Abstract
Background
Impairments in certain cognitive processes (e.g., working memory) are typically most pronounced in schizophrenia (SZ), intermediate in bipolar disorder (BP) and least in major depressive disorder (MDD). Given that working memory depends, in part, on neural circuitry that includes pyramidal cells in layer 3 (L3) and layer 5 (L5) of the dorsolateral prefrontal cortex (DLPFC), we sought to determine if transcriptome alterations in these neurons were shared or distinctive for each diagnosis.
Methods
Pools of L3 and L5 pyramidal cells in the DLPFC were individually captured by laser-microdissection from 19 matched tetrads of unaffected comparison, SZ, BP and MDD subjects and the mRNA was subjected to transcriptome profiling by microarray.
Results
In DLPFC L3 and L5 pyramidal cells, transcriptome alterations were numerous in SZ subjects, but rare in BP and MDD subjects. The leading molecular pathways altered in SZ subjects involved mitochondrial energy production and the regulation of protein translation. In addition, we did not find any significant transcriptome signatures related to psychosis or suicide.
Conclusions
In concert, these findings suggest that molecular alterations in DLPFC L3 and L5 pyramidal cells might be characteristic of the disease process(es) operative in individuals diagnosed with SZ and thus might contribute to the circuitry alterations underlying cognitive dysfunction in individuals with this disorder.
Keywords: bipolar disorder, major depression, microarray, prefrontal cortex, pyramidal neurons, schizophrenia
Introduction
Dysfunction of the dorsolateral prefrontal cortex (DLPFC) appears to be a core element of the disease process of schizophrenia (SZ) (1). This dysfunction is manifest as certain types of cognitive deficits, such as impairments in working memory (2). However, working memory deficits are also present in individuals with bipolar disorder (BP) or major depressive disorder (MDD) (3-5). The severity of these impairments typically differs across diagnoses, with impairments most pronounced in SZ, intermediate in BP and least in MDD (6, 7). These findings, in concert with evidence that certain genetic(8) and environmental risk factors (9) are shared among psychotic and mood disorders, suggest that these diagnoses might represent different points on a spectrum of disease in contrast to the long-standing view that they are distinct kinds of illnesses (10).
This spectrum of disease hypothesis can be tested, in part, by determining if alterations in the neurobiological substrate for working memory are shared among individuals with SZ, BP and MDD. In monkeys, working memory depends on task-specific patterns of activity in pyramidal cells (PCs) located in DLPFC layer 3 (L3) and layer 5 (L5) (11, 12). In the DLPFC of individuals with SZ, PCs in these layers exhibit morphological alterations such as smaller somal size, lower spine density and/or truncated dendritic trees (13-18). A lower density of dendritic spines on DLPFC L3 PCs was also detected in BP subjects (19) but not in those with MDD (16), although the latter did have smaller dendritic trees. At the molecular level, relative to unaffected comparison (UC) subjects, SZ subjects exhibited transcriptome alterations in DLPFC L3 and/or L5 PCs (20, 21) that were not detected in total grey matter samples, suggesting that cell type-specific analyses might reveal diagnosis-related patterns of molecular pathology not detected in transcriptome studies of cortical grey matter (22).
In this study we assessed gene expression profiles in DLPFC L3 and L5 PCs obtained from matched tetrads of SZ, BP, MDD and UC subjects to address two questions: 1) Relative to UC subjects, what transcriptome alterations are present in DLPFC L3 and L5 PCs from subjects with each diagnosis? 2) Are these alterations shared or distinctive for each diagnosis?
Methods and Materials
Human subjects
Brain specimens (n=76) were obtained during autopsies conducted at the Allegheny County Office of the Medical Examiner (Pittsburgh, PA) after consent was obtained from the next-of-kin. An independent committee of research clinicians made consensus DSM-IV diagnoses using information obtained from medical records and structured diagnostic interviews conducted with the decedent's family members (16). The same approach was used to confirm the absence of psychiatric diagnoses in the UC subjects. Subjects with SZ (n=19; 6 had schizoaffective disorder), BP (n=19; all had bipolar 1 disorder) or MDD (n=19) and UC subjects (n=19) were matched as tetrads for sex and as closely as possible for age (Table 1; subject details in Supplementary Table 1). All procedures were approved by the University of Pittsburgh's Committee for the Oversight of Research and Clinical Training Involving the Dead and Institutional Review Board for Biomedical Research.
Table 1.
Summary of subject characteristics.
| Subject Groups*** | ||||
|---|---|---|---|---|
|
| ||||
| UC | SZ | BP | MDD | |
| Number | 19 | 19 | 19 | 19 |
| Sex | 10 M, 9 F | 10 M, 9 F | 10 M, 9 F | 10 M, 9 F |
| Race | 18 W, 1 B | 13 W, 6 B | 19 W | 18 W, 1 B |
| Age (years) | 47.8 (10.4) | 45.1 (8.5) | 46.3 (9.5) | 45.2 (10.1) |
| PMI (hours) | 19.3 (5.3) | 20.1 (6.9) | 21.3 (6.6) | 20.1 (6.0) |
| Brain pH | 6.6 (0.2) | 6.6 (0.3) | 6.6 (0.2) | 6.6 (0.2) |
| RIN (frontal pole) | 8.0 (0.6) | 7.9 (0.7) | 8.0 (0.4) | 8.0 (0.5) |
| Storage time (months at -80°C) | 110 (43) | 111 (27) | 128 (26) | 126 (29) |
| Tobacco* | 6 | 12 | 13** | 7 |
| Antidepressants* | 0 | 10 | 12 | 7 |
| Benzodiazepines/Anticonvulsants* | 0 | 8 | 11 | 4 |
| Antipsychotics* | 0 | 16 | 7 | 2 |
Values are Mean (SD)
PMI: Post-Mortem Interval
RIN: RNA Integrity Number; obtained from a tissue block near the location of the tissue sections used for PC capture
Number of subjects with known use at time of death
In 4 BP subjects, tobacco use at time of death was unknown; however, given the high frequency of smoking BP subjects, they were included as smokers in all analyses.
