Abstract
Background
Early identification of the potential to adopt an unhealthy diet, long-term, could improve weight outcomes for patients having undergone bariatric surgery.
Objectives
We explored whether pre-surgical sociodemographic and lifestyle characteristics, together with the type of surgery, could predict 10-year changes in dietary intake following bariatric surgery.
Setting
Surgical departments and primary health care centers, nationwide.
Methods
Participants were from the Swedish Obese Subjects study, a matched (nonrandomized) prospective trial comparing bariatric surgery to standard care for obese patients. This study included the 1695 surgery patients with complete information on pre-surgery diet. Questionnaires were completed before and 0.5, 1–4, 6, 8, and 10 years after surgery. Analyses were conducted with linear mixed model.
Results
Dietary changes were observed in 1561, 1298, and 1243 participants, at the 2-, 6-, and 10-year follow-ups, respectively. Sex and treatment type predicted changes in energy, carbohydrate, protein, and fiber intake over the follow-up (P <.05). Furthermore, male sex, younger age, a sedentary behavior, and gastric bypass predicted increased alcohol consumption (P <.001). Two important phases for intervening bariatric patients’ diet were identified. The first was six months after surgery, when the maximal changes in diet were achieved. The second, stretched from the six-month until the 4-year after surgery, during which earlier commitments to dietary changes were largely abandoned.
Conclusions
Male sex and banding surgery in particular predicted unfavorable post-surgery changes in energy and macronutrient intake. Furthermore, gastric bypass, a younger age, and an unhealthy lifestyle pre-surgery, may predispose individuals to increased alcohol intake following surgery.
Keywords: Bariatric surgery, dietary change, macronutrients, energy intake, lifestyle, sociodemographic factors, surgery type, Swedish Obese Subjects
Introduction
Obesity (body mass index (BMI) ≥ 30.0 kg/m2) is a significant worldwide health problem that has been ranked as the sixth (1) most important factor in contributing to mortality and morbidity from chronic disease. Currently, bariatric surgery is the most effective treatment for morbidly obese individuals and for obese patients with metabolic conditions (e.g. type 2 diabetes). However, weight outcomes vary considerably with some patients losing 30% of their bodyweight 10-years after weight-loss surgery, while others experience weight gain(2).
Insufficient weight loss or weight regain following bariatric surgery may be provoked by physiological factors such as enlargement of the gastric pouch (3) or hormonal imbalance (e.g. increased ghrelin) (4). However, it is likely that poor adherence to post-surgical recommendations for dietary and physical activity is causal (5,6). For example, thus far, short-term studies have shown that weight regain after surgery is associated with excessive caloric intake and a poor quality diet (typically) rich in refined carbohydrates and fatty foods (6).
The early identification of patients prone to adopting unhealthy dietary patterns after bariatric surgery could help dieticians and other surgical team members to intervene to improve eventual weight outcomes by targeting intensive lifestyle counseling. Such interventions have been shown to improve weight maintenance among bariatric patients (7). Within the general population, certain socio-demographic and lifestyle characteristics, such as lengthier education and not-smoking correlate with higher quality diets (e.g. less fat and more dietary fiber/vitamins) (8). The evaluation of these characteristics prior to surgery could therefore serve as predictors of patients’ long-term quality of diet, post surgery.
In this study we aimed to describe long-term changes in dietary intake after bariatric surgery. Correlates were then sought for pre-surgery socio-demographic and lifestyle characteristics, and type of surgical procedure, with the aim of determining their value in predicting changes in dietary intake after surgery. We chose to focus on energy and macronutrient intake given their prominence in contributing to weight change.
Materials and Methods
The Swedish Obese Subjects (SOS) study is a prospective, matched, non-randomized, surgical intervention trial, comparing the long-term effects of bariatric surgery versus conventional obesity treatment (9). The current analysis concerns the surgical group alone (n = 2010). Recruitment campaigns were organized via media outlets, primary health care centers, and surgical departments. In total, participants were recruited over a 13.4-year period (September 1st 1987 to January 31st 2001) from 25 surgical departments and 480 primary health care centers, nationwide. Seven regional ethics committees approved the study protocol, with written or oral informed consent obtained from all participants. The SOS study is registered at clinicaltrials.gov with the identifier: NCT01479452.
Inclusion criteria included those aged 37–60 y with a BMI ≥ 34 kg/m2 for men and ≥ 38 kg/m2 for women. Exclusion criteria (9) were minimal and aimed to ensure that the patients were suitable for surgery. Of the eligible patients, 2010 constituted the surgical group, with 376, 1369, and 265 patients subsequently undergoing nonadjustable or adjustable banding, vertical banded gastroplasty (VBG), or gastric bypass, respectively. For adjustable banding, the Swedish Adjustable Gastric Band (Obtech Medical), comparable to the American lap band, was used. The current analyses were restricted to a 10-year follow-up given the low completion rate for later dietary questionnaires (15 and 20-year follow-up visits). Those, who were re-operated, or whose operation was reversed during the 10-year follow-up (n = 315) were excluded from analyses.
