Abstract
The functional integration of external and internal signals forms the basis of information processing and is essential for higher cognitive functions. This occurs in finely-tuned cortical microcircuits whose functions are balanced at the cellular level by excitatory glutamatergic pyramidal neurons and inhibitory γ-aminobutyric acid (GABA) interneurons. The balance of excitation and inhibition, from cellular processes to neural network activity, is characteristically disrupted in multiple neuropsychiatric disorders, including major depressive disorder (MDD), bipolar disorder (BPD), anxiety disorders, and schizophrenia (SCZ). Specifically, nearly three decades of research demonstrate a role for reduced inhibitory GABA level and function across disorders. In MDD, recent evidence from human postmortem and animal studies suggests a selective vulnerability of GABAergic interneurons that co-express the neuropeptide somatostatin (“SST cells/interneurons”). Advances in cell type-specific molecular genetics have now helped to elucidate several important roles for SST interneurons in cortical processing (regulation of pyramidal cell excitatory input) and behavioral control (mood and cognition). Here, we review evidence for altered inhibitory function arising from GABAergic deficits across disorders, and specifically in MDD. We then focus on properties of the cortical microcircuit, wherein SST-positive GABA interneuron deficits may disrupt functioning in several ways. Finally, we discuss the putative origins of SST cell deficits, as informed by recent research, and implications for therapeutic approaches. We conclude that deficits in SST interneurons represent a contributing cellular pathology, and therefore a promising target for normalizing altered inhibitory function in MDD and other disorders with reduced SST cell and GABA functions.
Keywords: Depression, Somatostatin, GABA, Microcircuit, Dimensional, Pathology
Cognitive-Emotional Disruption and Excitation-Inhibition Balance in Depression
Major depressive disorder (MDD) is characterized by low mood, anhedonia, and cognitive deficits relating to negative biases in attention, sensory processing, and memory (1). Several mechanisms have been hypothesized, including low monoamine levels (2), reduced neuroplasticity through altered glutamate or growth factor signaling (3), impaired neuroendocrine stress response regulation (4), and more recently, altered activity of corticolimbic brain regions driven by altered excitatory and inhibitory neuron function (5, 6). Although interesting insight has emerged from these hypotheses, limited progress has been made in drug development, whereby current treatments remain ineffective in half of treated patients (7).
Maintaining the balance between excitation and inhibition is a fundamental attribute of brain function that is necessary for information processing and higher cognitive functions. At a reductionist level, neural “information” consists of excitatory signals originating from principal glutamatergic neurons that are matched by proportional GABAergic interneuron inhibition. Changes in cellular components underlying excitation-inhibition balance may affect the detection and propagation of information across cortical microcircuits, brain regions, and neural networks. Initially adaptive, chronic changes in the excitation-inhibition balance, as hypothesized to occur in psychiatric disorders, can become maladaptive and support the emergence of clinical symptoms. For instance, human and animal studies have demonstrated altered GABA-related inhibitory function in neurodevelopmental disorders, and associated these changes with cognitive deficits in information processing (8, 9). Reduced GABA levels and function may similarly account for disrupted cognitive-emotional processes in MDD (10, 11). For instance, functional neuroimaging identified MDD-related hyperactivity in the default mode network (DMN), a set of brain regions including the dorsolateral prefrontal cortex (dlPFC), posterior and anterior cingulate cortices (ACC), amygdala, and hippocampus (12, 13). In healthy individuals, the DMN is active during internal focus (e.g., attending to personal thoughts) and deactivated during externally-oriented events (e.g. goal-directed behavior) (14), hence a failure to suppress DMN activity during external tasks may contribute to negative self-referential processes (e.g., rumination) in MDD (15). Studies have now identified reduced molecular markers of GABA function in DMN regions (16–21) and correlated GABA content with functional connectivity (22–25), together suggesting a GABA-related inhibitory deficit contributing to altered activity in DMN regions and symptom emergence in MDD.
GABA Levels and Related Neurophysiological Measures in Depression
Studies in human live and postmortem subjects suggest a predominant role for altered GABA function underlying inhibitory deficits across multiple psychiatric disorders, including MDD (26–28), BPD, SCZ (29), and stress-related disorders (30). Reduced cerebrospinal fluid GABA levels were first reported in MDD and SCZ over 35 years ago (31), and have been consistently found in MDD and BPD (32–34), and extended to plasma levels correlating with illness severity, use of medication, and genetic risk (34–36).
Imaging studies using proton magnetic resonance spectroscopy provided evidence of central GABA deficits in MDD. A 50% reduction in occipital cortex GABA levels was first reported in medication-free depressed patients and later found to be more severe in patients with persistent melancholic depression (37). Low GABA levels were consistently identified in brain regions responsible for emotional-cognitive processes that are disrupted in mood disorders, including the PFC (16, 17), amygdala (18), and ACC (19–21). These reductions were more robust in treatment resistant depression (TRD) (19), but normalized in remitted patients (38). Imaging studies further identified a role for GABA in antidepressant response as brain levels were elevated following selective serotonin reuptake inhibitor (SSRI) treatment (39, 40), transcranial magnetic stimulation (TMS) (41, 42), electroconvulsive therapy (43), and cognitive-behavioral therapy (44).
Evidence of cortical inhibitory deficits arising from GABA alterations comes from TMS studies. Using TMS, single or repetitive magnetic fields are applied to the cortex to excite or inhibit cortical activity, as measured by electromyography or electroencephalogram. TMS showed efficacy as an antidepressant treatment in TRD patients (45), and is also used to experimentally probe cortical inhibition, including short-interval cortical inhibition (SICI), long-interval cortical inhibition (LICI), and cortical silent period (CSP). SICI is similar in duration to GABAA receptor (GABAAR)-mediated inhibitory post-synaptic potentials (46, 47) and is lengthened by GABAA-acting drugs (48, 49), and is therefore considered to measure GABAAR-mediated neurotransmission. Conversely, the slower LICI and CSP time-courses resemble GABAB receptor (GABABR)-mediated post-synaptic potentials (50), and are increased by GABABR agonists (51, 52), suggesting a reflection of GABABR-mediated neurotransmission.
TMS studies in MDD patients demonstrated reduced SICI and CSP (27, 28). Although reduced cortical inhibition was not always reported (53), meta-analysis confirmed MDD-related deficits (54). Others found CSP deficits in MDD, but SICI reduction only in TRD, potentially implicating GABABR impairments in overall MDD pathophysiology, and GABAAR impairments in more severe MDD (27). Reflecting findings in MDD (41, 42), repetitive TMS increased cortical inhibition (55) and lengthened CSP (56) in healthy individuals, suggesting enhanced GABA neurotransmission.
Evidence across treatment modalities implicates cortical inhibition in antidepressant effects. For instance, electroconvulsive therapy increased SICI and CSP in non-medicated MDD patients (57). Pharmacological treatment with the SSRI, citalopram, rapidly increased SICI and CSP (58), whereas the tricyclic antidepressant clomipramine increased only SICI in MDD patients (59). Finally, nucleus accumbens deep brain stimulation also showed antidepressant efficacy in TRD patients (60), and increased SICI in subjects with epilepsy (61).
Cortical Microcircuit Organization and Function
How can GABA-related neurophysiological measures be linked to cellular and molecular dysfunction in depression? Here, we briefly review microcircuit roles of SST-expressing GABAergic interneurons, and then discuss putative functional implications.
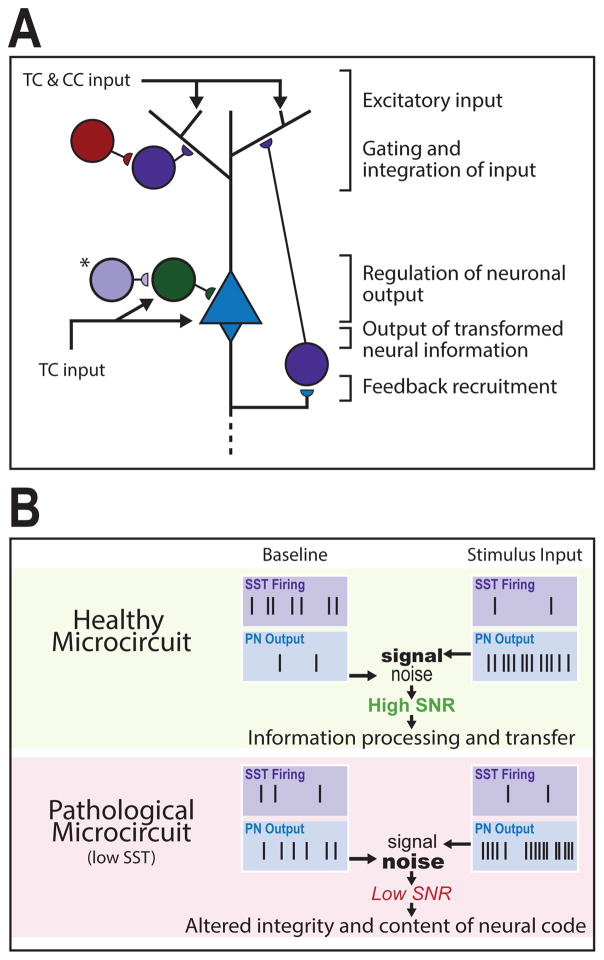
In the neocortex, external (e.g. sensory stimuli) and internal (e.g. past representations) information is coded by excitatory activation patterns that input onto pyramidal neurons (PNs). These signals are locally integrated, and, through interneuron inhibition, transformed into PN firing patterns. This neuronal output contributes to sensory processing and cognitive function (Figure 1). Studies suggest a compartmentalized integration of excitatory signals within PNs. Thalamic feed-forward excitation terminates onto the PN soma in L4-5, whereas cortico-cortical feedback excitation (alongside thalamocortical afferents) impinges separately onto PN distal dendrites (Figure 1A). These signals facilitate the integration of distinct information streams and combine to drive bursts of PN activity, forming the basis of neural coding (62, 63).
