Abstract
Context
Prevalence of type 2 diabetes mellitus (T2DM) in youth has increased rapidly in recent decades along with rises in childhood obesity. Disparities in risk and prevalence of T2DM are evident in Hispanic youth when compared with non-Hispanic whites. Targeted diabetes prevention programs have been recommended to reduce risk prior to adulthood in this population. This systematic review explores the effectiveness of lifestyle-based diabetes prevention interventions for Hispanic youth.
Evidence acquisition
PubMed, PsychInfo, Web of Science, and CENTRAL were searched from database inception to March 1, 2017, for studies that evaluated lifestyle-focused prevention trials targeting U.S. Hispanic youth under age 18 years. Fifteen publications met criteria for inclusion.
Evidence synthesis
Eleven of fifteen studies were RCTs; four were uncontrolled. Interventions were heterogeneous in intensity, content, and setting. Duration of most trials was 12–16 weeks. Mean age of participants ranged from 9.8 to 15.8 years, sample sizes were generally small, and the majority of participants were overweight (BMI ≥85th percentile). Three studies reported statistically significant reductions in mean BMI, four in BMI z-score, and six in fasting glucose/insulin. Study quality was moderate to high. Effect sizes were generally small to medium.
Conclusions
Evidence for the impact of lifestyle-based diabetes prevention interventions targeting U.S. Hispanic youth remains limited. Few interventions demonstrated success in reducing BMI and glucose regulation and follow-up times were brief. More studies are needed that recruit larger samples sizes, extend follow-up times, explore innovative delivery modalities, and examine effectiveness across sex and age.
Context
The prevalence of childhood obesity in the U.S. has risen markedly in recent decades, contributing to heightened risk for type 2 diabetes mellitus (T2DM) over the lifespan and increased public health costs. Insulin resistance, a cardinal etiologic component of T2DM, is common in the context of childhood obesity and is associated with development of metabolic syndrome, T2DM, and cardiovascular disease,1 and predicts risk of cardiometabolic morbidity and mortality during adulthood.1 Hispanic/Latino (hereafter referred to as “Hispanic”) youth are disproportionally affected by these trends, and prevalence of cardiometabolic risk factors in this population are concerning. In youth aged 8–16 years in the multi-site Study of Latino Youth, 19.9% were overweight, 26.5% were obese,2 and 16.5% had prediabetes or diabetes.2 Though the overall prevalence of T2DM in U.S. youth is low (e.g., 0.046% in 2009 in the SEARCH for Diabetes in Youth study3), prevalence has increased by 30% from 2001, and rates are significantly higher in Hispanic youth (0.079%) compared with their non-Hispanic white counterparts (0.017%).3 In the National Health and Nutrition Examination Survey, Hispanic youth exhibited higher prevalence of obesity than non-Hispanic white youth (2011–2014, 21.9% versus 14.7%)4 and metabolic syndrome (2001–2006, 11.2% versus 8.9%).5 Further, Hispanic children are more insulin resistant than non-Hispanic white children independent of levels of adiposity.6 Once diagnosed with T2DM, Hispanic individuals have poorer glucose control, more frequent organ and vascular complications, and increased depression, cardiovascular disease, and overall mortality.7–9 Thus, early prevention is critical.
Multiple RCTs have shown that lifestyle interventions such as the Diabetes Prevention Program that emphasize changes in diet and physical activity (PA) and incorporate behavior change strategies can delay or prevent T2DM in adults10–12 and reduce diabetes risk in youth.13–16 Many of these programs, however, have struggled to engage and retain minority youth,17–22 which may contribute to disparities in diabetes risk and outcomes. In attempt to more effectively reach high-risk individuals, tailored and adapted approaches have been recommended that modify content, language, mode of delivery, theoretical approach or other intervention components to improve engagement and outcomes.23–25 Although multiple interventions targeting Hispanic Americans have been created with this goal, it is unclear to what extent programs have targeted Hispanic youth, and if they have been effective in reducing risk for T2DM. No publications have systematically aggregated data on diabetes prevention interventions for Hispanic youth, or examined the extent to which existing programs were able to modify critical risk reduction outcomes (e.g., reduction in BMI or improved insulin and glucose regulation or both, as defined by fasting insulin or glucose).
The current systematic review examines all peer-reviewed publications of lifestyle-based diabetes prevention interventions targeting Hispanic youth. The primary aim is to investigate the effectiveness of these interventions in lowering risk for T2DM via reductions in adiposity, as measured by BMI, or glucose dysregulation —variables known to be associated with development of diabetes in youth.26,27 Secondarily, the review examines effectiveness in modifying health behaviors critical to diabetes risk in youth: PA and dietary intake.
Evidence Acquisition
Data Sources
This systematic literature review was conducted and reported in alignment with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.28 Search protocols were designed for PubMed, PsychInfo, the Cochrane Central Register of Controlled Trials, and Web of Science to capture all studies from database inception to March 1, 2017. Search terms included medical subject (MeSH) headings and keywords in three domains: disease focus (diabetes), intervention type (prevention, risk reduction), and population (Hispanic/Latino and children/adolescents). For example, the PubMed search included the following terms: [diabetes mellitus/prevention and control (MeSH) OR diabetes prevention (keyword) OR diabetes risk reduction (keyword)] AND [intervention (keyword) OR health promotion (MeSH)] AND [Hispanic Americans [MeSH] OR Hispanic (keyword) OR Latin*(keyword)] AND [child (MeSH) OR adolescent (MeSH) OR youth OR teen*]. The MeSH term [Hispanic Americans] includes all Hispanic nationalities (e.g., Mexican, Cuban, Puerto Rican). Hand searching of reference lists of included publications was conducted to identify additional eligible studies. Due to absence of peer-review and risk of bias, grey literature (e.g., dissertations, conference proceedings) was not included.
Study Selection
The following inclusion criteria were used:
study evaluated a behavioral lifestyle intervention (i.e., an intervention that targeted behavior change in nutrition/dietary intake or PA or both; not a pharmaceutical intervention);
the intervention aimed to reduce risk for T2DM (e.g., not simply a weight loss/obesity intervention);
study targeted and recruited primarily Hispanic youth participants (Hispanic youth comprised ≥50% of the sample); and
the publication reported an outcome measure of BMI or a glycemic regulation variable (e.g., fasting insulin, fasting glucose, oral glucose tolerance test [OGTT]) or both.
The criterion of ≥50% Hispanic youth in the sample was selected to capture studies that targeted and recruited large numbers of Hispanic youth, but allowed participants of other ethnicities as well (e.g., school-based interventions). Studies were excluded if participants had diabetes, if published in a language other than English or Spanish, or if they were secondary analyses of trials already included.
Data Extraction and Quality Assessment
Two reviewers independently conducted review of abstracts, data extraction, and quality appraisal. Screening and inclusion details are shown in the flow diagram (Figure 1). The following information was extracted from each publication: participant characteristics, study design and setting, intervention components, and select clinical and behavioral outcomes (BMI or glycemic regulation or both, PA, and dietary intake). Raw mean difference and Hedges' g effect sizes were calculated for changes in clinical outcomes (e.g., BMI, fasting glucose). Consistent with Cochrane systematic review guidelines,29 effect sizes were not aggregated in a meta-analysis because of the heterogeneity of study design, intervention content, and intervention intensity. The Effective Public Health Practice Project Quality Assessment Tool (EPHPP; available online at www.ephpp.ca/tools.html) was utilized to rate data quality.
Figure 1.