Subject groups did not differ in mean age, postmortem interval (PMI), brain pH, RNA integrity number (RIN) or tissue storage time at -80°C (all t71< 2.30; all p>0.14) or in race (χ2<3.2; p>0.07).
Laser microdissection
Brain tissue was prepared and pyramidal cell bodies were dissected as described previously (21). Sample collection is described in Supplementary Methods. Given the limited RNA quantity obtained from each pool of microdissected neurons, RIN values were not assessed in the samples of neurons used for microarray profiling. However, we have previously demonstrated that the Nissl staining and laser microdissection approach used here results in RIN >7 in all samples, values nearly identical to those obtained in tissue homogenates from the same subjects (21).
Microarray
For each sample, RNA was extracted using the QIAGEN® RNeasy Plus Micro kit (QIAGEN, Valencia). All RNA samples from the same tetrad were processed together. CDNA was synthesized and amplified using the Ovation Pico WTA System (Nugen, San Carlos), labeled using the Encore Biotin module (Nugen) and loaded on an Affymetrix GeneChip® U219 (Affymetrix, Santa Clara) which contains ∼49,000 probesets designed to assess the expression level of more than 20,000 transcripts in the human genome. Replicate samples were synthesized and loaded on microarrays independently, with the loading order randomized. No batch correction was performed as PCA did not detect any variance attributable to plate. Samples from two SZ subjects (see Supplementary Table 1) did not pass quality control following array processing; the reported data are from the remaining 17 subjects. For each of the 74 unique subject samples in each layer, expression intensities were extracted from Affymetrix Expression Console and normalized using Robust Multi-array Average Express (23). The data were deposited in GEO (GSE87610). The correlations between replicates were high (0.80<r<0.95), and thus the expression values of the replicate samples from each layer were averaged for data analysis.
Statistical analysis
Data filtering and detection of differentially-expressed transcripts within each diagnostic group
The Affymetrix control probesets were removed, as they have no biological relevance. In order to eliminate low expressing and non-informative probesets, we used a modification of a previously described filtering procedure based on a threshold determined by the contrast in expression levels of Y-chromosome genes (see Supplementary Methods, Supplementary Figure 1 and Supplementary Table 2) between male and female subjects (24). To detect differentially-expressed transcripts, we followed a previously reported procedure (21) to fit a random intercept model (25) for each diagnosis separately (Supplementary Methods) in order to account for the matched design and the potential impact of covariates including sex, age, RIN, brain pH, PMI, death by suicide, presence of psychosis, presence of mood diagnosis, use of antidepressant, antipsychotic, or benzodiazepines and/or anticonvulsant medications at time of death, and tobacco use at time of death (Supplementary Methods). The best model was determined through the Bayesian Information Criterion (BIC) and the p-value of diagnosis effect was assessed via likelihood ratio test. We randomly permutated samples 500 times to correct the p-value due to bias from model selection. The corrected p-value was adjusted for multiple comparisons using the Storey procedure (26).
Effects of clinical covariates
To explore the effects of suicide or psychosis, we used a linear regression model with suicide or psychosis as the main effect and diagnosis as a covariate (Supplementary Methods).
Pathway analysis
We used INGENUITY® Pathway Analysis software, treating all filtered probesets as background. Only pathways containing 15-300 genes, of which >10% were differentially expressed, were included. A right-tailed Fisher's exact test was applied to determine pathway enrichment.
Results
Differentially-expressed transcripts within each diagnostic group
Using paired or unpaired analysis, similar numbers of DEPs were found (Table 2). A Rank-Rank Hypergenomic Overlap (RRHO) plot (27) using all filtered probesets ranked by q value also indicated that paired and unpaired analysis generated very similar findings (Supplementary Figure 2).
Table 2.
Numbers of DEPs identified using paired or unpaired analysis at different FDR.
