Abstract
Introduction
This study investigated associations between maternal prepregnancy BMI and child behaviors at ages 9–11 years and examine interaction by race and gender.
Methods
The National Longitudinal Survey of Youth and the Children and Young Adults surveys are U.S.-based, ongoing longitudinal studies, initiated in 1979 and 1986, respectively. Mothers (n=2,952) reported pregnancy and child (n=5,660) developmental information at multiple time points. Child total, internalizing, and externalizing problems at ages 9–11 years were assessed using the Behavior Problems Index (BPI), collected biennially until 2012. Associations between prepregnancy BMI and child BPI outcomes were examined, as well as two- and three-way interactions by race and gender. Analyses were conducted in 2017.
Results
Boys whose mothers had higher prepregnancy weights exhibited higher total BPI and externalizing scores at ages 9–11 years versus those with normal-weight mothers. Boys with severely obese mothers had higher total BPI (mean difference=7.99, 95% CI=3.53, 12.46) and externalizing (mean difference=5.77, 95% CI=1.50, 10.04) scores. Prepregnancy underweight was associated with boys’ higher total BPI (mean difference=2.34, 95% CI=0.02, 4.66) and externalizing (mean difference=3.30, 95% CI=0.69, 5.91); these associations were not significant in sensitivity analyses. No associations emerged for girls or internalizing problems. Two-way interactions by race and three-way interactions by race and gender were not significant.
Conclusions
Maternal prepregnancy weight was associated with BPI level among boys. Boys with severely obese mothers exhibited markedly higher behavioral problems at ages 9–11 years versus those with normal-weight mothers, regardless of race. Maintaining healthy prepregnancy weight may be important for preventing boys’ deleterious behavior outcomes in middle childhood.
INTRODUCTION
The prevalence of both maternal obesity and child neurodevelopmental problems has increased in the U.S.1,2 Maternal weight, before and during pregnancy, is linked to child behavior. Two recent reviews support an association between high maternal weight and a range of neurodevelopmental and psychiatric problems, including cognitive deficits, attention deficit hyperactivity disorder, and internalizing problems.3,4
Many studies suggest that maternal prepregnancy obesity is associated with children’s externalizing problems, including attention deficit hyperactivity disorder.5–9 Less attention has focused on internalizing problems, (e.g., depression), but mixed evidence points to a similar association.10–13 Several gaps and challenges exist in the literature, however. First, comparing results is challenging because studies often adjust for different sets of covariates, raising questions about whether associations are the result of confounding due to unmeasured covariates.14 Second, although maternal underweight before or during pregnancy could influence children’s behaviors,15,16 through its associations with pregnancy complications including preterm birth,17 most attention has been focused on maternal obesity. Third, only one known study examined the modifying roles of both ethnicity and gender.18 Finally, many studies focus on young children, and do not capture the emergence of behavior problems later in childhood.3,4
To the authors’ knowledge, no studies of maternal BMI and child neurodevelopment have examined effect modification by race and gender simultaneously (three-way interaction). A study of children aged 10 years born to high-risk women suggested an association between maternal obesity and child behavior, with no effect modification by race or gender.18 By contrast, using the U.S. National Longitudinal Survey of Youth 1979 (NLSY79), Tanda et al.12 concluded that overweight and obese white, but not African American women, are at elevated risk for having children with behavioral problems at ages 8–9 years.12
The present study also uses the NLSY79 to investigate whether maternal prepregnancy BMI is associated with behavioral problems among school-age children. Effect modification is examined by race or gender, as well as by race and gender simultaneously. Because early puberty is a time when behavioral problems emerge, this study focuses on children aged 9–11 years. Potentially important covariates that often have been excluded from previous studies are included in the analysis.
METHODS
Study Population
The NLSY79 recruited participants using a multistage sampling design, and the weighted data are representative of the U.S. population in 1979. This analysis included female NLSY79 cohort participants (N=4,932) and their biological children, who were studied between 1986 and 2012 as part of the NLSY Children and Young Adults (NLSYCYA) cohort (N=11,512). Study details for both cohorts are available.19,20
Children were included if their mothers reported their prepregnancy weight and height and had complete questionnaire data at ages 9–11 years for the Behavior Problems Index (BPI) composite measures described below. Given previous NLSYCYA research showing black–white differences,12 only respondents classified as non-Hispanic black or non-Hispanic, non-black (the latter is 88% white) were included.21 Hispanic participants (n=1,168) were not included because cell sizes were too small to obtain reliable estimates for interaction by ethnicity. For example, there were only six Hispanic boys and 17 Hispanic girls whose mothers had severe (Class II/III) prepregnancy obesity. Also, this group was heterogeneous (69% Mexican or Mexican American, 15% Puerto Rican, and 6% other Hispanic, 4% Chicano, and 4% Cuban), limiting its generalizability. The Committee for Protection of Human Subjects at the University of California, Berkeley approved this study.