Baseline examinations were conducted 4 weeks prior to surgery with follow-up visits (0.5, 1, 2, 3, 4, 6, 8, and 10 years post surgery) scheduled according to the date of surgery (9). Follow-ups included outpatient clinical visits and questionnaires (mailed-in). Questionnaires queried socioeconomic status and lifestyle. Educational level was assessed using the question, “What is your education?” with 6 possible options (elementary, primary, vocational, high-school, college/university, or post graduate) combined into 3 categories to expand group size. These 3 categories were designated: low educational level (elementary/primary school), average (vocational/high school), or high (college/university or higher). In terms of physical activity, participants classified themselves as belonging to one of four groups for leisure-time activity, and one of five groups for work (10,11). Physical activity groups (leisure and work) were combined into a matrix that categorized participants into 5 activity classes: sedentary, light, moderate, high, and very high. Given the scarcity of participants in the latter two categories ((n <30) high /very high), these were combined with the “moderate” group for analysis. Current smoking habit was queried as follows: “Do you smoke daily?” Former smokers were identified by the question “Have you smoked before?”.
Dietary data were collected using a validated semi-quantitative diet questionnaire (12) that was completed 4-weeks prior to surgery and at all follow-up visits. The questionnaire was adapted from a diet history interview developed for the general Swedish population based on clinical experience with reporting problematic eating patterns in obese individuals (13). The questionnaire included 49 questions covering habitual dietary intake for the previous 3 months. Completed food questionnaires were linked to a food composition table, compiled according to Swedish food composition tables to provide energy and nutrient data.
In this study, calculations for total energy intake (kcal/d) and macronutrients (g/d; carbohydrates, total fat, protein, fiber, and alcohol) were used. To illustrate changes in macronutrients proportional to total energy intake, rather than absolute changes, carbohydrates, fat, protein and fiber was further calculated as a percentage of total energy intake (E%). Alcohol intake (g/d) was corrected for the confounding effect of energy intake using the residual method (14) in which an estimate of energy-adjusted alcohol intake is the residual from a regression model, with total energy intake as the independent variable and absolute alcohol intake is the dependent variable. Thus, the residual value is an estimate of alcohol intake, uncorrelated with total energy intake.
Statistical analyses
Data were analyzed with R statistical software version 3.2.5 (15). Linear mixed models (lmer – procedure in the lme4 package) (16) including repeated measures with a random intercept and an unstructured covariance matrix were used to analyze associations between sociodemographic and lifestyle variables, surgery type, and 10-year changes in energy and macronutrient intake. This analytical design can accommodate missing data.
First, changes in energy and macronutrient intake between each follow-up point and baseline were calculated as percentages, and then used as outcome variables. Participants’ age (</> the median age of 46.5 years), sex (male/female), educational level (low/average/high), smoking habit (current/former/never), physical activity level (sedentary/light/moderate), and treatment type (banding/gastric bypass/VBG), were used as main predictors. Time (years since surgery) was used as a categorical explanatory variable in the model, with each follow-up point representing its own category. The main predictors were initially entered univariably (to the model) as fixed effects with or without an interaction term with time (association with changes in dietary intake over time). The final model was adjusted for age and sex (when not used as the main predictor), and pre-surgical energy/macronutrient intake.
The statistical significance of interactions between the main predictors and time were derived from ANOVA tables (ANOVA procedure in package base) (15). Based on the mixed model, least square means and asymptotic confidence intervals (CIs) were calculated by categories of predictor variables for each time point (lsmeans procedure/lsmeans package) (17). Statistical significance for differences between categories of predictor variables at each time point were derived using Tukey’s multiplicity adjusted P-values (Tukey’s Honestly Significant Difference; pairs procedure in package lsmeans) (17). A P-value of <.05 was considered to indicate statistical significance.
Results
Patient characteristics are presented in Table 1. For the 1695 participants included in the analysis, information on dietary intake was available for 92.0% (n = 1561), 76.6% (n = 1298), and 73.3% (n = 1243) at 2-, 6-, and 10-year follow-ups, respectively. At baseline, self-reported energy and alcohol intake differed between the sexes, with men reporting higher alcohol consumption (Supplementary Table 1). Other than a trend for younger participants to report lower energy intake at baseline vs. older participants, differences in pre-surgery energy and macronutrient intake were small when grouped by sociodemographic and lifestyle factors.
Table 1.
Baseline characteristics in surgically treated participants (n = 1695) in the Swedish Obese Subjects Study
| Characteristics | Mean / % | sd / n |
|---|---|---|
| Male, % | 30.2 | 512 |
| Age, y | 47.3 | 5.9 |
| Treatment type | ||
| Gastric bypass, % | 15.5 | 270 |
| Banding, % | 15.9 | 263 |
| VBG, % | 68.6 | 1162 |
| Current smokers, % | 43.7 | 740 |
| Low education, % * | 43.9 | 744 |
| Sedentary behavior, % † | 49.1 | 833 |
| Height, cm | 168.8 | 9.2 |
| Weight, kg | 121.1 | 16.7 |
| BMI, kg/m2 | 42.5 | 4.5 |
| WC, cm | 125.8 | 11.0 |
BMI, body mass index; VBG, vertical banded gastroplasty; WC, waist circumference
A low educational level is comparable to elementary school.
The sedentary activity group includes both leisure-time and work activity.
Post surgery, patients reported their lowest energy intake at the 0.5-year follow-up (Supplementary Table 2). At this point, the reported energy intake was, on average, 35–45% lower than at baseline. At the 1–4-year follow-ups, participants reported increased energy intake compared to their six-month follow-up value. Following the 4-year follow-up, relatively stable energy intakes were reported. At the end of follow-up, the reported energy intake was 15–25% lower than at baseline. When comparing the first, 0.5-year follow-up values vs. those at baseline, the relative proportion of energy derived from carbohydrates, fiber, and protein had increased, with decreased energy derivation from fat and alcohol (Supplementary Table 2). These changes were partly or entirely lost by the 4 to 6-year follow-ups.