Figure 1. Cortical microcircuit roles and pathological consequences of low SST cell function.
(A) Excitatory signals originate through thalamocortical (TC) and cortico-cortical (CC) projections that converge upon pyramidal neurons (PNs). Somatostatin-expressing interneurons (SST interneurons) primarily regulate gating and integration of dendritic input through PN feedback recruitment. Vasoactive intestinal peptide-expressing (VIP) interneurons inhibit supragranular SST interneurons to facilitate excitatory input. TC input targets the PN soma and parvalbumin-expressing (PV) interneurons simultaneously, generating low-frequency PN output that is regulated by PV feed-forward inhibition. (*), In layer 4 a distinct SST interneuron population targets PV interneurons for somatic disinhibition of PN output. Dendrite- and soma-level activity combines to drive PN output, which is propagated across cortical layers and brain regions. (B) In a healthy microcircuit (top) at baseline, SST interneurons exhibit high spontaneous activity that maintains sparse PN activation. Upon stimulus input, SST interneurons reduce firing (e.g. through VIP-SST inhibition) resulting in sustained and ordered bursts of PN output. The difference between activated and baseline PN output patterns represents a signal-to-noise ratio (SNR) that facilitates coherent information processing and transfer. In a pathological microcircuit (bottom), low SST function is predicted to result in increased PN baseline activity (i.e., increased noise) and decreased regulation of stimulus-induced PN firing (i.e., altered signal) through altered input gating, disynaptic inhibition, and other disinhibitory roles, hence leading to low SNR and translating into altered integrity and content of neural code.
The input, output, and integration of excitatory signals is regulated by interconnected inhibitory GABAergic interneurons with heterogeneous morphology, distribution, electrophysiological properties, connectivity, and molecular identities. Nearly all interneurons belong to one of three non-overlapping classes based on co-expression of markers for the calcium-binding protein parvalbumin (PV; ~40%), the neuropeptide somatostatin (SST; ~30%), or the ionotropic serotonin receptor 5HT3aR (~30%), including vasoactive intestinal peptide (VIP)-expressing interneurons (64) (Figure 1A).
SST Interneurons consist mainly of translaminar distal dendrite-targeting Martinotti cells, with low-threshold regular-spiking properties and high spontaneous activity levels, distributed throughout L2-6 (65). In the hippocampus and neocortex, SST interneurons mediate feedback and lateral inhibition, contributing to maintain sparse activity of PNs at rest (e.g. in L2/3), gate converging cortico-cortical and thalamic input from L1 signal streams (63, 66), and regulate microcircuit gain (67). Additionally, L4 non-Martinotti SST interneurons preferentially target local PV interneurons, and receive thalamic afferents, thus mostly exerting PN disinhibitory functions (68).
PV Interneurons have basket or chandelier cell morphologies, target PN perisomatic region with fast-spiking properties, and are distributed across L2-6 (65). PV interneurons regulate PN spiking output through thalamic feed-forward afferents simultaneously targeting PV interneurons and PNs. Recruitment of PV interneuron feed-forward inhibition is delayed compared to direct PN innervation, providing a short window for PN signal summation that facilitates coincidence detection and firing synchrony (69), the latter function supported further by PV-PV reciprocal connections (70). Regulation of thalamic sensory input positions PV interneurons to enhance stimulus selectivity (or “tuning”) of neuronal ensembles (71, 72). Rapid and massively divergent PV interneuron inhibition prevents saturation of PN sensory responses, thus also contributing to gain modulation (73, 74).
VIP Interneurons are 5HT3aR-expressing cells with double-bouquet, bipolar, and bi-tufted cell morphologies, and non-fast-spiking properties. These cells are highly distributed in L2/3, preferentially excited by cortico-cortical afferents, and mainly target other interneurons (75). Activated VIP interneurons inhibit SST interneurons, hence release inhibitory tone on PN dendrites, and facilitating excitatory input, which may contribute to top-down modulation of sensory information by behavioral state (76, 77).
GABA and SST interneuron-related Microcircuit Deficits in Depression
Although glutamate alterations are commonly reported in MDD (78), studies more consistently demonstrate GABA-related deficits. Postmortem studies found PFC and amygdala reductions in mRNA and protein levels of the GABA-synthesizing gene, GAD67, in MDD (18, 79). Reductions in neuron and glial cell size and density were identified across layers in the dlPFC of MDD subjects (80). An MDD-related reduction in size and density of neurons immunoreactive for calbindin, a calcium-binding protein co-expressed with SST, was also reported (65), in contrast to less pronounced PV changes (17). Reduced calbindin cell density was further reported in the occipital cortex (81).
Postmortem evidence suggests a selective vulnerability of SST cells in MDD (18, 82–84). MDD-related SST mRNA reductions were reported in dlPFC, amygdala, and ACC (83, 84). Follow-up investigation in the ACC demonstrated reduced SST expression per cell across all cortical layers (83), suggesting a common origin despite SST cell heterogeneity. A putative causal role for reduced SST cell function is supported by work showing that Sst knockout mice recapitulated several MDD hallmarks, including elevated depressive-/anxiety-like behaviors, increased corticosterone, and reduced expression of brain-derived neurotrophic factor (Bdnf) and Gad67 genes, although, these changes were not seen in SstHz mice, possibly due to compensatory cellular adaptations (85). Note that SST is co-released with GABA, has pre- and post-synaptic physiological and neuronal roles (See review (86)), and when infused into rodent corticolimbic brain regions, has anxiolytic- and antidepressant-like effects (87–90). However, in light of cortical inhibitory deficits in MDD (27, 28), we consider SST as a marker for susceptible cells, and focus on SST interneurons as a GABAergic entity that is altered in MDD.
In mice, acute pharmacogenetic inhibition of PFC SST interneurons increased behavioral emotionality, but chronic blockade had the opposite effect (91), supporting a role for SST cells in regulating emotionality, but also suggesting network adaptations recruiting other brain regions. Conversely, PV and VIP changes were either not, or inconsistently reported in MDD (17, 92); although see (93) for reduced ACC PV expression in MDD. In mice, SST cell function was also associated with fear learning and working memory, and with deficits in these cognitive dimensions (94, 95). Reduced SST cell function was further demonstrated to mediate cognitive impairments in Alzheimer’s disease models (96).
Non-neuronal cells are also essential for cortical function. As microcircuit processing depends highly on temporal signal conductance, oligodendrocyte-mediated myelination can impact dendritic integration, synaptic plasticity, and synchronization of network activity. Indeed, postmortem MDD studies identified decreased size of PFC oligodendrocytes, associated with altered myelination (97), and putative reductions in amygdala oligodendrocyte numbers (98). Animal studies increasingly highlight a role for oligodendrocytes in mood regulation (99). Astrocytes also influence microcircuit function through modulation of synaptic transmission via calcium signaling, release of glutamate and purinergic neurotransmitters, and neurotransmitter recycling (100). Further, circuit connectivity depends on the formation of functional neuronal assemblies mediated by astrocytes and microglia that are similarly altered in MDD. Specifically, density of astrocytes and expression of astrocyte-specific markers were decreased in MDD patients (101), whereas microglial activation was increased (102).
A Pathophysiological Model of Low SST Cell Function
Neural code is derived from excitatory signals that converge onto PNs and undergo modulation or “fine-tuning” by GABAergic interneurons (Figure 1A), with the resulting output signals being then propagated across cortical layers and brain regions. This code is partly derived from the relative change between baseline and stimulus-induced PN outputs, where the difference represents a signal-to-noise ratio contributing to encode neural information (103). In an SST interneuron deficient system, several outcomes are predicted (Figure 1B): i) Reduced SST interneuron dendritic inhibition increases baseline PN activity, namely in L2/3; ii) Given their role in disynaptic inhibition and superficial layer synaptic integration, impaired SST interneuron function reduces gating of associative feedback projections; iii) VIP-mediated inhibition of SST cells leads to normal or enhanced PN disinhibition; iv) An increased baseline activity, combined with possibly normal, increased, or altered PN excitatory output patterns, reduces the signal-to-noise ratio, resulting in reduced detection accuracy and altered encoding of neural content; v) The resulting altered information is propagated across brain areas. Although not discussed here, the impact of reduced SST cell function is likely to be exacerbated by glial deficits, which are predicted to increase processing errors by cortical microcircuits, i.e., oligodendrocyte myelination deficits may alter spike timing or network synchrony (104, 105), whereas astrocyte dysfunction may reduce regulation of neurotransmission or spiking (106, 107), and disrupt glutamate/GABA cycling (108).
In support of these predictions, in vitro and in vivo calcium imaging studies demonstrated that L5 Martinotti (SST+) cells control PN population firing via disynaptic inhibition, and computational modeling of the data suggested that dendritic inhibition regulates microcircuit gain and cortical sensory response dynamics (63). Combined whole-cell recordings and calcium imaging in the mouse barrel cortex, an area of sensorimotor integration, demonstrated reduced SST interneuron firing and greater (L1) dendritic excitability of L2/3 PNs during active behavior (109). Optogenetic silencing of SST interneurons increased PN activity and burst firing, supporting a SST cell-mediated top-down integration of behaviorally relevant information (109), which in a system with reduced dendritic inhibition is predicted to impact perception (62). In contrast to these findings, L4 SST interneuron silencing decreased local PN activity due to SST-PV connections (68), suggesting that regulation of neuronal output may be layer-dependent. Increased PN burst firing from SST interneuron silencing was also demonstrated in hippocampal CA1 (110) and cortical V1 (111).