Measures
The NLSY79 women reported their heights in 1985, which were regression calibrated to National Health and Nutrition Examination Survey data.22 Prepregnancy weight was recalled in 1986 for prior pregnancies and, after 1986, reported at the interview following each pregnancy. Prepregnancy BMI (kg/m2) was categorized as: underweight (BMI <18.5), normal weight (BMI=18.5–24.9), overweight (BMI=25.0–29.9), obese Class I (BMI=30.0–34.9), and obese Classes II/III (BMI ≥35).23
In the NLSYCYA, behavioral problems were assessed biennially for children aged 4–14 years using maternal report of the BPI, a widely used 28–item questionnaire, to determine whether they exhibited specific behaviors in the past 3 months.24,25 The BPI was originally developed using items from the Child Behavior Checklist that were determined to be common in the population and that discriminated between children who were and were not receiving psychological services.26 Responses were often true, sometimes true, and not true. Raw scores were calculated for total problems and two composite measures (externalizing and internalizing problems) based on Child Behavior Checklist guidelines.27 Scores were computed for children with complete BPI data. Standardized scores were created (mean=100, SD=15) using population-based age and gender norms from the Child Health Supplement of the National Health Interview Survey 1981 data.28 Higher scores indicated worse problems. The reliability of the total BPI (α=0.90) was similar to past studies (α=0.89).26
Covariates were selected a priori, taking previous research and temporal ordering into account, and included maternal Armed Forces Qualification Test score (<33%, 33%–66%, >66%; a proxy for IQ), parity (nulliparous, parous), and, at delivery: maternal age (≤19 years, 20–29 years, ≥30 years), education (<12 years, 12–15 years, ≥16 years), marital status (married, unmarried), employment status (full-time, part-time, unemployed), decade of birth (1970–1979, 1980–1989, 1990–1999, ≥2000) and annual equivalized household income29 in year 2000 dollars. Maternal race and child gender were potential effect modifiers.
Statistical Analysis
Analyses were conducted in 2017 using R, version 3.2.2 and Stata, version 14.0.30 Sample characteristics were examined stratified by prepregnancy BMI. Rao–Scott31 chi-square tests and linear regression models were used to test for differences in covariate distributions by exposure. Linear regression analyses were conducted to estimate crude mean differences in BPI total and composite scores between prepregnancy BMI categories in the full sample, as well as in gender subsets when interactions with gender were significant. Main effects were significant at p<0.05 and interactions at p<0.10. Multivariate linear regression models were used to estimate mean BPI differences compared to children of mothers of normal prepregnancy weight, adjusted for covariates.32 In one set of models, Wald tests were used to assess gender by BMI interactions. A second set of models was estimated with race by BMI interactions. Three-way interactions (prepregnancy BMI X race X gender) were assessed in a final set of models. All regression models were weighted. Robust SEs were calculated to account for NLSY79’s complex survey design, as well as within-family correlation.
To address covariate missingness (0%–22.1%; median, 3.4%), multiple imputation by chained equations was used for multivariate models.33 The imputation model included BPI measures, prepregnancy BMI, covariates, and quintiles of the sampling weights to reduce bias from the sampling design.34 Fifty data sets were imputed using a 50-step iterative process, and estimates, variances, and test statistics were pooled.
Because the sample consisted in part of siblings, a sensitivity analysis was conducted in the sibling subsample (n=1,823 mothers, 4,531 children) using fixed effects regression to control for unmeasured shared familial characteristics. These models consider maternal- and family-level factors that are constant across a mother’s pregnancies, such as genetic and some environmental factors. Implicit in this approach, only the children of mothers who changed BMI categories between pregnancies (n=672 mothers, 1,794 children) contributed to the estimates of the associations. SEs were adjusted for clustering at the highest level of sampling.
RESULTS
Table 1 describes characteristics for the 5,660 mother–child dyads, stratified by prepregnancy BMI and accounting for clustered sampling and oversampling of blacks and low-income whites. Total raw percentages are included to illustrate the ethnic diversity of the unweighted sample.
Table 1.
Maternal and Child Characteristics of 5,660 Mother-Child Dyads in the NLSYCYA Cohort Ages 9–11 Years by Maternal Prepregnancy BMI
| Characteristics | Total (raw) | Total (weighted) (n=5660) | Under weight (n=518) | Normal weight (n=3708) | Over weight (n=927) | Obese I (n=330) | Obese II/III (n=177) | p-value |
|---|---|---|---|---|---|---|---|---|
| Total | 8.2 | 65.8 | 16.5 | 6.0 | 3.5 | |||
| Maternal characteristics | ||||||||
