Abstract
Background
Though the use of adjuvant chemotherapy in patients with pathologically node-positive (pN+) atypical carcinoid tumor of the lung is an accepted practice, controversy exists regarding its utilization in node-negative (pN0) patients. Our aim was to determine if a survival advantage exists in patients receiving chemotherapy post-operatively for pN0 or pN+ atypical carcinoid tumors of the lung.
Methods
Adult patients treated with lobectomy or pneumonectomy for pulmonary atypical carcinoid tumor were identified using the National Cancer Data Base (NCDB), 2006–2011. Propensity scoring (4:1 nearest neighbor algorithm) and survival analysis were employed to examine the association between adjuvant chemotherapy and pathologically node positive (pN+) vs. pathologically node negative (pN0) atypical carcinoid tumors.
Results
Of the total 581 patients identified with a diagnosis of atypical carcinoid of the lung, 363 (62.5%) were found to be node negative at the time of surgery and 218 (37.5%) had node positive disease. Adjuvant chemotherapy was used in 15 (4.1%) patients with pN0 disease and 89 (40.8%) with pN+. Unadjusted survival, at 12-months and 60-months, was similar between pN+ patients who were treated with adjuvant chemotherapy vs. those who received surgery alone (Adjuvant chemotherapy: 12mo–98.9% 60 mo–47.9% vs. Surgery alone: 12mo–98.4%, 60mo–67.1%, p=0.46) as well as for propensity-matched pN0 (Adjuvant chemotherapy: 12mo–86.7%, 60mo–73.3% vs. Surgery alone: 12mo–87.9%, 60mo–72.3% p=0.54).
Conclusions
In a national-level analysis, the use of adjuvant chemotherapy post-operatively in patients with pN+ and pN0 disease conferred no survival advantage; further study is needed to determine proper chemotherapy utilization for these patients.
Bronchopulmonary carcinoid tumors are relatively rare and often indolent in nature tumors that account for roughly 2% of primary lung cancers. Carcinoid tumors exist on a spectrum of neuroendocrine tumors of the lung that arise from Kulchitsky cells with shared pathologic and biochemical characteristics. Other cancers classified as neuroendocrine include small cell lung cancers, gastrointestinal carcinoids, thymic carcinoids and Merkel cell carcinomas [1–3]. Carcinoid tumors are classified either as typical (<2 mitoses/2mm2 of viable tumor) or atypical (2–10 mitoses, necrosis, or architectural disruption) [4]. Atypical carcinoids have an aggressive course with 5- and 10-year survival rates in the range of 35–44%. This is attributed to the higher frequency of patients presenting with advanced stage as well as higher rates of recurrence compared to typical carcinoids [5].
Management of patients with typical carcinoid is founded on surgical resection [6–8], however due to the aggressive nature of atypical tumors clinicians often employ adjuvant chemotherapy for high-risk disease with large and locally advanced tumors (stage IIB/IIIA). This therapeutic regimen is based on data that demonstrated a 20% response rate to any chemotherapy [2]. While patients with pulmonary carcinoid tumors have been included in institutional and large-scale studies, very little data exist on the role of adjuvant chemotherapy in node negative atypical carcinoid tumors [9–11]. Studies on the use of adjuvant therapy in pathologic node-positive disease (pN+) are similarly lacking [12]. To help define the role of adjuvant therapy for atypical pulmonary carcinoids, we undertook a national retrospective study of pathologically node-negative and node-positive patients who either had surgery alone or received adjuvant treatment postoperatively between 2006 and 2011. We hypothesized that adjuvant chemotherapy does not confer a survival advantage compared to surgery alone for pN0 patients, but would enhance survival for those with pN+ disease.
Patients and Methods
Data Source
The Duke University Institutional Review Board granted exempt status for this retrospective analysis of the National Cancer Data Base (NCDB). The National Cancer Data Base (NCDB) Participant User File for 2006 through 2011 was utilized for this retrospective analysis. The NCDB is a nationwide database, consisting of data from over 1,500 Commission on Cancer institutions and is jointly administered by the American Cancer Society and the American College of Surgeons Commission on Cancer. It is estimated to capture records from more than 30 million patients and approximately 70% of all new cancer diagnoses in the United States annually [13].
Patient Selection
Patients treated with lobectomy or pneumonectomy (Facility Oncology Data Standards Procedure codes 30–33 and 55–65) for a diagnosis of pulmonary atypical carcinoid tumor were identified using International Classification of Diseases for Oncology, 3rd Edition (ICD-O-3) topography codes C34.0–3 and C34.8–9 and histology code 8249, defined as carcinoid tumors with atypical features. Patients with a previous cancer diagnosis, evidence of metastatic disease, receipt of neoadjuvant chemotherapy or missing information regarding pathologic nodal status or use of chemotherapy were excluded a priori from analysis. As our primary analysis focused on the role of adjuvant therapy in this patient population, only patients surviving at least 30 days post-operatively were included for analysis.