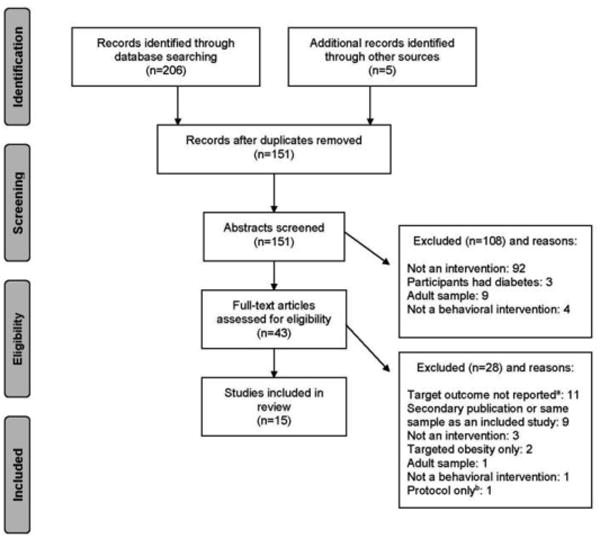
Flow diagram: screening and inclusion of publications.
aOutcomes that were inclusion criteria for this review (BMI, glucose).
bPublication described protocol but not outcomes of study.
Evidence Synthesis
Study Characteristics
The search strategy identified 151 unique publications, and 15 studies were included in the final review (Figure 1). Characteristics of included studies are presented in Table 1 and clinical outcomes are presented in Table 2. Eleven studies were RCTs and four were uncontrolled. Samples were mostly convenience or clinic-referred. Sample sizes were generally small, varying from N=15 to N=102, with the exception of two large community-based trials that included N=1,41930 and N=4,60331 children across multiple schools. Mean age of participants ranged from 9.8 to 15.8 years. In addition to age, eight studies measured and reported Tanner stage, an assessment of physical maturation ranging from 1 (pre-adolescent) to 5 (post-puberty/sexual maturity). Of these, four studies contained samples of youth in stages 3–532–35 and four reported youth in stages 4–5 only.36–39 Three studies recruited only females33,36,37 and two recruited only males.34,35 The majority of participants were overweight or obese (age- and sex-specific BMI ≥85th or 95th percentile, respectively). Twelve of 15 studies used risk-based inclusion criteria, either BMI percentile (generally, BMI ≥85th percentile) or the American Diabetes Association consensus panel risk score. One intervention included a corresponding intervention group of parents40; child data were extracted for this review.
Table 1. Study Characteristics.
| Reference | Participantsa | Mean age (years + SD) or range | Setting | Lifestyle component s addressed | Behavior change strategies reported | Frequency; modality; duration |
|---|---|---|---|---|---|---|
| RCTs | ||||||
| Davis et al. (2007) | N=23 Females; BMI ≥85th percentile | 14.2±1.7 |
Group 1: Home Group 2: Clinic |
Nutrition |
All: CD, GS, IE, NE, PI Group 2: +MI |
Weekly 90 minute sessions; Individual (home) or group (clinic) sessions; 12 weeks |
| Davis et al. (2009a) | N=54 Grade 9–12; BMI ≥85th percentile | 15.5±1.0 | Clinic |
Group 1: Nutrition Group 2: Nutrition, Physical activity |
Group 1: NE, MI, FC Group 2: NE, MI, FC, ST |
Weekly 90 minute sessions; Group; 16 weeks Group 2: +two 60 minute sessions per week |
| Davis et al. (2009b) | N=41 Females grade 9–12; BMI ≥85th percentile | 15.2±1.1 | Clinic |
Group 1: Nutrition Groups 2 – 3: Nutrition, Physical activity |
Group 1: NE+MI; Group 2: NE+MI+ST; Group 3: NE+MI+ST + aerobic PA |
Weekly 90 minute sessions; Group; 16 weeks Groups 2, 3: +Two 60 minute PA sessions per week |
| Davis et al. (2011) | N=38 Females aged 14–18 years; BMI ≥85th percentile | 15.8±1.1 | Clinic | Physical activity |
Group 1: ST+aerobic PA; Group 2: ST+aerobic PA+MI |
Weekly 60–90 minute sessions; Group; 16 weeks Group 2: + Eight individual and eight group MI sessions |
| Foster et al. (2010) | N=4,603 Sixth grade at baseline; 54% Hispanic; 50% BMI ≥85th percentile | 11.3±0.6 | School | Nutrition, Physical activity | GS, NE, PA, PAE, SE, PI, SM | Weekly educational and PA sessions of varying length; Group; 2.5 years (sixth–eighthgrade) |
| Kelly et al. (2015) | N=26 Males grade 9–12; with BMI >=95th percentile | 15.48±0.2 | Home | Physical activity | GS, MI, PAE, ST | Two weekly 1 hour sessions + 1 monthly personal training session + weekly phone calls; Individual; 16 weeks |
| Patrick et al. (2013) | N=101 Age 12–16 years; with Internet access; 74.3% Hispanic; High-risk for diabetesb | Range: 12–16 | Online/mobile | Nutrition, Physical activity | GS, NE, PAE, PI, SE | Three intervention arms: Group 1: website + weekly emails Group 2: website + monthly 90 minute groups, two monthly phone calls Group 3: website + weekly texts |
| Rosenbaum et al. (2007) | N=73 Dominican Republic background | 13.6±0.2 | School | Nutrition, Physical activity | DE, NE, PA, PAE | Weekly 45 minute sessions; Group; 14 weeks |
| Shaibi et al. (2006) | N=22 Males; BMI ≥85th percentile; Tanner stage ≥3 | 15.1±0.5 | Community | Physical activity | ST | Two weekly 60 minute sessions; Individual; 16 weeks |
| Treviño et al. (2004) | N=1,419 Fourth graders; 80% Mexican-American | 9.7±0.5 | School | Nutrition, Physical activity | CD, DE, GS, NE, PA, PI, SE | Daily (5 days/week); 50 group sessions over 7 months |
| Weigensberg et al. (2014) | N=35 Grades 9–12; BMI ≥95th percentile | Range: 14–17 | Clinic | Nutrition, Physical activity | NE, IE, PAE, PA, SE + guided imagery | Weekly; 90 minute group sessions; 12 weeks |
| Uncontrolled trials | ||||||
| Coleman et al. (2010) | N=62 At risk for T2DM (family history of T2DM and/or BMI ≥85th percentile) | 7.5±3.1 | School | Nutrition, Physical activity | CD, DE, NE, PA, PI | Weekly 90 minute sessions; Group; 10 weeks |
| Shaibi et al. (2010) | N=102c BMI ≥85th percentile | 11.8±3.0 | Clinic | Nutrition, Physical activity | CC, DE, GS, NE, PA, SE | NR; Group; NR |
| Shaibi et al. (2012) | N=15 Age 14–16 years; BMI ≥85th percentile | 15.0±0.9 | Community | Nutrition, Physical activity | CC, CD, DE, NE, PI, SE, PA | Weekly; Lifestyle education group class + three 60 minute PA sessions; 12 weeks |
| Van der Heijden et al. (2010) | N=29 Sedentaryd; 15 obese (BMI ≥95th percentile), 14 lean (BMI <85th percentile) | 15.6±0.4 (obese) 15.1±0.3 (lean) | Clinic | Physical activity | Aerobic PA | Two 30 minute group sessions + two 30 minute individual sessions per week; 12 weeks |
Hispanic heritage/nationality of participants (e.g., Mexican, Puerto Rican) is listed if reported in the publication.
As defined by the American Diabetes Association expert consensus panel, i.e., overweight (BMI >85th percentile for age and sex, weight and height >85th percentile, or weight >120% of ideal for height) plus any two of the following risk factors: family history of T2DM in a first- or second-degree relative, race/ethnicity (American Indian, African-American, Hispanic, Asian/Pacific Islander), or signs of insulin resistance (acanthosis nigricans, hypertension, dyslipidemia, polycystic ovary syndrome).
Only 50 with outcome data
<45 minute light to moderate PA/week.