| Race/ethnicity, % | 0.235 | |||||||
| White/other | 63.9 | 81.4 | 81.1 | 82.2 | 79.3 | 79.1 | 79.2 | |
| Black | 36.1 | 18.6 | 18.9 | 17.8 | 20.7 | 20.9 | 20.8 | |
| Maternal education,a % | <0.001 | |||||||
| <12 years | 18.8 | 14.0 | 25.6 | 13.3 | 12.0 | 13.0 | 13.4 | |
| 12–15 years | 44.4 | 43.3 | 47.0 | 43.1 | 43.0 | 41.5 | 43.7 | |
| ≥16 years | 36.8 | 42.7 | 27.4 | 43.6 | 44.9 | 45.6 | 42.9 | |
| Maternal employment,a % | <0.01 | |||||||
| Unemployed | 38.5 | 33.3 | 42.1 | 32.3 | 33.7 | 31.0 | 34.5 | |
| Part-time employment | 27.8 | 28.2 | 32.2 | 28.8 | 26.1 | 27.1 | 21.4 | |
| Employed | 33.7 | 38.5 | 25.7 | 38.9 | 40.2 | 41.9 | 44.2 | |
| Married at birth year,a % | 65.7 | 75.7% | 65.3 | 76.4 | 79.1 | 74.4 | 71.5 | <0.01 |
| Age at birth, % | ||||||||
| <20 years | 19.9 | 12.9 | 28.0 | 14.0 | 7.3 | 3.0 | 0.8 | <0.001 |
| 20–29 years | 57.3 | 55.8 | 55.2 | 56.6 | 56.3 | 52.1 | 46.9 | |
| ≥30 years | 22.8 | 31.3 | 16.9 | 29.5 | 36.5 | 44.9 | 52.4 | |
| Parous, % | 54.1 | 55.7% | 48.1 | 52.9 | 65.0 | 67.7 | 60.5 | <0.001 |
| AFQT score percentilea, mean (SD) | 40.1 (28.2) | 48.5 (28.4) | 39.8 (26.6) | 49.6 (28.4) | 49.3 (28.6) | 47.3 (27.1) | 46.0 (28.7) | <0.01 |
| Family background | ||||||||
| Income at birth year,a,b mean (SD) | 9.6 (1.3) | 9.9 (1.2) | 9.5 (1.3) | 9.9 (1.2) | 9.9 (1.0) | 9.9 (0.9) | 9.9 (1.0) | <0.001 |
| Child characteristics | ||||||||
| Female, % | 50.0 | 49.2% | 48.2 | 49.3 | 48.0 | 50.0 | 53.0 | 0.810 |
| Age, % | 0.542 | |||||||
| 60–71 months | 8.3 | 7.5 | 8.2 | 8.0 | 5.9 | 6.2 | 6.0 | |
| 72–83 months | 44.9 | 44.7 | 41.4 | 44.4 | 45.9 | 48.3 | 46.3 | |
| 84–95 months | 46.8 | 47.8 | 50.4 | 47.6 | 48.2 | 45.4 | 47.7 | |
| Year of birth, % | <0.001 | |||||||
| 1970–1979 | 16.9 | 10.6 | 20.4 | 11.8 | 6.1 | 2.4 | 0.2 | |
| 1980–1989 | 54.8 | 51.0 | 57.2 | 52.5 | 48.2 | 42.1 | 34.4 | |
| 1990–1999 | 26.7 | 36.3 | 21.9 | 34.1 | 42.6 | 49.8 | 58.0 | |
| 2000–2009 | 1.7 | 2.2 | 0.4 | 1.6 | 3.1 | 5.6 | 7.4 | |
| Behavior | ||||||||
| Total BPI score, mean (SD) | 106.6 (16.2) | 105.5 (15.7) | 107.2 (15.9) | 105.0 (15.4) | 105.9 (15.7) | 105.8 (15.6) | 109.3 (19.3) | 0.104 |
| Externalizing BPI score, mean (SD) | 110.6 (16.8) | 109.5 (16.4) | 111.8 (16.8) | 108.8 (16.1) | 109.9 (16.4) | 110.8 (17.5) | 111.4 (18.1) | <0.05 |
| Internalizing BPI score, mean (SD) | 108.2 (15.9) | 107.9 (15.7) | 108.7 (16.0) | 107.8 (15.5) | 107.6 (15.8) | 108.1 (16.1) | 110.3 (17.3) | 0.711 |
Notes: Boldface indicates statistical significance (p<0.05).
Missingness in these variables ranged from 3.4%–22.1%
Equivalized family income in year 2000 dollars
AFQT; Armed Forces Qualifying Test; BPI, Behavior Problems Index; NLSYCYA, National Longitudinal Survey of Youth Children/Young Adults
Sixty-six percent of the mothers were normal weight, 8% underweight, and 10% obese, of whom 3.5% were Class II/III. Maternal characteristics varied by prepregnancy BMI. More underweight mothers gave birth in the 1980s, whereas more obese Class II/III women gave birth in the 1990s, reflecting increases in BMI with maternal age and parity. Underweight women were younger, less likely to be married, and had the lowest education, income, and Armed Forces Qualifying Test scores (Table 1).
Complete data on exposure and outcome were available for 74% of the eligible sample. The analyzed sample included younger (13% vs 10% aged <20 years, p<0.001), nulliparous (44% vs 34%, p<0.001), and employed (33% vs 38%, p=0.011) mothers and fewer children born after 1990 (39% vs 50%, p<0.001) than those excluded. Other characteristics were similar.
In unadjusted analyses in the full sample, maternal prepregnancy underweight was related to higher total BPI and externalizing scores (not shown). These associations differed between boys and girls, such that maternal Class II/III obesity was significantly associated with total BPI and externalizing among boys, but not girls (Appendix Table 1). There were no significant associations with internalizing problems.
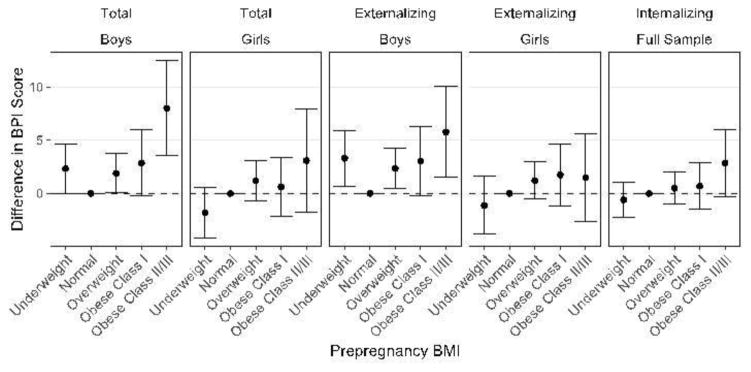
In the adjusted models for the full sample, prepregnancy BMI by gender interactions for total and externalizing scales were significant (p=0.07, 0.09, respectively). There were no associations between prepregnancy BMI and internalizing scores, and no two-way interaction with gender (p=0.36). Figure 1 shows adjusted results stratified by gender for total and externalizing outcomes. Children of the same gender whose mothers had normal prepregnancy BMI were the reference category. Among boys, higher prepregnancy BMI was associated with poorer total and externalizing scores; total scores were 7.99 points higher (95% CI=3.53, 12.46) and externalizing scores 5.77 points higher (95% CI=1.50, 10.04) in sons of mothers who were obese Class II/III prepregnancy compared with the reference group. Boys whose mothers were underweight also had increased total (2.34, 95% CI=0.02, 4.66) and externalizing (3.30, 95% CI=0.69, 5.91) scores. There were no significant associations for girls. As there was no gender interaction for internalizing, the graph is not stratified by gender for this outcome.
Figure 1.
