB) accounts for a considerable burden of pediatric morbidity and mortality worldwide1. Due to non-specific signs and symptoms, paucibacillary disease and difficulty in obtaining adequate specimens for microbiologic investigation diagnosing pulmonary TB in children remains challenging, and development of novel diagnostic tools is a research priority. Children are at particular risk of progression to disseminated and severe forms of TB1.
Ultrasonography findings suggestive of abdominal TB have been reported to occur in a substantial proportion of both pediatric and adult patients for whom chest radiography (CXR) does not indicate TB2,3. Abdominal lymphadenopathy and splenic micro-abscesses are considered diagnostic of TB in particular clinical and epidemiologic settings4. A point-of-care ultrasound protocol for focused assessment with sonography for HIV-associated TB (FASH) has been developed for adults and has successfully been implemented in emergency settings in South Africa5,6.
Abdominal ultrasound data in children with pulmonary TB are limited. FASH has not been systematically studied in children but may be a valuable tool to support diagnosis and assess extent of disease. This study was performed to investigate the prevalence of ultrasound findings suggestive of abdominal TB in children with pulmonary TB and the association of HIV-infection with these.
Materials and Methods
This secondary analysis reviewed ultrasound reports available for children hospitalized at Red Cross War Memorial Children’s Hospital in Cape Town, South Africa, who participated in a TB diagnostic study7 in 2008–2013 and who had an abdominal ultrasound. Children up to 13 years of age were enrolled if they met the following inclusion criteria: cough, plus one or more of the following criteria: i) failure to thrive or loss of weight in the past 3 months; ii) a positive tuberculin skin test (TST, positive: >10 mm in HIV-uninfected and >5 mm in HIV-infected); iii) CXR suggestive of active TB; iv) household or close TB contact. Exclusion criteria were TB treatment or prophylaxis for more than 72 hours. Diagnostic TB work-up comprised history taking, physical examination, CXR, TST, and repeated induced sputum for smear microscopy, liquid culture and GeneXpert®. Children were categorized as ‘definite TB’ (culture or GeneXpert® positive), ‘TB negative’ (culture or GeneXpert® negative and documented resolution of symptoms and signs after 3 months in the absence of TB treatment), or ‘clinical TB’ (microbiologically negative but clinically diagnosed and treated for TB). CXRs were interpreted by two independent readers blinded to clinical data including ultrasound findings; in case of discrepant readings a third reader was added and diagnosis based on majority opinion. HIV testing was done in all children unless their HIV status was known. The study was approved by the Research Ethics Committee of the Faculty of Health Sciences, University of Cape Town and written informed consent was obtained from a parent or legal guardian.
Children with definite or clinical pulmonary TB who underwent a formal abdominal ultrasound examination at the hospital’s pediatric radiology department were included. The indication for the abdominal ultrasound examination was determined by the individual child’s attending physician. The ultrasound equipment consisted of a Philips iU22 and Toshiba Aplio 400 machine. A curvilinear probe 5.0 – 8.0 MHz was used for infants; and a curvilinear probe 2.5 – 6.2 MHz for children older than 6 months. For the evaluation of splenic micro-abscesses, a linear probe 5 – 12.6 MHz was employed. The standard abdominal imaging protocol included evaluation of the liver, portal vein, hepatic veins, hepatic artery, common bile duct, gallbladder, aorta, inferior vena cava, pancreas, spleen, right and left kidney, bladder, and pelvis. Lymphadenopathy was evaluated in the peri-pancreatic region, porta hepatis, splenic hilar region and mesenterium and defined as lymph nodes larger than 10 mm in short axis. Splenic micro-abscesses are defined as multiple focal hypoechoic lesions throughout the spleen parenchyma usually measuring 0.5 to 1.0 cm and best visualized with a linear high frequency transducer. Copies of images were obtained selectively. Ultrasound examinations were performed by consultant radiologists, radiology registrars under supervision, qualified sonographers, and student sonographers under supervision. Ultrasound data that were extracted from radiology reports comprised presence or absence of abdominal lymphadenopathy and splenic micro-abscesses (see Figures, Supplemental Digital Content 1 and 2). Data were analyzed using SPSS (Macintosh) Version 22.0 (Armonk, NY, IBM Corp.); Chi Square test was used two-sided at α = 0.05.
