Abstract
Objectives
Individuals taking opioids for an extended period of time may become physically dependent, and will therefore experience opioid withdrawal should they stop taking the medication. Previous work in animal and human models has shown that the serotonin (5-HT3) receptor may be implicated in opioid withdrawal. In this study, we investigated if ondansetron, a 5-HT3-receptor antagonist, could reduce the symptoms of opioid withdrawal after chronic opioid exposure in humans.
Methods
In this double-blinded, randomized crossover study, thirty-three chronic back pain patients (N=33) were titrated onto sustained-release oral morphine for 30 days. Following titration, participants attended two study sessions, one week apart, in which opioid withdrawal was induced with intravenous naloxone, with or without 8mg intravenous ondansetron pretreatment. Opioid withdrawal symptoms were assessed by a blinded research assistant (objective opioid withdrawal score - OOWS) and by the research participant (subjective opioid withdrawal score - SOWS).
Results
Clinically significant signs of withdrawal were observed during both the ondansetron (ΔOOWS = 3.58 ± 2.22, p < .0001; ΔSOWS = 12.48 ± 11.18, p < .0001) and placebo sessions (ΔOOWS = 3.55 ± 2.39, p < .0001; ΔSOWS = 12.21 ± 10.72, p < .0001), but no significant differences were seen between the treatment sessions in either the OOWS or SOWS scores.
Conclusion
We hypothesized that ondansetron would reduce opioid withdrawal symptoms in human subjects, but found no difference in withdrawal severity between ondansetron and placebo sessions. These findings suggest that more investigation may be necessary to determine if 5-HT3-receptor antagonists are suitable treatment options for opioid withdrawal.
Introduction
Prescription opioids are a commonly used therapy for management of moderate to severe pain. Consequently, they are currently the most prescribed class of medications in the United States. While opioids can effectively control chronic pain, their utility is limited by their high potential for misuse. It is estimated that one-fourth of all 18- to 25-year-olds will misuse prescription opioid analgesics in their lifetime. Opioid addiction is a growing concern, with a 300% increase in opioid prescriptions between 1999 and 2010 (Batses & Brennan, 2013). Prescription opioid misuse is also a significant economic burden with costs totaling $78.5 billion in the United States in 2013 alone (Florence et al., 2016). Addressing the components of opioid dependence is a necessary step in decreasing the public health and economic burdens associated with opioid therapy.
Opioid withdrawal (OW) is a physiological withdrawal state that occurs upon reduction or cessation of opioid use after physical dependence has developed. OW symptoms include hyperalgesia, insomnia, tachycardia, fever, chills, and other flu-like symptoms. The severity of OW symptoms is a major contributor to the addictive potential of prescription and illicit opioids, as users continue taking the drugs to avoid withdrawal symptoms. Several medications are currently available for the treatment of OW, including clonidine, methadone, and buprenorphine. However, each of these treatments is limited by significant drawbacks, including severe side effects, narrow therapeutic windows and their own potential for misuse.
Although current methods of treating OW are problematic, recent studies have identified a novel therapeutic target. Our pilot study demonstrated that in an acute human physical dependence model, pretreatment with the 5-HT3 receptor antagonist (5-HT3-RA) ondansetron, used to treat chemotherapy-associated nausea and vomiting, reduced objective measures of OW by up to 76 percent in humans (Chu et al., 2009). 5-HT3-RAs are an ideal candidate for OW treatment because they are a non-opioid, non-addicting medication with a wide therapeutic window and low side effect profile. Moreover, 5-HT3-RAs could be employed safely in non-monitored outpatient settings. Haplotype-based computational genetic mapping in mice demonstrated that morphine-induced physical dependence is associated with decreased 5-HT3 protein expression and down-regulation of the Htr3a gene, which encodes subunit A of the 5-HT3 receptor in brainstem nuclei (Chu et al., 2009). Here, we investigated in this double-blinded, randomized, placebo-controlled crossover study whether acute ondansetron infusion could reduce the objective and subjective measures of opioid withdrawal in subjects taking morphine for chronic back pain.
Methods
Study Participants
Chronic back pain patients were recruited from the San Francisco Bay Area via recruitment posters, newspaper advertisements, and radio and television announcements. Patient history, physical examination, and the Opioid Risk Tool (ORT) (Webster & Webster, 2005) were administered during the patient intake exam to ensure that each subject met study inclusion criteria, were able to tolerate the OW protocol and were at no or low risk for addiction. Study measure data was collected by TR, EC, HO, EE, ME, JS, ZS, and AC; patient history and physical exam were conducted by LC; vital signs were collected by the study nurse. A board-certified addiction expert and chronic pain physician were available for consultation regarding patients to be included in the study. Patients at high risk for addiction (ORT score higher than 8) were excluded and patients with medium risk (ORT score of 4 to 7) were then evaluated using the Screener and Opioid Assessment for Patients with Pain-Revised (SOAPP-R). Eligible patients exhibited nonmalignant moderate to severe chronic low-back pain defined as average visual analogue scale (VAS; Price et al., 1983) ≥ 4 (on a scale of 1–10) for the previous two weeks and pain for the past six months currently treated with up to 40 mg morphine equivalents per day. Patients were then excluded if their SOAPP-R score was ≥11. Overall exclusion criteria included: a) history of cardiovascular disease, b) history of peripheral neuropathic pain, scleroderma, or other condition that would preclude cold water forearm immersion, c) history of addictive disease, d) chronic low-back pain lasting less than 6 months, e) history of cardiac arrhythmia, f) history of hepatic disease, g) use of steroid or nerve-stimulating medications, h) any condition precluding opioid use, and i) pregnancy.