Adjusted mean differences in BPI score associated with prepregnancy BMI in NLSYCYA children stratified by gender.
p-values for interaction: 0.07 (total problems); 0.09 (externalizing problems)
BPI, Behavior Problems Index; NLSYCYA, National Longitudinal Survey of Youth Children/Young Adults
There were no two-way interactions with race/ethnicity for total, externalizing, and internalizing problems (p=0.70, 0.81, 0.87, respectively), nor were there three-way interactions (p=0.16, 0.29, 0.31).
Fixed-effects sensitivity analysis was conducted to address the fact that the study included some siblings in the same family. Results suggested associations with maternal Class II/III obesity in boys that were slightly larger in magnitude compared with those from the standard multivariate analysis (Appendix Table 2). Total problems scores increased 10.56 points and 10.74 points in sons of women with Class II/III obesity prepregnancy, accounting for characteristics shared by siblings. However, estimates were marginally significant (p<0.10), likely because of reduced power in this smaller sample. The associations with prepregnancy underweight were similar in magnitude to those from standard analysis, but were no longer significant for total and externalizing problems (p=0.480, 0.207, respectively).
DISCUSSION
In this large sample of black and white mothers and their children aged 9–11 years, severe maternal prepregnancy obesity was associated with higher total and externalizing behavior problems for boys, but not girls. The heavier mothers were when they entered pregnancy, the higher the risk for behavior problem outcomes for their sons. Maternal prepregnancy underweight was also associated with higher total and externalizing problems for boys, although this finding was attenuated and not significant in the fixed-effects analysis, and requires replication. There were no significant associations for girls or for internalizing problems, and there were no differences across race. This study is the first to document gender differences in these associations, and one of a handful of studies to show that prepregnancy underweight, in addition to obesity, may be problematic.15,16
The finding that boys are at special risk adds to previous literature linking maternal prepregnancy weight with children’s externalizing problems. A study of three Nordic cohorts (N=12,566) suggested that prepregnancy overweight/obesity was associated with teacher-rated attention deficit hyperactivity disorder symptoms at ages 7–12 years,8 but did not test for gender differences. Similarly, a U.S. study of 1,311 mother–child pairs showed that Class II/III prepregnancy obesity was associated with psychosocial problems at age 6 years,35 but children were not followed to older ages, the sample comprised predominantly white families (87%), and interaction by gender was not examined. A recent study of 511 high-risk black and white Pennsylvania women reported that maternal obesity was associated with behavioral and emotional problems at age 10 years, but found no differences by race or gender.18 The current findings build on this work by revealing gender differences, and showing risk associated with maternal prepregnancy underweight.
Tanda and colleagues12 also analyzed school-aged children in the NLSY cohorts, and concluded that there was an association between prepregnancy obesity and BPI outcomes at ages 8–9 years among white, but not black, children.12 By contrast, the current study suggested a significant two-way interaction with gender but not by race. There are several possible explanations for differences in results between these two analyses of the NLSY79 cohort. First, the current study included a larger sample (5,660 vs 3,395) because multiple imputation was used to resolve missing data issues rather than omitting participants, and preterm and low birth weight children were not excluded. The age groups were not identical as the current study focused on slightly older children. In addition, the previous study included birth weight, gestational age, and child’s weight status as covariates, whereas these were excluded from the current analyses because they are potential mediators on the causal path. A unique aspect of this study was the use of fixed effects analysis to confirm that results were not solely attributable to unmeasured confounding among siblings. Therefore, although both studies suggest an association between maternal obesity and child development in the NLSY79 and NLSYCYA cohorts, these differences in study methods may explain differences in results.
Research suggests that exposures in utero are associated with poor neurocognitive, behavioral, and emotional outcomes in offspring.36–40 Prepregnancy obesity appears to confer negative effects for a developing fetus. Few studies, however, have focused on factors that mediate associations between prepregnancy weight and children’s behaviors. Obesity during pregnancy creates inflammation and causes metabolic changes that may affect gene expression and alter a fetus’s developing brain.41–44 In addition, lifestyle or behavioral factors associated with maternal obesity (e.g., prenatal diet, substance abuse, breastfeeding) or psychosocial factors (e.g., maternal mental health, stress) may influence child development.45,46 A recent review pointed to unhealthy diet during pregnancy and maternal distress as two leading risk factors for children’s poor neurodevelopment.47 One study tested several prepartum and postpartum mediators, including gestational weight gain, gestational diabetes, breastfeeding, postpartum depression, and birth weight, but none of these factors explained associations between maternal obesity and child outcomes.35 Research in this area is nascent, and future investigation is warranted.
In the current study, there was some evidence that maternal prepregnancy underweight—in addition to overweight—may be linked to boys’ problems, although this requires replication. Maternal underweight may reflect poor nutrition and stress during pregnancy.48 These factors may be linked to pregnancy behaviors, such as tobacco or other substance use or dieting behaviors, which also impact fetal development,15 and may continue after pregnancy, impacting children’s behaviors in early life and beyond. Unfortunately, these exposures were not well-measured in the NLSY, which precluded investigation into their roles as mechanisms. Future research that documents behaviors before, during, and after pregnancy among diverse samples and tracks child outcomes downstream is warranted. Finally, no known studies have investigated whether child BMI or pubertal status may mediate these associations downstream. This examination was beyond the current scope, but represents an important area for future research.
This study suggests gender differences in the association between maternal BMI and total behavior and externalizing problems. Metabolic exposures in utero may differentially influence a developing fetus. In studies of famine and nutrition, male fetuses consistently exhibit stronger risk due to undernutrition, and greater benefits from adequate nutrition, compared with girls.49–51 During times of stress, boys are subject to higher fetal death rates than girls.51,52 One suggested mechanism for stress-induced fetal programming of sex ratios is glucocorticoid exposure in utero because of maternal stress.51 Animal and human studies examining other environmental exposures also support sex differences in risk; for example, only male offspring appear to be at higher risk for behavior problems when exposed in utero to the chemical bisphenol A.53 As such, boys may be more susceptible to certain insults in utero.