NA, not assessed; NR, assessed, but not reported; CC, cultural constructs related to health; CD, cooking demonstrations/recipes/meal planning; DE, diabetes education; FC, financial compensation/gift cards; GS, goal setting; IE, intuitive eating; MI, motivational interviewing; NE, nutrition education/counseling; PA, physical activity; PAE, physical activity education; PI, parental involvement; SE, self-esteem/self-efficacy building; SM, social marketing; ST, strength training
Table 2. Raw Mean Differences and Hedges' g Effect Sizesa for Clinical Outcomes.
| Study | BMI (kg/m2) | Effect size, g (95% CI) |
BMI z-score | Effect size, g (95% CI) |
|---|---|---|---|---|
| Adiposity | ||||
| Intervention versus comparison/controlb | ||||
| Davis et al. (2007) | +1.2 | 0.16 (−0.66, 0.98) | +0.1 | 0.21 (−0.61, 1.03) |
| Davis et al. (2009a) | −1.7 (N) +1.0 (N+ST) |
−0.23 (−0.89, 0.42) 0.13 (−0.55, 0.12) |
−0.1 (N) +0.1 (N+ST) |
−0.18 (−0.83, 0.47) 0.18 (−0.51, 0.86) |
| Davis et al. (2009b) |
−4.6% (N+CAST vs N+ST)c |
– |
−7.7% (N+CAST vs N+ST)c |
– |
| Davis et al. (2011) | NS | – | NS | – |
| Foster et al. (2010) | NR | – | −0.01 | -0.01 (−0.07, 0.05) |
| Kelly et al. (2015) | +3.8 | 0.74 (−0.05, 1.54) | NR | – |
| Patrick et al. (2013) | NR | – | −0.1 (W) −0.2 (WG) −0.1 (WSMS) |
−0.22 (−0.77, 0.33) −0.44 (−0.99, 0.12) −0.22 (−0.78, 0.34) |
| Rosenbaum et al. (2007) | −0.8 | −0.48 (−0.98, 0.01) | NR | – |
| Shaibi et al. (2006) | −2.2 | −1.17 (−2.07, −0.26) | NR | – |
| Trevino et al. (2004) | NR | – | NR | – |
| Weigensberg et al. (2014) | NR | – | NR | – |
| Pre versus post intervention | ||||
| Davis et al. (2007) | −0.1 (Group-based) −0.1 (Home-based) |
−0.01 (0.41, − 0.81) − 0.02 (0.43, − 0.85) |
−0.1e (Group-based) −0.1e (Home-based) |
−0.24 (−1.04, 0.56) −0.20 (−1.03, 0.65) |
| Davis et al. (2009a) | −0.1 (N) 0.0 (N+ST) |
−0.02 (−0.62, 0.59) 0.00 (−0.67, 0.67) |
0.0 (N) 0.0 (N+ST) |
0.00 (−0.60, 0.60) 0.00 (−0.67, 0.67) |
| Davis et al. (2009b) | +0.3 (N) +1.1 (N+ST) −0.5 (N+CAST) |
– – – |
+0.02 (N) +0.08 (N+ST) −0.05 (N+CAST) |
– – – |
| Davis et al (2011) | NR | – | NR | – |
| Foster et al. (2010) | NR | – | −0.05 | −0.05 (−0.11, 0.01) |
| Kelly et al (2015) | +1.3 | 0.22 (−0.55, 0.99) | NR | – |
| Patrick et al. (2013) | NR | – | −0.1 (W) −0.2 (WG) −0.1 (WSMS) |
−0.24 (−0.79, 0.30) −0.49 (−1.04, 0.06) −0.24 (−0.81, 0.32) |
| Rosenbaum et al. (2007) | −0.7 | −0.48 (−0.88, −0.08) | NR | – |
| Shaibi et al. (2006) | +0.3 | 0.18 (−0.66, 1.02) | NR | – |
| Trevino et al. (2004) | NR | – | NR | – |
| Coleman et al. (2010) | −0.2 | −0.04 (−0.39, 0.32) | +0.01 | 0.01 (−0.34, 0.36) |
| Shaibi et al. (2010) | −1.1 | −2.18 (−2.68, −1.69) | NR | – |
| Shaibi et al. (2012) | −0.5 | −0.29 (−1.01, 0.42) | −0.1 | −0.62 (−1.35, 0.12) |
| Van der Heijden et al. (2010) | −0.3 (Obese youth) +0.1 (Lean youth) |
−0.27 (−0.98, 0.45) 0.12 (−0.62, 0.86) |
NR NR |
– – |
| Weigensberg et al. (2014) | +0.8 | 0.13 (−0.59, 0.84) | +0.04 | 0.10 (−0.62, 0.81) |
|
|
||||
| Glucose regulation |
Fasting glucose (mg/dL) |
Effect size, g (95% CI) |
Fasting insulin (μU/mL) |
Effect size, g (95% CI) |
|
|
||||
| Intervention versus control group | ||||
| Davis et al. (2007) | −0.9 | −0.14 (−0.96, 0.68) | +2.6 | 0.33 (−0.49, 1.15) |
| Davis et al. (2009a) | +2.7 (N) −0.2 (N+ST) |
0.37 (−0.29, 1.02) −0.02 (−0.71, 0.66) |
−1.8 (N) +1.7 (N+ST) |
−0.10 (−0.75, 0.55) 0.10 (−0.58, 0.78) |
| Davis et al. (2009b) | −10.3% (N+CAST vs N)c | – | NS | |
| Davis et al. (2011) | NS | – | 24% decrease vs 6% increase (CT vs control)c | – |
| Foster et al. (2010) | −0.08 | −0.10 (−0.15, −0.04) | −0.5 | −0.03 (−0.09, 0.02) |
| Kelly et al. (2015) | +2.4 | 0.44 (−0.34, 1.22) | NR | – |
| Patrick et al. (2013) | NR | – | NR | – |
| Rosenbaum et al. (2007) | 0 | 0.00 (−0.49, 0.49) | +1.0 | 0.33 (−0.75, 0.55) |
| Shaibi et al. (2006) | +2.1 | 1.03 (0.12, 1.95) | −7.6 | −2.81 (−4.02, −1.60) |
| Trevino et al. (2004) | −2.5 | −0.25 (−0.36, −0.15) | NR | – |
| Weigensberg et al. (2014) | NR | – | NR | – |
| Pre versus post intervention | ||||
| Davis et al. (2007) | +0.9 (Home-based) −3.6 (Group-based) |
0.10 (−0.73, 0.94) −0.65 (−1.47, 0.17) |
−3.3 (Home-based) 0.0 (Group-based) |
−0.40 (−1.25, 0.44) 0.00 (−0.80, 0.80) |
| Davis et al (2009a) | −0.8 (N) −2.4 (N+ST) |
−0.12 (−0.73, 0.48) −0.29 (−0.97, 0.38) |
−1.8 (N) −3.9 (N+ST) |
−0.11 (−0.72, 0.49) −0.30 (−0.98, 0.37) |
| Davis et al. (2009b) | +2.5 (N) −3.6 (N+ST) − 4.3 (N+CAST) |
– – – |
+4.5 (N) −0.8 (N+ST) −0.5 (N+CAST) |
– – – |
| Davis et al. (2011) | NR | – | NR | – |
| Foster et al. (2010) | 0 | 0.00 (−0.06, 0.06) | +3.80 | 0.29 (0.23, 0.35) |
| Kelly et al. (2015) | +4.4 | 0.67 (−0.12, 1.46) | NR | – |
| Patrick et al. (2013) | NR | – | NR | – |
| Coleman et al. (2010) | NR | – | NR | – |
| Rosenbaum et al. (2007) | −1.0 | −0.99 (−1.41, −0.57) | −1.0 | −0.33 (−0.73, 0.07) |
| Shaibi et al. (2006) | +2.5 | 1.30 (0.38, 2.22) | −1.3 | −0.54 (−1.39, 0.31) |
| Trevino et al. (2004) | −0.2 | −0.02 (−0.13, 0.08) | NR | – |
| Shaibi et al. (2010) | −0.6 | −0.50 (−0.89, −0.10) | −5.9 | −2.53 (−3.05, −2.00) |
| Shaibi et al. (2012) | NR | – | NR | – |
| Van der Heijden et al. (2010) | 0.0 (Obese youth) −1.0 (Lean youth) |
0.00 (−0.72, 0.72) −0.97 (−1.75, −0.19) |
−3.6 (Obese youth) −0.6 (Lean youth) |
−1.37 (−2.17, −0.58) −0.65 (−1.41, 0.11) |
| Weigensberg et al. (2014) | NR | – | NR | – |
Notes: Boldface indicates statistical significance (p<0.05) as reported by study authors.