Patient history and physical exam were conducted to look for the “red flags” of chronic back pain and to identify and exclude patients with neurological complications (Cohen et al., 2008). In particular, the physical exam used the following tests to identify possible neurological complications: straight leg raise tests, examination of hips, palpation of the back, trunk strength (time flexors and extensors maintain isomeric contraction), and walking on toes and heels (Carragee & Hannibal, 2004; Atlas & Nardin, 2003; Malliou et al., 2006; Jensen, 2004; Clark, 2002). If patients exhibited any abnormal findings they were excluded from the study and were referred to see their primary physician for further management.
This study was registered in the clinicaltrials.gov database (NCT01549652). The Institutional Review Board (Stanford University) approved this protocol (NIH # 19821). Prior to study enrollment written informed consent was collected from all participants.
Titration
Before starting study medications baseline data for all measures of the study sessions were assessed: Beck Depression Inventory (Beck et al., 1961), Roland-Morris Disability Questionnaire (Roland & Morris, 1983), State-Trait Anxiety Inventory (Speilberger et al., 1970), Brief Pain Inventory (Tan et al., 2004), Profile of Mood States (McNair et al., 1971), Objective Opioid Withdrawal Scale (Handelsman et al., 1987), Subjective Opioid Withdrawal Scale (Handelsman et al., 1987), and vitals. Afterwards, subjects underwent a titration protocol to either begin, or switch from their current opioid regimen to sustained-release oral morphine (Purdue Pharma, Stamford, CT, 2007), starting at 30 mg/day and increasing by 15 mg/day every 2 days until 1) adequate analgesia was achieved, 2) side effects limited further titration, or 3) the maximum dose of 120 mg/day was reached. To facilitate adherence to this protocol subjects recorded least, average, and worst pain scores on a 0–10 scale and rated any potential side effects such as sedation, nausea, vomiting, light-headedness, dry mouth, and loss of appetite. Researchers contacted participants daily to discuss pain levels and side effects; a physician adjusted the dose of opioid medication as necessary. Researchers continued daily contact until pain was well treated or the maximum tolerated dose of opioid medication was achieved. This protocol was safe and reasonably well tolerated. Complaints of nausea or vomiting were treated by reducing the dose of opioid medication and/or treating with 10 mg doses of metoclopramide (Schwarz Pharma Mfg., Inc., Seymour, IN, 2004). Docusate sodium 100 mg soft gel capsules (Apothecon Pharmaceuticals Pvt. Ltd., Vadodara, Gujarat, India, 1993) were given for complaints of constipation associated with opioid use, as well as instruction to increase water intake. After the last study session was completed, patients were titrated back to their original starting dose, either to their previous prescription opioid regimen or back to no opioids. The final titration was closely monitored in the same fashion as initial titration, reducing 15mg/day every two days. A registered nurse supervised the final titration schedules and alerted the PI and addiction expert to any adverse events or side effects. In rare instances where subjects showed mild OW symptoms, titration off the opioid was slowed down.
Study Sessions
Following one month of oral morphine therapy subjects participated in two study sessions one week apart. Thirty minutes prior to naloxone challenge, participants were randomized to receive pre-treatment of either ondansetron (Hospira, Inc., Lake Forest, IL, 2004) (8mg) or placebo (0.9% saline solution, Hospira Inc., Lake Forest, IL, 2004) during the first withdrawal study session and the opposite pre-treatment during the second withdrawal study session one week later. The T-Max (time to reach C-Max, lowest concentration of a drug before the next dose is administered) of 8mg intravenous ondansetron is 7 minutes, thus we concluded that thirty minutes would be sufficient time to allow the ondansetron to take full effect (Simpson & Hicks, 1996). Intravenous ondansetron was selected over oral administration, as the intravenous route is the most reliable method to quickly establish precise serum plasma levels of ondansetron in a clinically relevant manner prior to precipitated OW. Furthermore, Colthup et al. (1991), found no difference in the mean half-life of ondansetron between oral and intravenous 8mg administration. A single, 8mg dose of ondansetron was selected, as only one opportunity to induce withdrawal existed per study session. Studying multiple doses or a dose response would have necessitated prolonged exposure to opioids and more than two withdrawal sessions which was determined to be unfavorable for study participants.
Prior to naloxone administration, participants were informed of the effects of naloxone and what they should expect to experience during withdrawal induction, for example, flu like symptoms. OW was induced with 0.4mg/70kg IV naloxone (Hospira, Inc., Lake Forest, IL, 2004) which was selected based on the work of Compton et al. (2003), in which IV naloxone challenge in an acute physical dependence model using healthy volunteers was shown to produce transient and dose-dependent OW symptoms. If significant withdrawal symptoms were not observed (OOWS score < 6) after first naloxone dose, subjects were given a second 0.8mg/70kg IV naloxone dose. Between these two study sessions, participants returned to their titrated dose of morphine. Overall study and session timeline are illustrated in Figure 1.
Figure 1.