| Diagnostic | Paired analysis | Unpaired analysis | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| FDR | ||||||||||
|
| ||||||||||
| 0.05 | 0.1 | 0.2 | 0.3 | 0.5 | 0.05 | 0.1 | 0.2 | 0.3 | 0.5 | |
| BP-L3 | 0 | 0 | 2 | 2 | 2 | 0 | 0 | 0 | 0 | 0 |
| BP-L5 | 0 | 0 | 0 | 4 | 5 | 0 | 0 | 3 | 3 | 33 |
| MDD-L3 | 0 | 0 | 0 | 0 | 17 | 0 | 0 | 0 | 4 | 4 |
| MDD-L5 | 0 | 0 | 0 | 0 | 4 | 0 | 0 | 0 | 0 | 4 |
| SZ-L3 | 77 | 331 | 1337 | 3123 | 8358 | 70 | 326 | 1268 | 2850 | 7532 |
| SZ-L5 | 23 | 302 | 1309 | 3205 | 8614 | 61 | 288 | 1441 | 2997 | 8510 |
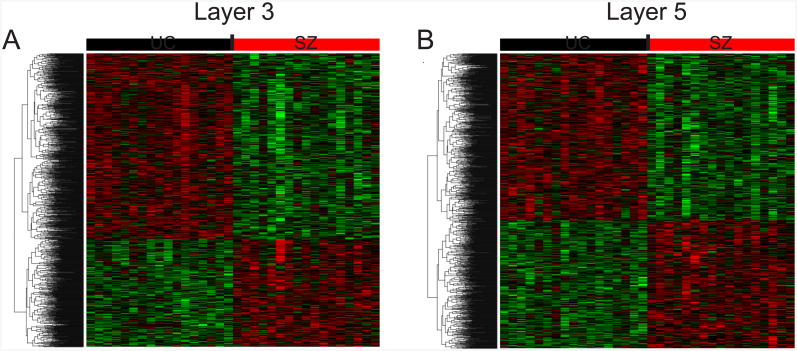
At 20% FDR, the number of DEPs in PCs from SZ subjects was similar to that found previously with a 5% FDR in a larger sample (21). Therefore, given the smaller sample size for each diagnostic group, we chose to identify DEPs by using a previously developed random intercept model with covariate selection and a 20% FDR as described in Supplementary Methods. This approach revealed numerous DEPs in L3 (n=1,339) and L5 (n=1,309) PCs in all three diagnostic groups relative to matched UC subjects (Table 2). All of these DEPs were from SZ subjects (Figure 1), with the exception of 2 DEPs in L3 PCs from BP subjects. Even using a 50% FDR, the number of DEPs for BP and MDD subjects was extremely low (Table 2). The majority of DEPS were under-expressed in SZ subjects (63% and 57% in L3 and L5 PCs, respectively), and most DEPs differed across layers; only 13.3% and 13.6% of DEPs in L3 and L5 PCs, respectively, were also differentially expressed in the other layer. For BP, the 2 DEPs in L3 PCs were under-expressed and were not shared by SZ subjects.
Figure 1.
Heat maps representing all DEPs for SZ subjects in L3 PCs (A) and L5 PCs (B) compared to UC subjects. Heat maps were built using the expression values for each DEP in each subject after adjusting for covariate effects. Each row represents a probe set ordered by hierarchical clustering results; each column represents a subject ordered by tetrad number.
Effects of clinical covariates
Among the SZ subjects, 29% (388/1337) and 31% (401/1309) of the DEPs in L3 and PCs, respectively, showed a significantly different effect in subjects with schizoaffective disorder (SA) relative to those with “pure” SZ. This transcriptome difference between SZ and SA subjects was quite similar to our prior findings (21). Statistical correction for the SA effect resulted in the SA subjects clustering more tightly with “pure” SZ subjects by PCA analysis (Supplementary Figure 3). However, possibly due to the small size of the subgroup, we did not detect any significant transcriptome signatures specific to SA subjects.
Because psychosis was present in a subset of BP (n=11) and MDD (n=5) subjects, we determined if these 16 subjects had a transcriptome signature that differed from the other 22 mood disorder subjects. After controlling for effects of diagnostic group, no psychosis-related DEPs were detected in either L3 or L5 PCs from the mood disorder subjects, even when the FDR was relaxed to 30%.
Because death by suicide was shared among a subset of subjects across diagnostic groups (6 SZ, 8 BP and 7 MDD subjects), we determined if these 21 subjects had a transcriptome signature that differed from the remaining psychiatric subjects. After controlling for the effects of diagnostic group, only 16 DEPs in L3 PCs and none in L5 PCs were significantly related to suicide (q<0.05). After relaxing the FDR to 10%, 53 DEPs related to suicide were detected in L3 PCs (Supplementary Table 3), but these DEPs did not cluster in any known molecular pathways.
Validation of microarray results
Given that DEPs were almost exclusively found in SZ subjects, we focused on validating those findings. Microarray studies have traditionally been validated by assessing a small set of transcripts using qPCR, with recent studies typically demonstrating very high validation rates (28). Consistent with this high reliability of microarray findings, in our recent microarray study of DLPFC L3 and L5 PCs in SZ (21), 16 of 18 transcripts tested were correlated between microarray and qPCR results. Here we sought to validate the results of the current study by comparisons to our previous study of DLPFC L3 and L5 PCs that used a largely different cohort of SZ and UC subjects (only 4 pairs in common) and a different Affymetrix microarray platform. Of the top 25 differentially-expressed genes (DEGs) (ranked by q-value) found in L3 or L5 PCs here, the majority (63.6% and 78.6% in L3 and L5, respectively) of those represented on the prior array platform were also differentially expressed in that study. Similar results were found for the top 100 DEGs (56.4% and 81% of DEGs in L3 and L5, respectively, were also DEGs in the previous study). In addition, of the DEPs detected at 20% FDR in L3 and L5 PCs in the present study that were also represented on the prior array platform, the direction of the disease effect was significantly correlated (L3 PCs: R=0.61, p<0.0001; L5 PCs: R=0.59, p<0.0001) across studies, with 79.3%-92.5% of the DEPs down- or up-regulated in a given layer in the current study changed in the same direction in that layer in the prior study. These similarities across different microarray platforms in largely separate subject cohorts demonstrate the technical and biological reliability of the findings.