Effect sizes were calculated if publication provided sufficient data (e.g., group means, SDs.
All comparisons are between specified group and control group unless otherwise noted.
Percentage reported because publication did not provide raw data.
CAST, combined aerobic and strength training; N, nutrition education only; NR, not reported; NS, not significant and data not reported; N+ST, nutrition education plus strength training; W, website-only intervention group; WG, website + group meetings; WSMS, website + text content
Interventions
Detailed information about each intervention is presented in Appendix Table 1. Interventions took place across multiple settings: clinics,32,36–39,41 schools,30,31,40,42 community settings,35,43 participant homes,34 and online.44 One intervention compared parallel home- and clinic-based interventions.33 Most interventions targeted nutrition and increased PA, but four studies targeted PA only,34,35,37,38 and one study targeted nutrition only.33 All utilized face to face delivery of content, except for two arms of a web-based intervention.44 Interventions generally involved weekly contact with youth and varied in duration from 10 weeks to 2.5 years, the majority lasting 12–16 weeks. Follow-up assessments occurred at the post-intervention time point in all but one study, which measured outcomes after 1 year.41 Ten studies utilized a group modality, two provided individual sessions,34,35 one involved both group and individual,38 and two compared outcomes across group and individual arms.33,44 The following behavior change strategies were common: guided PA/PA education (13 studies), nutrition education/counseling (ten studies), diabetes-specific education (five studies), motivational interviewing (five studies), goal setting (six studies), self-efficacy/self-esteem building (six studies), and parent engagement (six studies). Two studies reported no behavior change strategies aside from PA training.35,38
Clinical Outcomes
Reduction of risk for diabetes was measured by a variety of outcomes: changes in weight, BMI, cardiovascular fitness, total fat and lean muscle mass, and multiple outcomes of glucose regulation or insulin sensitivity (e.g., fasting glucose, fasting insulin, OGTT, homeostatic model assessment of insulin resistance [HOMA-IR], and insulin sensitivity measured by frequency-sampled intravenous glucose tolerance test [FSIVGTT]). In line with the a priori inclusion criteria of this review, data were extracted for outcomes of BMI and glucose regulation variables. Given the heterogeneity in variables measured, the four most commonly reported outcomes were selected for presentation in Table 2: BMI, BMI z-score, fasting glucose, and fasting insulin.
Twelve of 15 studies measured participant BMI (kg/m2). Of these, three (two RCTs) reported a statistically significant reduction in mean BMI post intervention or compared with the control group.36,41,42,41,42 Within-group changes in BMI were small across studies, ranging from −0.1 to −1.1 kg/m2. Eight studies reported changes in BMI z-scores. Of these, three RCTs31,33,36 and one uncontrolled study42 reported significant reductions. Changes in within-group BMI z-scores were also small, ranging from −0.1 to +0.04. Six studies evaluated changes in age- and sex-adjusted BMI percentile; one reported significant intervention effects (change of −1.3%).43
Of ten studies that reported fasting glucose outcomes, two RCTs reported statistically significant reductions compared with controls.30,36 Mean differences in fasting glucose ranged from −0.2 to −4.3 mg/dL. Nine studies reported fasting insulin outcomes, and four resulted in significant reduction in insulin either between31,37 or within38,41 groups. Mean reduction in fasting insulin across studies ranged from −0.5 to −5.9 μU/mL. Of five interventions that calculated HOMA-IR scores32,33,37–39 two achieved significant changes compared with the control group (21% versus 4% decrease)33 or baseline (mean change −0.8; results not shown in Table 2).38 Seven studies measured post-load 2-hour glucose via OGTT, but only one43 reported an improved tolerance compared with baseline (10.8% reduction). Four studies conducted FSIVGTT for direct assessment of insulin sensitivity. One of these,35 a resistance training intervention, reported significant differences compared with baseline and controls (increase in insulin sensitivity of 0.9 ×10−4 min−1* μU−1 mL−1).
Behavioral Outcomes
As a secondary aim of this review, changes in PA and dietary intake were extracted and results are presented in Table 3. Eight studies examined self-reported changes in dietary intake30,32,33,36,37,39,43,44 and six reported significant changes compared with controls32,36,39,44 or baseline33,43(Table 3). Tools utilized to assess dietary intake were 3-day dietary intake records, a self-administered food frequency questionnaire44 and the Brief Dietary Assessment Tool for Hispanics.43 Statistically significant changes were reported in each of the following outcomes: reduction in amount of sugar consumed,33,36 reduction in carbohydrate intake,32,33 reduction in dietary fat,32,43 reduction in total energy consumed,32,33 increase in dietary fiber,30,33 and increase in fruit and vegetable consumption compared with controls.44
Table 3. Physical Activity and Dietary Behavior Outcomes of Diabetes Prevention Interventions for U.S. Hispanic Youth.
| Reference | PA outcome; measure | PA findings | Dietary outcome; measure | Dietary findings |
|---|---|---|---|---|
| RCTs | ||||
| Davis et al. (2007) | None | N/A | Dietary intake; 3-day diet recordsa | Reduced intake of added sugar (33%), sugary beverages (66%), refined carbohydrates (35%), and total energy (22%); increased dietary fiber (46%) in both group and individual format |
| Davis et al. (2009a) | None | N/A | Dietary intake; 3-day dietary recordsa | N+ST group: reduced total energy intake (20%), carbohydrate intake (18%), and dietary fat intake (24%) compared to controls |
| Davis et al. (2009b) | Light and MVPA; 7-day ActiGraph accelerometer wear, 3-DPAR | No significant changes | Dietary intake; 3-day diet recordsa | Group (1): significant reduction in added sugar (38.7%); Group (3): significant reductions in total sugar consumed (45.7%) |
| Davis et al. (2011) | Light and MVPA; 7-day ActiGraph accelerometer wear | No significant changes | Dietary intake; 3-day diet recordsa | No significant changes |
| Foster et al. (2010) | None | N/A | None | N/A |
| Kelly et al. (2015) | None | N/A | None | N/A |
| Patrick et al. (2013) | PA; 7-day PA recall interview | No significant changes | Dietary intake; Self-administered food frequency questionnaire | Group (2): increased fruit and vegetable intake compared to controls |
| Rosenbaum et al. (2007) | None | N/A | None | N/A |
| Shaibi et al. (2006) | None | N/A | None | N/A |
| Trevino et al. (2004) | None | N/A | Dietary fiber % kcal from saturated fat; three 24-hr dietary recalls administered by staff | Increase in dietary fiber consumed compared to controls |
| Weigensberg et al. (2014) | MVPA; 3-DPAR | Moderate PA increased by 29% compared to controls | Dietary intake; 3-day diet recordsa | Decrease in total energy intake compared to controls |
| Uncontrolled trials | ||||
| Coleman et al. (2010) | None | N/A | None | N/A |
| Shaibi et al. (2010) | None | N/A | None | N/A |
| Shaibi et al. (2012) | MVPA; 3-DPAR | Daily MVPA increase of 26.1%; 67% of sample (post) versus 47% (pre) met CDC recommendations of 60 minutes MVPA per day | Dietary intake; Brief Dietary Assessment Tool for Hispanics | Reduction in servings of fat consumed per day from 3.3 (±0.3) to 2.0 (±0.2) |
| Van der Heijden et al. (2010) | None | N/A | None | N/A |
Notes: Boldface indicates statistical significance (p<0.05).
3-day diet recalls were self-administered and then clarified by study staff.
3DPAR, 3-Day Physical Activity Recall questionnaire; Kcal, kilocalories; MVPA, moderate to vigorous physical activity; PA, physical activity, N/A, not applicable
Five studies measured pre- and post-intervention PA (Table 3). Three assessed PA by self-report alone39,43,44; two utilized self-report and 7-day accelerometry measures.36,37 Observed changes in PA ranged from no change/non-significant changes to a 29% increase in daily moderate intensity PA compared with controls, based on self-report.39 Because of the heterogeneity in components of PA and dietary intake reported, effect sizes could not be calculated for these outcomes.