Ultimately, we predict a two-fold impact of reduced SST cell function: altered integrity of internal information through impaired cortico-cortical signaling, and simultaneously decreased coding (and transfer) of external information through reduced L4 thalamocortical input integration. At the psychological and symptom levels, this may manifest in shifting attention away from external focus towards internal focus, potentially underlying rumination symptoms in MDD (see review (10)). However, much remains to be elucidated regarding both the role of SST interneurons in normal microcircuit functions and whether chronically low SST cell function in psychiatric disorders leads to cellular or network-level adaptations. Several sophisticated genetic, optogenetic, and chemogenetic techniques now exist (e.g., see: 97, 117, 118), allowing fine control of distinct SST cell populations (113), and facilitating high-resolution imaging and electrophysiological recording. However, current experiments have mainly focused on short-term interneuron manipulations, whereas longer-term deficits may be more informative of human pathology (91). Another issue lies in SST cell heterogeneity, as the extent to which the expression of overlapping neurochemical markers (e.g., calbindin, calretinin, neuropeptide Y) or morphologies (e.g., Martinotti vs. non-Martinotti) bias towards functionally distinct properties is unclear (114). It also remains to be seen how upstream neuromodulators may affect microcircuit function. For example, acetylcholine and serotonin can activate or inhibit SST interneurons through direct and indirect (VIP-mediated) pathways (115, 116), which may drive brain state-dependent changes in microcircuit function (71, 117).
Origins of SST Deficits
Reduced SST expression or cell number/density is reported across several neuropsychiatric (e.g. MDD, SCZ, BPD) and neurodegenerative disorders (e.g. Alzheimer’s, Parkinson’s, and Huntington’s diseases) (118). The fact that these findings span multiple brain regions and, at least in MDD, all cells across cortical layers, suggests a cell type-specific vulnerability affecting most, if not all, SST interneurons.
Several etiological factors contributing to SST cell deficits have been identified from preclinical and postmortem MDD studies. Mouse models of depression using unpredictable chronic mild stress (UCMS) or elevated glucocorticoid exposure recapitulated SST and GABA-related deficits (85). Sex differences have also been implicated, as MDD-related SST reductions are more severe in females (82, 83), who are twice as likely to develop mood disorders (119). Reduced neurotrophic support was also implicated, as human and animal studies demonstrated that decreased gene expression of SST or GABA-synthesizing enzymes occurred downstream from BDNF signaling deficits (18, 93).
Age strongly predicts SST expression levels, which decline over time (82, 120). Cross-sectional postmortem studies demonstrated progressive early reductions of SST in SCZ and MDD (120, 121), and BDNF-dependent genes in MDD (93), suggesting that accelerated brain molecular aging may contribute to SST cell vulnerability (120). Similarly, rodent chronic stress models showed altered SST cell function from disrupted cellular homeostatic mechanisms, including endoplasmic reticulum (ER) and mitochondrial-related oxidative stress (85, 118). ER stress is a form of cellular stress implicated in normal aging and neurodegenerative disorders, resulting from allostatic overload or extracellular stimuli impairing ER protein translation, leading to an accumulation of unfolded proteins. ER stress is countered by the unfolded-protein response signaling pathway that is mediated by protein kinase RNA-like endoplasmic reticulum (Perk)-mediated phosphorylation of eukaryotic initiation factor 2α (Eif2a). SST cell-specific suppression of Eif2a signaling was observed in UCMS and in chronically-elevated corticosterone mouse models, including highly correlated Sst and Eif2a expression. Notably, inhibition of Eif2a phosphorylation via PERK reduced behavioral emotionality in UCMS-exposed mice (85), together suggesting that altered proteostasis may contribute to SST cell-selective vulnerability.
Excess ER load can also lead to reactive oxygen species accumulation, which is linked to inflammatory processes and cell death, and implicated in MDD pathophysiology (102). Notably, neuronal nitric oxide synthase and NADPH diaphorase, two reactive oxygen species-producing enzymes, are heavily expressed in SST interneurons, supporting the involvement of stress-inflammation processes in SST cell-specific vulnerability (122).
Reports of low SST expression or cell function across disorders suggests a dimensional contribution to psychopathology, converging at the cellular level on disrupted microcircuit information processing in cortical and cortical-like brain regions. How disrupted information processing manifests at the symptom level may depend on the biological context and brain region affected. For instance, preclinical studies have consistently demonstrated altered emotionality, cognitive deficits, and neuroendocrine changes associated with low SST and SST cell function (85, 91, 94–96). Importantly, reduced SST cell function may occur in different biological contexts across disorders, wherein interactions with distinct (e.g. cholinergic deficits in Alzheimer’s disease) and/or overlapping pathophysiological deficits (e.g. low GABA or reduced BDNF-TRKB signaling in MDD, BPD, and SCZ) may lead to distinct clinical symptoms (123, 124). This is further impacted by interacting biological (e.g. age, sex) and environmental (e.g. stress) factors, together contributing to the heterogeneous association of a single pathology (i.e. low SST/SST cell) with multiple symptomatic presentations.
Target Engagement and Therapeutic Approach
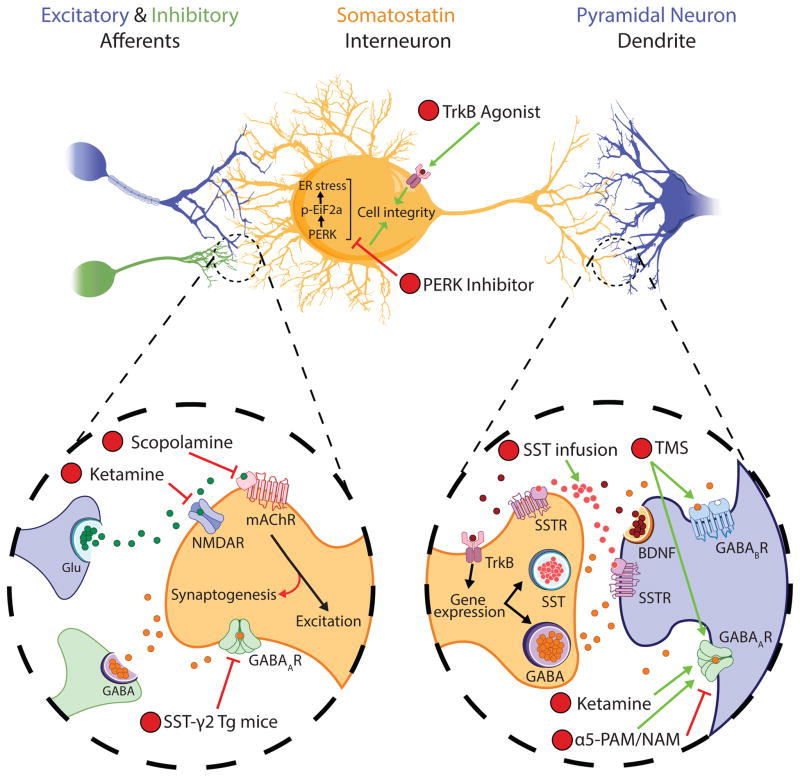
Following evidence of GABA and SST disruptions, studies have investigated the potential of monitoring and remediating these deficits for antidepressant activity (Figure 2). First, recent insights into the mechanisms underlying TMS-induced cortical inhibition suggest utility for this tool to diagnose and treat MDD-related SST cellular deficits. In rodents, single-pulse TMS inhibition of sensory-evoked L5 PN dendritic activity was demonstrated to occur via recruitment of L1-L2/3 dendrite-targeting interneurons acting through GABAAR- and GABABR-mediated inhibition (125), hence implicating both SST and neurogliaform interneurons (126, 127). Notably, SST interneuron inhibition is thought to occur mainly through α5-subunit-containing GABAARs, due to their preferential localization to extrasynaptic distal dendritic regions where they regulate tonic inhibition (128, 129), but also through GABABRs (130). Therefore, considering well-characterized SST cell deficits, and lacking evidence of neurogliaform cell pathology, MDD-related deficits in SICI and LICI/CSP may reflect reduced dendrite-level inhibition via low SST interneuron function through GABAARs and GABABRs, respectively (27, 28, 54). Similarly, increased GABA and cortical inhibitory function across antidepressant treatment modalities may reflect SST interneuron remediation, implicating these cells in the pathophysiology, diagnosis, and treatment of MDD.
Figure 2. Targeting SST-expressing GABA interneuron signaling as an antidepressant strategy.
(1) Preclinical studies demonstrate that antidepressant-like effects of the rapid-acting antidepressant scopolamine depends upon expression of mAChRs specifically in mPFC SST interneurons (134). (2) Further evidence suggests that the antidepressant-like effects of the NMDAR antagonist, ketamine (2), may similarly occur through potentiation of GABA interneuron function. These drugs may converge on antagonism of receptors that drive excitatory activity of SST interneurons, resulting in a rapid cortical glutamatergic surge (through acute inhibition of SST interneurons) that feeds-back on a longer time-scale to promote SST interneuron function (e.g. through synaptogenesis or potentiation of α5-GABAA receptor (α5-GABAAR) function (135, 136)). (3) Similarly, transgenic (Tg) mice heterozygous for SST interneuron γ2-GABAAR-subunit knockout demonstrate an antidepressant- and anxiolytic-like behavioral profile, putatively resulting from disinhibition of SST interneuron function (112). Experimental compounds that promote SST cell integrity, e.g., by increasing brain-derived neurotrophic factor (BDNF)-TrKB signaling (4) or inhibiting protein kinase RNA-like endoplasmic reticulum (PERK) and therefore endoplasmic reticulum (ER) stress (5), have shown antidepressant-like action in rodent stress models (85, 149). (6) Interventions that potentiate the post-synaptic modulators of SST interneuron inhibition also show potential for monitoring and remediating SST interneuron deficits. Notably, pharmacologic α5-GABAAR positive and negative allosteric modulation (α5-PAM/NAM) show antidepressant-like activity in mice (139, 146). (7) In human, transcranial magnetic stimulation (TMS) has efficacy in patients with treatment-resistant depression (45), and was recently demonstrated in rodent to induce cortical inhibition through recruitment of dendrite-targeting supragranular interneurons acting through GABAAR- and GABABR-mediated neurotransmission, putatively implicating SST interneurons in the antidepressant effects of TMS (125). (8) Finally, direct infusion of SST or SST agonists into corticolimbic brain regions produces antidepressant-like effects in rodents (87–90). Glu, glutamate; GABA, γ-aminobutyric acid; NMDAR, N-methyl-D-aspartate receptor (NR2B subunit-containing); mAChR, muscarinic acetylcholine receptor (m1 subtype); p-Eif2a, phosphorylated eukaryotic initiation factor 2α; TrKB, tropomyosin receptor kinase B; SSTR, somatostatin receptor (pre- and post-synaptic).