Future research should examine whether the gender differences reported here for ages 9–11 years persist into adolescence or shift as children get older. In previous results from NLSY79, maternal prepregnancy overweight/obesity and excessive gestational weight gain predicted girls’ earlier menarche,54 which has been linked to poor behavioral and emotional outcomes in adolescence.55–58 Even though the current study did not show associations between maternal BMI and internalizing problems, adolescence is also a time when anxiety and depression become more prevalent, particularly among girls.59 Longer-term research may reveal gender differences at older ages, and therefore research examining pregnancy factors, adolescent outcomes, and gender is warranted.
Limitations
This study was observational; therefore, the possibility of unmeasured confounding remains. Pregnancy-related weight data relied on maternal self-report. However, studies have shown that self-reported and clinically assessed prepregnancy weights are highly correlated,7,60 and reliability between self-reported weights within 2 years of the pregnancy and prepregnancy weight has been shown previously in NLSY.61 Child outcomes also relied on maternal report, which may reflect biases that vary by race and gender. The study included siblings, and although fixed-effects models accounted for shared variance among siblings to the extent that confounders are consistent across pregnancies, three-way interactions could not be tested in these models because of small sample sizes. Hispanics were also omitted from this investigation given small cell sizes. Future studies should include measured or validated reports of maternal weights, adequate samples of Hispanics and other racial/ethnic groups, additional reporters (such as teachers) and objective measures of child problems assessed by trained professionals (such as diagnostic testing), and adequate measures of mediators.
Study strengths include longitudinal follow-up of a large, longitudinal, intergenerational sample and assessment of effect modification by gender during an understudied and important developmental period.62,63 The validated survey instrument allowed investigation of total, externalizing, and internalizing problems, instead of limiting this investigation to a single outcome.
CONCLUSIONS
Prepregnancy Class II and Class III obesity have recently doubled and tripled, respectively, in the U.S.1, and the behavioral problems studied here have implications for poor mental health trajectories through adolescence and into adulthood.64,65 In this large U.S. sample of children aged 9–11 years, maternal prepregnancy weight, particularly severe obesity, was related to markedly higher behavioral problems in boys. These results add new evidence that attaining and maintaining a healthy weight before pregnancy is crucial for children’s development.
Supplementary Material
Acknowledgments
The authors thank Stephanie Leonard and the University of California, Berkeley National Longitudinal Survey of Youth – Adverse Childhood Events team for their helpful comments on analytic strategies and other aspects of the manuscript. This research was supported by Award Number R01MD006104 from the National Center on Minority Health and Health Disparities. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center on Minority Health and Health Disparities or NIH. NIH had no role in study design; collection, analysis, and interpretation of data; writing the report; or the decision to submit the report for publication. This research was conducted with restricted access to Bureau of Labor Statistics data. The views expressed here do not reflect the views of the Bureau of Labor Statistics.
JD collaborated on the conceptualization of the paper, guided the statistical approach, and interpreted the results. She oversaw all aspects of the study and the writing and takes full and final responsibility for the paper. LS prepared, analyzed, and interpreted the data. She assisted with writing the methods and results sections and wrote sections of the introduction and conclusion. LP helped determine the appropriate analytic approach and assisted with analyses; she wrote sections of the methods and results. HK assisted with the original conceptualization of the paper and conducted early analyses and writing; she provided critical feedback on the final paper. BA is the senior author on the paper; she secured funding for the study and has been involved in all aspects of the paper from conceptualization to analyses to writing. All authors critically read, provided feedback, and edited the final paper.
No financial disclosures were reported by the authors of this paper.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.National Academy of Medicine, National Research Council. Weight Gain During Pregnancy: Reexamining the Guidelines. Washington, DC: National Academies Press; 2009. [PubMed] [Google Scholar]
- 2.U.S. Environmental Protection Agency. America’s Children and the Environment. 3. 2013. Neurodevelopmental Disorders; pp. 233–253. Publisher unknown. [Google Scholar]
- 3.Van Lieshout RJ. Role of maternal adiposity prior to and during pregnancy in cognitive and psychiatric problems in offspring. Nutr Rev. 2013;71(suppl 1):95–101. doi: 10.1111/nure.12059. https://doi.org/10.1111/nure.12059. [DOI] [PubMed] [Google Scholar]
- 4.Van Lieshout RJ, Taylor VH, Boyle MH. Pre-pregnancy and pregnancy obesity and neurodevelopmental outcomes in offspring: a systematic review. Obes Rev. 2011;12(5):548–559. doi: 10.1111/j.1467-789X.2010.00850.x. https://doi.org/10.1111/j.1467-789X.2010.00850.x. [DOI] [PubMed] [Google Scholar]