Quality of Evidence
A summary of evidence quality is presented in Appendix Table 2. Utilizing the data quality rating protocol of the EPHPP, the following characteristics were evaluated to generate a global rating for each study: selection bias, study design, confounders, blinding, assessment methods, and attrition. Four publications received a strong global rating of evidence quality30,41,43,44; the remaining studies received a moderate global rating. The included studies were strong on study design (11 RCTs), control of confounders, and assessment methodology. Quality ratings were often low for selection bias, attrition, and lack of blinding.
Discussion
Of the 15 interventions surveyed in this review, six resulted in reductions in either BMI or BMI z-score, and six reported changes in fasting glucose or fasting insulin. The 11 RCTs presented the strongest evidence for behavioral interventions, and reported significant changes in BMI/BMI z-score (n=3) and fasting glucose or insulin (n=4). Although evidence quality was moderate to strong, results must be interpreted cautiously as samples sizes were mostly small, and long-term effects of interventions are unknown. Considering these factors, evidence for feasibility of lifestyle-based diabetes prevention programs targeting Hispanic youth exists, but support for efficacy in reducing risk for diabetes remains limited.
It is worth noting that while effect sizes were small to medium for most interventions, incremental differences in glucose regulation can correspond to clinically meaningful changes in disease risk. Risk for incident diabetes increases markedly across the A1c range of 5.0 to 6.5%.45 Though risk associated with small changes in fasting glucose in adolescents is less established, mean fasting glucose at baseline in many samples was 90–95 mg/dL, very close to the cut point for impaired fasting glucose/prediabetes (100–125 mg/dL). A mean increase of up to 4.4 mg/dL was observed in some groups over the course of a 16-week intervention, meaning that youth are exhibiting rapid movement towards prediabetes without intervention, or despite intervention in some cases (e.g.,34). In these high-risk scenarios, interventions that result in reduction of even a few mg/dL in mean fasting glucose may prevent or stall the development of prediabetes and further pathophysiological changes.
Intervention Completeness
Despite strong evidence supporting the necessity of addressing both PA and dietary intake in lifestyle interventions for effective reduction of diabetes risk,10–12,15,16 multiple interventions addressed only one of these domains,33–35,37,38 and some studies reported using few or no behavior change strategies. Future interventions should target both PA and nutrition using empirically supported strategies (e.g., setting achievable goals, reducing barriers, increasing self-efficacy) to provide youth an optimal chance of realizing and maintaining difficult behavior change.
Sample Heterogeneity
A challenge in the detection of intervention effects on glucose regulation in pediatric populations is sample heterogeneity in age, sex, and level of metabolic risk, as these factors affect adiposity and glucose metabolism in complex ways. Most samples included children of both sexes who spanned multiple Tanner stages. Age and pubertal stage can affect outcomes through physiological and behavioral pathways,1,26 including increases in growth hormone secretion and changes in adiposity,1 and these may differ by sex. Growth hormones affect both adiposity and glucose regulation, and adiposity is associated with increased fasting insulin and insulin resistance.1 Insulin resistance varies with pubertal level or Tanner stage, increasing roughly 25%–50% during early to mid puberty and returning to normal levels at the end of pubertal development.26 This timeline has been shown to vary by sex,46 with serum insulin concentrations peaking at earlier ages and remaining higher in females than males prior to age 18 years. Eight of the reviewed studies measured Tanner stage, but only three controlled for it in analyses30,33,36 and none stratified analyses by stage. Further, few studies presented sex differences in outcomes.30,39,44 Because of fluctuations in insulin resistance that occur during pubertal ages,26,47 such as transient decline in insulin sensitivity,48 sex and age/pubertal stage should be controlled for in analyses and considered in interpretation of intervention effects on glucose regulation. Including adiposity as a covariate, on the other hand, as was done by four author groups33,35,36,39 may be over controlling, as changes in adiposity may represent an intermediate stage in the causal pathway through which lifestyle interventions lead to changes in glucose metabolism.
Regarding health behaviors, older adolescents are likely to have autonomy with PA and dietary intake, whereas younger children and adolescents rely heavily on caregivers for facilitation of activities and food.49,50 A lifestyle intervention focused on motivation, goal setting, and decision making may therefore have more immediate success in older adolescents, whereas an intervention that prioritizes caretaker engagement may have stronger effects in younger adolescents. Studies with samples that fall across the spectrum of increasing autonomy and do not explore age as a moderator or effect modifier, as many of the studies in this review, may have difficulty detecting effects that vary by age.
Participants exhibited varying levels of metabolic risk, including normal weight, overweight with no metabolic dysregulation, and overweight/obese plus metabolic dysregulation (e.g., low high-density lipoprotein cholesterol, triglyceridemia). As glucose dysregulation progresses gradually, and is often not observed at clinical levels until adulthood, post-intervention changes may be difficult to detect in adolescents with low duration of exposure to glucose dysregulation (and little resulting β cell damage).47,51 However, given that β cell decline is thought to progress more rapidly in youth than adults once dysregulation begins,47,52,53 early prevention and intervention efforts are critically important.
Outcome Selection and Measurement
Although it is possible that many of the interventions reviewed were simply not effective, or not of appropriate intensity to affect BMI or insulin resistance, the choice of outcome may also have obscured true intervention effects. Fasting glucose and fasting insulin, surrogate markers of insulin resistance, were the most commonly reported outcomes in the included studies. These variables are not consistently correlated with insulin sensitivity in adolescents, however.54,55 Fasting insulin has been shown to have low accuracy as a measure of insulin sensitivity, with correlations between fasting insulin and the gold standard euglycemic hyperinsulinemic clamp ranging from 0.42–0.91.54 Similarly, changes in insulin sensitivity detected by the FSIVGTT or OGTT after a lifestyle intervention for overweight youth were not detected by the HOMA-IR.55 The Insulin Resistance in Children Consensus Group56 recommends use of the clamp technique in intervention trials, names the FSIVGTT and steady-state plasma glucose methods as reliable and valid alternatives, and recommends fasting glucose and insulin only in large epidemiological studies.54,56 However, the clamp, FSIVGTT, and steady-state plasma glucose are time- and resource-intensive and burdensome for participants, as they require repeated intravenous blood sampling and testing done in a closely monitored clinic setting. As illustrated by the infrequent use of these measures in the trials in this review, the trade-off between validity of measure and feasibility/participant acceptability may not be worthwhile. Nonetheless, given that most youth will not develop clinical levels of diabetes pathology for many years, the lack of direct measurement of insulin sensitivity and other pathophysiological processes (e.g., insulin secretion, β cell function) may conceal or confound true intervention effects.
Despite trends of using either BMI z-score or BMI percentile for youth adiposity outcomes,57–59 multiple studies presented simple BMI without adjustment for age and sex. BMI z-scores and percentiles are generally preferred over raw BMI due to the fluctuations in adiposity and growth that differ by sex over the course of adolescence26,46,47,57 and because those measures are highly correlated with Dual-Energy X-ray Absorptiometry-measured adiposity60 and fat mass z-score change,61 though some researchers continue to report BMI without adjustment.62,63
Behavioral Outcomes
In the 11 studies that targeted dietary changes, outcomes were promising. Six of eight studies that measured dietary intake showed significant post-intervention increases in healthy eating. These outcomes are impressive considering the multiple domains of influence on adolescent eating behavior (e.g., school food availability, caregiver income and food provision, peer influences). Interventions appear to have been more successful in influencing renunciation of unhealthful foods (e.g., reduction in dietary sugar, sugary beverages, carbohydrates, or fat) than uptake of healthy foods (e.g., vegetables, fruit). All measurements were based on participant self-report, however, and social desirability bias may have affected responses.