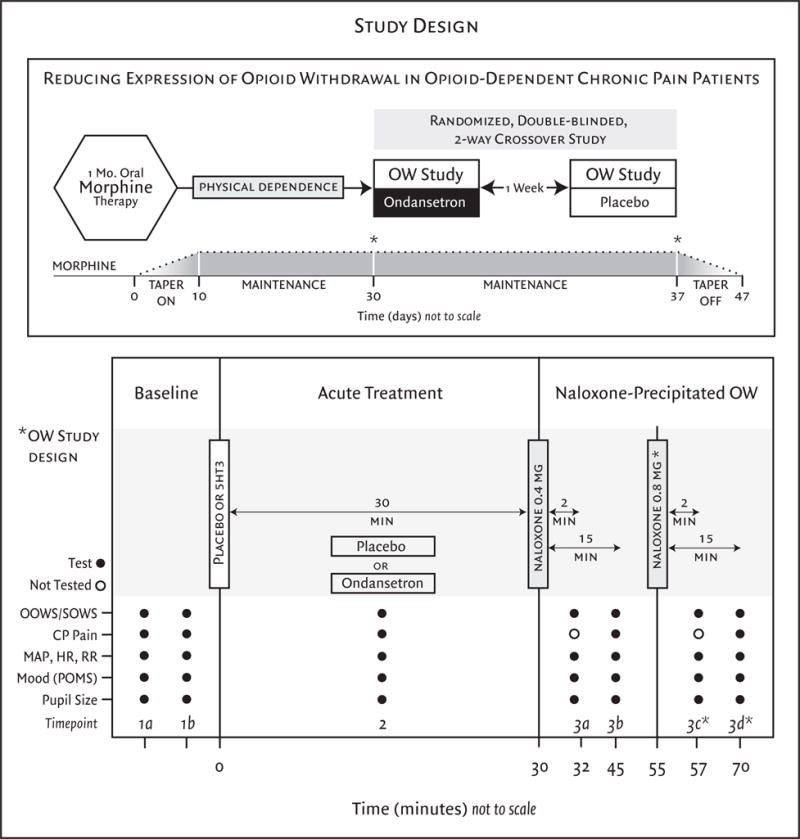
This randomized double-blinded, placebo-controlled, 2-way crossover study enrolled opioid-naive patients with chronic low back pain (<30 mg morphine-equivalent use per day). After one month of oral morphine therapy patients entered a two-visit crossover experiment that employed naloxone-precipitated withdrawal, with or without ondansetron pretreatment immediately prior to the naloxone challenge. *A second naloxone dose and subsequent measures at time points 3c and 3d did not occur for every patient, only those who received an OOWS score less than 6 after the first withdrawal and consented. OOWS=objective opioid withdrawal scale, SOWS=subjective opioid withdrawal scale, CP pain=cold pressor tolerance/threshold, MAP=mean arterial pressure, HR=heart rate, RR=respiratory rate, POMS=profile of mood states.
Data Collection
The following data points were measured at a baseline session before morphine titration as well as at both withdrawal study sessions (ondansetron or placebo). All data was collected at the Stanford University School of Medicine.
Primary Outcome Measure: Objective Opioid Withdrawal Scale (OOWS)
Originally developed by Handelsman et al. (1987), the OOWS score is a well-characterized measure of OW in humans. The OOWS is a 13-item instrument documenting physically observable signs of withdrawal, which are rated as present (1) or absent (0) during the observation period. The instrument was completed by a trained research assistant who was blinded to study design, treatment assignment and stage of study. OOWS was assessed five or seven times during the study sessions: twice before pretreatment, 15 minutes after pretreatment, and 2 and 15 minutes after one or two naloxone administrations. Second naloxone administration was given if OOWS score < 6 after first naloxone administration.
Secondary Outcome Measures
Several secondary surveys were implemented to assess other signs and symptoms of opioid withdrawal. The SOWS score is composed of 16 subjective symptoms rated on a scale of 0 to 4 (0=not at all, 4=extremely) based on what subjects were experiencing at the time of testing. An additional subjective measure of mood was taken through the Profile of Mood States (POMS), which has been associated with OW (Price et al., 1975). SOWS and POMS were assessed five or seven times during the study sessions: twice before pretreatment, 15 minutes after pretreatment, and 2 and 15 minutes after one or two naloxone administrations.
The Beck Depression Inventory is a self-report questionnaire measuring the severity of depression and yields a single score between 0 and 63; higher scores indicate more severe depression. The Roland-Morris Questionnaire asks about how back pain affects their daily activities. The State-Trait Anxiety Inventory is a 40-question assessment of anxiety and mood. The Brief Pain Inventories (Short Form) is a 9-item multi-dimensional pain and functionality survey (Cleeland, 1989). These four surveys were completed at the beginning of each study session. OOWS & SOWS have both been shown to be sensitive tools in the measurement of withdrawal scores in opioid users (Sarkar et al., 2016; Chu et al., 2009).
Physical vital signs
Heart rate (HR), respiratory rate (RR) and pupil diameter (PD) before & changes after precipitated OW were measured. Heart rate was monitored by electrocardiogram and respiratory rate was counted by direct observation over a period of thirty seconds. Pupil diameter was measured using a research-caliber digital automated pupillometer (PRL-200, NeurOptics). Vital signs were measured at the same five or seven time points as OOWS and SOWS.
Statistical Analysis
Statistical analysis was performed using SAS® software version 9.4 (SAS Institute, Cary, NC). Students’ t-tests for paired samples with Bonferroni correction for multiple comparisons were used to compare differences between the outcome measures (OOWS, SOWS, POMS) for crossover treatment arms (placebo vs. ondansetron). A Students’ t-test for paired samples was used as only a single post-naloxone withdrawal time point (15 minutes) was selected for analysis. This single time point was selected based on the work of Compton et al. (2004) who noted increased severity of objective and subjective withdrawal symptoms on physiological parameters at 15 minutes compared to 5 minutes. We tested for carryover effects between study sessions using Grizzle’s 2-stage model (Senn, 1993). As no carryover effect was detected, the pooled data from all periods was used to estimate the treatment effect.
Assigned participant numbers for patients who discontinued the study at any point in time were carried over to the next enrolled participant (e.g. patient 10 discontinues the study and the next patient to enroll in the study is assigned patient number 10a and so on.) Completer analysis was conducted as data from discontinued participants was not used in the final analysis.