Advances in understanding the mechanisms of rapid-acting antidepressants further implicate SST interneurons in putative therapeutic activity. Ketamine, an NR2B-subtype NMDAR antagonist, and scopolamine, a mAChR antagonist, have converging mechanisms associated with a rapid “glutamate surge”, downstream activation of neurotrophic support pathways, and subsequent synaptogenesis, together with lasting antidepressant action (131, 132) (reviewed in: (133)). How these effects impinge on GABAergic cells are still being elucidated. However, it was recently demonstrated in mice that the antidepressant-like effects of scopolamine depend partially on mAChR antagonism specifically in mPFC SST cells (134), suggesting a mechanism involving PN disinhibition via suppression of SST-mediated inhibition. At first this seems inconsistent with evidence of MDD-related SST reductions and that low SST cell function is pro-depressive-like in animals (85, 91). However, rapid-acting antidepressant response is observed following drug clearance (134). Therefore, one possible explanation is that an immediate glutamatergic surge feeds-back to enhance GABAergic interneuron function on a longer scale (e.g., synaptogenesis), potentially through excitatory feedback afferents that preferentially recruit SST interneurons. Indeed, rapid-acting antidepressants increased recycling of both glutamate and GABA in rats (131). This idea is further supported by γ2-subunit knockout mice (exhibiting mild GABAAR deficits) that demonstrate elevated behavioral emotionality and a homeostatic-like reduction of glutamate synaptic function and receptor cell surface expression (135). Specifically, ketamine-treated γ2-heterozygous mice demonstrated reversal of glutamatergic synaptic deficits together with enhanced pre- and post-synaptic GABA function and antidepressant-like behavioral changes, suggesting that ketamine promotes GABAergic innervation in a GABA-deficient system (135). More specifically, work in cultured cortical and hippocampal murine neurons showed that ketamine selectively potentiated extrasynaptic GABAAR–mediated tonic inhibition (136), a function fulfilled largely by α5-GABAARs, hence providing a putative mechanism reconciling acute SST cell blockade by rapid-acting antidepressants, with prolonged cellular effects ultimately leading to increased SST cell function. Notably, rapid-acting antidepressant mechanisms may also depend on subcortical structures, as ventral hippocampus inactivation prevented sustained antidepressant-like effects of ketamine in rats (137).
Recent preclinical work supports the potentiation of SST interneuron function as an antidepressant strategy. Increasing SST interneuron inhibitory input onto target neurons (through genetic deletion of the γ2-subunit gene restricted to SST cells) demonstrated antidepressant- and anxiolytic-like activity in mice (112). Directly enhancing post-synaptic targets of SST interneurons may represent an alternative strategy. Based on restricted corticolimbic distribution of α5-GABAARs (138), and that these receptors partially mediate SST interneuron inhibition, SH-053-2′F-R-CH3, a compound with α5-selective positive allosteric modulation activity demonstrated antidepressant-like properties in female mice exposed to chronic stress (139). However, others found anxiolytic-, but not antidepressant-like effects of benzodiazepines, which act as GABAAR positive allosteric modulators, mediated specifically by α5-GABAARs in rodents (140). Although depressive- and anxiety-like behaviors are closely related in animal models (141), a thorough behavioral characterization of SST interneuron potentiation will provide further insight on how deficits in this system contribute to symptom emergence. Interestingly, GABA-elevating pharmacological treatment (e.g. the anticonvulsant lamotrigine) showed evidence of antidepressant/anxiolytic action in BPD and MDD patients (142). Benzodiazepines also showed efficacy comparable to tricyclic antidepressants in some studies (143), and may improve treatment response in TRD patients when combined with traditional pharmacotherapy (144). However, utility of benzodiazepines in treating MDD is not established and may be outweighed by a significant side-effect profile and abuse liability (145).
Note that others have also shown antidepressant-like action of an α5-selective negative allosteric modulator in chronic stress-exposed rats (146), potentially reflecting a feedback mechanism similar to that hypothesized for rapid-acting antidepressants. Alternatively, this may reflect a putative inverted-U effect for α5-GABAAR function, wherein both high and low function may have therapeutic potential. However, reducing α5-GABAAR function is predicted to worsen the pathology associated with low SST cell function; hence it is potentially associated with higher risk for long-term detrimental effects.
Preserving SST cell integrity may represent an additional antidepressant strategy. BDNF is selectively decreased in rodents following chronic stress and reversed by antidepressant treatment (147). BDNF signaling significantly contributes to SST cell markers (18, 93) and is necessary for rapid-acting antidepressant response (148). Chronic administration of 7,8-DHF, a BDNF receptor (TrkB) agonist, reversed elevated behavioral emotionality in UCMS-exposed mice and increased downstream expression of synaptic proteins that are reduced in MDD (149). Cell survival, dendritic growth, and increased neurotrophic pathway gene expression may therefore be critical to maintaining healthy SST interneuron function (see review: (150)).
Conclusion
Reduced SST expression and cell function is a replicated pathology in MDD which extends to other psychiatric and neurodegenerative disorders, suggesting a dimensional and potentially combinatorial contribution to symptoms across disorders (124). Evidence supports a role for SST interneurons in the antidepressant efficacy of current treatments and in experimental manipulations with fast-acting antidepressant-like activity (Figure 2). Together, this offers a unique opportunity for gaining insight into mechanisms underlying psychopathology, and for novel drug development in MDD and other brain disorders characterized by low SST cell function.
Acknowledgments
This work was supported by National Institute of Mental Health (NIMH) R01 MH077159, The Brain & Behavior Research Foundation (NARSAD), and by the Campbell Family Mental Health Research Institute. ES is co-inventor on a US provisional patent application that covers compounds modulating the function of GABA neurons.
Footnotes
DISCLOSURES
The other authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Disner SG, Beevers CG, Haigh EAP, Beck AT. Neural mechanisms of the cognitive model of depression. Nat Rev Neurosci. 2011;12:467–477. doi: 10.1038/nrn3027. [DOI] [PubMed] [Google Scholar]
- 2.Hirschfeld RM. History and evolution of the monoamine hypothesis of depression. J Clin Psychiatry. 2000;61(Suppl 6):4–6. [PubMed] [Google Scholar]
- 3.Wang J, Jing L, Toledo-Salas J-C, Xu L. Rapid-onset antidepressant efficacy of glutamatergic system modulators: The neural plasticity hypothesis of depression. Neurosci Bull. 2015;31:75–86. doi: 10.1007/s12264-014-1484-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pariante CM, Lightman SL. The HPA axis in major depression: classical theories and new developments. Trends Neurosci. 2008;31:464–468. doi: 10.1016/j.tins.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 5.Diener C, Kuehner C, Brusniak W, Ubl B, Wessa M, Flor H. A meta-analysis of neurofunctional imaging studies of emotion and cognition in major depression. Neuroimage. 2012;61:677–685. doi: 10.1016/j.neuroimage.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 6.Thompson SM, Kallarackal AJ, Kvarta MD, Van Dyke AM, LeGates TA, Cai X. An excitatory synapse hypothesis of depression. Trends Neurosci. 2015;38:279–294. doi: 10.1016/j.tins.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gaynes BN, Warden D, Trivedi MH, Wisniewski SR, Fava M, Rusha J. What did STAR*D teach us? Results from a large-scale, practical, clinical trial for patients with depression. Psychiatr Serv. 2009;60:1439–45. doi: 10.1176/ps.2009.60.11.1439. [DOI] [PubMed] [Google Scholar]