- 5.Antoniou EE, Fowler T, Reed K, Southwood TR, McCleery JP, Zeegers MP. Maternal pre-pregnancy weight and externalising behaviour problems in preschool children: a UK-based twin study. BMJ Open. 2014;4(10) doi: 10.1136/bmjopen-2014-005974. https://doi.org/10.1136/bmjopen-2014-005974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buss C, Entringer S, Davis EP, et al. Impaired executive function mediates the association between maternal pre-pregnancy body mass index and child ADHD symptoms. PloS One. 2012;7(6) doi: 10.1371/journal.pone.0037758. https://doi.org/10.1371/journal.pone.0037758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hinkle SN, Sharma AJ, Schieve LA, Ramakrishnan U, Swan DW, Stein AD. Reliability of gestational weight gain reported postpartum: a comparison to the birth certificate. Matern Child Health J. 2013;17(4):756–765. doi: 10.1007/s10995-012-1057-0. https://doi.org/10.1007/s10995-012-1057-0. [DOI] [PubMed] [Google Scholar]
- 8.Rodriguez A, Miettunen J, Henriksen TB, et al. Maternal adiposity prior to pregnancy is associated with ADHD symptoms in offspring: evidence from three prospective pregnancy cohorts. Int J Obes (Lond) 2008;32(3):550–557. doi: 10.1038/sj.ijo.0803741. https://doi.org/10.1038/sj.ijo.0803741. [DOI] [PubMed] [Google Scholar]
- 9.Van Lieshout RJ, Robinson M, Boyle MH. Maternal pre-pregnancy body mass index and internalizing and externalizing problems in offspring. Can J Psychiatry. 2013;58(3):151–159. doi: 10.1177/070674371305800305. https://doi.org/10.1177/070674371305800305. [DOI] [PubMed] [Google Scholar]
- 10.Rodriguez A. Maternal pre-pregnancy obesity and risk for inattention and negative emotionality in children. J Child Psychol Psychiatry. 2010;51(2):134–143. doi: 10.1111/j.1469-7610.2009.02133.x. https://doi.org/10.1111/j.1469-7610.2009.02133.x. [DOI] [PubMed] [Google Scholar]
- 11.Robinson M, Zubrick SR, Pennell CE, et al. Pre-pregnancy maternal overweight and obesity increase the risk for affective disorders in offspring. J Dev Orig Health Dis. 2013;4(1):42–48. doi: 10.1017/S2040174412000578. https://doi.org/10.1017/S2040174412000578. [DOI] [PubMed] [Google Scholar]
- 12.Tanda R, Salsberry PJ. Racial differences in the association between maternal prepregnancy obesity and children’s behavior problems. J Dev Behav Pediatr. 2014;35(2):118–127. doi: 10.1097/DBP.0000000000000007. https://doi.org/10.1097/DBP.0000000000000007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van Lieshout RJ, Schmidt LA, Robinson M, Niccols A, Boyle MH. Maternal pre-pregnancy body mass index and offspring temperament and behavior at 1 and 2 years of age. Child Psychiatry Hum Dev. 2013;44(3):382–390. doi: 10.1007/s10578-012-0332-z. https://doi.org/10.1007/s10578-012-0332-z. [DOI] [PubMed] [Google Scholar]
- 14.Brion MJ, Zeegers M, Jaddoe V, et al. Intrauterine effects of maternal prepregnancy overweight on child cognition and behavior in 2 cohorts. Pediatrics. 2011;127(1):202–211. doi: 10.1542/peds.2010-0651. https://doi.org/10.1542/peds.2010-0651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Polańska K, Muszyński P, Sobala W, Dziewirska E, Merecz-Kot D, Hanke W. Maternal lifestyle during pregnancy and child psychomotor development - Polish Mother and Child Cohort study. Early Hum Dev. 2015;91(5):317–325. doi: 10.1016/j.earlhumdev.2015.03.002. https://doi.org/10.1016/j.earlhumdev.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 16.Hinkle SN, Schieve LA, Stein AD, Swan DW, Ramakrishnan U, Sharma AJ. Associations between maternal prepregnancy body mass index and child neurodevelopment at 2 years of age. Int J Obes (London) 2012;36(10):1312–1319. doi: 10.1038/ijo.2012.143. https://doi.org/10.1038/ijo.2012.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wise LA, Palmer JR, Heffner LJ, Rosenberg L. Prepregnancy body size, gestational weight gain, and risk of preterm birth in African-American women. Epidemiology. 2010;21(2):243–252. doi: 10.1097/EDE.0b013e3181cb61a9. https://doi.org/10.1097/EDE.0b013e3181cb61a9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pugh SJ, Hutcheon JA, Richardson GA, et al. Gestational weight gain, prepregnancy body mass index and offspring attention-deficit hyperactivity disorder symptoms and behaviour at age 10. BJOG. 2016;123(13):2094–2103. doi: 10.1111/1471-0528.13909. https://doi.org/10.1111/1471-0528.13909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Center for Human Resource Research. NLSY79 User’s Guide. Columbus, OH: Ohio State University; 2008. [Google Scholar]
- 20.Center for Human Resource Research. NLSY79 Child and Young Adult Data User’s Guide. Columbus, OH: Ohio State University; 2010. [Google Scholar]
- 21.Bureau of Labor Statistics. [Accessed August 24, 2016];National Longitudinal Study of Youth: Household, Geography, & Contextual Variables. www.nlsinfo.org/content/cohorts/nlsy79/topical-guide/household/race-ethnicity-immigration-data.
- 22.Burkhauser RV, Cawley J. Beyond BMI: the value of more accurate measures of fatness and obesity in social science research. J Health Econ. 2008;27(2):519–529. doi: 10.1016/j.jhealeco.2007.05.005. https://doi.org/10.1016/j.jhealeco.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 23.WHO. [Accessed May 2, 2017];Global database on body mass index: BMI classification. www.assessmentpsychology.com/icbmi.htm.
- 24.Achenbach TM, Edelbrock CS. Behavioral problems and competencies reported by parents of normal and disturbed children aged four through sixteen. Monogr Soc Res Child Dev. 1981;46(1):1–82. https://doi.org/10.2307/1165983. [PubMed] [Google Scholar]
- 25.Peterson JL, Zill N. Marital disruption, parent-child relationships, and behavior problems in children. J Marriage Fam. 1986;48(2):295–307. https://doi.org/10.2307/352397. [Google Scholar]
- 26.Zill N. Behavior problem scales developed from the 1981 Child Health Supplement to the National Health Interview Survey. Washington, DC: Child Trends; 1985. [Google Scholar]
- 27.Guttmannova K, Szanyi JM, Cali PW. Internalizing and externalizing behavior problem scores: cross-ethnic and longitudinal measurement invariance of the Behavior Problem Index. Educ Psychol Meas. 2008;68(4):676–694. https://doi.org/10.1177/0013164407310127. [Google Scholar]
- 28.Minnesota Population Center and State Health Access Data Assistance Center, University of Minnesota. [Accessed August 24, 2016];Integrated Health Interview Series: Version 4.0. www.ihis.us.