Sample Size Calculation
Our sample size analysis was based on data from a preliminary study in which we compared changes in OOWS scores after naloxone-precipitated opioid withdrawal with either ondansetron or placebo pretreatment. The differences in OOWS scores for ondansetron vs. placebo pretreatment were computed for each patient. From these scores the mean and standard deviation were computed. To compute the sample size, we aimed for a 20% change in OOWS score for the treatment effect, with a power of 80% and an alpha of 0.05, yielding a target of 33 patients.
Results
Patient demographics and baseline characteristics
Of the 57 patients initially enrolled in the study, 20 males and 13 females (n=33) completed the study (Table 1, Table 2, Figure 2). Data from participants who discontinued the study were not included in the final analysis. Failure during morphine titration was broadly defined and included individuals who could not maintain the required dose during the 30 days, those who preferred their old medication and switched back, those who did not achieve adequate analgesia or those who left the study during the titration period. Discontinued participants included 17 males and 7 females made up of 22 Caucasian and 2 Asian participants. Mean age (46.3 years), weight (96.1kg) height (174.1cm), baseline Beck Depression Inventory score (4.58) and baseline Roland-Morris score (8.66) were noted for discontinued participants.
Table 1.
Demographic and baseline characteristics of patients participating in the study.
| Demographic and Baseline Characteristics of the Patients. * | |
|---|---|
|
| |
| Characteristic | Result |
|
| |
| Sex (no.) | |
| Male | 20 |
| Female | 13 |
|
| |
| Age (yr.) | 42.06 ± 13.36 |
|
| |
| Weight (kg) | 88.09 ± 19.86 |
|
| |
| Height (m) | 173.94 ± 9.01 |
|
| |
| Body Mass Index | 29.16 ± 6.43 |
|
| |
| Race (no.) | |
| Caucasian | 22 |
| African-American | 5 |
| Asian | 2 |
| Hispani | 2 |
| Other | 2 |
|
| |
| Baseline Average Pain § | 5.12 ± 1.24 |
|
| |
| Pre-existing Opioid Therapy (no.) | |
| Opioid naïve in past 5 years | 10 |
| Some opioid exposure in past 5 years | 10 |
| Either chronic or intermittent opioid use in past 5 years | 2 |
| Chronic opioid use in past 5 years | 1 |
| Current chronic opioid use | 8 |
|
| |
| Average Pre-existing Opioid Dose (morphine equivalents mg/day) | 3.15 ± 8.90 |
|
| |
| Average Opioid Dose Among Pre-existing Opioid Users (morphine equivalents mg/day) | 21.25 ± 13.15 |
|
| |
| Baseline Beck Depression Inventory† | 4.36 ± 4.23 |
|
| |
| Baseline Roland-Morris Disability Index‡ | 6.97 ± 4.03 |
Plus-minus values are means±SD.
Intensity of pain was as reported by patients on a visual-analogue scale labeled “no pain” at 0 mm and “worst pain imaginable” at 100 mm.
Beck Depression Inventory is a single score ranging from 0 to 63; higher scores indicate more severe depression.
Roland-Morris Disability Index yields a score between 0 and 24; higher scores indicate more pronounced disability.
Table 2.
Reasons given for discontinued patients. Discontinued patients either chose to end their participation, or study coordinators chose to drop the patients due to nonadherence. Data from discontinued patients were not included in the analysis.
| Primary reasons for discontinuation. | |
|---|---|
| Reason for discontinuation | Number of subjects |
| Study drug titration failure | 11 |
| Discontinued study session due to severe withdrawal effects | 6 |
| Did not return to final study session due to severe withdrawal effects | 2 |
| Discontinued study session due to problem with infusion | 1 |
| Other | 4 |
Figure 2.
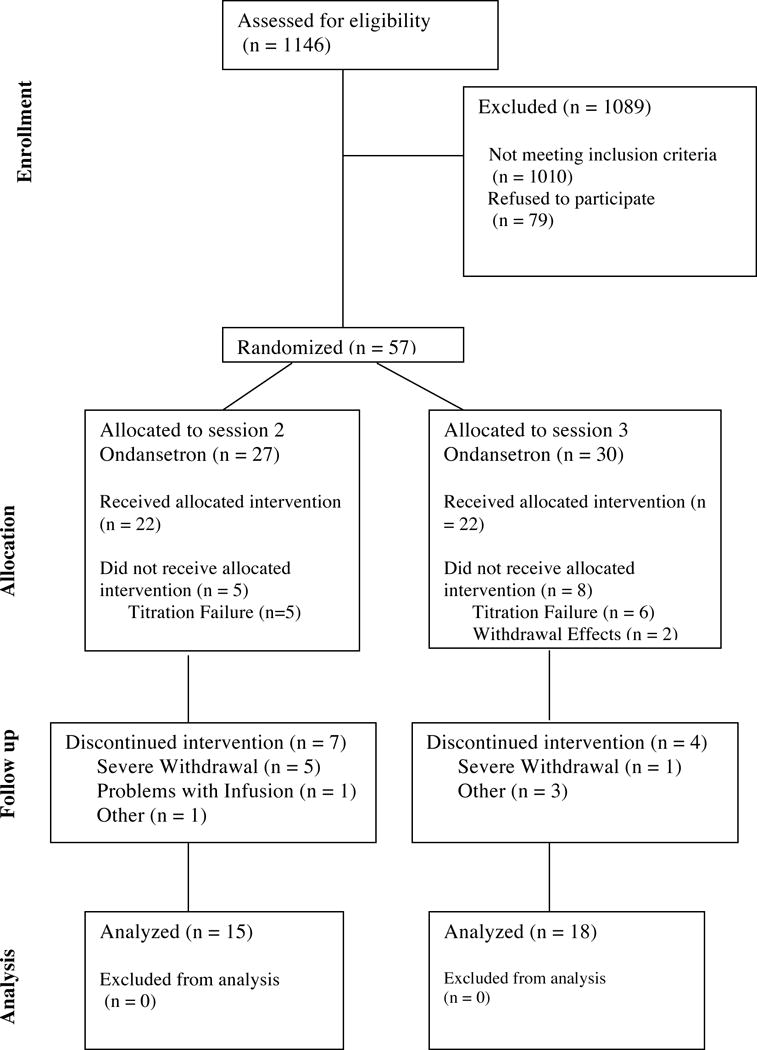
CONSORT diagram
Mean pre-existing opioid dose for opioid users (n = 31) was 21.3 ± 13.2 mg morphine equivalents/day. Pre-existing opioid dose was not found to have an effect on treatment outcomes. Baseline average pain as measured by VAS score (scale 1–10) was 5.12 ± 1.24. Baseline degree of disability as measured by the Roland-Morris Disability Index (scale 1–24) was 6.97 ± 4.03. Baseline Beck Depression Inventory was 4.36 ± 4.23. (Table 1)
The mean number of 15mg morphine pills taken over the course of the study was 173.1 ± 74.2. The final mean dose of morphine achieved was 89.0 ± 31.9 mg/day for a mean of 39.3 ± 3.9 days. A total of 2 patients maintained 30 mg/day, 3 maintained 45 mg/day, 5 maintained 60 mg/day, 2 maintained 75 mg/day, 3 maintained 90 mg/day, 3 maintained 105 mg/day and 12 maintained 120 mg/day. Data on opioid dose was not available for 3 patients. No adverse events occurred during the course of the study.