Self-reported changes in total energy intake were neither modulated by background characteristics nor lifestyle habits over follow-up (P >.05 for all interactions, Fig. 1). However, sex and age seemed to correlate with levels of energy intake post surgery (Fig. 1). Women reported a 10.7% lower energy intake at the 0.5-year follow-up vs. men (P <.001). Subsequently, both sexes increased their energy intake during follow-up, although women still reported a 6.5% lower energy intake than men at the 10-year follow-up (P =.002). Furthermore, participants younger than 46.5 years (the median age of our cohort) reported at least a 3.5% lower energy intake compared to older participants, until year-4 of follow-up (22.2% in the younger vs. 18.5% in the older participants; P =.047), after which this difference reduced to less than 3.0% (P >.05).
Fig 1.
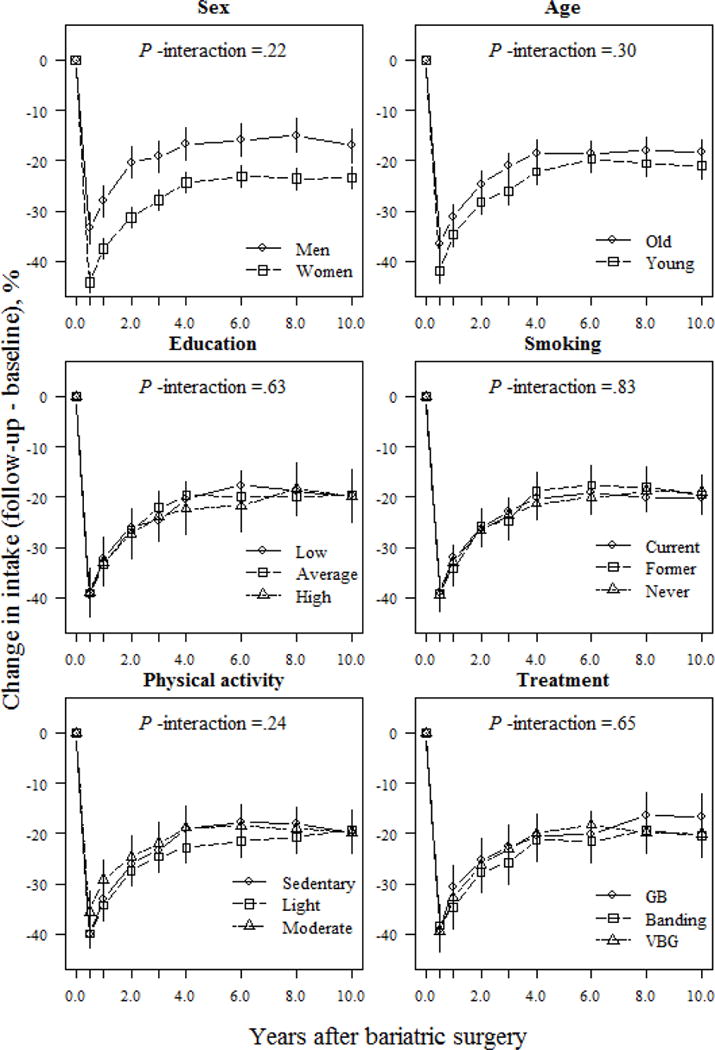
Relative changes (means and 95% CIs) in energy intake (kcal/d) based on a mixed model for surgically treated individuals, grouped according to baseline sex, age, education, smoking and physical activity, and treatment type. The model was adjusted for sex and baseline age (except when examined as the main predictor), and baseline energy intake. P-values for the interactions between subgroups and changes in energy intake over time were derived from ANOVA tables (F-statistics). GB, gastric bypass; VBG, vertical banded gastroplasty.
Sex appeared to be a major factor modulating changes in macronutrient composition. Women reported larger and better-maintained changes in carbohydrate, protein, and fiber intake over follow-up than men (Figs. 2, 4, and 5). At the 10-year follow-up, women reported a 3.5% and 20.2% (P <.001; Figs. 2 and 5) higher relative proportion of energy from carbohydrates and fiber, respectively, than men. Furthermore, at the end of follow-up, women reported a 1.4% increase, and men, a 1.5% decrease, in their relative proportion of energy derived from protein, vs. baseline values (absolute difference 2.9%; P =.005; Fig. 4).
Fig 2.