Results
A total of 102 South African children [median age 37 months (interquartile range (IQR) 17;73] were included. Thirty-four (33%) children were HIV-infected with a median CD4 percentage of 17% (IQR 8;26); and 24 (71%) were receiving antiretroviral treatment. Thirty-seven (36%) children had definite pulmonary TB and 65 (64%) had clinical pulmonary TB. All but one isolate belonged to the Mycobacterium tuberculosis complex and all but two isolates were susceptible to rifampin and isoniazid. 13/92 (14%) children had received previous treatment for latent TB infection (LTBI). HIV status and median age did not differ significantly between children with definite and clinical TB.
Abdominal lymphadenopathy was present in 46/100 (46%) children. Splenic micro-abscesses were reported in 24/97 (25%) children; Table 1. Abdominal lymphadenopathy was located in the epigastrium in 25 (56%), in the para-aortal or para-caval regions in 16 (36%), in the splenic hilum in 7 (16%), and in the lower abdomen in 5 (11%) of the children.
Table 1.
Abdominal ultrasound findings in children by TB category and by HIV status
| Definite TB n = 37 (36%) |
Clinical TB n = 65 (64%) |
Total n = 102 (100%) |
p value | HIV-infected n = 34 (33%) |
HIV-uninfected n = 67 (66%) |
p value | |
|---|---|---|---|---|---|---|---|
| Abdominal lymphadenopathy, n (%) | 23 (64) | 23 (36) | 46 (46) | 0.007 | 15 (44) | 31 (48) | n.s. |
| Splenic micro-abscesses, n (%) | 16 (44) | 8 (13) | 24 (25) | 0.001 | 12 (38) | 12 (19) | 0.041 |
Abdominal lymphadenopathy or splenic micro-abscesses were significantly more common in children with definite pulmonary TB than in children with clinical pulmonary TB (p < 0.007, table 1). Splenic micro-abscesses were associated with the presence of concurrent abdominal lymphadenopathy (p < 0.001); 19/46 (41%) children with abdominal lymphadenopathy also had splenic micro-abscesses. Both abdominal lymphadenopathy and splenic micro-abscesses were associated with clinical signs of abdominal distention (p < 0.001 and p 0.04, respectively). While splenic micro-abscesses were associated with HIV-infection (p 0.041), abdominal lymphadenopathy was not. Median CD4 cell counts did not differ between HIV-infected children with and without splenic micro-abscesses. Neither age, nor sex, nor previous treatment for LTBI was associated with abdominal ultrasound findings.
CXR reports were available for 98/102 children; of those, 56 (57%) were interpreted as suggestive of TB and 15 (15%) as not suggestive of TB; in 27 (28%), CXR interpretation was inconclusive. Of 32 children with clinical TB and with a CXR not suggestive or inconclusive for TB, 13 (40%) had either abdominal lymphadenopathy or splenic micro-abscesses.
Duration of treatment was lengthened in 30% of children for whom these data were available; neither in HIV-uninfected nor in HIV-infected children duration of treatment was associated with abdominal ultrasound findings.
Discussion
To our knowledge, this is the largest evaluation of abdominal ultrasound findings of TB in a well-defined cohort of children with pulmonary TB. Our data support the observation that concurrent pulmonary TB and abdominal TB are common in children3 and thereby highlight the risk of extra-pulmonary TB (EPTB) or disseminated TB in children with pulmonary disease.
Consistent with reports on abdominal TB in adults and children3,8, abdominal lymphadenopathy was the most common finding. Splenic micro-abscesses were also a common feature, especially in children with abdominal lymphadenopathy. Children with definite TB were more likely to have concurrent abdominal lymphadenopathy or splenic micro-abscesses compared with children without microbiologic confirmation. This may indicate poor host control (uncontrolled disease)9, suggesting that clinicians should consider possible extra-thoracic spread in children with confirmed pulmonary TB.
A considerable proportion (40%) of children without microbiologic confirmation and who had a CXR which was not interpreted as suggestive of TB had either abdominal lymphadenopathy or splenic micro-abscesses supporting that abdominal ultrasound may play an adjunctive role in the investigation of children with suspected pulmonary TB particularly in high-risk populations such as HIV-infected children 3.