Pathway analysis
The DEPs in SZ were then identified as a smaller set of differentially-expressed genes (DEGs; 1,090 and 1,053 DEGs in L3 and L5 PCs, respectively) and subjected to Ingenuity Pathway Analysis. In both layers, the two gene pathways that were most altered (defined by level of statistical significance) involved oxidative phosphorylation and mitochondrial dysfunction (Table 3). The percentage of genes altered in both pathways, and the statistical significance of the pathway finding, was larger in L3 than in L5 PCs, consistent with our previous study showing a greater oxphos-mitochondrial signature in L3 than L5 PCs in SZ (21). The EIF2 (Eukaryotic Initiation Factor 2) regulation pathway and the p70S6K (p70 S6 kinase) signaling and mTOR (mechanistic target of rapamycin) signaling pathways were also significant in both layers, suggesting that SZ is associated with alterations in protein synthesis and stress-related regulation of translation initiation in DLPFC PCs. In MDD and BP subjects, no biological pathways reached statistical significance.
Table 3.
Top 10 gene pathways (ranked by p-value) altered in L3 or L5 pyramidal cells for SZ**.
| Name of pathway | Number of genes in pathway | L3 | L5 | ||
|---|---|---|---|---|---|
|
| |||||
| DEGs in pathway* | p-value | DEGs in pathway* | p-value | ||
| Oxidative phosphorylation | 95 | 41 (43.2%) | < 10-24 | 27 (28.4%) | < 10-10 |
| Mitochondrial dysfunction | 155 | 49 (31.6%) | < 10-22 | 32 (20.6%) | < 10-8 |
| EIF2 signaling | 182 | 38 (20.9%) | < 10-10 | 31 (17%) | < 10-6 |
| Protein Ubiquitination Pathway | 254 | 34 (13.4%) | < 10-5 | ||
| Regulation of eIF4 and p70S6K Signaling | 151 | 21 (13.9%) | <0.001 | 24 (15.9%) | < 10-5 |
| mTOR Signaling | 193 | 24 (12.4%) | <0.001 | 25 (13%) | <0.001 |
| CDK5 Signaling | 98 | 15 (15.3%) | <0.001 | ||
| Pyrimidine Deoxyribonucleotides De Novo Biosynthesis I | 23 | 6 (26.1%) | <0.01 | ||
| Pyrimidine Ribonucleotides Interconversion | 30 | 6 (20%) | <0.01 | ||
| Gluconeogenesis I | 24 | 6 (25%) | <0.01 | ||
| Cdc42 Signaling | 121 | 15 (12.4%) | <0.01 | ||
| Endoplasmic Reticulum Stress Pathway | 21 | 5 (23.8%) | <0.01 | ||
| TCA Cycle II (Eukaryotic) | 23 | 5 (21.7%) | 0.010 | 6 (26.1%) | <0.01 |
| Pyrimidine Ribonucleotides De Novo Biosynthesis | 32 | 6 (18.8%) | 0.011 | ||
Number and % of differentially expressed genes (DEGs) in each pathway.
No significantly altered pathways were detected in either layer for subjects with bipolar or major depressive disorder.
Discussion
Consistent with our prior study (21), we found that SZ is associated with multiple transcriptome alterations in pools of individually-dissected DLPFC L3 and L5 PCs. The leading molecular pathways altered in SZ, which were conserved across L3 and L5 PCs, involved mitochondrial energy production and the regulation of protein translation. In contrast, even at relaxed FDR, very few DEPs were found, and no pathways were altered, in BP or MDD. In addition, no transcriptome signatures related to psychosis or suicide were found. Together, these findings suggest that molecular alterations in DLPFC PCs might be characteristic of the disease process(es) operative in individuals diagnosed with SZ.
Transcriptome alterations in DLPFC L3 and L5 PCs are more pronounced in SZ than mood disorders
Transcriptome alterations in DLPFC PCs were almost exclusively found in SZ subjects, with a greater magnitude and statistical significance of gene pathway alterations in L3 than in L5 PCs. These findings suggest that 1) gene expression alterations in DLPFC PCs are characteristic of SZ relative to mood disorders and 2) DLPFC L3 PCs are especially affected in schizophrenia. This interpretation is consistent with previous reports that alterations in soma size (17) and dendritic spine density (18) in SZ are more pronounced in L3 than L5 PCs.
The expression alterations detected in SZ here are in agreement with our previous findings in a mostly separate and larger SZ cohort where DLPFC L3 and L5 PC transcriptomes were determined using a different microarray platform (21). Despite these differences, 89.3% of DEPs detected in L3 PCs from SZ subjects showed concordant differences across studies relative to UC subjects. Furthermore, in both studies, the most significant transcript alterations involved lower expression of nuclear genes involved in mitochondria and oxidative phosphorylation pathways required for energy production. We cannot ascertain whether these transcriptome alterations predict alterations in the levels of function of the cognate proteins. However, both mitochondria activity and oxidative phosphorylation are tightly controlled processes (29) and even limited alterations in their function are likely to affect cell function.
The much greater number of transcripts altered in the SZ relative to the other subject groups suggests that cell type-specific patterns of gene expression alterations may differentiate SZ from mood disorders. Consistent with this interpretation, relative to BP or MDD subjects, SZ subjects had a greater number of gene expression alterations in the thalamic mediodorsal nucleus and the expression deficits in SZ were related to proteasome and translation initiation, similar to some of those reported here (30). Similarly, mitochondria-related gene expression alterations were detected in hippocampal dentate granule neurons from SZ but not from BP or MDD subjects (31). Furthermore, in other studies the number of genes with shared patterns of altered expression across diagnoses was modest and far smaller than the number of genes selectively altered in each disorder (30, 32, 33). These differences in gene expression alterations across diagnoses appear to be similar to findings from genome wide association studies; although certain risk loci have been associated with both SZ and BP, most risk loci associated with one diagnosis were not significantly associated with the other diagnosis (8, 34-36).