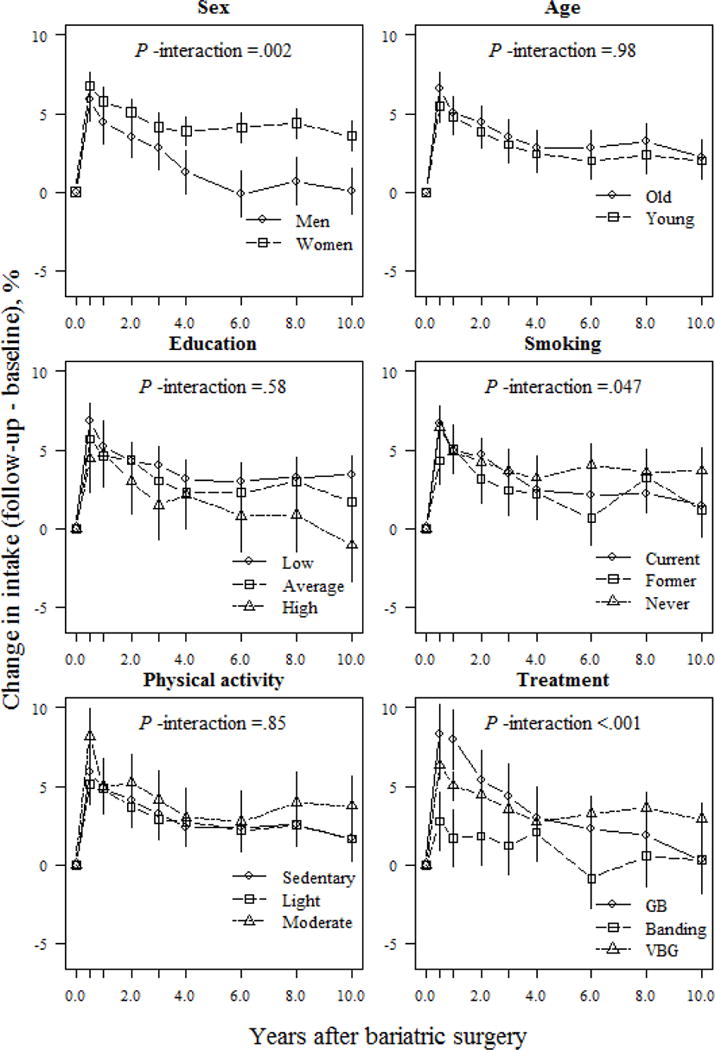
Relative changes (means and 95% CIs) in carbohydrate intake (E%) based on a mixed model for surgically treated individuals, grouped according to baseline sex, age, education, smoking and physical activity, and treatment type. The model was adjusted for sex and baseline age (except when examined as the main predictor) and baseline carbohydrate intake. P-values for the interactions between subgroups and changes in carbohydrate intake over time were derived from ANOVA tables (F-statistics). E% denotes percentage of total energy intake; GB, gastric bypass; VBG, vertical banded gastroplasty.
Fig 4.
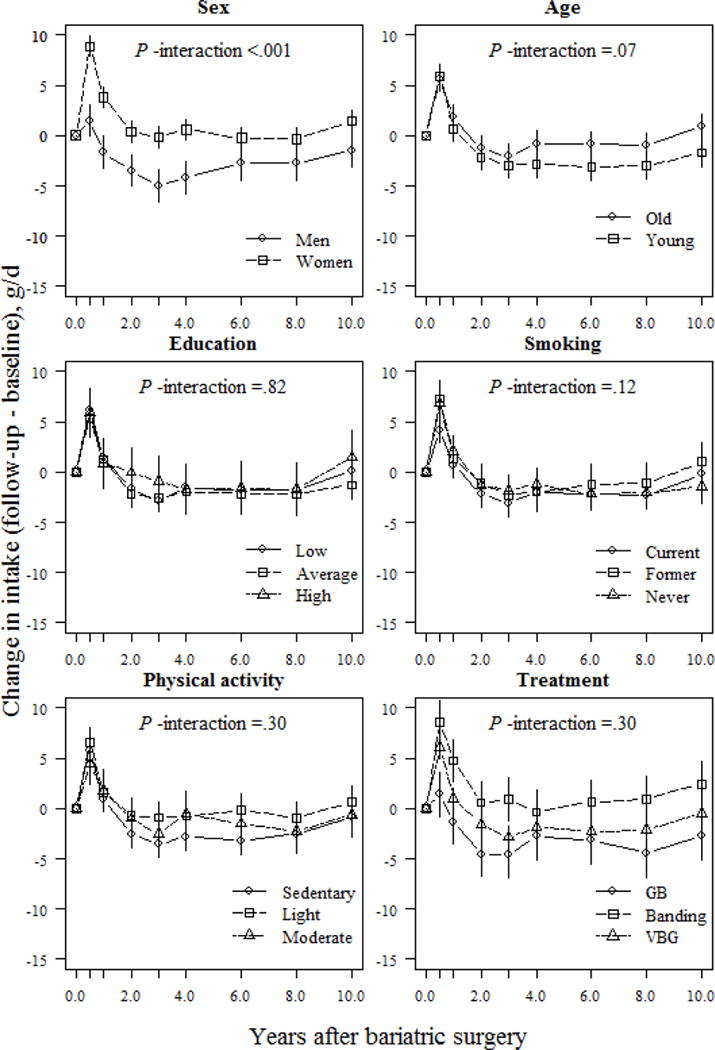
Relative changes (means and 95% CIs) in protein intake (E%) based on mixed model among surgically treated individuals, grouped according to baseline sex, age, education, smoking and physical activity, and treatment type. The model was adjusted for sex and baseline age (except when examined as the main predictor) and baseline protein intake. P-values for interactions between the subgroups and changes in protein intake over time were derived from ANOVA tables (F-statistics). E% denotes percentage of total energy intake; GB, gastric bypass; VBG, vertical banded gastroplasty.
Fig 5.