While HIV is a risk factor for EPTB including abdominal lymphadenopathy in adults10, abdominal lymphadenopathy was not associated with HIV-infection in children in a smaller retrospective study3. Our data confirm this. In contrast, a novel finding was the significant association of splenic micro-abscesses with HIV-infection in our study.
Our data are limited by the selective referral of children for abdominal ultrasound precluding any calculation of the true prevalence of concurrent abdominal TB in children presenting with pulmonary TB. Furthermore, we did not have ultrasound data from a control group of children with non-TB respiratory disease. Abdominal features suggestive of EPTB were not confirmed by microbiology; precluding definite knowledge on their etiology. Abdominal lymphadenopathy is a common finding in children11–13; however, the high TB prevalence in this setting, stringent inclusion criteria, strict case definitions as well as microbiologic confirmation of pulmonary TB in half of the patients with abdominal lymphadenopathy and concurrence with splenic micro-abscesses as a further sonographic feature of EPTB in 41% of patients suggest that enlarged lymph nodes in this cohort most likely reflect tuberculous lymphadenitis.
This study suggests that detection of features of abdominal TB by ultrasound may be a useful investigation for defining the extent of TB in children with pulmonary TB, particularly in HIV-infected children, and with implications for treatment as extensive or severe TB should be treated with a four drug regimen in South Africa. As portable point-of-care ultrasound devices become more affordable, ultrasound is an imaging modality that may be increasingly useful in resource limited areas, where radiography may not be widely available. A prospective larger evaluation of the usefulness of abdominal ultrasound5 in a cohort of HIV-infected and HIV-uninfected children is warranted.
Supplementary Material
Figure 1.
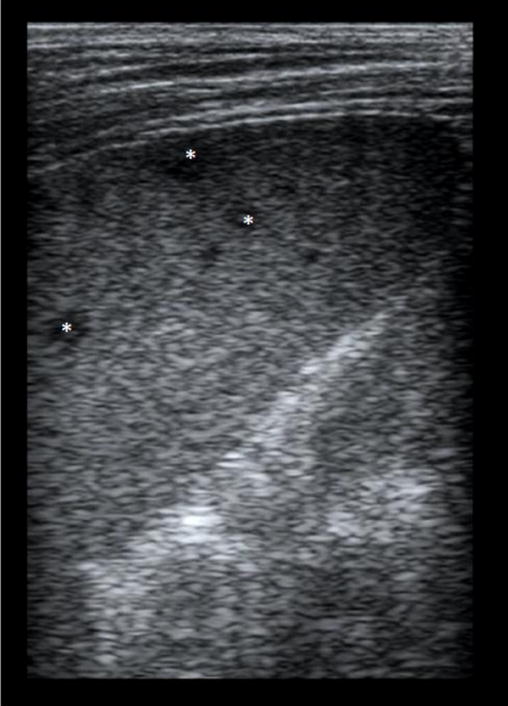
Splenic micro-abscesses (*) in a 12 year old HIV-infected boy.
Figure 2.
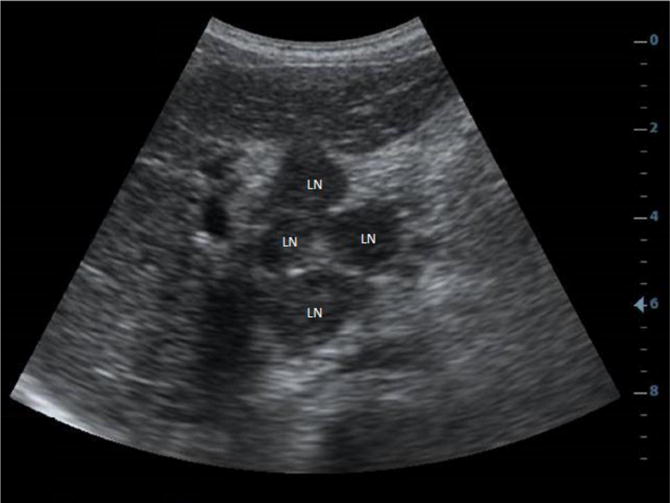
Multiple enlarged lymph nodes (LN) in the peri-pancreatic region in a 10 year old HIV-uninfected girl.