Surprisingly, despite the focus on PA modification in all but one intervention,33 fewer than half of the studies measured daily or routine PA, and only one39 detected significant changes post-intervention. A small number of studies reported cardiovascular fitness level,30,38 or strength32,35 instead of PA, citing the lower reliability and validity of self-report measures of PA in children. Although objective measures of PA are preferable to self-report, omission of measurement of daily or routine PA is also problematic, as the presumption of lifestyle interventions is that dietary and PA changes will influence glucose metabolism. If PA is not measured, conclusions regarding the mechanism of action of the intervention are limited. Furthermore, sedentary behavior was not measured by any study in this review, but represents an important component of the adolescent activity spectrum. Because sedentary behavior is associated with elevated fasting insulin regardless of moderate to vigorous PA, if sedentary behavior is high, substantive changes in insulin resistance may not be observed even if PA is increasing. Investigators planning new interventions should strongly consider objective measurement of both PA and sedentary behavior via accelerometry.
Targeting and Tailoring
Despite targeting Hispanic/minority youth, few studies reported cultural tailoring or adaptation of intervention content to improve engagement. Only four studies30,33,40,41 described culturally-informed adaptation, and in most cases this was limited in scope, for example, incorporation of Mexican food recommendations and recipes,40 or inclusion of “bilingual culturally and contextually relevant themes.”30 Only one intervention was developed through a community-based participatory research approach that included Hispanic community members,41 and only one intervention incorporated youth feedback in creation of intervention content.44 An obvious area for improvement of intervention salience and engagement is to incorporate members of the target demographic in intervention development processes, and provide delivery modalities tailored to youth, such as mobile applications.
Limitations
This is the first systematic review to synthesize outcomes from diabetes prevention interventions targeting Hispanic youth. PRISMA guidelines were followed throughout the review process from literature search to reporting of results. A comprehensive search strategy was utilized across multiple databases, and data quality was assessed via the rigorous, structured EPHPP protocol. A notable strength of the review is the quality of data included; all evidence was moderate to strong. Limitations include the inclusion of uncontrolled trials and trials with small sample sizes. As most interventions were pilot or exploratory in nature, many were not powered to detect the clinical outcomes examined. In addition, interventions with null results may not have been included due to publication bias. Lastly, results may not be generalizable to all heterogeneous groups of Hispanic youth. Though most studies did not specify Hispanic heritage or family nationality, the majority of studies were conducted in southern California and likely involved youth of primarily Mexican background.
Conclusions
Given the heterogeneity of intervention content, lack of stratification by sex and pubertal development level, deficits in power, and small sample sizes of interventions to date, the effectiveness of lifestyle-based diabetes prevention programs for Hispanic youth has not been conclusively demonstrated. The completion of 15 lifestyle interventions in Hispanic youth lends evidence for the feasibility and relevance of targeted interventions. Nonetheless, high attrition rates indicate a need for improved tailoring and engagement strategies, recognition and reduction of barriers to completion, and novel delivery modalities. Future interventions should assess participant engagement, solicit feedback about barriers and facilitators of participation, and publish these results. New interventions would be served by employing socioecological and systems-based approaches that recognize the multiple domains of influence on adolescent behavior (e.g., family, school, peer, community). Given the prevalence of Internet and mobile device use among adolescents (92% of teens go online daily; 71% of Hispanic teens have access to a smartphone),64 intervention designers should consider innovative mobile and online delivery formats with strategic elements that boost user engagement.
Supplementary Material
Acknowledgments
Authors Jessica L. McCurley and Margaret A. Crawford were supported during the preparation of this article by a research and training grant from NIH/National Heart, Lung, and Blood Institute (5T32HL079891 − 06). Author Jessica L. McCurley was additionally supported by a fellowship from the Fogarty International Center and University of California Global Health Institute (R25 TW009343). Linda C. Gallo was supported by grants from NIH/National Institute of Nursing Research (1 R01 NR015754 − 02), NIH/National Diabetes, Digestive, and Kidney Institute (1 R01 DK106209 − 01; 1 R18 DK104250 − 02; P30 DK111022 − 01), and NIH/National Center for Research Resources (5 ULI TR001114 − 04).
Footnotes
No financial disclosures were reported by the authors of this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pinhas-Hamiel O, Zeitler P. Acute and chronic complications of type 2 diabetes mellitus in children and adolescents. Lancet. 2007;369(9575):1823–1831. doi: 10.1016/S0140-6736(07)60821-6. https://doi.org/10.1016/S0140-6736(07)60821–6. [DOI] [PubMed] [Google Scholar]
- 2.Isasi CR, Parrinello CM, Ayala GX, et al. Sex Differences in Cardiometabolic Risk Factors among Hispanic/Latino Youth. J Pediatr. 2016;176:121–127. doi: 10.1016/j.jpeds.2016.05.037. https://doi.org/10.1016/j.jpeds.2016.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dabelea D, Mayer-Davis EJ, Saydah S, et al. Prevalence of type 1 and type 2 diabetes among children and adolescents from 2001 to 2009. JAMA. 2014;311(17):1778–1786. doi: 10.1001/jama.2014.3201. https://doi.org/10.1001/jama.2014.3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ogden CL, Carroll MD, Lawman HG, et al. Trends in Obesity Prevalence Among Children and Adolescents in the United States, 1988-1994 Through 2013-2014. JAMA. 2016;315(21):2292–2299. doi: 10.1001/jama.2016.6361. https://doi.org/10.1001/jama.2016.6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnson WD, Kroon JJM, Greenway FL, Bouchard C, Ryan DH, Katzmarzyk PT. Prevalence of risk factors for metabolic syndrome in adolescents: National Health and Nutrition Examination Survey, 2001-2006. Arch Pediatr Adolesc Med. 2009;163(4):373–377. doi: 10.1001/archpediatrics.2009.3. https://doi.org/10.1001/archpediatrics.2009.3. [DOI] [PubMed] [Google Scholar]
- 6.Goran M, Bergman RN, Cruz ML, Watanabe R. Insulin Resistance and Associated Compensatory Responses in African-American and Hispanic Children. Diabetes Care. 2002;25(12):2184–2190. doi: 10.2337/diacare.25.12.2184. https://doi.org/10.2337/diacare.25.12.2184. [DOI] [PubMed] [Google Scholar]
- 7.U.S. CDC. National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States. 2014 www.cdc.gov/diabetes/pubs/statsreport14/national-diabetes-report-web.pdf.