- 8.Chao H-T, Chen H, Samaco RC, Xue M, Chahrour M, Yoo J, et al. Dysfunction in GABA signalling mediates autism-like stereotypies and Rett syndrome phenotypes. Nature. 2010;468:263–9. doi: 10.1038/nature09582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yizhar O, Fenno LELE, Prigge M, Schneider F, Davidson TJTJ, O’Shea DJDJ, et al. Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature. 2011;477:171–178. doi: 10.1038/nature10360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Northoff G, Sibille E. Why Are Cortical GABA Neurons Relevant to Internal Focus in Depression? A cross-level model linking cellular, biochemical, and neural network findings. Mol Psychiatry. 2015;73:389–400. doi: 10.1038/mp.2014.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luscher B, Fuchs T. GABAergic control of depression-related brain states. Adv Pharmacol. 2015;8:1699–1712. doi: 10.1016/bs.apha.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hamilton JP, Furman DJ, Chang C, Thomason ME, Dennis E, Gotlib IH. Default-Mode and Task-Positive Network Activity in Major Depressive Disorder: Implications for Adaptive and Maladaptive Rumination. Biol Psychiatry. 2011;70:327–333. doi: 10.1016/j.biopsych.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sheline YI, Barch DM, Price JL, Rundle MM, Vaishnavi SN, Snyder AZ, et al. The default mode network and self-referential processes in depression. Proc Natl Acad Sci. 2009;106:1942–1947. doi: 10.1073/pnas.0812686106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anticevic A, Cole MW, Murray JD, Corlett PR, Wang X-J, Krystal JH. The role of default network deactivation in cognition and disease. Trends Cogn Sci. 2012;16:584–92. doi: 10.1016/j.tics.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nejad AB, Fossati P, Lemogne C. Self-referential processing, rumination, and cortical midline structures in major depression. Front Hum Neurosci. 2013;7:666. doi: 10.3389/fnhum.2013.00666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hasler G, van der Veen JW, Tumonis T, Meyers N, Shen J, Drevets WC. Reduced prefrontal glutamate/glutamine and gamma-aminobutyric acid levels in major depression determined using proton magnetic resonance spectroscopy. Arch Gen Psychiatry. 2007;64:193–200. doi: 10.1001/archpsyc.64.2.193. [DOI] [PubMed] [Google Scholar]
- 17.Rajkowska G, O’Dwyer G, Teleki Z, Stockmeier CA, Miguel-Hidalgo JJ. GABAergic neurons immunoreactive for calcium binding proteins are reduced in the prefrontal cortex in major depression. Neuropsychopharmacology. 2007;32:471–482. doi: 10.1038/sj.npp.1301234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guilloux J, Douillard-guilloux G, Kota R, Wang X, Martinowich K, Tseng GC, et al. Molecular evidence for BDNF- and GABA-related dysfunctions in the amygdala of female subjects with Major Depression. Mol Psychiatry. 2012;17:1130–1142. doi: 10.1038/mp.2011.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Price RB, Shungu DC, Mao X, Nestadt P, Kelly C, Collins KA, et al. Amino acid neurotransmitters assessed by proton magnetic resonance spectroscopy: relationship to treatment resistance in major depressive disorder. Biol Psychiatry. 2009;65:792–800. doi: 10.1016/j.biopsych.2008.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ham B-J, Sung Y, Kim N, Kim SJ, Kim JE, Kim DJ, et al. Decreased GABA levels in anterior cingulate and basal ganglia in medicated subjects with panic disorder: A proton magnetic resonance spectroscopy (1H-MRS) study. Prog Neuro-Psychopharmacology Biol Psychiatry. 2007;31:403–411. doi: 10.1016/j.pnpbp.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 21.Gabbay V, Mao X, Klein RG, Ely BA, Babb JS, Panzer AM, et al. Anterior Cingulate Cortexγ-Aminobutyric Acid in Depressed Adolescents. Arch Gen Psychiatry. 2012;69:139. doi: 10.1001/archgenpsychiatry.2011.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Northoff G, Walter M, Schulte RF, Beck J, Dydak U, Henning A, et al. GABA concentrations in the human anterior cingulate cortex predict negative BOLD responses in fMRI. 2007:10. doi: 10.1038/nn2001. [DOI] [PubMed] [Google Scholar]
- 23.Hu Y, Chen X, Gu H, Yang Y. Resting-State Glutamate and GABA Concentrations Predict Task-Induced Deactivation in the Default Mode Network. J Neurosci. 2013:33. doi: 10.1523/JNEUROSCI.1973-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kapogiannis D, Reiter DA, Willette AA, Mattson MP. Posteromedial cortex glutamate and GABA predict intrinsic functional connectivity of the default mode network. Neuroimage. 2013;64:112–119. doi: 10.1016/j.neuroimage.2012.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wiebking C, Duncan NW, Tiret B, Hayes DJ, Marjan`ska M, Doyon J, et al. GABA in the insula — a predictor of the neural response to interoceptive awareness. Neuroimage. 2014;86:10–18. doi: 10.1016/j.neuroimage.2013.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sanacora G, Mason GF, Rothman DL, Behar KL, Hyder F, Petroff OAC, et al. Reduced Cortical γ-Aminobutyric Acid Levels in Depressed Patients Determined by Proton Magnetic Resonance Spectroscopy. Arch Gen Psychiatry. 1999;56:1043. doi: 10.1001/archpsyc.56.11.1043. [DOI] [PubMed] [Google Scholar]
- 27.Levinson AJ, Fitzgerald PB, Favalli G, Blumberger DM, Daigle M, Daskalakis ZJ. Evidence of Cortical Inhibitory Deficits in Major Depressive Disorder. Biol Psychiatry. 2010;67:458–464. doi: 10.1016/j.biopsych.2009.09.025. [DOI] [PubMed] [Google Scholar]
- 28.Bajbouj M, Lisanby SH, Lang UE, Danker-Hopfe H, Heuser I, Neu P. Evidence for Impaired Cortical Inhibition in Patients with Unipolar Major Depression. Biol Psychiatry. 2006;59:395–400. doi: 10.1016/j.biopsych.2005.07.036. [DOI] [PubMed] [Google Scholar]
- 29.Lewis DA, Sweet RA. Schizophrenia from a neural circuitry perspective: advancing toward rational pharmacological therapies. J Clin Invest. 2009;119:706–16. doi: 10.1172/JCI37335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hasler G, van der Veen JW, Geraci M, Shen J, Pine D, Drevets WC. Prefrontal Cortical Gamma-Aminobutyric Acid Levels in Panic Disorder Determined by Proton Magnetic Resonance Spectroscopy. Biol Psychiatry. 2009;65 doi: 10.1016/j.biopsych.2008.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gold BI, Bowers MB, Roth RH, Sweeney DW. GABA levels in CSF of patients with psychiatric disorders. Am J Psychiatry. 1980;137:362–364. doi: 10.1176/ajp.137.3.362. [DOI] [PubMed] [Google Scholar]
- 32.Gerner RH, Fairbanks L, Anderson GM, Young JG, Scheinin M, Linnoila M, et al. CSF neurochemistry in depressed, manic and schizophrenic patients compared with that of normal controls. Am J Psychiatry. 1984;141:1533–1540. doi: 10.1176/ajp.141.12.1533. [DOI] [PubMed] [Google Scholar]
- 33.Kasa K, Otsuki S, Yamamoto M, Sato M, Kuroda H, Ogawa N. Cerebrospinal fluid gamma-aminobutyric acid and homovanillic acid in depressive disorders. Biol Psychiatry. 1982;17:877–83. [PubMed] [Google Scholar]
- 34.Berrettini WH, Nurnberger JIJ, Hare TA, Simmons-Alling S, Gershon ES, Post RM. Reduced plasma and CSF gamma-aminobutyric acid in affective illness: effect of lithium carbonate. Biol Psychiatry. 1983;18:185–194. [PubMed] [Google Scholar]
- 35.Petty F, Kramer GL, Gullion CM, John Rush A. Low plasma γ-aminobutyric acid levels in male patients with depression. Biol Psychiatry. 1992;32:354–363. doi: 10.1016/0006-3223(92)90039-3. [DOI] [PubMed] [Google Scholar]
- 36.Bjork JM, Moeller FG, Kramer GL, Kram M, Suris A, Rush AJ, Petty F. Plasma GABA levels correlate with aggressiveness in relatives of patients with unipolar depressive disorder. Psychiatry Res. 2001;101:131–136. doi: 10.1016/s0165-1781(01)00220-7. [DOI] [PubMed] [Google Scholar]
- 37.Sanacora G, Gueorguieva R, Epperson CN, Wu Y-T, Appel M, Rothman DL, et al. Subtype-specific alterations of gamma-aminobutyric acid and glutamate in patients with major depression. Arch Gen Psychiatry. 2004;61:705–13. doi: 10.1001/archpsyc.61.7.705. [DOI] [PubMed] [Google Scholar]
- 38.Hasler G, Neumeister A, Van Der Veen JW, Tumonis T, Bain EE, Shen J, et al. Normal prefrontal gamma-aminobutyric acid levels in remitted depressed subjects determined by proton magnetic resonance spectroscopy. Biol Psychiatry. 2005;58:969–973. doi: 10.1016/j.biopsych.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 39.Sanacora G, Mason GF, Rothman DL, Krystal JH. Increased Occipital Cortex GABA Concentrations in Depressed Patients After Therapy With Selective Serotonin Reuptake Inhibitors. 2002 doi: 10.1176/appi.ajp.159.4.663. http://dx.doi.org/101176/appi.ajp1594663. [DOI] [PubMed]
- 40.Bhagwagar Z, Wylezinska M, Taylor M, Jezzard P, Matthews PM, Cowen PJ. Increased Brain GABA Concentrations Following Acute Administration of a Selective Serotonin Reuptake Inhibitor. Am J Psychiatry. 2004;161:368–370. doi: 10.1176/appi.ajp.161.2.368. [DOI] [PubMed] [Google Scholar]
- 41.Dubin M, Mao X, Gordon R, Kang G, Liston C, Shungu D. TMS over the left dorsolateral prefrontal cortex increases GABA concentration in the ventromedial prefrontal cortex in major depression. Compr Psychiatry. 2014;55:e46–e47. [Google Scholar]
- 42.Dubin MJ, Mao X, Banerjee S, Goodman Z, Lapidus KAB, Kang G, et al. Elevated prefrontal cortex GABA in patients with major depressive disorder after TMS treatment measured with proton magnetic resonance spectroscopy. J Psychiatry Neurosci. 2016;41:E37–45. doi: 10.1503/jpn.150223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sanacora G, Mason GF, Rothman DL, Hyder F, Ciarcia JJ, Ostroff RB, et al. Increased cortical GABA concentrations in depressed patients receiving ECT. Am J Psychiatry. 2003;160:577–579. doi: 10.1176/appi.ajp.160.3.577. [DOI] [PubMed] [Google Scholar]