Acknowledgments
Source of Funding: NIH RO1 HD058971, MRC South Africa, NRF South Africa. SB and CCH were funded by a Marie Curie People grant and SB is currently participant in the BIH-Charité Clinical Scientist Program funded by Charité – Universitätsmedizin Berlin and the Berlin Institute of Health. HZ is funded by the MRC South Africa.
Footnotes
Conflicts of Interest
None of the authors declare any conflict of interest.
References
- 1.Seddon JA, Shingadia D. Epidemiology and disease burden of tuberculosis in children: a global perspective. Infect Drug Resist. 2014;7:153–65. doi: 10.2147/IDR.S45090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heller T, Goblirsch S, Bahlas S, et al. Diagnostic value of FASH ultrasound and chest X-ray in HIV-co-infected patients with abdominal tuberculosis. Int J Tuberc Lung Dis. 2013;17:342–4. doi: 10.5588/ijtld.12.0679. [DOI] [PubMed] [Google Scholar]
- 3.Scheepers S, Andronikou S, Maputaka A, Donald P. Abdominal lymphadenopathy in children with tuberculosis presenting with respiratory symptoms. Ultrasound. 2011;19:134–139. [Google Scholar]
- 4.Malik A, Saxena NC. Ultrasound in abdominal tuberculosis. Abdom Imaging. 2003;28:574–9. doi: 10.1007/s00261-002-0061-z. [DOI] [PubMed] [Google Scholar]
- 5.Heller T, Wallrauch C, Goblirsch S, Brunetti E. Focused assessment with sonography for HIV-associated tuberculosis (FASH): a short protocol and a pictorial review. Crit Ultrasound J. 2012;4:21. doi: 10.1186/2036-7902-4-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Hoving DJ, Lamprecht HH, Stander M, et al. Adequacy of the emergency point-of-care ultrasound core curriculum for the local burden of disease in South Africa. Emerg Med J. 2013;30:312–5. doi: 10.1136/emermed-2012-201358. [DOI] [PubMed] [Google Scholar]
- 7.Nicol MP, Workman L, Isaacs W, et al. Accuracy of the Xpert MTB/RIF test for the diagnosis of pulmonary tuberculosis in children admitted to hospital in Cape Town, South Africa: a descriptive study. Lancet Infect Dis. 2011;11:819–24. doi: 10.1016/S1473-3099(11)70167-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pereira JM, Madureira AJ, Vieira A, Ramos I. Abdominal tuberculosis: imaging features. Eur J Radiol. 2005;55:173–80. doi: 10.1016/j.ejrad.2005.04.015. [DOI] [PubMed] [Google Scholar]
- 9.Wiseman CA, Gie RP, Starke JR, et al. A proposed comprehensive classification of tuberculosis disease severity in children. Pediatr Infect Dis J. 2012;31:347–52. doi: 10.1097/INF.0b013e318243e27b. [DOI] [PubMed] [Google Scholar]
- 10.Jones BE, Young SM, Antoniskis D, Davidson PT, Kramer F, Barnes PF. Relationship of the manifestations of tuberculosis to CD4 cell counts in patients with human immunodeficiency virus infection. Am Rev Respir Dis. 1993;148:1292–7. doi: 10.1164/ajrccm/148.5.1292. [DOI] [PubMed] [Google Scholar]
- 11.Vayner N, Coret A, Polliack G, Weiss B, Hertz M. Mesenteric lymphadenopathy in children examined by US for chronic and/or recurrent abdominal pain. Pediatr Radiol. 2003;33:864–7. doi: 10.1007/s00247-003-0985-7. [DOI] [PubMed] [Google Scholar]
- 12.Sivit CJ, Newman KD, Chandra RS. Visualization of enlarged mesenteric lymph nodes at US examination. Clinical significance Pediatr Radiol. 1993;23:471–5. doi: 10.1007/BF02012457. [DOI] [PubMed] [Google Scholar]
- 13.Karmazyn B, Werner EA, Rejaie B, Applegate KE. Mesenteric lymph nodes in children: what is normal? Pediatr Radiol. 2005;35:774–7. doi: 10.1007/s00247-005-1462-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