- 8.Lanting LC, Joung IM, Mackenbach JP, Lamberts SW, Bootsma AH. Ethnic differences in mortality, end-stage complications, and quality of care among diabetic patients: a review. Diabetes Care. 2005;28(9):2280–2288. doi: 10.2337/diacare.28.9.2280. https://doi.org/10.2337/diacare.28.9.2280. [DOI] [PubMed] [Google Scholar]
- 9.American Diabetes Association. Executive summary: Standards of medical care in diabetes--2014. Diabetes Care. 2014;37(Suppl 1):S5–13. doi: 10.2337/dc14-S005. https://doi.org/10.2337/dc14-S005. [DOI] [PubMed] [Google Scholar]
- 10.Diabetes Prevention Program Research Group. 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet. 2009;374(9702):1677–1686. doi: 10.1016/S0140-6736(09)61457-4. https://doi.org/10.1016/S0140-6736(09)61457-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diabetes Prevention Program Research Group. Reduction in the Incidence of Type 2 Diabetes with lifestyle intervention or metformin. N Eng J Med. 2002;346(6):393–403. doi: 10.1056/NEJMoa012512. https://doi.org/10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tuomilehto J, Lindstrom J, Eriksson JG, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Eng J Med. 2001;344(18):1343–1350. doi: 10.1056/NEJM200105033441801. https://doi.org/10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- 13.Raman A, Ritchie LD, Lustig RH, Fitch MD, Hudes ML, Fleming SE. Insulin resistance is improved in overweight African American boys but not in girls following a one-year multidisciplinary community intervention program. J Pediatr Enocrinol Metab. 2010;23(1–2):109–120. doi: 10.1515/jpem.2010.23.1-2.109. https://doi.org/10.1515/jpem.2010.23.1-2.109. [DOI] [PubMed] [Google Scholar]
- 14.Burnet D, Plaut A, Courtney R, Chin MH. A practical model for preventing type 2 diabetes in minority youth. Diabetes Educ. 2002;28(5):779–795. doi: 10.1177/014572170202800519. https://doi.org/10.1177/014572170202800519. [DOI] [PubMed] [Google Scholar]
- 15.Savoye M, Nowicka P, Shaw M, et al. Long-term results of an obesity program in an ethnically diverse pediatric population. Pediatrics. 2011;127(3):402–410. doi: 10.1542/peds.2010-0697. https://doi.org/10.1542/peds.2010-0697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Savoye M, Caprio S, Dziura J, et al. Reversal of Early Abnormalities in Glucose Metabolism in Obese Youth: Results of an Intensive Lifestyle Randomized Controlled Trial. Diabetes Care. 2014;37(2):317–324. doi: 10.2337/dc13-1571. https://doi.org/10.2337/dc13-1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schoeppe S, Oliver M, Badland HM, Burke M, Duncan MJ. Recruitment and retention of children in behavioral health risk factor studies: REACH strategies. Int J Behav Med. 2014;21(5):794–803. doi: 10.1007/s12529-013-9347-5. https://doi.org/10.1007/s12529-013-9347-5. [DOI] [PubMed] [Google Scholar]
- 18.Smith KL, Straker LM, McManus A, Fenner AA. Barriers and enablers for participation in healthy lifestyle programs by adolescents who are overweight. BMC Pediatrics. 2014;14:53. doi: 10.1186/1471-2431-14-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nguyen TT, Jayadeva V, Cizza G, et al. Challenging recruitment of youth with type 2 diabetes into clinical trials. J Adolesc Health. 2014;54(3):247–254. doi: 10.1016/j.jadohealth.2013.08.017. https://doi.org/10.1016/j.jadohealth.2013.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oster NV, Welch V, Schild L, Gazmararian JA, Rask K, Spettell C. Differences in Self-Management Behaviors and Use of Preventive Services among Diabetes Management Enrollees by Race and Ethnicity. Dis Manag. 2006;9(3):167–175. doi: 10.1089/dis.2006.9.167. https://doi.org/10.1089/dis.2006.9.167. [DOI] [PubMed] [Google Scholar]
- 21.Onwudiwe NC, Mullins D, Winston RA, et al. Barriers to self-management of diabetes: A qualitative study among low-income minority diabetics. Ethn Dis. 2011;21:27–32. [PubMed] [Google Scholar]
- 22.Zeh P, Sandhu HK, Cannaby AM, Sturt JA. Cultural barriers impeding ethnic minority groups from accessing effective diabetes care services: a systematic review of observational studies. Divers Equal Health Care. 2014;11(1):9–33. [Google Scholar]
- 23.Branscum P, Sharma M. A systematic analysis of childhood obesity prevention interventions targeting Hispanic children: lessons learned from the previous decade. Obes Rev. 2011;12(5):e151–158. doi: 10.1111/j.1467-789X.2010.00809.x. https://doi.org/10.1111/j.1467-789X.2010.00809.x. [DOI] [PubMed] [Google Scholar]
- 24.Vivian EM. Strategies and considerations for community-based participatory research in the prevention of type 2 diabetes in youth. Diabetes Spectr. 2010;23(4):213–215. https://doi.org/10.2337/diaspect.23.4.213. [Google Scholar]
- 25.Wang Y, Tussing L. Culturally appropriate approaches are needed to reduce ethnic disparity in childhood obesity. J Am Diet Assoc. 2004;104(11):1664–1666. doi: 10.1016/j.jada.2004.08.035. https://doi.org/10.1016/j.jada.2004.08.035. [DOI] [PubMed] [Google Scholar]
- 26.Goran MI, Gower BA. Longitudinal study on pubertal insulin resistance. Diabetes. 2001;50(11):2444–2450. doi: 10.2337/diabetes.50.11.2444. https://doi.org/10.2337/diabetes.50.11.2444. [DOI] [PubMed] [Google Scholar]
- 27.Bluher S, Molz E, Wiegand S, et al. Body mass index, waist circumference, and waist-to-height ratio as predictors of cardiometabolic risk in childhood obesity depending on pubertal development. J Clin Endocr Metab. 2013;98(8):3384–3393. doi: 10.1210/jc.2013-1389. https://doi.org/10.1210/jc.2013-1389. [DOI] [PubMed] [Google Scholar]
- 28.Moher D, Liberati A, Tetzlaff J, Altman DG PRISMA Group. Preferred reporting items for systematic reviews and meta-anlayses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. https://doi.org/10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cochrane Collaboration. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. 2011 http://handbook.cochrane.org.
- 30.Trevino RP, Yin Z, Hernandez A, Hale DE, Garcia OA, Mobley C. Impact of the Bienestar school-based diabetes mellitus prevention program on fasting capillary glucose levels: a randomized controlled trial. Arch Pediatr Adolesc Med. 2004;158(9):911–917. doi: 10.1001/archpedi.158.9.911. https://doi.org/10.1001/archpedi.158.9.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Foster GD, Linder B, Baranowski T, et al. A school-based intervention for diabetes risk reduction. N Engl J Med. 2010;363(5):443–453. doi: 10.1056/NEJMoa1001933. https://doi.org/10.1056/NEJMoa1001933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Davis JN, Kelly LA, Lane CJ, et al. Randomized control trial to improve adiposity and insulin resistance in overweight Latino adolescents. Obesity. 2009;17(8):1542–1548. doi: 10.1038/oby.2009.19. https://doi.org/10.1038/oby.2009.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Davis JN, Ventura EE, Alexander KE, et al. Feasibility of a home-based versus classroom-based nutrition intervention to reduce obesity and type 2 diabetes in Latino youth. Int J Pediatr Obes. 2007;2(1):22–30. doi: 10.1080/17477160601133077. https://doi.org/10.1080/17477160601133077. [DOI] [PubMed] [Google Scholar]
- 34.Kelly LA, Loza A, Lin X, et al. The effect of a home-based strength training program on type 2 diabetes risk in obese Latino boys. J Pediatr Endocr Metab. 2015;28(3–4):315–322. doi: 10.1515/jpem-2014-0470. https://doi.org/10.1515/jpem-2014-0470. [DOI] [PubMed] [Google Scholar]
- 35.Shaibi GQ, Cruz ML, Ball GDC, et al. Effects of resistance training on insulin sensitivity in overweight Latino adolescent males. Med Sci Sports Exerc. 2006;38(7):1208–1215. doi: 10.1249/01.mss.0000227304.88406.0f. https://doi.org/10.1249/01.mss.0000227304.88406.0f. [DOI] [PubMed] [Google Scholar]
- 36.Davis JN, Tung A, Chak SS, et al. Aerobic and strength training reduces adiposity in overweight Latina adolescents. Med Sci Sports Exerc. 2009;41(7):1494–1503. doi: 10.1249/MSS.0b013e31819b6aea. https://doi.org/10.1249/MSS.0b013e31819b6aea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Davis JN, Gyllenhammer LE, Vanni AA, et al. Startup circuit training program reduces metabolic risk in Latino adolescents. Med Sci Sports Exerc. 2011;43(11):2195–2203. doi: 10.1249/MSS.0b013e31821f5d4e. https://doi.org/10.1249/MSS.0b013e31821f5d4e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van der Heijden GJ, Wang ZJ, Chu ZD, et al. A 12-week aerobic exercise program reduces hepatic fat accumulation and insulin resistance in obese, Hispanic adolescents. Obesity. 2010;18(2):384–390. doi: 10.1038/oby.2009.274. https://doi.org/10.1038/oby.2009.274. [DOI] [PubMed] [Google Scholar]