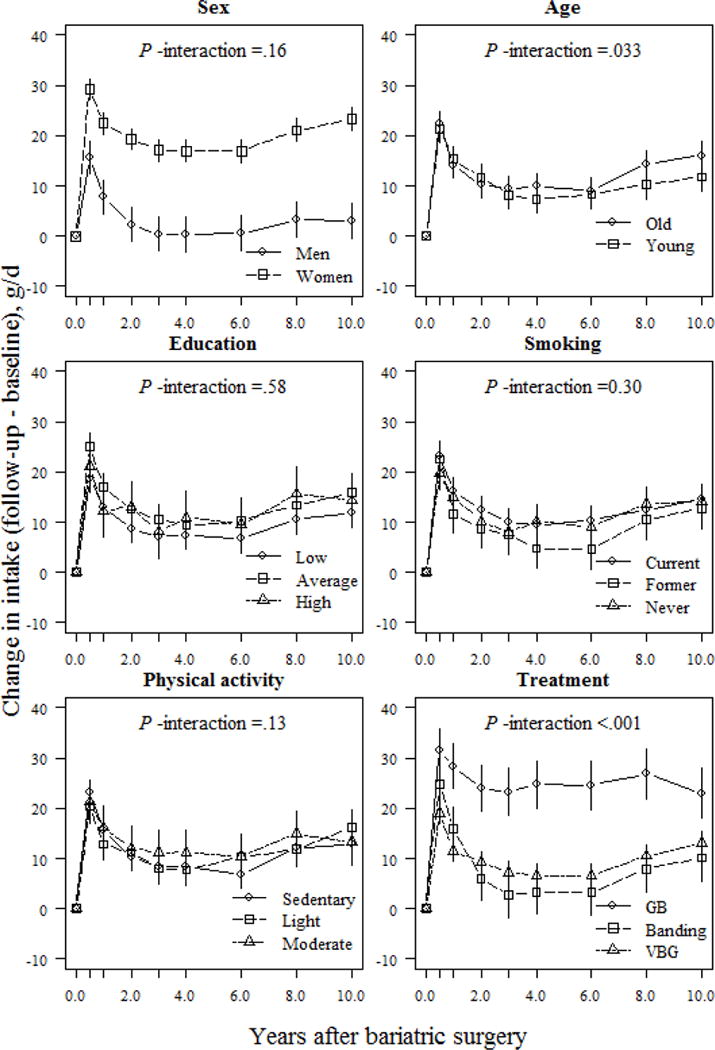
Relative changes (means and 95% CIs) in fiber intake (E%) based on a mixed model for surgically treated individuals, grouped according to baseline sex, age, education, smoking and physical activity, and treatment type. The model was adjusted for sex and baseline age (except when examined as the main predictor) and baseline fiber intake. P-values for the interactions between subgroups and changes in fiber intake over time were derived from ANOVA tables (F-statistics). E% denotes percentage of total energy intake; GB, gastric bypass; VBG, vertical banded gastroplasty.
Treatment type also influenced changes in macronutrient composition. At the 1-year follow-up, banding patients reported a 1.7% increase in their relative proportion of energy derived from carbohydrates, whereas VBG and gastric bypass patients reported increases of 5.0% and 8.0%, respectively (P <.05 between-group differences; Fig. 2). However, at the 10-year follow-up, VBG patients had maintained a nearly 3.0% increase in their relative proportion of energy derived from carbohydrates, whereas banding and gastric bypass patients maintained a <1.0% increase compared to initial values (P =.049 and P =.068 for comparisons between VBG and banding / gastric bypass, respectively; Fig. 2). Gastric bypass patients demonstrated the largest increase in relative proportion of energy derived from fiber vs. other operations, which was maintained over follow-up (absolute difference 12.9% and 10.0% between gastric bypass and banding/VBG, respectively; P <.001; Fig. 5). Changes in self-reported protein and fat intake were not modulated by treatment type over follow-up (P >.05 for interaction; Figs. 3 and 4). However, banding patients had the highest relative intake of energy from protein (absolute difference 5.2%, P =.006; Fig. 4) until the end of follow-up, and also the highest relative intake of energy from fat until 6 years after surgery (absolute difference 5.4%; P =.002; Fig. 3) compared to gastric bypass patients.
Fig 3.
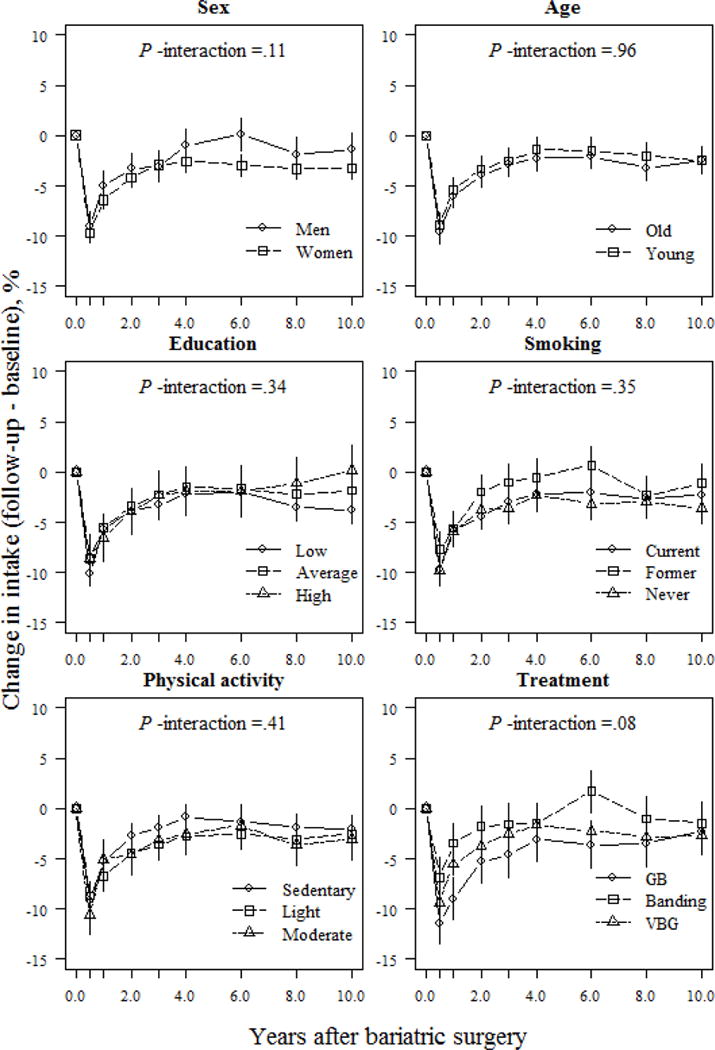
Relative changes (means and 95% CIs) in fat intake (E%) based on a mixed model for surgically treated individuals, grouped according to baseline sex, age, education, smoking and physical activity, and treatment type. The model was adjusted for sex and baseline age (except when examined as the main predictor) and baseline fat intake. P-values for the interactions between subgroups and changes in fat intake over time were derived from ANOVA tables (F-statistics). E% denotes percentage of total energy intake; GB, gastric bypass; VBG, vertical banded gastroplasty.