- 29.Rehkopf DH, Krieger N, Coull B, Berkman LF. Biologic risk markers for coronary heart disease: nonlinear associations with income. Epidemiology. 2010;21(1):38–46. doi: 10.1097/EDE.0b013e3181c30b89. https://doi.org/10.1097/EDE.0b013e3181c30b89. [DOI] [PubMed] [Google Scholar]
- 30.R Core Team. R: A language and environment for statistical computing. [Accessed August 24, 2016];The R Project website. Published 2013. www.R-project.org/
- 31.Rao JNK, Scott AJ. On chi-squared tests for multiway contigency tables with proportions estimated from survey data. Ann Stat. 1984;12(1):46–60. https://doi.org/10.1214/aos/1176346391. [Google Scholar]
- 32.Lumley T. Analysis of complex survey samples. J Stat Softw. 2004;9(1):1–19. https://doi.org/10.18637/jss.v009.i08. [Google Scholar]
- 33.van Buuren S, Groothuis-Oudshoorn K. Mice: multivariate imputation by chained equations in R. J Stat Softw. 2011;45(3):1–67. https://doi.org/10.18637/jss.v045.i03. [Google Scholar]
- 34.Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55. https://doi.org/10.1093/biomet/70.1.41. [Google Scholar]
- 35.Jo H, Schieve LA, Sharma AJ, Hinkle SN, Li R, Lind JN. Maternal prepregnancy body mass index and child psychosocial development at 6 years of age. Pediatrics. 2015;135(5):1198–1209. doi: 10.1542/peds.2014-3058. https://doi.org/10.1542/peds.2014-3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Antonelli MC, Pallarés ME, Ceccatelli S, Spulber S. Long-term consequences of prenatal stress and neurotoxicants exposure on neurodevelopment. Prog Neurobiol. doi: 10.1016/j.pneurobio.2016.05.005. In press. Online May 25, 2016. https://doi.org/10.1016/j.pneurobio.2016.05.005. [DOI] [PubMed]
- 37.Bilbo SD, Tsang V. Enduring consequences of maternal obesity for brain inflammation and behavior of offspring. FASEB J. 2010;24(6):2104–2115. doi: 10.1096/fj.09-144014. https://doi.org/10.1096/fj.09-144014. [DOI] [PubMed] [Google Scholar]
- 38.Cabaj JL, McDonald SW, Tough SC. Early childhood risk and resilience factors for behavioural and emotional problems in middle childhood. BMC Pediatr. 2014;14(1) doi: 10.1186/1471-2431-14-166. https://doi.org/10.1186/1471-2431-14-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Galéra C, Côté SM, Bouvard MP, et al. Early risk factors for hyperactivity-impulsivity and inattention trajectories from age 17 months to 8 years. Arch Gen Psychiatry. 2011;68(12):1267–1275. doi: 10.1001/archgenpsychiatry.2011.138. https://doi.org/10.1001/archgenpsychiatry.2011.138. [DOI] [PubMed] [Google Scholar]
- 40.Wakschlag LS, Pickett KE, Cook E, Benowitz NL, Leventhal BL. Maternal smoking during pregnancy and severe antisocial behavior in offspring: a review. Am J Public Health. 2002;92(6):966–974. doi: 10.2105/ajph.92.6.966. https://doi.org/10.2105/AJPH.92.6.966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Denison FC, Roberts KA, Barr SM, Norman JE. Obesity, pregnancy, inflammation, and vascular function. Reproduction. 2010;140(3):373–385. doi: 10.1530/REP-10-0074. https://doi.org/10.1530/REP-10-0074. [DOI] [PubMed] [Google Scholar]
- 42.Edlow AG, Vora NL, Hui L, Wick HC, Cowan JM, Bianchi DW. Maternal obesity affects fetal neurodevelopmental and metabolic gene expression: a pilot study. PloS One. 2014;9(2):88661. doi: 10.1371/journal.pone.0088661. https://doi.org/10.1371/journal.pone.0088661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ou X, Thakali KM, Shankar K, Andres A, Badger TM. Maternal adiposity negatively influences infant brain white matter development. Obesity (Silver Spring) 2015;23(5):1047–1054. doi: 10.1002/oby.21055. https://doi.org/10.1002/oby.21055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jonakait GM. The effects of maternal inflammation on neuronal development: possible mechanisms. Int J Dev Neurosci. 2007;25(7):415–425. doi: 10.1016/j.ijdevneu.2007.08.017. https://doi.org/10.1016/j.ijdevneu.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 45.Amir LH, Donath S. A systematic review of maternal obesity and breastfeeding intention, initiation and duration. BMC Pregnancy Childbirth. 2007;4(7):9. doi: 10.1186/1471-2393-7-9. https://doi.org/10.1186/1471-2393-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.LaCoursiere DY, Barrett-Connor E, O’Hara MW, Hutton A, Varner MW. The association between prepregnancy obesity and screening positive for postpartum depression. BJOG. 2010;117(8):1011–1018. doi: 10.1111/j.1471-0528.2010.02569.x. https://doi.org/10.1111/j.1471-0528.2010.02569.x. [DOI] [PubMed] [Google Scholar]
- 47.Monk C, Georgieff MK, Osterholm EA. Maternal prenatal distress and poor nutrition – mutually influencing risk factors affecting infant neurocognitive development. J Child Psychol Psychiatry. 2013;54(2):115–130. doi: 10.1111/jcpp.12000. https://doi.org/10.1111/jcpp.12000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Neggers Y, Goldenberg RL. Some thoughts on body mass index, micronutrient intakes and pregnancy outcome. J Nutr. 2003;133(5 suppl 2):1737–1740. doi: 10.1093/jn/133.5.1737S. [DOI] [PubMed] [Google Scholar]