Primary outcome measures
Objective Opioid Withdrawal Scale (OOWS)
Patients demonstrated clinically significant signs of withdrawal during both the ondansetron (Δ OOWS = 3.6 ± 2.2, p < .0001) and placebo sessions (Δ OOWS = 3.6 ± 2.4, p < .0001) as measured by OOWS. No significant differences were observed between the ondansetron and placebo sessions in either the OOWS withdrawal scores (p = 0.87) or OOWS pre-withdrawal scores (p = 0.77; Figure 3A). Sixteen participants did not display sufficient signs of withdrawal (OOWS score <6) after the first dose of naloxone (0.4 mg/70kg). These sixteen participants consented to receive a second dose of naloxone (0.8 mg/70kg), which then produced an OOWS score of ≥ 6 indicating sufficient withdrawal for study purposes; there was no statistically significant difference in OOWS scores between the two sessions. Individual analysis of each OOWS subcategory showed no statistical difference between ondansetron and placebo withdrawal sessions (Figure 3B).
Figure 3.
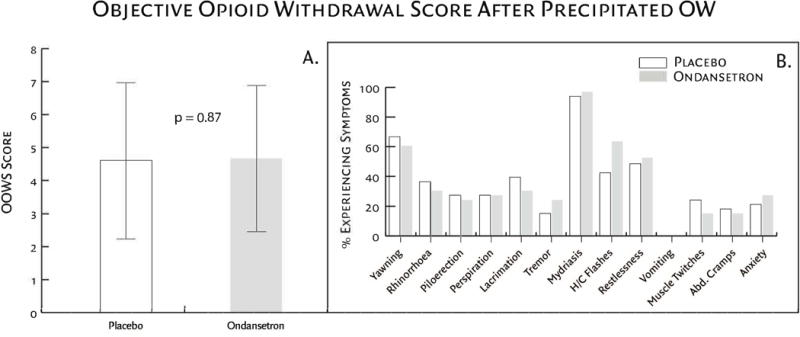
(Fig. 3A) Participant response to naloxone-precipitated withdrawal; no significant differences were observed between treatment groups (p = 0.87). (Fig. 3B) OOWS components of participant response to naloxone-precipitated withdrawal. This objective scale consists of 13 physically observable signs associated with opioid withdrawal assessed by the research assistant. Results are graphically displayed as a percentage of participants demonstrating the signs. No statistical difference was found between OOWS subcategories and treatment group.
Secondary Outcome Measures
Subjective Opioid Withdrawal Scale (SOWS)
Participants reported clinically significant symptoms of withdrawal during both the ondansetron (Δ SOWS = 12.5 ± 11.2, p < .0001) and placebo sessions (Δ SOWS = 12.2 ± 10.7, p < .0001) as measured by SOWS. No significant differences were observed between the ondansetron and placebo sessions in either the SOWS withdrawal scores (p = 0.92) or SOWS pre-withdrawal scores (p = 0.80). Individual analysis of each SOWS subcategory showed no statistical difference between ondansetron and placebo withdrawal sessions.
Profile of Mood States (POMS)
Patients demonstrated clinically significant changes in mood profile during both the ondansetron (Δ POMS = 29.3 ± 31.3, p < .0001) and placebo sessions (Δ POMS = 28.3 ± 37.1, p < .0001) as measured by POMS. No significant differences were observed between the ondansetron and placebo sessions in either the POMS withdrawal scores (p = 0.91) or POMS pre-withdrawal scores (p = 0.36). Individual analysis of each POMS subcategory showed no statistical difference between ondansetron and placebo withdrawal sessions. Furthermore, we did not see a statistically significant change in mood after one month of morphine treatment (ΔPOMStitration = −1.9 ± 16.8, p = 0.50).
Other Secondary Surveys
Self-reported pain as measured by VAS pain scores decreased significantly over the course of morphine treatment. Clinically and statistically significant decreases were observed in the reported least pain (Δ =−1.5 ± 1.8, p < .0001), average pain (Δ =−2.7 ± 2.2, p < .0001), and most pain (Δ =−3.2 ± 2.5, p < .0001) between the baseline and immediately prior to the first withdrawal session. Self-reported degree of disability as measured by the Roland-Morris Disability Index decreased significantly over the course of morphine treatment (Δ =−2.6 ± 4.6, p = 0.003). Psychological state and mood as measured by the Beck Depression Inventory improved slightly over the course of morphine treatment, however this improvement was not statistically significant (−0.4 ± 3.4, p = 0.47) (Table 3).