Changes in reported alcohol intake were modulated by sex, age, physical activity, and treatment type (Fig. 6). At the 10-year follow-up, men reported an average 2.2 g/d increase over baseline, with women reporting a lower, 0.16 g/d increase (P <.001). Furthermore, from the 6-year follow-up onwards, the younger participants tended to report higher alcohol intake vs. older participants, but this difference was not clinically meaningful (absolute difference between age groups <1 g/d at the 10-y follow-up; P =.012). Regarding lifestyle factors, participants categorized in the sedentary group reported a 1.9 g/d increase over their initial intake, whereas those in the light or moderate activity group reported <1.0 g/d change vs. their initial intake during the 10-y follow-up (absolute differences between sedentary and light/moderate groups were 1.26 g/d and 2.07 g/d, respectively; P <.01). Of the surgical treatments, gastric bypass patients reported a 3.8 g/d increase of their initial intake at the 10-year follow-up. In comparison, banding patients reported a 1.1 g/d increase and VBG patients a 0.5 g/d increase over their initial intake (P <.001 for difference between gastric bypass and banding / VBG).
Fig 6.
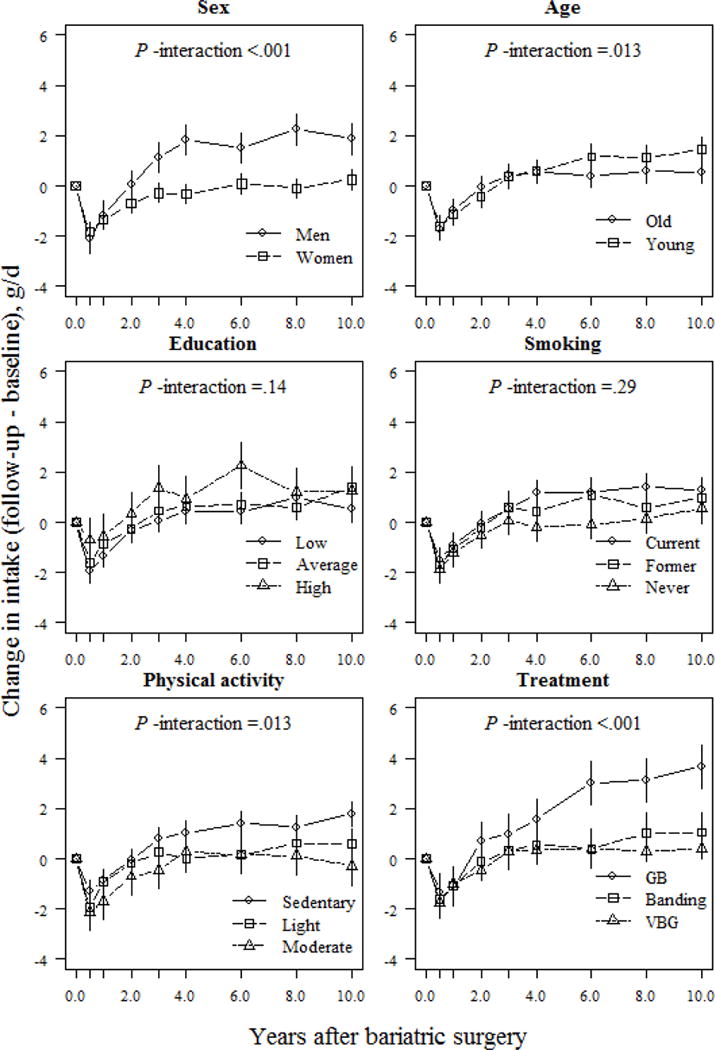
Changes (means and 95% CIs) in alcohol intake (g/d) based on a mixed model of surgically treated individuals, grouped according to baseline sex, age, education, smoking and physical activity, and treatment type. The model was adjusted for sex and baseline age (except when examined as the main predictor), baseline alcohol intake, and total energy intake. P-values for interactions between subgroups and changes in alcohol intake over time were derived from ANOVA tables (F-statistics). GB, gastric bypass; VBG, vertical banded gastroplasty.
Discussion
We observed decreased energy intake and a favorable move towards a healthier macronutrient composition in the first 6 months after bariatric surgery. Unfortunately, these changes tended to fade during the first 4 years following surgery. Women reported larger changes in energy intake and macronutrient composition, and maintained these changes to a better degree than men. Along with sex, treatment type was another main determinant for changes in macronutrient composition. Rather than switching to a more favorable macronutrient intake, subjects reported increased alcohol intake over the 10-year follow-up that was more pronounced in men, younger participants, sedentary participants, and those having undergone gastric bypass.
We identified two important phases in terms of dietary change following bariatric surgery (at six months and before the 4-year follow-up) that could be targeted to improve outcomes. For the first phase, support and encouragement from the surgical team and registered dieticians could encourage patients to adhere to dietary recommendations. During phase 2, self-monitoring of dietary intake and weight, as well as education and targeted lifestyle interventions could help patients to maintain the good practices adopted shortly after surgery (7).