Gene expression alterations are related to diagnosis and not to psychosis, antipsychotic medications or suicide
Psychosis, a core element of current diagnostic formulations of SZ, is present in some individuals with BP or MDD. Furthermore, at least some psychotic features are thought to emerge from cognitive disturbances that are shared, albeit to different degrees, across all three diagnoses (3-5, 37). The idea that psychosis might have a conserved molecular substrate across diagnoses is supported by previous reports of certain transcripts that were altered in SZ and psychotic BP subjects but not BP subjects without psychosis (38), or that were altered in SZ and showed more pronounced alterations in mood disorder subjects with, than without, psychotic features (39). However, here we did not find any significant mRNA expression alterations in L3 or L5 PCs in subjects with psychosis after controlling for effects of diagnosis.
Because many subjects with psychotic BP or MDD were treated with antipsychotic medications, these findings suggest that the gene expression alterations in SZ are not due to antipsychotic medications. This interpretation is consistent with evidence in antipsychotic-exposed monkeys that these medications did not mimic gene expression changes observed in DLPFC L3 or L5 PCs in SZ (20, 21). However, we cannot definitely exclude the possibility that antipsychotic medications account for some of our findings.
Suicide has been reported to be associated with various gene expression alterations (40-43). We uncovered a small number of DEPs significantly related to suicide, mainly in L3 PCs, but these results did not reveal any molecular pathways associated with suicide independent of diagnosis.
It should be noted that we cannot exclude the possibility that our study is underpowered to uncover transcriptome signatures associated with psychosis, antipsychotic medications or suicide in DLPFC PCs.
Functional and diagnostic significance
The transcriptome differences in DLPFC L3 and L5 PCs detected here have implications for our understanding of both disease processes and diagnostic approaches in psychiatry. From the perspective of disease processes, the prominence in SZ of alterations in the expression of nuclear gene products that mitochondria need to make energy is consistent with long-standing observations that individuals with SZ are not fully able to activate the requisite DLPFC circuitry when faced with cognitive demands (44). The presence of more marked alterations of mitochondrial-related gene expression in L3 than in L5 PCs is consistent with the hypothesis that energy production is down-regulated in L3 PCs secondary to lower excitatory drive to these neurons due to their deficits in dendritic spines (21, 45). Although DLPFC L5 PCs appear to have a normal complement of dendritic spines in SZ (18), the fact that they receive excitatory inputs from hypoactive L3 PCs would be expected to result in a down-regulation of mitochondrial energy production but to a lower degree than in L3 PCs (46), consistent with the findings of the present study. Furthermore, the interpretation that spine deficits lead to lower mitochondrial-related gene expression rather than vice versa is supported by recent findings that leading risk genes for SZ may cause a complement-mediated loss of dendritic spines (47) or encode for proteins that regulate the actin cytoskeleton required for spine formation and maintenance (48, 49). In contrast, our findings suggest that cortical circuits involving DLPFC L3 and L5 PCs are less affected in mood disorders.
From the perspective of diagnosis, additional studies are needed to determine the implications of our findings for psychiatric nosology. It is possible that our findings of more gene expression alterations in SZ than mood disorders is indexing severity of illness. However, given that diagnostic differences remained significant after accounting for the effects of measures of disease severity such as psychosis and suicide suggests that our findings are more likely to reflect diagnosis-associated differences in fundamental disease processes. Molecular profiling of additional cell types in other brain circuits across diagnostic categories may help to distinguish between these alternatives.
Supplementary Material
Supplementary Figure S1. Y chromosome-linked filtering of non-informative probesets. The x axis represents the average expression for a subset of Y chromosome-linked probesets for male subjects. The y axis represents the enrichment in fold change of the expression of the Y chromosome-linked probesets in male subjects compared to female subjects. The inflection point of a lowess curve fitted to the scatter plot was then determined. The mean (MEAN) and standard deviation (SD) of fold enrichment (y-axis) was calculated for probes with average male expression (x-axis) below 3. The x-axis cut-point (filtering threshold) was chosen to correspond to the fold enrichment at MEAN+3*SD (y-axis) on the fitted curve.
Supplementary Figure S2: Rank-rank hypergeometric overlap (RRHO) plot of all Y-chromosome filtered probesets (n= 27,298). X-axis (left to right) and Y-axis (bottom to top) are ranked by differential expression p-value from 0 to 1. Each dot in the heatmap represents an enrichment level via log10 (p-value) of a hypergeometric test and the corresponding X-axis and Y-axis represent varying p-value cutoffs. Increasing enrichment levels are depicted using colors from blue to red. The color scale legend is indicated on the right hand side of each comparison. Please note that the scale differs for each comparison due to variations in the p-value distribution for SZ, BP and MDD. First row: unpaired (regression and paired (RIM) analysis comparison. Following rows: transcriptome-wide comparisons of SZ vs BP, SZ vs MDD and BP vs MDD for L3 and L5 PCs through RRHO.
Supplementary Figure S3. Principal component analysis for DEPs in L3 and L5 PCs for SZ subjects with or without a diagnosis of schizoaffective disorder (SA). The data points for SA subjects are shown before and after correction by the statistical analysis model used in this study. The subjects with a diagnosis of pure SZ are represented by gray squares; the subjects with a diagnosis of SA before correction for the effect of mood are represented by open circles and after correction by black circles. Note that after correction, SA subjects cluster more tightly with all other SZ subjects.