- 49.Song S. Does famine influence sex ratio at birth? Evidence from the 1959–1961 Great Leap Forward Famine in China. Proc Biol Sci. 2012;279(1739):2883–2890. doi: 10.1098/rspb.2012.0320. https://doi.org/10.1098/rspb.2012.0320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mora JO, Sanchez R, De Paredes B, Guillermo Herrera M. Sex related differences of nutritional supplementation during pregnancy on fetal growth. Early Hum Dev. 1981;5:243–251. doi: 10.1016/0378-3782(81)90032-3. https://doi.org/10.1016/0378-3782(81)90032-3. [DOI] [PubMed] [Google Scholar]
- 51.Navara KJ. Programming of offspring sex ratios by maternal stress in humans: assessment of physiological mechanisms using a comparative approach. J Comp Physiol B. 2010;180(6):785–796. doi: 10.1007/s00360-010-0483-9. https://doi.org/10.1007/s00360-010-0483-9. [DOI] [PubMed] [Google Scholar]
- 52.Catalano RF, Bruckner T, Gould J, Eskenazi B, Anderson E. Sex ratios in California following the terrorist attacks of September 11, 2001. Hum Reprod. 2005;20(5):1221–1227. doi: 10.1093/humrep/deh763. https://doi.org/10.1093/humrep/deh763. [DOI] [PubMed] [Google Scholar]
- 53.Mustieles V, Pérez-Lobato R, Olea N, Fernández MF. Bisphenol A: Human exposure and neurobehavior. Neurotoxicology. 2015;49:174–184. doi: 10.1016/j.neuro.2015.06.002. https://doi.org/10.1016/j.neuro.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 54.Deardorff J, Berry-Millett R, Rehkopf DH, Luecke E, Lahiff M, Abrams B. Maternal pre-pregnancy BMI, gestational weight gain, and age at menarche in daughters. Matern Child Health J. 2013;17(8):1391–1398. doi: 10.1007/s10995-012-1139-z. https://doi.org/10.1007/s10995-012-1139-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mendle J, Turkheimer E, Emery RE. Detrimental psychological outcomes associated with early pubertal timing in adolescent girls. Dev Rev. 2007;27(2):151–171. doi: 10.1016/j.dr.2006.11.001. https://doi.org/10.1016/j.dr.2006.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stice E, Presnell K, Bearman SK. Relation of early menarche to depression, eating disorders, substance abuse, and comorbid psychopathology among adolescent girls. Dev Psychol. 2001;37(5):608–619. doi: 10.1037//0012-1649.37.5.608. https://doi.org/10.1037/0012-1649.37.5.608. [DOI] [PubMed] [Google Scholar]
- 57.Deardorff J, Hayward C, Wilson KA, Bryson S, Hammer LD, Agras S. Puberty and gender interact to predict social anxiety symptoms in early adolescence. J Adolesc Health. 2007;41(1):102–104. doi: 10.1016/j.jadohealth.2007.02.013. https://doi.org/10.1016/j.jadohealth.2007.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Deardorff J, Gonzales NA, Christopher FS, Roosa MW, Millsap R. Early puberty and adolescent pregnancy: the influence of alcohol use. Pediatrics. 2005;116(6):1451–1456. doi: 10.1542/peds.2005-0542. https://doi.org/10.1542/peds.2005-0542. [DOI] [PubMed] [Google Scholar]
- 59.Nolen-Hoeksema S, Girgus JS. The emergence of gender differences in depression during adolescence. Psychol Bull. 1994;115(3):424–443. doi: 10.1037/0033-2909.115.3.424. https://doi.org/10.1037/0033-2909.115.3.424. [DOI] [PubMed] [Google Scholar]
- 60.Lederman SA, Paxton A. Maternal reporting of prepregnancy weight and birth outcome: consistency and completeness compared with the clinical record. Matern Child Health J. 1998;2(2):123–126. doi: 10.1023/a:1022996924094. https://doi.org/10.1023/A:1022996924094. [DOI] [PubMed] [Google Scholar]
- 61.Ranchod YK, Headen IE, Petito LC, Deardorff JK, Rehkopf DH, Abrams BF. Maternal childhood adversity, prepregnancy obesity and gestational weight gain. Am J Prev Med. 2016;50(4):463–469. doi: 10.1016/j.amepre.2015.08.032. https://doi.org/10.1016/j.amepre.2015.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Paus T, Keshavan M, Giedd JN. Why do many psychiatric disorders emerge during adolescence? Nat Rev Neurosci. 2008;9(12):947–957. doi: 10.1038/nrn2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lacourse E, Côté S, Nagin DS, Vitaro F, Brendgen M, Tremblay RE. A longitudinal-experimental approach to testing theories of antisocial behavior development. Dev Psychopathol. 2002;14(4):909–924. doi: 10.1017/s0954579402004121. https://doi.org/10.1017/S0954579402004121. [DOI] [PubMed] [Google Scholar]
- 64.Holbrook JR, Cuffe SP, Cai B, et al. Persistence of parent-reported ADHD symptoms from childhood through adolescence in a community sample. J Atten Disord. 2014;20(1):11–20. doi: 10.1177/1087054714539997. https://doi.org/10.1177/1087054714539997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kessler RC, Amminger GP, Aguilar-Gaxiola S, Alonso J, Lee S, Ustün TB. Age of onset of mental disorders: a review of recent literature. Curr Opin Psychiatry. 2007;20(4):359–364. doi: 10.1097/YCO.0b013e32816ebc8c. https://doi.org/10.1097/YCO.0b013e32816ebc8c. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.