Statistical Analysis
Patients were initially grouped by pathologic lymph node status (positive versus negative). Both groups were then stratified by the use of adjuvant chemotherapy. Baseline characteristics and outcomes between groups were compared using Pearson’s chi-square test for discrete variables and Kruskal-Wallis analysis of variance for continuous variables. To control for potential confounding in the use of chemotherapy we developed propensity scores, which we defined as the probability of being treated with chemotherapy following resection, conditional on the following variables: patient age, sex, race, Charlson/Deyo comorbidity score, tumor size, extent of resection (pneumonectomy vs. lobectomy), treatment facility type (academic or community hospital), and hospital length of stay. Patients were matched based on propensity scores using a 4:1 nearest neighbor algorithm (MatchIt: Nonparametric Preprocessing for Parametric Casual Inference). A 1:1 ratio was selected for matching of the pN+ cohort due to equivalency in treatment group sizes. Balance was assessed using standardized differences, and adjusted outcomes between the propensity-matched groups were then compared. Long-term survival among both the unadjusted and propensity-matched groups was estimated using the Kaplan-Meier method and the log-rank test.
Results are reported as median (IQR), proportions (%) and odds ratios (OR, 95% CI) as applicable. P-values <0.05 indicate statistical significance, and we controlled for type I error at the level of the comparison. All statistical analyses were performed using R (The R Foundation for Statistical Computing, version 3.1.2, Vienna, Austria).
RESULTS
Baseline Characteristics
We identified a total of 581 patients with a diagnosis of atypical carcinoid tumor. Overall, 363 patients (62%) had node-negative (pN0) disease, while 218 patients (38%) had positive lymph node metastasis (pN+). We further subdivided these patients into those who received adjuvant chemotherapy and those who underwent surgery alone. Of the pN0 patients, 15 (4%) received post-operative chemotherapy while 348 (96%) underwent surgery alone. 89 (41%) pN+ patients received adjuvant therapy and 129 (59%) had surgery only. There were no significant differences in demographic, clinical, and tumor characteristics including age, gender, Charlson-Deyo comorbidity score, income status, tumor size, and type of treatment facility between pN0 patients who received adjuvant therapy and those who had surgery alone (Table 1). PN+ patients who underwent adjuvant therapy tended to be younger (57 vs. 63, p=0.06) and travel longer distances (18 vs. 11 miles, p=0.005) for their care compared to those who had surgery alone, though rates of treatment at community, comprehensive cancer center, and academic medical centers were similar (Table 1).
Table 1.
Unadjusted Patient and Tumor Characteristics by pathological nodal status, NCDB 2006–2011
| pN0 Disease | pN+ Disease | |||||||
|---|---|---|---|---|---|---|---|---|
| Overall | Adjuvant Chemo (n = 15) | Surgery alone (n = 348) | P-value | Overall | Adjuvant Chemo (n = 89) | Surgery alone (n = 129) | P-value | |
| Patient characteristics | ||||||||
| Age, yrs (IQR) | 61 (53, 69) | 61 (53.5, 70) | 61 (53, 69) | 0.98 | 62 (52, 71) | 57 (50, 69) | 63 (55, 72) | 0.006 |
| Female | 219 (60.3%) | 6 (40%) | 213 (61.2%) | 0.17 | 146 (67%) | 55 (61.8%) | 91 (70.5%) | 0.23 |
| Race | 0.71 | 0.53 | ||||||
| White | 314 (88%) | 14 (93.3%) | 300 (87.7%) | 194 (89.8%) | 80 (92%) | 114 (88.4%) | ||
| Black | 30 (8.4%) | 1 (6.7%) | 29 (8.5%) | 22 (10.2%) | 7 (8%) | 15 (11.6%) | ||
| Other | 13 (3.6%) | 0 (0%) | 13 (3.8%) | 0 (0%) | 0 (0%) | 0 (0%) | ||
| Charlson-Deyo Score | 0.57 | 0.05 | ||||||
| 0 | 228 (62.8%) | 8 (53.3%) | 220 (63.2%) | 143 (65.6%) | 66 (74.2%) | 77 (59.7%) | ||