Supplementary Table S1. Full demographic data for UC, SZ, BP and MDD subjects.
Supplementary Table S2. Probesets/genes used in the Y-linked filtering.
Supplementary Table S3. Complete list of significant DEGs related to suicide.
Acknowledgments
The authors thank Mary L. Brady, Carol Sue Johnston, Mary Ann Kelly, Kiley Laing, Kelly Rogers, Amy Truong and Vishal Patel for excellent technical assistance. David A. Lewis currently receives investigator-initiated research support from Pfizer and has recently served as a consultant in the areas of target identification and validation and new compound development to Sunovion.
Grant Support: This work was supported by National Institutes of Health Grants MH103204 and MH043784 and a grant from Bristol-Myers Squibb.
Footnotes
Disclosures: All other authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Goldman-Rakic PS. Working memory dysfunction in schizophrenia. J Neuropsychiatry Clin Neurosci. 1994;6:348–357. doi: 10.1176/jnp.6.4.348. [DOI] [PubMed] [Google Scholar]
- 2.Kahn RS, Keefe RS. Schizophrenia is a cognitive illness: time for a change in focus. JAMA Psychiatry. 2013;70:1107–1112. doi: 10.1001/jamapsychiatry.2013.155. [DOI] [PubMed] [Google Scholar]
- 3.Millan MJ, Agid Y, Brune M, Bullmore ET, Carter CS, Clayton NS, et al. Cognitive dysfunction in psychiatric disorders: characteristics, causes and the quest for improved therapy. Nat Rev Drug Discov. 2012;11:141–168. doi: 10.1038/nrd3628. [DOI] [PubMed] [Google Scholar]
- 4.Vohringer PA, Barroilhet SA, Amerio A, Reale ML, Alvear K, Vergne D, et al. Cognitive impairment in bipolar disorder and schizophrenia: a systematic review. Front Psychiatry. 2013;4:87. doi: 10.3389/fpsyt.2013.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barch DM, Sheffield JM. Cognitive impairments in psychotic disorders: common mechanisms and measurement. World Psychiatry. 2014;13:224–232. doi: 10.1002/wps.20145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reichenberg A, Harvey PD, Bowie CR, Mojtabai R, Rabinowitz J, Heaton RK, et al. Neuropsychological function and dysfunction in schizophrenia and psychotic affective disorders. Schizophr Bull. 2009;35:1022–1029. doi: 10.1093/schbul/sbn044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bora E, Pantelis C. Meta-analysis of Cognitive Impairment in First-Episode Bipolar Disorder: Comparison With First-Episode Schizophrenia and Healthy Controls. Schizophr Bull. 2015;41:1095–1104. doi: 10.1093/schbul/sbu198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gatt JM, Burton KL, Williams LM, Schofield PR. Specific and common genes implicated across major mental disorders: a review of meta-analysis studies. J Psychiatr Res. 2015;60:1–13. doi: 10.1016/j.jpsychires.2014.09.014. [DOI] [PubMed] [Google Scholar]
- 9.Mizrahi R. Social Stress and Psychosis Risk: Common Neurochemical Substrates? Neuropsychopharmacology. 2016;41:666–674. doi: 10.1038/npp.2015.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuswanto CN, Sum MY, Sim K. Neurocognitive Functioning in Schizophrenia and Bipolar Disorder: Clarifying Concepts of Diagnostic Dichotomy vs. Continuum. Front Psychiatry. 2013;4:162. doi: 10.3389/fpsyt.2013.00162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arnsten AF. The neurobiology of thought: the groundbreaking discoveries of Patricia Goldman-Rakic 1937-2003. Cereb Cortex. 2013;23:2269–2281. doi: 10.1093/cercor/bht195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldman-Rakic PS. Cellular basis of working memory. Neuron. 1995;14:477–485. doi: 10.1016/0896-6273(95)90304-6. [DOI] [PubMed] [Google Scholar]
- 13.Black JE, Kodish IM, Grossman AW, Klintsova AY, Orlovskaya D, Vostrikov V, et al. Pathology of layer V pyramidal neurons in the prefrontal cortex of patients with schizophrenia. Am J Psychiatry. 2004;161:742–744. doi: 10.1176/appi.ajp.161.4.742. [DOI] [PubMed] [Google Scholar]
- 14.Garey LJ, Ong WY, Patel TS, Kanani M, Davis A, Mortimer AM, et al. Reduced dendritic spine density on cerebral cortical pyramidal neurons in schizophrenia. J Neurol Neurosurg Psychiatry. 1998;65:446–453. doi: 10.1136/jnnp.65.4.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rajkowska G, Selemon LD, Goldman-Rakic PS. Neuronal and glial somal size in the prefrontal cortex: a postmortem morphometric study of schizophrenia and Huntington disease. Arch Gen Psychiatry. 1998;55:215–224. doi: 10.1001/archpsyc.55.3.215. [DOI] [PubMed] [Google Scholar]
- 16.Glantz LA, Lewis DA. Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Arch Gen Psychiatry. 2000;57:65–73. doi: 10.1001/archpsyc.57.1.65. [DOI] [PubMed] [Google Scholar]
- 17.Pierri JN, Volk CLE, Auh S, Sampson A, Lewis DA. Decreased somal size of deep layer 3 pyramidal neurons in the prefrontal cortex of subjects with schizophrenia. Arch Gen Psychiatry. 2001;58:466–473. doi: 10.1001/archpsyc.58.5.466. [DOI] [PubMed] [Google Scholar]
- 18.Kolluri N, Sun Z, Sampson AR, Lewis DA. Lamina-specific reductions in dendritic spine density in the prefrontal cortex of subjects with schizophrenia. Am J Psychiatry. 2005;162:1200–1202. doi: 10.1176/appi.ajp.162.6.1200. [DOI] [PubMed] [Google Scholar]