- 44.Sanacora G, Fenton LR, Fasula MK, Rothman DL, Levin Y, Krystal JH, Mason GF. Cortical γ-Aminobutyric Acid Concentrations in Depressed Patients Receiving Cognitive Behavioral Therapy. Biol Psychiatry. 2006;59 doi: 10.1016/j.biopsych.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 45.Carpenter LL, Janicak PG, Aaronson ST, Boyadjis T, Brock DG, Cook IA, et al. Transcranial magnetic stimulation (TMS) for major depression: A multisite, naturalistic, observational study of acute treatment outcomes in clinical practice. Depress Anxiety. 2012;29:587–596. doi: 10.1002/da.21969. [DOI] [PubMed] [Google Scholar]
- 46.McCormick DA. GABA as an inhibitory neurotransmitter in human cerebral cortex. J Neurophysiol. 1989;62:1018–27. doi: 10.1152/jn.1989.62.5.1018. [DOI] [PubMed] [Google Scholar]
- 47.Sanger TD, Garg RR, Chen R. Interactions between two different inhibitory systems in the human motor cortex. J Physiol. 2001;530:307–317. doi: 10.1111/j.1469-7793.2001.0307l.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Di Lazzaro V, Pilato F, Dileone M, Tonali PA, Ziemann U. Dissociated effects of diazepam and lorazepam on short-latency afferent inhibition. J Physiol. 2005;569:315–323. doi: 10.1113/jphysiol.2005.092155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Király J, Premoli I, Zipser C, Belardinelli P, Ziemann U, Müller-Dahlhaus F. Characterization of GABAA-receptor mediated neurotransmission in the human cortex by paired-pulse TMS-EEG. Clin Neurophysiol. 2016:127. doi: 10.1016/j.clinph.2015.11.146. [DOI] [PubMed] [Google Scholar]
- 50.Roick H, von Giesen HJ, Benecke R. On the origin of the postexcitatory inhibition seen after transcranial magnetic brain stimulation in awake human subjects. Exp Brain Res. 1993;94:489–498. doi: 10.1007/BF00230207. [DOI] [PubMed] [Google Scholar]
- 51.Siebner HR, Dressnandt J, Auer C, Conrad B. Continuous intrathecal baclofen infusions induced a marked increase of the transcranially evoked silent period in a patient with generalized dystonia. Muscle Nerve. 1998;21:1209–12. doi: 10.1002/(sici)1097-4598(199809)21:9<1209::aid-mus15>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 52.Premoli I, Rivolta D, Espenhahn S, Castellanos N, Belardinelli P, Ziemann U, Müller-Dahlhaus F. Characterization of GABAB-receptor mediated neurotransmission in the human cortex by paired-pulse TMS-EEG. Neuroimage. 2014;103:152–62. doi: 10.1016/j.neuroimage.2014.09.028. [DOI] [PubMed] [Google Scholar]
- 53.Steele JD, Glabus MF, Shajahan PM, Ebmeier KP. Increased cortical inhibition in depression: a prolonged silent period with transcranial magnetic stimulation (TMS) Psychol Med. 2000;30:565–70. doi: 10.1017/s0033291799002032. [DOI] [PubMed] [Google Scholar]
- 54.Radhu N, de Jesus DR, Ravindran LN, Zanjani A, Fitzgerald PB, Daskalakis ZJ. A meta-analysis of cortical inhibition and excitability using transcranial magnetic stimulation in psychiatric disorders. Clin Neurophysiol. 2013;124:1309–1320. doi: 10.1016/j.clinph.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 55.Chen R-S, Classen J, Gerloff C, Celnik P, Wassermann EM, Hallett M, Cohen LG. Depression of motor cortex excitability by low-frequency transcranial magnetic stimulation. Neurology. 1997;48:1398–1403. doi: 10.1212/wnl.48.5.1398. [DOI] [PubMed] [Google Scholar]
- 56.Daskalakis ZJ, Möller B, Christensen BK, Fitzgerald PB, Gunraj C, Chen R. The effects of repetitive transcranial magnetic stimulation on cortical inhibition in healthy human subjects. Exp Brain Res. 2006;174:403–412. doi: 10.1007/s00221-006-0472-0. [DOI] [PubMed] [Google Scholar]
- 57.Bajbouj M, Lang UE, Niehaus L, Hellen FE, Heuser I, Neu P. Effects of right unilateral electroconvulsive therapy on motor cortical excitability in depressive patients. J Psychiatr Res. 2006;40:322–327. doi: 10.1016/j.jpsychires.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 58.Robol E, Fiaschi A, Manganotti P. Effects of citalopram on the excitability of the human motor cortex: a paired magnetic stimulation study. J Neurol Sci. 2004;221:41–46. doi: 10.1016/j.jns.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 59.Manganotti P, Bortolomasi M, Zanette G, Pawelzik T, Giacopuzzi M, Fiaschi A. Intravenous clomipramine decreases excitability of human motor cortex. A study with paired magnetic stimulation. J Neurol Sci. 2001;184:27–32. doi: 10.1016/s0022-510x(00)00495-0. [DOI] [PubMed] [Google Scholar]
- 60.Bewernick BH, Hurlemann R, Matusch A, Kayser S, Grubert C, Hadrysiewicz B, et al. Nucleus Accumbens Deep Brain Stimulation Decreases Ratings of Depression and Anxiety in Treatment-Resistant Depression. Biol Psychiatry. 2010;67:110–116. doi: 10.1016/j.biopsych.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 61.Molnar GF, Sailer A, Gunraj CA, Cunic DI, Wennberg RA, Lozano AM, Chen R. Changes in motor cortex excitability with stimulation of anterior thalamus in epilepsy. Neurology. 2006;66:566–571. doi: 10.1212/01.wnl.0000198254.08581.6b. [DOI] [PubMed] [Google Scholar]
- 62.Larkum M. A cellular mechanism for cortical associations: An organizing principle for the cerebral cortex. Trends Neurosci. 2013:36. doi: 10.1016/j.tins.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 63.Murayama M, Pérez-Garci E, Nevian T, Bock T, Senn W, Larkum ME. Dendritic encoding of sensory stimuli controlled by deep cortical interneurons. Nature. 2009;457:1137–1141. doi: 10.1038/nature07663. [DOI] [PubMed] [Google Scholar]
- 64.Rudy B, Fishell G, Lee S, Hjerling-Leffler J. Three groups of interneurons account for nearly 100% of neocortical GABAergic neurons. Dev Neurobiol. 2011;71:45–61. doi: 10.1002/dneu.20853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gonchar Y, Burkhalter A. Three distinct families of GABAergic neurons in rat visual cortex. Cereb cortex. 1997;7:347–358. doi: 10.1093/cercor/7.4.347. [DOI] [PubMed] [Google Scholar]
- 66.Larkum ME, Nevian T, Sandler M, Polsky A, Schiller J. Synaptic integration in tuft dendrites of layer 5 pyramidal neurons: a new unifying principle. Science. 2009;325:756–760. doi: 10.1126/science.1171958. [DOI] [PubMed] [Google Scholar]
- 67.Phillips EA, Hasenstaub AR. Asymmetric effects of activating and inactivating cortical interneurons. Elife. 2016:5. doi: 10.7554/eLife.18383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xu H, Jeong HY, Tremblay R, Rudy B. Neocortical Somatostatin-Expressing GABAergic Interneurons Disinhibit the Thalamorecipient Layer 4. Neuron. 2013;77:155–167. doi: 10.1016/j.neuron.2012.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gabernet L, Jadhav SP, Feldman DE, Carandini M, Scanziani M. Somatosensory integration controlled by dynamic thalamocortical feed-forward inhibition. Neuron. 2005;48:315–327. doi: 10.1016/j.neuron.2005.09.022. [DOI] [PubMed] [Google Scholar]
- 70.Galarreta M, Hestrin S. Electrical and chemical synapses among parvalbumin fast-spiking GABAergic interneurons in adult mouse neocortex. Proc Natl Acad Sci U S A. 2002;99:12438–43. doi: 10.1073/pnas.192159599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Phillips WA, Larkum ME, Harley CW, Silverstein SM. The effects of arousal on apical amplification and conscious state. Neurosci Conscious. 2016;2016:niw015. doi: 10.1093/nc/niw015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Merchant H, de Lafuente V, Peña-Ortega F, Larriva-Sahd J. Functional impact of interneuronal inhibition in the cerebral cortex of behaving animals. Prog Neurobiol. 2012;99:163–178. doi: 10.1016/j.pneurobio.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 73.Pouille F, Marin-Burgin A, Adesnik H, Atallah BV, Scanziani M. Input normalization by global feedforward inhibition expands cortical dynamic range. Nat Neurosci. 2009;12:1577–85. doi: 10.1038/nn.2441. [DOI] [PubMed] [Google Scholar]
- 74.El-Boustani S, Sur M. Response-dependent dynamics of cell-specific inhibition in cortical networks in vivo. Nat Commun. 2014;5:5689. doi: 10.1038/ncomms6689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pfeffer CK, Xue M, He M, Huang ZJ, Scanziani M. Inhibition of inhibition in visual cortex: the logic of connections between molecularly distinct interneurons. Nat Neuro. 2013:16. doi: 10.1038/nn.3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang S, Xu M, Kamigaki T, Hoang Do JP, Chang W-C, Jenvay S, et al. Long-range and local circuits for top-down modulation of visual cortex processing. Science (80-) 2014:345. doi: 10.1126/science.1254126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yang GR, Murray JD, Wang X-J. A dendritic disinhibitory circuit mechanism for pathway-specific gating. Nat Commun. 2016;7:12815. doi: 10.1038/ncomms12815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sanacora G, Treccani G, Popoli M. Towards a glutamate hypothesis of depression: An emerging frontier of neuropsychopharmacology for mood disorders. Neuropharmacology. 2012;62:63–77. doi: 10.1016/j.neuropharm.2011.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Karolewicz B, Maciag D, O’Dwyer G, Stockmeier CA, Feyissa AM, Rajkowska G, et al. Reduced level of glutamic acid decarboxylase-67 kDa in the prefrontal cortex in major depression. Int J Neuropsychopharmacol. 2010;13:411–20. doi: 10.1017/S1461145709990587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rajkowska G, Miguel-Hidalgo JJJ, Wei J, Dilley G, Pittman SD, Meltzer HY, et al. Morphometric evidence for neuronal and glial prefrontal cell pathology in major depression. Biol Psychiatry. 1999;45:1085–1098. doi: 10.1016/s0006-3223(99)00041-4. [DOI] [PubMed] [Google Scholar]