Table 3.
Secondary measures results, reported as mean ± SD at the beginning of each session, participants were asked to complete the Roland-Morris Disability Index, the Beck Depression Inventory, and Pain and Hyperalgesia Questionnaires. Mean score changes (from baseline at session 0 to session 2) and standard deviations are reported here. All differences except the Beck Depression Inventory are significant (significance at p < 0.05).
| Secondary outcome measures. | ||
|---|---|---|
| Outcome | Change from baseline to session 2 | P Value |
| Least VAS Pain | −1.48 ± 1.81 | <.0001 |
| Average VAS Pain | −2.68 ± 2.23 | <.0001 |
| Worst VAS Pain | −3.18 ± 2.54 | <.0001 |
| Roland-Morris Disability Index | −2.59 ± 4.56 | .003 |
| Beck Depression Inventory | −.44 ± 3.36 | .47 |
Discussion
Major Findings
Based on pilot data from healthy human volunteers we hypothesized that ondansetron, a 5-HT3 receptor antagonist (5-HT3-RA), would reduce opioid withdrawal (OW) symptoms in human subjects. In chronic opioid users receiving acute ondansetron or placebo infusion thirty minutes prior to naloxone-induced withdrawal, we found no difference in withdrawal severity between ondansetron and placebo sessions. None of our measures of withdrawal (OOWS, SOWS, and POMS) showed any significant difference between sessions (Figure 1). Analysis of individual sub scores within each survey (e.g. anxiety, lacrimation, yawning, etc.) yielded no difference between sessions. These data stand in contrast to our previous pilot data in healthy human volunteers.
Possible Explanations of Results
Several key design differences between this study and our pilot study may help to explain this discrepancy. In our pilot study, we utilized the acute opioid physical dependence (APD) model (Compton et al., 2004), in which healthy subjects receive morphine infusion followed by IV naloxone administration 120 minutes later. In the current study, we utilized a chronic model of physical dependence in which chronic back pain patients received morphine for 30 days before ondansetron administration and naloxone challenge. It is possible that acute opioid infusion does not replicate the physiologic conditions created by chronic opioid use and that the mechanism for chronic opioid dependence is different from that of acute opioid dependence. The response to naloxone challenge may differ according to the length of opioid use. We feel that the more chronic treatment approach is the better model based on the route and duration of opioid administration.
Another key difference between this study and our previous study is the severity of the naloxone-precipitated withdrawal. The mean withdrawal OOWS score for the placebo group in this study was larger than that of our pilot study. It is conceivable that 5-HT3-RAs are effective only up to a certain threshold of withdrawal severity. The paradigm involving chronic opioid administration may simply result in too intense an opioid withdrawal response to be blocked by the dose of ondansetron selected. Furthermore, in our pilot study withdrawal was precipitated utilizing 10mg/70kg naloxone, whereas the present study utilized a minimum dose of 0.4 mg/70kg or a maximum dose of 0.8 mg/70kg naloxone.
Finally, it is also possible that chronic pain patients and healthy volunteers react differently to ondansetron. Moreover, chronic pain patients may react differently to naloxone challenge. Both of these distinctions might again be explained by mechanistic differences between chronic and acute opioid dependence. Either of these possibilities could explain why the effects of ondansetron were seen in the pilot study but not in this study.
One important similarity between our previous work involving one month of opioid therapy (Chu et al., 2012) and this study is a decrease in VAS pain scores and degree of disability (as measured by the Roland-Morris Disability Index). This consistency points to the construct validity of those two secondary measures. Compared to our previous back pain study the average final morphine dose was similar. Ballantyne & LaForge (2007) suggest that opioid physical dependence can occur three days after sustained opioid use, well within the 39.3±3.9 days of sustained opioid use in this study. Furthermore, studies using animal models suggest that physical dependence severity increases with increasing daily morphine dose (Weeks & Collins, 1979). The daily average morphine dose in this study was 89.0±31.9 mg/day producing statistically significant changes in OOWS and SOWS scores from baseline suggesting physical dependence was achieved in our study population.
Second Naloxone Dose
A total of sixteen (n=16) participants required a second dose of naloxone to achieve an OOWS score of ≥ 6. While our administered dose of naloxone (0.4mg/70kg initial and 0.8mg/70kg secondary if necessary) was lower than the 10mg/70kg dose used by Compton et al., (2004) and in our pilot study (Chu et al., 2009) we observed statistically significant subjective and objective signs of OW suggesting our selected doses were effective. Interestingly, there is currently no universally agreed upon dose of naloxone for use in rescue situations suggesting that little is known concerning individual variation in response to naloxone dosing (Connors & Nelson, 2016).
One possible individual variation may involve acutely depressed individuals. Lautenbacher et al. (1994) observed that individuals with acute depression experience decreased pain sensitivity which is not affected by naloxone administration. Decreased pain sensitivity in acutely depressed individuals may attenuate symptoms of OW necessitating a second naloxone dose. Differences in response to naloxone between gender and opioid dose was examined in a retrospective analysis by Chopra et al. (2008) who found that females on low-dose methadone exhibited larger naloxone-induced increases on Adjective Rating Scale (ARS) and Addiction Research Center Inventory (ARCI) subscales compared to men. Males on high-dose methadone by comparison showed a modest but more sustained naloxone-induced increase on ARS and ARCI compared to females. Both men and women on low-dose methadone exhibited larger, but shorter lasting naloxone-induced increases on VAS while high doses showed a smaller but longer lasting naloxone-induced increase on VAS. In the present study, both males and females were titrated onto a standard opioid dose suggesting that the need for 16 participants to receive a second naloxone dose was not likely due to opioid dose. No significant difference was observed in final dose of morphine maintained by those who did not receive a second 0.8mg/70kg naloxone dose at either SS2 or SS3 (n = 16) and those who did receive a second 0.8mg/70kg naloxone dose at both SS2 and SS3 (n = 14; p = 0.63). Furthermore, no significant difference in age (p = 0.95), weight (p = 0.51), or height (p = 0.92) was observed between those who required a second naloxone dose and those who did not. Data on final morphine dose was not available for three (n = 3) participants.