- 39.Weigensberg MJ, Lane CJ, Avila Q, et al. Imagine HEALTH: results from a randomized pilot lifestyle intervention for obese Latino adolescents using Interactive Guided ImagerySM. BMC Complement Altern Med. 2014;14:28. doi: 10.1186/1472-6882-14-28. https://doi.org/10.1186/1472-6882-14-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Coleman KJ, Ocana LL, Walker C, et al. Outcomes from a culturally tailored diabetes prevention program in Hispanic families from a low-income school: Horton Hawks Stay Healthy (HHSH) Diabetes Educ. 2010;36(5):784–792. doi: 10.1177/0145721710377360. https://doi.org/10.1177/0145721710377360. [DOI] [PubMed] [Google Scholar]
- 41.Shaibi GQ, Greenwood-Ericksen MB, Chapman CR, Konopken Y, Ertl J. Development, implementation, and effects of community-based diabetes prevention program for obese Latino youth. J Prim Care Community Health. 2010;1(3):206–212. doi: 10.1177/2150131910377909. https://doi.org/10.1177/2150131910377909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rosenbaum M, Nonas C, Weil R, et al. School-based intervention acutely improves insulin sensitivity and decreases inflammatory markers and body fatness in junior high school students. J Clin Endocrin Metab. 2007;92(2):504–508. doi: 10.1210/jc.2006-1516. https://doi.org/10.1210/jc.2006-1516. [DOI] [PubMed] [Google Scholar]
- 43.Shaibi GQ, Konopken Y, Hoppin E, Keller CS, Ortega R, Castro FG. Effects of a culturally grounded community-based diabetes prevention program for obese Latino adolescents. Diabetes Educ. 2012;38(4):504–512. doi: 10.1177/0145721712446635. https://doi.org/10.1177/0145721712446635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Patrick K, Norman GJ, Davila EP, et al. Outcomes of a 12-month technology-based intervention to promote weight loss in adolescents at risk for type 2 diabetes. J Diabetes Sci Technol. 2013;7(3):759–770. doi: 10.1177/193229681300700322. https://doi.org/10.1177/193229681300700322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang X, Gregg EW, Williamson DF, et al. A1C level and future risk of diabetes: a systematic review. Diabetes Care. 2010;33(7):1665–1673. doi: 10.2337/dc09-1939. https://doi.org/10.2337/dc09-1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ford ES, Li C, Imperatore G, Cook S. Age, sex, and ethnic variations in serum insulin concenrtration among U.S. youth. Diabetes Care. 2006;29(12):2605–2611. doi: 10.2337/dc06-1083. https://doi.org/10.2337/dc06-1083. [DOI] [PubMed] [Google Scholar]
- 47.Nadeau KJ, Anderson BJ, Berg EG, et al. Youth-Onset Type 2 Diabetes Consensus Report: Current Status, Challenges, and Priorities. Diabetes Care. 2016;39(9):1635–1642. doi: 10.2337/dc16-1066. https://doi.org/10.2337/dc16-1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Goran MI, Shaibi GQ, Weigensberg MJ, Davis JN, Cruz ML. Deterioration of insulin sensitivity and beta-cell function in overweight Hispanic children during pubertal transition: A longitudinal assessment. Int J Pediatr Obes. 2006;1(3):139–145. doi: 10.1080/17477160600780423. https://doi.org/10.1080/17477160600780423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Poti JM, Popkin BM. Trends in energy intake among U.S. children by eating location and food source, 1977-2006. J Am Diet Assoc. 2011;111(8):1156–1164. doi: 10.1016/j.jada.2011.05.007. https://doi.org/10.1016/j.jada.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reicks M, Banna J, Cluskey M, et al. Influence of Parenting Practices on Eating Behaviors of Early Adolescents during Independent Eating Occasions: Implications for Obesity Prevention. Nutrients. 2015;7(10):8783–8801. doi: 10.3390/nu7105431. https://doi.org/10.3390/nu7105431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nolan CJ, Damm P, Prentki M. Type 2 diabetes across generations: from pathophysiology to prevention and management. Lancet. 2011;378(9786):169–181. doi: 10.1016/S0140-6736(11)60614-4. https://doi.org/10.1016/S0140-6736(11)60614-4. [DOI] [PubMed] [Google Scholar]
- 52.Rascati K, Richards K, Lopez D, Cheng LI, Wilson J. Progression to insulin for patients with diabetes mellitus on dual oral antidiabetic therapy using the U.S. Department of Defense Database. Diabetes Obes Metab. 2013;15(10):901–905. doi: 10.1111/dom.12103. https://doi.org/10.1111/dom.12103. [DOI] [PubMed] [Google Scholar]
- 53.Kahn SE, Lachin JM, Zinman B, et al. Effects of rosiglitazone, glyburide, and metformin on beta-cell function and insulin sensitivity in ADOPT. Diabetes. 2011;60(5):1552–1560. doi: 10.2337/db10-1392. https://doi.org/10.2337/db10-1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schwartz B, Jacobs DR, Jr, Moran A, Steinberger J, Hong CP, Sinaiko AR. Measurement of insulin sensitivity in children: comparison between the euglycemic-hyperinsulinemic clamp and surrogate measures. Diabetes Care. 2008;31(4):783–788. doi: 10.2337/dc07-1376. https://doi.org/10.2337/dc07-1376. [DOI] [PubMed] [Google Scholar]
- 55.Ortega R, Hoppin E, Konopken Y, Ryder J, Shaibi GQ. Fitness, Activity, And Adherence In A Diabetes Prevention Program For Overweight And Obese Latino Youth. Med Sci Sports Exer. 2011;43(5):902–903. https://doi.org/10.1249/01.MSS.0000402522.37391.3b. [Google Scholar]
- 56.Levy-Marchal C, Arslanian S, Cutfield W, et al. Insulin resistance in children: Consensus, perspective, and future directions. J Clin Endocrinol Metab. 2010;95(12):5189–5198. doi: 10.1210/jc.2010-1047. https://doi.org/10.1210/jc.2010-1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Flegal KM, Ogden CL. Childhood obesity: are we all speaking the same language? Adv Nutr. 2011;2(2):159S–166S. doi: 10.3945/an.111.000307. https://doi.org/10.3945/an.111.000307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Smith LR, Chadwick P, Radley D, et al. Assessing the short-term outcomes of a communitybased intervention for overweight and obese children: The MEND 5-7 programme. BMJ Open. 2013;3(5):e002607. doi: 10.1136/bmjopen-2013-002607. https://doi.org/10.1136/bmjopen-2013-002607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Robertson W, Fleming J, Kamal A, et al. Randomised controlled trial evaluating the effectiveness and cost-effectiveness of ‘Families for Health’, a family-based childhood obesity treatment intervention delivered in a community setting for ages 6 to 11 years. Health Technol Assess. 2017;21(1):1–180. doi: 10.3310/hta21010. https://doi.org/10.3310/hta21010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mei Z, Grummer-Strawn LM, Pietrobelli A, Goulding A, Goran M, Dietz WH. Validity of body mass index compared with other body-composition screening indexes for the assessment of body fatness in children and adolescents. Am J Clin Nutr. 2002;75(6):978–985. doi: 10.1093/ajcn/75.6.978. [DOI] [PubMed] [Google Scholar]
- 61.Kakinami L, Henderson M, Chiolero A, Cole TJ, Paradis G. Identifying the best body mass index metric to assess adiposity change in children. Arch Dis Child. 2014;99(11):1020–1024. doi: 10.1136/archdischild-2013-305163. https://doi.org/10.1136/archdischild-2013-305163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cole TJ, Faith MS, Pietrobelli A, Heo M. What is the best measure of adiposity change in growing children: BMI, BMI %, BMI z-score or BMI centile? Eur J Clin Nutr. 2005;59(3):419–425. doi: 10.1038/sj.ejcn.1602090. https://doi.org/10.1038/sj.ejcn.1602090. [DOI] [PubMed] [Google Scholar]
- 63.Field AE, Laird N, Steinberg E, Fallon E, Semega-Janneh M, Yanovski JA. Which Metric of Relative Weight Best Captures Body Fatness in Children? Obes Res. 2003;11(11):1345–1352. doi: 10.1038/oby.2003.182. https://doi.org/10.1038/oby.2003.182. [DOI] [PubMed] [Google Scholar]
- 64.Lenhart A. Pew Research Center; 2015. Teens, social media, and technology: Overview 2015. www.pewinternet.org/2015/04/09/teens-social-media-technology-2015/ [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