- 81.Maciag D, Hughes J, O’Dwyer G, Pride Y, Stockmeier CA, Sanacora G, Rajkowska G. Reduced Density of Calbindin Immunoreactive GABAergic Neurons in the Occipital Cortex in Major Depression: Relevance to Neuroimaging Studies. Biol Psychiatry. 2010;67:465–470. doi: 10.1016/j.biopsych.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tripp A, Kota RS, Lewis DA, Sibille E. Reduced somatostatin in subgenual anterior cingulate cortex in major depression. Neurobiol Dis. 2011;42:116–124. doi: 10.1016/j.nbd.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Seney M, Tripp A, McCune S, Lewis D, Sibille E. Laminar and cellular analyses of reduced somatostatin gene expression in the subgenual anterior cingulate cortex in Major Depression. Neurobiol Dis. 2015;2:213–219. doi: 10.1016/j.nbd.2014.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Douillard-Guilloux G, Lewis D, Seney ML, Sibille E. Decrease in somatostatin-positive cell density in the amygdala of females with major depression. Depress Anxiety. 2016:1–11. doi: 10.1002/da.22549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lin LCL, Sibille E. Somatostatin, neuronal vulnerability and behavioral emotionality. Mol Psychiatry. 2015;20:377–387. doi: 10.1038/mp.2014.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Martel G, Dutar P, Epelbaum J, Viollet C. Somatostatinergic systems: an update on brain functions in normal and pathological aging. Front Endocrinol (Lausanne) 2012;3:154. doi: 10.3389/fendo.2012.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Engin E, Stellbrink J, Treit D, Dickson CT. Anxiolytic and antidepressant effects of intracerebroventricularly administered somatostatin: Behavioral and neurophysiological evidence. Neuroscience. 2008;157:666–676. doi: 10.1016/j.neuroscience.2008.09.037. [DOI] [PubMed] [Google Scholar]
- 88.Engin E, Treit D. Anxiolytic and antidepressant actions of somatostatin: The role of sst2 and sst3 receptors. Psychopharmacology (Berl) 2009;206:281–289. doi: 10.1007/s00213-009-1605-5. [DOI] [PubMed] [Google Scholar]
- 89.Yeung M, Engin E, Treit D. Anxiolytic-like effects of somatostatin isoforms SST 14 and SST 28 in two animal models (Rattus norvegicus) after intra-amygdalar and intra-septal microinfusions. Psychopharmacology (Berl) 2011;216:557–567. doi: 10.1007/s00213-011-2248-x. [DOI] [PubMed] [Google Scholar]
- 90.Prévôt TD, Gastambide F, Viollet C, Henkousl N, Martel G, Epelbaum J, et al. Roles of Hippocampal Somatostatin Receptor Subtypes in Stress Response and Emotionality. Neuropsychopharmacology. 2016 doi: 10.1038/npp.2016.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Soumier A, Sibille E. Opposing effects of acute versus chronic blockade of frontal cortex somatostatin-positive inhibitory neurons on behavioral emotionality in mice. Neuropsychopharmacology. 2014;39:2252–62. doi: 10.1038/npp.2014.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sibille E, Morris H, Kota R, Lewis DA. GABA-related transcripts in the dorsolateral prefrontal cortex in mood disorders. J Neuropsychopharmacol. 2011;14:721–734. doi: 10.1017/S1461145710001616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tripp A, Oh H, Guilloux J-P, Martinowich K, Lewis DA, Sibille E. Brain-derived neurotrophic factor signaling and subgenual anterior cingulate cortex dysfunction in major depressive disorder. Am J Psychiatry. 2012;169:1194–202. doi: 10.1176/appi.ajp.2012.12020248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kim D, Jeong H, Lee J, Ghim J-W, Her ES, Lee S-H, Jung MW. Distinct Roles of Parvalbumin- and Somatostatin-Expressing Interneurons in Working Memory. Neuron. 2016;92:902–915. doi: 10.1016/j.neuron.2016.09.023. [DOI] [PubMed] [Google Scholar]
- 95.Lovett-Barron M, Kaifosh P, Kheirbek MA, Danielson N, Zaremba JD, Reardon TR, et al. Dendritic inhibition in the hippocampus supports fear learning. Science. 2014;343:857–63. doi: 10.1126/science.1247485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Schmid LC, Mittag M, Poll S, Steffen J, Wagner J, Geis H-R, et al. Dysfunction of Somatostatin-Positive Interneurons Associated with Memory Deficits in an Alzheimer’s Disease Model. Neuron. 2016;92:114–125. doi: 10.1016/j.neuron.2016.08.034. [DOI] [PubMed] [Google Scholar]
- 97.Rajkowska G, Mahajan G, Maciag D, Sathyanesan M, Iyo AH, Moulana M, et al. Oligodendrocyte morphometry and expression of myelin – Related mRNA in ventral prefrontal white matter in major depressive disorder. J Psychiatr Res. 2015;65:53–62. doi: 10.1016/j.jpsychires.2015.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bowley MP, Drevets WC, Ongür D, Price JL. Low glial numbers in the amygdala in major depressive disorder. Biol Psychiatry. 2002;52:404–12. doi: 10.1016/s0006-3223(02)01404-x. [DOI] [PubMed] [Google Scholar]
- 99.Edgar N, Sibille E. A putative functional role for oligodendrocytes in mood regulation. Transl Psychiatry. 2012;2:e109. doi: 10.1038/tp.2012.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ma Z, Stork T, Bergles DE, Freeman MR. Neuromodulators signal through astrocytes to alter neural circuit activity and behaviour. Nature. 2016;539:428–432. doi: 10.1038/nature20145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rajkowska G, Miguel-Hidalgo J. Gliogenesis and Glial Pathology in Depression. CNS Neurol Disord - Drug Targets. 2007;6:219–233. doi: 10.2174/187152707780619326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Miller AH, Maletic V, Raison CL. Inflammation and Its Discontents: The Role of Cytokines in the Pathophysiology of Major Depression. Biol Psychiatry. 2009;65:732–741. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Murray JD, Anticevic A, Gancsos M, Ichinose M, Corlett PR, Krystal JH, Wang X-J. Linking microcircuit dysfunction to cognitive impairment: effects of disinhibition associated with schizophrenia in a cortical working memory model. Cereb Cortex. 2014;24:859–72. doi: 10.1093/cercor/bhs370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pajevic S, Basser PJ, Fields RD. Role of myelin plasticity in oscillations and synchrony of neuronal activity. Neuroscience. 2014;276:135–147. doi: 10.1016/j.neuroscience.2013.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fields RD, Woo DH, Basser PJ. Glial regulation of the neuronal connectome through local and long-distant communication. Neuron. 2015:86. doi: 10.1016/j.neuron.2015.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wang F, Smith NA, Xu Q, Fujita T, Baba A, Matsuda T, et al. Astrocytes modulate neural network activity by Ca2+-dependent uptake of extracellular K+ Sci Signal. 2012;5:ra26. doi: 10.1126/scisignal.2002334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Halassa MM, Haydon PG. Integrated Brain Circuits: Astrocytic Networks Modulate Neuronal Activity and Behavior. Annu Rev Physiol. 2010;72:335–355. doi: 10.1146/annurev-physiol-021909-135843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Banasr M, Chowdhury GMI, Terwilliger R, Newton SS, Duman RS, Behar KL, Sanacora G. Glial pathology in an animal model of depression: reversal of stress-induced cellular, metabolic and behavioral deficits by the glutamate-modulating drug riluzole. Mol Psychiatry. 2010;15:501–11. doi: 10.1038/mp.2008.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gentet LJ, Kremer Y, Taniguchi H, Huang ZJ, Staiger JF, Petersen CC. Unique functional properties of somatostatin-expressing GABAergic neurons in mouse barrel cortex. Nat Neurosci. 2012;15:607–612. doi: 10.1038/nn.3051. [DOI] [PubMed] [Google Scholar]