To our knowledge, this study is the first to report changes in energy and macronutrient intake for up to 10 years after surgery, inclusive of the main macronutrients, fiber, and alcohol. Previous studies with shorter follow-ups (<3 years) and a predominance of Roux-en-Y gastric bypass (RYGB) patients have reported similar patterns of energy intake post surgery, but with inconsistent data in terms of macronutrients (18–20). We could identify no previous study that has explored pre-surgery sociodemographic and lifestyle factors as predictors for long-term energy and macronutrient intake. A possible explanation for this is omission is that most studies assessing dietary intake in bariatric patients have involved a small sample size (n <100) with a predominance of female participants and a single type of surgical intervention; these conditions rule out stratified analyses. Currently, limited data exist for differences in dietary intake between surgery types (18,21,22), studies reporting either no differences in energy and macronutrient intakes between treatments (18,21) or lower relative proportion of energy from fat (22) over 1 to 6-years follow-ups. Furthermore, it is generally known that protein malnutrition is more common after RYGB (23).
We observed an increase in self-reported alcohol consumption during the follow-up, especially in men, younger participants, those reporting sedentary behavior, and for those having had a gastric bypass operation. However, the absolute increase in alcohol consumption was, on average, small, with only a minority of the operated population exceeding “low risk” consumption-levels. Most previous studies have explored alcohol problems and alcohol abuse, which have been also reported in SOS study participants (24). Our results support the earlier findings, which have associated male sex, a younger age, and smoking with an increased risk of alcohol use disorder 2 years after surgery (25). Furthermore, our results are in line with the general knowledge that RYGB is associated with increased post-surgery alcohol consumption (26).
The strengths of our study include its prospective design with an exceptionally long follow-up with repeated dietary data collection. Furthermore, a large sample size allowed stratification by several background factors, which has not been previously attempted. Our use of the linear mixed model as the main analytic strategy allowed us to cope with missing data and to take into account changes in association between the outcome and predictors over time.
Because the SOS study was initiated almost 30 years ago, the majority of the patients were operated with surgical procedures that are now obsolete. This is a possible limitation that may affect the contemporary relevance of our results. However, the need for careful and intensive follow-up during the first four years is likely relevant irrespective of surgical technique. Another limitation of the study is that dietary intake and physical activity were self-reported. Thus, inaccurate reporting may have affected our analysis. In particular, obese individuals and women are prone to misreport their dietary intake (27). However, the dietary questionnaire used in the SOS study was validated against laboratory measurements for energy and protein intake, and has been previously validated for energy intake in both obese and nonobese individuals (12). Further, the (Saltin-Grimby) scale for physical activity has been validated (10).
Conclusions
In our study, men in particular, and banding patients, experienced difficulties in adhering to recommendations for energy restriction and macronutrient intake after bariatric surgery. Furthermore, gastric bypass, a younger age, and an unhealthy lifestyle, pre-surgery, seemed to predispose individuals to increased alcohol consumption post-surgery. Two important phases for intervening in dietary intake after bariatric surgery were identified, at six months after surgery, when the greatest positive changes in diet were achieved, and then before the 4-year mark, after which most of these behavioral gains were lost. During these phases, patients who risk the stable adoption of a healthy diet should be targeted for intensive lifestyle counseling in order to improve long-term weight outcome.
Supplementary Material
Acknowledgments
Source of funding: This study was supported by the Finnish Cultural Foundation (via the Finnish Post Doc Pool; grand awarded to NK), the Swedish Research Council (K2012-55X-22082-01, K2013-54X-11285-19, and K2013-99X-22279-01), the Sahlgrenska University Hospital ALF research grant, Diabetesfonden, National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health, under Award Number R01DK105948 (the content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health), and the VINNOVA-VINNMER program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Clinical trial registry number and website: This trial was registered at clinicaltrials.gov with identifier: NCT01479452.
Disclosures
The authors have no commercial associations that would be a conflict of interest in relation to this article.