Limitations
A possible limitation of this study was the usage of a single, 8mg dose of ondansetron. As mentioned previously, multiple or varying doses of ondansetron would have required prolonged morphine usage and extended withdrawal sessions; 8mg ondansetron is commonly given to prevent chemotherapy related nausea and vomiting (Plosker & Milne, 1992). Tramer et al. (1997), found no dose response relationship between 1 mg and 8 mg for intravenous ondansetron administration to prevent postoperative nausea. They did find a difference between 8mg and 16mg indicating that a higher dose might have been more effective for our model. Due to the rapid onset of symptoms after naloxone-precipitated withdrawal, it is possible that ondansetron did not have sufficient time to establish its therapeutic properties leading to a possible false-negative result (type 2 error). However, as previously discussed the T-Max of 8mg intravenous ondansetron is 7 minutes with a half-life 3 to 3.5 hours, thus we believe that the 30-minute gap between intravenous ondansetron administration and naloxone-precipitated withdrawal was sufficient time for ondansetron to establish its therapeutic effect (Simpson & Hicks, 1996). To attenuate this possibility, future studies may consider examining ondansetron in spontaneous rather than naloxone-precipitated withdrawal.
Our relatively small sample size of 33 participants may also be considered as a limitation of this study. Another limitation was the lack of biochemical data pertaining to serum morphine levels. While blood samples were collected from all study participants, analysis was deemed unnecessary as OOWS and SOWS pre and post withdrawal scores were indicative of participant compliance to the study protocol. Furthermore, a registered nurse counted the number of morphine tablets at the beginning and end of each study session, which further indicated protocol compliance.
Conclusion
The discrepancy between the results of this study and our pilot data warrants further investigation. Future studies might examine the effects of oral ondansetron given concurrently with morphine treatment, rather than after the development of opioid dependence. If ondansetron prevented or reduced OW symptoms in this setting it could be easily implemented clinically to prevent or attenuate the drawbacks associated with OW and PD. As previously mentioned, another consideration is to study spontaneous rather than naloxone-precipitated withdrawal using daily oral ondansetron treatment after cessation of opioid administration. Most patients do not, after all, undergo precipitated withdrawal though this model does provide robust responses that can be quantified easily. We believe that 5-HT3 receptor antagonists should be studied further for their potential to address the problems associated with opioid withdrawal and physical dependence.
Acknowledgments
This study was funded by a grant awarded to LC from the National Institutes of Health (NIH, 1 RO1 DA029078-01A1), and the Stanford University School of Medicine Department of Anesthesiology.
Declaration of Interest:
This work was supported by a grant from the National Institutes of Health (NIH, 1 RO1 DA029078-01A1), and the Stanford University School of Medicine Department of Anesthesiology.
Footnotes
There are no conflicts of interest to report.
Clinical Trials registration: NCT01549652
References
- Atlas SJ, Nardin RA. Evaluation and treatment of low back pain: an evidence-based approach to clinical care. Muscle Nerve. 2003;27:265–284. doi: 10.1002/mus.10311. [DOI] [PubMed] [Google Scholar]
- Ballantyne JC, LaForge KS. Opioid dependence and addiction during opioid treatment of chronic pain. Journal of Pain. 2007;129(3):235–255. doi: 10.1016/j.pain.2007.03.028. [DOI] [PubMed] [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Betses M, Brennan T. Abusive prescribing of controlled substances—a pharmacy view. N Engl J Med. 2013;369:989–991. doi: 10.1056/NEJMp1308222. [DOI] [PubMed] [Google Scholar]
- Florence CS, Zhou C, Luo F, Xu L. The economic burden of prescription opioid dose, abuse, and dependence in the United States, 2013. Medical Care. 2016;54(10):901–906. doi: 10.1097/MLR.0000000000000625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carragee EJ, Hannibal M. Diagnostic evaluation of low back pain. Orthop Clin North Am. 2004;35:7–16. doi: 10.1016/S0030-5898(03)00099-3. [DOI] [PubMed] [Google Scholar]
- Chopra MP, Feldman Z, Mancino MJ, Oliveto A. Sex and opioid maintenance dose influence response to naloxone in opioid-dependent humans: A retrospective analysis. Pharmacol Biochem Behav. 2008;90(4):787–796. doi: 10.1016/j.pbb.2008.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu LF, Liang DY, Li X, et al. From mouse to man: the 5-HT3 receptor modulates physical dependence on opioid narcotics. Pharmacogenet Genomics. 2009;19:193–205. doi: 10.1097/FPC.0b013e328322e73d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu LF, D’Arcy N, Brady C, et al. Analgesic tolerance without demonstrable opioid-induced hyperalgesia: a double blinded, randomized, placebo-controlled trial of sustained release morphine for treatment of chronic nonradicular low-back pain. Pain. 2012;153:1583–1592. doi: 10.1016/j.pain.2012.02.028. [DOI] [PubMed] [Google Scholar]