- 19.Konopaske GT, Lange N, Coyle JT, Benes FM. Prefrontal cortical dendritic spine pathology in schizophrenia and bipolar disorder. JAMA Psychiatry. 2014;71:1323–1331. doi: 10.1001/jamapsychiatry.2014.1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Datta D, Arion D, Corradi JP, Lewis DA. Altered Expression of CDC42 Signaling Pathway Components in Cortical Layer 3 Pyramidal Cells in Schizophrenia. Biol Psychiatry. 2015;78:775–785. doi: 10.1016/j.biopsych.2015.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arion D, Corradi JP, Tang S, Datta D, Boothe F, He A, et al. Distinctive transcriptome alterations of prefrontal pyramidal neurons in schizophrenia and schizoaffective disorder. Mol Psychiatry. 2015;20:1397–1405. doi: 10.1038/mp.2014.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fromer M, Roussos P, Sieberts SK, Johnson JS, Kavanagh DH, Perumal TM, et al. Gene expression elucidates functional impact of polygenic risk for schizophrenia. Nat Neurosci. 2016;19:1442–1453. doi: 10.1038/nn.4399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Irizarry RA, Hobbs B, Collin F, Beazer-Barclay YD, Antonellis KJ, Scherf U, et al. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics. 2003;4:249–264. doi: 10.1093/biostatistics/4.2.249. [DOI] [PubMed] [Google Scholar]
- 24.Galfalvy HC, Erraji-Benchekroun L, Smyrniotopoulos P, Pavlidis P, Ellis SP, Mann JJ, et al. Sex genes for genomic analysis in human brain: internal controls for comparison of probe level data extraction. BMC Bioinformatics. 2003;4:37. doi: 10.1186/1471-2105-4-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang X, Lin Y, Song C, Sibille E, Tseng GC. Detecting disease-associated genes with confounding variable adjustment and the impact on genomic meta-analysis: with application to major depressive disorder. BMC Bioinformatics. 2012;13:52. doi: 10.1186/1471-2105-13-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Storey JD. A direct approach to false discovery rates. J R Stat Soc. 2002;64:479–498. [Google Scholar]
- 27.Plaisier SB, Taschereau R, Wong JA, Graeber TG. Rank-rank hypergenomic overlap: identification of statistically significant overlap between gene-expression signatures. Nucleic Acids Res. 2010;38 doi: 10.1093/nar/gkq636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Horvath S, Janka Z, Mirnics K. Analyzing schizophrenia by DNA microarrays. Biol Psychiatry. 2011;69:157–162. doi: 10.1016/j.biopsych.2010.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Popa-Wagner A, Mitran S, Sivanesan S, Chang E, Buga AM. ROS and brain diseases: the good, the bad, and the ugly. Oxid Med Cell Longev. 2013;2013:963520. doi: 10.1155/2013/963520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chu TT, Liu Y, Kemether E. Thalamic transcriptome screening in three psychiatric states. J Hum Genet. 2009;54:665–675. doi: 10.1038/jhg.2009.93. [DOI] [PubMed] [Google Scholar]
- 31.Altar CA, Jurata LW, Charles V, Lemire A, Liu P, Bukhman Y, et al. Deficient hippocampal neuron expression of proteasome, ubiquitin, and mitochondrial genes in multiple schizophrenia cohorts. Biol Psychiatry. 2005;58:85–96. doi: 10.1016/j.biopsych.2005.03.031. [DOI] [PubMed] [Google Scholar]
- 32.Shao L, Vawter MP. Shared gene expression alterations in schizophrenia and bipolar disorder. Biol Psychiatry. 2008;64:89–97. doi: 10.1016/j.biopsych.2007.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kohen R, Dobra A, Tracy JH, Haugen E. Transcriptome profiling of human hippocampus dentate gyrus granule cells in mental illness. Transl Psychiatry. 2014;4:e366. doi: 10.1038/tp.2014.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang J, Perlis RH, Lee PH, Rush AJ, Fava M, Sachs GS, et al. Cross-disorder genomewide analysis of schizophrenia, bipolar disorder, and depression. Am J Psychiatry. 2010;167:1254–1263. doi: 10.1176/appi.ajp.2010.09091335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chang LC, Jamain S, Lin CW, Rujescu D, Tseng GC, Sibille E. A conserved BDNF, glutamate-and GABA-enriched gene module related to human depression identified by coexpression meta-analysis and DNA variant genome-wide association studies. PLoS One. 2014;9:e90980. doi: 10.1371/journal.pone.0090980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao Z, Xu J, Chen J, Kim S, Reimers M, Bacanu SA, et al. Transcriptome sequencing and genome-wide association analyses reveal lysosomal function and actin cytoskeleton remodeling in schizophrenia and bipolar disorder. Mol Psychiatry. 2015;20:563–572. doi: 10.1038/mp.2014.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ivleva EI, Morris DW, Osuji J, Moates AF, Carmody TJ, Thaker GK, et al. Cognitive endophenotypes of psychosis within dimension and diagnosis. Psychiatry Res. 2012;196:38–44. doi: 10.1016/j.psychres.2011.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guidotti A, Auta J, Davis JM, Gerevini VD, Dwivedi Y, Grayson DR, et al. Decrease in reelin and glutamic acid decarboxylase67 (GAD67) expression in schizophrenia and bipolar disorder. Arch Gen Psychiatry. 2000;57:1061–1069. doi: 10.1001/archpsyc.57.11.1061. [DOI] [PubMed] [Google Scholar]