References
- 1.Lim SS, Vos T, Flaxman AD, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2224–60. doi: 10.1016/S0140-6736(12)61766-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Karlsson J, Taft C, Rydén A, Sjöström L, Sullivan M. Ten-year trends in health-related quality of life after surgical and conventional treatment for severe obesity: the SOS intervention study. Int J Obes. 2007;31(8):1248–61. doi: 10.1038/sj.ijo.0803573. [DOI] [PubMed] [Google Scholar]
- 3.Yimcharoen P, Heneghan HM, Singh M, et al. Endoscopic findings and outcomes of revisional procedures for patients with weight recidivism after gastric bypass. Surg Endosc. 2011;25(10):3345–52. doi: 10.1007/s00464-011-1723-0. [DOI] [PubMed] [Google Scholar]
- 4.Bohdjalian A, Langer FB, Shakeri-Leidenmühler S, et al. Sleeve gastrectomy as sole and definitive bariatric procedure: 5-year results for weight loss and ghrelin. Obes Surg. 2010;20(5):535–40. doi: 10.1007/s11695-009-0066-6. [DOI] [PubMed] [Google Scholar]
- 5.Sjöström L, Lindroos AK, Peltonen M, et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med. 2004;351(26):2683–93. doi: 10.1056/NEJMoa035622. [DOI] [PubMed] [Google Scholar]
- 6.Freire RH, Borges MC, Alvarez-Leite JI, Toulson Davisson Correia MI. Food quality, physical activity, and nutritional follow-up as determinant of weight regain after Roux-en-Y gastric bypass. Nutrition. 2012;28(1):53–8. doi: 10.1016/j.nut.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 7.Rudolph A, Hilbert A. Post-operative behavioural management in bariatric surgery: a systematic review and meta-analysis of randomized controlled trials. Obes Rev. 2013;14(4):292–302. doi: 10.1111/obr.12013. [DOI] [PubMed] [Google Scholar]
- 8.Drake I, Gullberg B, Ericson U, et al. Development of a diet quality index assessing adherence to the Swedish nutrition recommendations and dietary guidelines in the Malmö Diet and Cancer cohort. Public Health Nutr. 2011;14(5):835–45. doi: 10.1017/S1368980010003848. [DOI] [PubMed] [Google Scholar]
- 9.Sjöström L. Review of the key results from the Swedish Obese Subjects (SOS) trial - a prospective controlled intervention study of bariatric surgery. J Intern Med. 2013;273(3):219–34. doi: 10.1111/joim.12012. [DOI] [PubMed] [Google Scholar]
- 10.Saltin B, Grimby G. Physiological analysis of middleaged and old former athletes. Comparison with still active athletes of the same ages. Circulation. 1968;38(6):1104–15. doi: 10.1161/01.cir.38.6.1104. [DOI] [PubMed] [Google Scholar]
- 11.Larsson I, Lissner L, Näslund I, Lindroos AK. Leisure and occupational physical activity in relation to body mass index in men and women. Scand J Nutr. 2004;48(4):165–72. [Google Scholar]
- 12.Lindroos AK, Lissner L, Sjöström L. Validity and reproducibility of a self-administered dietary questionnaire in obese and non-obese subjects. Eur J Clin Nutr. 1993;47(7):461–81. [PubMed] [Google Scholar]
- 13.Abrahamsson M, Isaksson B. Swedish title: Förenklad metodik vid kostvaneundersökningar (In English: simplified method for dietary surveys) Näringsforskning. 1988;32:93–9. [Google Scholar]
- 14.Willett W, Stampfer MJ. Total energy intake: implications for epidemiologic analyses. Am J Epidemiol. 1986;124(1):17–27. doi: 10.1093/oxfordjournals.aje.a114366. [DOI] [PubMed] [Google Scholar]
- 15.R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2016. Internet: https://www.R-project.org/ (accessed 10 Sep 2016) [Google Scholar]
- 16.Bates D, Maechler M, Bolker B, Walker S. Fitting Linear Mixed-Effects Models Using lme4. J Stat Softw. 2015;67(1):1–48. [Google Scholar]
- 17.Lenth RV. Least-Squares Means: The R Package lsmeans. J Stat Softw. 2016;69(1):1–33. [Google Scholar]
- 18.Brolin RE, Robertson LB, Kenler HA, Cody RP. Weight loss and dietary intake after vertical banded gastroplasty and Roux-en-Y gastric bypass. Ann Surg. 1994;220(6):782–90. doi: 10.1097/00000658-199412000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sarwer DB, Wadden TA, Moore RH, et al. Preoperative eating behavior, postoperative dietary adherence, and weight loss after gastric bypass surgery. Surg Obes Relat Dis. 2008;4(5):4640–6. doi: 10.1016/j.soard.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Giusti V, Theytaz F, Di Vetta V, Clarisse M, Suter M, Tappy L. Energy and macronutrient intake after gastric bypass for morbid obesity: a 3-y observational study focused on protein consumption. Am J Clin Nutr. 2016;103(1):18–24. doi: 10.3945/ajcn.115.111732. [DOI] [PubMed] [Google Scholar]
- 21.Moizé V, Andreu A, Flores L, et al. Long-term dietary intake and nutritional deficiencies following sleeve gastrectomy or Roux-En-Y gastric bypass in a mediterranean population. J Acad Nutr Diet. 2013;113(3):400–10. doi: 10.1016/j.jand.2012.11.013. [DOI] [PubMed] [Google Scholar]
- 22.le Roux CW, Bueter M, Theis N, et al. Gastric bypass reduces fat intake and preference. Am J Physiol Regul Integr Comp Physiol. 2011;301(4):R1057–66. doi: 10.1152/ajpregu.00139.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stein J, Stier C, Raab H, Weiner R. Review article: The nutritional and pharmacological consequences of obesity surgery. Aliment Pharmacol Ther. 2014;40(6):582–609. doi: 10.1111/apt.12872. [DOI] [PubMed] [Google Scholar]
- 24.Svensson PA, Anveden Å, Romeo S, et al. Alcohol consumption and alcohol problems after bariatric surgery in the Swedish obese subjects study. Obesity. 2013;21(12):2444–51. doi: 10.1002/oby.20397. [DOI] [PubMed] [Google Scholar]
- 25.King WC, Chen JY, Mitchell JE, et al. Prevalence of alcohol use disorders before and after bariatric surgery. JAMA. 2012;307(23):2516–25. doi: 10.1001/jama.2012.6147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spadola CE, Wagner EF, Dillon FR, Trepka MJ, De La Cruz-Munoz N, Messiah SE. Alcohol and drug use among postoperative bariatric patients: a systematic review of the emerging research and its implications. Alcohol Clin Exp Res. 2015;39(9):1582–601. doi: 10.1111/acer.12805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mattisson I, Wirfält E, Aronsson CA, et al. Misreporting of energy: prevalence, characteristics of misreporters and influence on observed risk estimates in the Malmö Diet and Cancer cohort. Br J Nutr. 2005;94(5):832. doi: 10.1079/bjn20051573. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