- Clark JD. Chronic pain prevalence and analgesic prescribing in a general medical population. J Pain Symptom Manage. 2002;23:131–137. doi: 10.1016/s0885-3924(01)00396-7. [DOI] [PubMed] [Google Scholar]
- Cleeland C. Measurement of pain by subjective report. In: Chapman CR, Loeser JD, editors. Issues in pain Measurement. New York: Raven press; 1989. pp. 391–403. [Google Scholar]
- Cohen SP, Argoff CE, Carragee EJ. Management of low back pain. BMJ. 2008;337:a2718. doi: 10.1136/bmj.a2718. [DOI] [PubMed] [Google Scholar]
- Colthrup PV, Felgate CC, Palmer JL, Scully NL. Determination of ondansetron in plasma and its pharmacokinetics in the young and elderly. J Pharm Sci. 1991;80(9):868–871. doi: 10.1002/jps.2600800913. [DOI] [PubMed] [Google Scholar]
- Compton P, Athanasos P, Elashoff D. Withdrawal hyperalgesia after acute opioid physical dependence in nonaddicted humans: a preliminary study. Journal of Pain. 2003;4(9):511–519. doi: 10.1016/j.jpain.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Compton P, Miotto K, Elashoff D. Precipitated opioid withdrawal across acute physical dependence induction methods. Pharmacol Biochem Behav. 2004;77:263–268. doi: 10.1016/j.pbb.2003.10.017. [DOI] [PubMed] [Google Scholar]
- Connors NJ, Nelson LS. The evolution of recommended naloxone dosing for opioid overdose by medical specality. J Med Toxicol. 2016;12(4):276–281. doi: 10.1007/s13181-016-0559-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handelsman L, Cochrane KJ, Aronson MJ, Ness R, Rubinstein KJ, Kanof PD. Two new rating scales for opiate withdrawal. Am J Drug Alcohol Abuse. 1987;13:293–308. doi: 10.3109/00952998709001515. [DOI] [PubMed] [Google Scholar]
- Jensen S. Back pain-clinical assessment, Aust Farm Physician. 2004;33:397–401. [PubMed] [Google Scholar]
- Lautenbacher S, Roscher S, Strain D, Fassbender K, Krumrey K, Krieg JC. Pain perception in depression: Relationship to symptomatology and naloxone-sensitive mechanisms. Psychosom Med. 1994;56(4):345–352. doi: 10.1097/00006842-199407000-00010. [DOI] [PubMed] [Google Scholar]
- Malliou P, Gioftsidou A, Beneka A, Godolias G. Measurements and evaluations in low back pain patients. Scand J Med Sci Sports. 2006;16:219–230. doi: 10.1111/j.1600-0838.2005.00504.x. [DOI] [PubMed] [Google Scholar]
- McNair DM, Lorr M, Droppleman LF. Manual for the profile of mood states (POMS) San Diego, CA: Educational and Industrial Testing Services; 1971. [Google Scholar]
- Plosker GL, Milne RJ. Ondansetron: a pharmacoeconomic and quality-of-life evaluation of its antiemetic activity in patients receiving cancer chemotherapy. Pharmacoeconomics. 1992;2(4):285–304. doi: 10.2165/00019053-199202040-00005. [DOI] [PubMed] [Google Scholar]
- Price BB, Moran S, Crunican MA, Rothenberg S, Cutter HS. Mood, primary heroin withdrawal, and acute methadone administration. Int J Addict. 1975;10:613–631. doi: 10.3109/10826087509026739. [DOI] [PubMed] [Google Scholar]
- Price DD, McGrath PA, Rafii A, Buckingham B. The validation of visual analogue scales as ratio scale measures for chronic and experimental pain. Pain. 1983;17(1):45–56. doi: 10.1016/0304-3959(83)90126-4. [DOI] [PubMed] [Google Scholar]
- Roland M, Morris R. A study of the natural history of low back pain. Part 1: Development of a reliable and sensitive measure of disability in low-back pain. Spine. 1983;8(2):141–144. doi: 10.1097/00007632-198303000-00004. [DOI] [PubMed] [Google Scholar]
- Sarkar S, Subramaniam E, Konthoujam J. A novel approach to detoxification of intravenous buprenorphine dependence. Indian J Psychiatry. 2016;58(2):152–156. doi: 10.4103/0019-5545.183793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senn S. Crossover trials in clinical research. New York: John Wiley & Sons; 1993. [Google Scholar]
- Simpson KH, Hicks FM. Clinical pharmacokinetics of ondansetron. A review, J Pharm Pharmacol. 1996;48(8):774–781. doi: 10.1111/j.2042-7158.1996.tb03973.x. [DOI] [PubMed] [Google Scholar]
- Speilberger CD, Gorsuch RL, Lushene RE. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologist Press; 1970. [Google Scholar]
- Tan G, Jensen MP, Thornby JI, Shanti BF. Validation of the brief pain inventory for chronic nonmalignant pain. The Journal of Pain. 2004;5(2):133–137. doi: 10.1016/j.jpain.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Tramer MR, Reynolds DJ, Moore RA, McQuay HJ. Efficacy, dose-response, and safety of ondansetron in prevention of postoperative nausea and vomiting: a quantitative systematic review of randomized placebo-controlled trials. Anesthesiology. 1997;87(6):1277–1289. doi: 10.1097/00000542-199712000-00004. [DOI] [PubMed] [Google Scholar]
- Webster LR, Webster RM. Predicting aberrant behaviors in opioid-treated patients: preliminary validation of the Opioid Risk Tool. Pain med. 2005;6:432–442. doi: 10.1111/j.1526-4637.2005.00072.x. [DOI] [PubMed] [Google Scholar]
- Weeks JR, Collins RJ. Dose and physical dependence as factors in the self-administration of morphine by rats. Psychopharmacology. 1979;65:171–177. doi: 10.1007/BF00433045. [DOI] [PubMed] [Google Scholar]