- 110.Royer S, Zemelman BV, Losonczy A, Kim J, Chance F, Magee JC, Buzsáki G. Control of timing, rate and bursts of hippocampal place cells by dendritic and somatic inhibition. Nat Neurosci. 2012;15:769–75. doi: 10.1038/nn.3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hamm JP, Yuste R. Somatostatin Interneurons Control a Key Component of Mismatch Negativity in Mouse Visual Cortex. Cell Rep. 2016;16:597–604. doi: 10.1016/j.celrep.2016.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Fuchs T, Jefferson SJ, Hooper A, Yee P-H, Maguire J, Luscher B. Disinhibition of somatostatin-positive GABAergic interneurons results in an anxiolytic and antidepressant-like brain state. Mol Psychiatry. 2016 doi: 10.1038/mp.2016.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ma Y, Hu H, Berrebi AS, Mathers PH, Agmon A. Distinct subtypes of somatostatin-containing neocortical interneurons revealed in transgenic mice. J Neurosci. 2006;26:5069–82. doi: 10.1523/JNEUROSCI.0661-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tremblay R, Lee S, Rudy B. GABAergic Interneurons in the Neocortex: From Cellular Properties to Circuits. Neuron. 2016;91:260–292. doi: 10.1016/j.neuron.2016.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Fanselow EE, Richardson KA, Connors BW. Selective, State-Dependent Activation of Somatostatin-Expressing Inhibitory Interneurons in Mouse Neocortex. J Neurophysiol. 2008:100. doi: 10.1152/jn.90691.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Jackson J, Ayzenshtat I, Karnani MM, Yuste R. VIP+ interneurons control neocortical activity across brain states. J Neurophysiol. 2016;115:3008–17. doi: 10.1152/jn.01124.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Neske GT, Connors BW. Distinct Roles of SOM and VIP Interneurons during Cortical Up States. Front Neural Circuits. 2016;10:52. doi: 10.3389/fncir.2016.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lin LC, Sibille E. Reduced brain somatostatin in mood disorders: A common pathophysiological substrate and drug target? Front Pharmacol. 2013;4:1–12. doi: 10.3389/fphar.2013.00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kessler RC. Epidemiology of women and depression. J Affect Disord. 2003;74:5–13. doi: 10.1016/s0165-0327(02)00426-3. [DOI] [PubMed] [Google Scholar]
- 120.Douillard-Guilloux G, Guilloux J-P, Lewis DA, Sibille E. Anticipated brain molecular aging in major depression. Am J Geriatr Psychiatry. 2013;21:450–60. doi: 10.1016/j.jagp.2013.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Morris HM, Hashimoto T, Lewis DA. Alterations in Somatostatin mRNA Expression in the Dorsolateral Prefrontal Cortex of Subjects with Schizophrenia or Schizoaffective Disorder. Cereb Cortex. 2008;18:1575–1587. doi: 10.1093/cercor/bhm186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Jaglin XH, Hjerling-Leffler J, Fishell G, Batista-Brito R. The origin of neocortical nitric oxide synthase-expressing inhibitory neurons. Front Neural Circuits. 2012;6:1–16. doi: 10.3389/fncir.2012.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sibille E, French B. Biological substrates underpinning diagnosis of major depression. Int J Neuropsychopharmacol. 2013;16:1893–1909. doi: 10.1017/S1461145713000436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sibille E. (In press) Reduced Somatostatin expression or Somatostatin-positive GABA neurons: a shared pathology across brain disorders. Biol Psychiatry. 2017 doi: 10.1016/j.biopsych.2016.12.002. [DOI] [PubMed] [Google Scholar]
- 125.Murphy SC, Palmer LM, Nyffeler T, Müri RM, Larkum ME. Transcranial magnetic stimulation (TMS) inhibits cortical dendrites. Elife. 2016;5:1–12. doi: 10.7554/eLife.13598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Jiang X, Shen S, Cadwell CR, Berens P, Sinz F, Ecker AS, et al. Principles of connectivity among morphologically defined cell types in adult neocortex. Science (80-) 2015:350. doi: 10.1126/science.aac9462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Palmer L, Murayama M, Larkum M. Inhibitory Regulation of Dendritic Activity in vivo. Front Neural Circuits. 2012;6:26. doi: 10.3389/fncir.2012.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Brickley SG, Mody I. Extrasynaptic GABAA Receptors: Their Function in the CNS and Implications for Disease. Neuron. 2012;73:23–34. doi: 10.1016/j.neuron.2011.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ali AB, Thomson AM. Synaptic 5 Subunit-Containing GABAA Receptors Mediate IPSPs Elicited by Dendrite-Preferring Cells in Rat Neocortex. Cereb Cortex. 2008;18:1260–1271. doi: 10.1093/cercor/bhm160. [DOI] [PubMed] [Google Scholar]
- 130.Urban-Ciecko J, Fanselow EE, Barth AL. Neocortical Somatostatin Neurons Reversibly Silence Excitatory Transmission via GABAb Receptors. Curr Biol. 2015;25:722–731. doi: 10.1016/j.cub.2015.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Chowdhury GMI, Zhang J, Thomas M, Banasr M, Ma X, Pittman B, et al. Transiently increased glutamate cycling in rat PFC is associated with rapid onset of antidepressant-like effects. Mol Psychiatry. 2017;22:120–126. doi: 10.1038/mp.2016.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Li N, Lee B, Liu R-J, Banasr M, Dwyer JM, Iwata M, et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329:959–64. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wohleb ES, Gerhard D, Thomas A, Duman RS. Molecular and Cellular Mechanisms of Rapid-Acting Antidepressants Ketamine and Scopolamine. Curr Neuropharmacol. 2017;15:11–20. doi: 10.2174/1570159X14666160309114549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wohleb ES, Wu M, Gerhard DM, Taylor SR, Picciotto MR, Alreja M, Duman RS. GABA interneurons mediate the rapid antidepressant-like effects of scopolamine. J Clin Invest. 2016;126:2482–2494. doi: 10.1172/JCI85033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ren Z, Pribiag H, Jefferson SJ, Shorey M, Fuchs T, Stellwagen D, Luscher B. Bidirectional Homeostatic Regulation of a Depression-Related Brain State by Gamma-Aminobutyric Acidergic Deficits and Ketamine Treatment. Biol Psychiatry. 2016;80:457–468. doi: 10.1016/j.biopsych.2016.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wang D-S, Penna A, Orser BA. (In Press) Ketamine Increases the Function of γ-Aminobutyric Acid Type A Receptors in Hippocampal and Cortical Neurons. Anesthesiology. 2016 doi: 10.1097/ALN.0000000000001483. [DOI] [PubMed] [Google Scholar]
- 137.Carreno FR, Donegan JJ, Boley AM, Shah A, DeGuzman M, Frazer A, Lodge DJ. Activation of a ventral hippocampus-medial prefrontal cortex pathway is both necessary and sufficient for an antidepressant response to ketamine. Mol Psychiatry. 2015;21:1–13. doi: 10.1038/mp.2015.176. [DOI] [PubMed] [Google Scholar]
- 138.Sur C, Fresu L, Howell O, McKernan RM, Atack JR. Autoradiographic localization of α5 subunit-containing GABAA receptors in rat brain. Brain Res. 1999;822:265–270. doi: 10.1016/s0006-8993(99)01152-x. [DOI] [PubMed] [Google Scholar]
- 139.Piantadosi SC, French BJ, Poe MM, Timić T, Marković BD, Pabba M, et al. Sex-Dependent Anti-Stress Effect of an α5 Subunit Containing GABAA Receptor Positive Allosteric Modulator. Front Pharmacol. 2016;7:446. doi: 10.3389/fphar.2016.00446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Behlke LM, Foster RA, Liu J, Benke D, Benham RS, Nathanson A, et al. A Pharmaco-Genetic “Restriction-of-Function” Approach Reveals Evidence for Anxiolytic-Like Actions Mediated by α5-Containing GABAA Receptors in Mice. Neuropsychopharmacology. 2016;003:1–10. doi: 10.1038/npp.2016.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kalueff AV, Wheaton M, Murphy DL. What ’ s wrong with my mouse model? Advances and strategies in animal modeling of anxiety and depression. Behav Brain Res. 2007;179:1–18. doi: 10.1016/j.bbr.2007.01.023. [DOI] [PubMed] [Google Scholar]
- 142.Calabrese JR, Bowden CL, McElroy SL, Cookson J, Andersen J, Paul E, Keck J, et al. Spectrum of Activity of Lamotrigine in Treatment-Refractory Bipolar Disorder. Am J Psychiatry. 1999 doi: 10.1176/ajp.156.7.1019. [DOI] [PubMed] [Google Scholar]
- 143.Petty F, Trivedi MH, Fulton M, John Rush A. Benzodiazepines as antidepressants: Does GABA play a role in depression? Biol Psychiatry. 1995;38:578–591. doi: 10.1016/0006-3223(95)00049-7. [DOI] [PubMed] [Google Scholar]
- 144.Morishita S. Clonazepam as a therapeutic adjunct to improve the management of depression: A brief review. Hum Psychopharmacol. 2009 Apr;:24. doi: 10.1002/hup.1015. [DOI] [PubMed] [Google Scholar]
- 145.Anderson I, Ferrier I, Baldwin R, Cowen P, Howard L, Lewis G, et al. Evidence-based guidelines for treating depressive disorders with antidepressants: A revision of the 2000 British Association for Psychopharmacology guidelines. J Psychopharmacol. 2008;22:343–396. doi: 10.1177/0269881107088441. [DOI] [PubMed] [Google Scholar]
- 146.Fischell J, Van Dyke AM, Kvarta MD, LeGates TA, Thompson SM. Rapid Antidepressant Action and Restoration of Excitatory Synaptic Strength After Chronic Stress by Negative Modulators of Alpha5-Containing GABAA Receptors. Neuropsychopharmacology. 2015;40:2499–509. doi: 10.1038/npp.2015.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Duman RS, Voleti B. Signaling pathways underlying the pathophysiology and treatment of depression: novel mechanisms for rapid-acting agents. Trends Neurosci. 2012;35:47–56. doi: 10.1016/j.tins.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng P, et al. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature. 2011;475:91–5. doi: 10.1038/nature10130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zhang M-W, Zhang S, Li Z-H, Han F. 7,8-Dihydroxyflavone reverses the depressive symptoms in mouse chronic mild stress. Neurosci Lett. 2016;635:33–38. doi: 10.1016/j.neulet.2016.10.035. [DOI] [PubMed] [Google Scholar]
- 150.Duman RS, Aghajanian GK, Sanacora G, Krystal JH. Synaptic plasticity and depression: new insights from stress and rapid-acting antidepressants. Nat Med. 2016;22:238–249. doi: 10.1038/nm.4050. [DOI] [PMC free article] [PubMed] [Google Scholar]