- 39.Kimoto S, Zaki MM, Bazmi HH, Lewis DA. Altered Markers of Cortical gamma-Aminobutyric Acid Neuronal Activity in Schizophrenia: Role of the NARP Gene. JAMA Psychiatry. 2015;72:747–756. doi: 10.1001/jamapsychiatry.2015.0533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Garbett K, Gal-Chis R, Gaszner G, Lewis DA, Mirnics K. Transcriptome alterations in the prefrontal cortex of subjects with schizophrenia who committed suicide. Neuropsychopharmacol Hung. 2008;10:9–14. [PubMed] [Google Scholar]
- 41.Dean B, Gibbons AS, Boer S, Uezato A, Meador-Woodruff J, Scarr E, et al. Changes in cortical N-methyl-d-aspartate receptors and post-synaptic density protein 95 in schizophrenia, mood disorders and suicide. Aust N Z J Psychiatry. 2016;50:275–283. doi: 10.1177/0004867415586601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhao J, Qi XR, Gao SF, Lu J, van Wamelen DJ, Kamphuis W, et al. Different stress-related gene expression in depression and suicide. J Psychiatr Res. 2015;68:176–185. doi: 10.1016/j.jpsychires.2015.06.010. [DOI] [PubMed] [Google Scholar]
- 43.Turecki G. The molecular bases of the suicidal brain. Nat Rev Neurosci. 2014;15:802–816. doi: 10.1038/nrn3839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weinberger DR, Berman KF, Zec RF. Physiologic dysfunction of dorsolateral prefrontal cortex in schizophrenia. I. Regional cerebral blood flow evidence. Arch Gen Psychiatry. 1986;43:114–124. doi: 10.1001/archpsyc.1986.01800020020004. [DOI] [PubMed] [Google Scholar]
- 45.Gonzalez-Burgos G, Cho RY, Lewis DA. Alterations in cortical network oscillations and parvalbumin neurons in schizophrenia. Biol Psychiatry. 2015;77:1031–1040. doi: 10.1016/j.biopsych.2015.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Glausier JR, Lewis DA. Dendritic spine pathology in schizophrenia. Neuroscience. 2013;251:90–107. doi: 10.1016/j.neuroscience.2012.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sekar A, Bialas AR, de Rivera H, Davis A, Hammond TR, Kamitaki N, et al. Schizophrenia risk from complex variation of complement component 4. Nature. 2016;530:177–183. doi: 10.1038/nature16549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fromer M, Pocklington AJ, Kavanagh DH, Williams HJ, Dwyer S, Gormley P, et al. De novo mutations in schizophrenia implicate synaptic networks. Nature. 2014;506:179–184. doi: 10.1038/nature12929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Purcell SM, Moran JL, Fromer M, Ruderfer D, Solovieff N, Roussos P, et al. A polygenic burden of rare disruptive mutations in schizophrenia. Nature. 2014;506:185–190. doi: 10.1038/nature12975. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure S1. Y chromosome-linked filtering of non-informative probesets. The x axis represents the average expression for a subset of Y chromosome-linked probesets for male subjects. The y axis represents the enrichment in fold change of the expression of the Y chromosome-linked probesets in male subjects compared to female subjects. The inflection point of a lowess curve fitted to the scatter plot was then determined. The mean (MEAN) and standard deviation (SD) of fold enrichment (y-axis) was calculated for probes with average male expression (x-axis) below 3. The x-axis cut-point (filtering threshold) was chosen to correspond to the fold enrichment at MEAN+3*SD (y-axis) on the fitted curve.
Supplementary Figure S2: Rank-rank hypergeometric overlap (RRHO) plot of all Y-chromosome filtered probesets (n= 27,298). X-axis (left to right) and Y-axis (bottom to top) are ranked by differential expression p-value from 0 to 1. Each dot in the heatmap represents an enrichment level via log10 (p-value) of a hypergeometric test and the corresponding X-axis and Y-axis represent varying p-value cutoffs. Increasing enrichment levels are depicted using colors from blue to red. The color scale legend is indicated on the right hand side of each comparison. Please note that the scale differs for each comparison due to variations in the p-value distribution for SZ, BP and MDD. First row: unpaired (regression and paired (RIM) analysis comparison. Following rows: transcriptome-wide comparisons of SZ vs BP, SZ vs MDD and BP vs MDD for L3 and L5 PCs through RRHO.
Supplementary Figure S3. Principal component analysis for DEPs in L3 and L5 PCs for SZ subjects with or without a diagnosis of schizoaffective disorder (SA). The data points for SA subjects are shown before and after correction by the statistical analysis model used in this study. The subjects with a diagnosis of pure SZ are represented by gray squares; the subjects with a diagnosis of SA before correction for the effect of mood are represented by open circles and after correction by black circles. Note that after correction, SA subjects cluster more tightly with all other SZ subjects.
Supplementary Table S1. Full demographic data for UC, SZ, BP and MDD subjects.
Supplementary Table S2. Probesets/genes used in the Y-linked filtering.
Supplementary Table S3. Complete list of significant DEGs related to suicide.