| 1 | 102 (28.1%) | 6 (40%) | 96 (27.6%) | 56 (25.7%) | 19 (21.3%) | 37 (28.7%) | ||
| ≥2 | 33 (9.1%) | 1 (6.7%) | 32 (9.2%) | 19 (8.7%) | 4 (4.5%) | 15 (11.6%) | ||
| Education census tract | 0.5 | 0.88 | ||||||
| Bottom | 52 (14.6%) | 2 (13.3%) | 50 (14.7%) | 31 (14.3%) | 11 (12.4%) | 20 (15.6%) | ||
| Second | 87 (24.5%) | 6 (40%) | 81 (23.8%) | 47 (21.7%) | 21 (23.6%) | 26 (20.3%) | ||
| Third | 131 (36.9%) | 5 (33.3%) | 126 (37.1%) | 92 (42.4%) | 38 (42.7%) | 54 (42.2%) | ||
| Top | 85 (23.9%) | 2 (13.3%) | 83 (24.4%) | 47 (21.7%) | 19 (21.3%) | 28 (21.9%) | ||
| Income census tract | 0.84 | 0.7 | ||||||
| Bottom | 48 (13.5%) | 3 (20%) | 45 (13.2%) | 29 (13.4%) | 10 (11.2%) | 19 (14.8%) | ||
| Second | 86 (24.2%) | 4 (26.7%) | 82 (24.1%) | 46 (21.2%) | 21 (23.6%) | 25 (19.5%) | ||
| Third | 96 (27%) | 4 (26.7%) | 92 (27.1%) | 76 (35%) | 29 (32.6%) | 47 (36.7%) | ||
| Top | 125 (35.2%) | 4 (26.7%) | 121 (35.6%) | 66 (30.4%) | 29 (32.6%) | 37 (28.9%) | ||
| Insurance status | 0.61 | 0.05 | ||||||
| Private | 184 (50.7%) | 7 (46.7%) | 177 (50.9%) | 104 (47.7%) | 50 (56.2%) | 54 (41.9%) | ||
| Medicare/Medicaid | 160 (44.1%) | 6 (40%) | 154 (44.3%) | 107 (49.1%) | 34 (38.2%) | 73 (56.6%) | ||
| Other Government | 2 (0.6%) | 0 (0%) | 2 (0.6%) | 1 (0.5%) | 1 (1.1%) | 0 (0%) | ||
| Unknown | 8 (2.2%) | 1 (6.7%) | 7 (2%) | 2 (0.9%) | 1 (1.1%) | 1 (0.8%) | ||
| Uninsured | 9 (2.5%) | 1 (6.7%) | 8 (2.3%) | 4 (1.8%) | 3 (3.4%) | 1 (0.8%) | ||
| Tumor characteristics | ||||||||
| Tumor size (mm) | 22 (15, 34) | 30 (19, 45) | 22 (15, 32.2) | 0.08 | 30 (20, 36.8) | 27 (20, 35) | 30 (20, 40) | 0.38 |
| Pathologic stage | 0.72 | 0.31 | ||||||
| Stage 1 | 201 (92.6%) | 8 (100%) | 193 (92.3%) | 1 (0.7%) | 0 (0%) | 1 (1.2%) | ||
| Stage 2 | 14 (6.5%) | 0 (0%) | 14 (6.7%) | 79 (54.1%) | 30 (48.4%) | 49 (58.3%) | ||
| Stage 3 | 2 (0.9%) | 0 (0%) | 2 (1%) | 66 (45.2%) | 32 (51.6%) | 34 (40.5%) | ||
| Facility characteristics | ||||||||
| Distance traveled (miles, IQR) | 11.7 (4.8, 31.6) | 9.8 (4.4, 18.1) | 11.8 (4.9, 31.7) | 0.48 | 14 (5.2, 28.8) | 18 (7.8, 32.1) | 10.6 (4.6, 22.8) | 0.005 |
| Treatment facility | 0.56 | 0.64 | ||||||
| Community Program | 16 (4.4%) | 1 (6.7%) | 15 (4.3%) | 7 (3.2%) | 4 (4.5%) | 3 (2.3%) | ||
| Comprehensive Community Program | 169 (46.7%) | 5 (33.3%) | 164 (47.3%) | 115 (53%) | 45 (51.1%) | 70 (54.3%) | ||
| Academic Program | 177 (48.9%) | 9 (60%) | 168 (48.4%) | 95 (43.8%) | 39 (44.3%) | 56 (43.4%) | ||
Percentages have been rounded and may not add up to 100 %
NCDB National Cancer Data Base, pN0 pathologically node negative, pN+ pathologically node positive,
IQR Interquartile Range
Short-Term Perioperative Outcomes
Those pN0 patients who received surgery followed by adjuvant therapy were noted to have modestly longer lengths of stay in the hospital (6 [IQR 4, 13] vs. 5 [IQR 4,7] days, p=0.046) compared to those who received surgical resection alone. All other short-term outcomes including surgical margin status, 30-day readmission, and 30- and 90-day mortality were similar between the two groups of pN0 patients (Table 2). For patients with node-negative disease, after multivariable adjustment for patient age and comorbidity burden, increasing tumor size remained independently associated with higher odds of treatment with adjuvant therapy (p=0.04).
Table 2.
Unadjusted short-term outcomes by pathological nodal status, NCDB 2006–2011
| Variable | pN0 Disease | pN+ Disease | ||||||
|---|---|---|---|---|---|---|---|---|
| Overall | Adjuvant Chemo (n = 15) | Surgery alone (n = 348) | P-value | Overall | Adjuvant Chemo (n = 89) | Surgery alone (n = 129) | P-value | |
| Nodes examined (median, IQR) | 7 (4, 13) | 8 (2.5, 19.5) | 7 (4, 13) | 0.68 | 10 (6, 17) | 10 (6, 17) | 10 (5, 18) | 0.57 |
| Margins | 0.94 | 0.08 | ||||||
| R0 | 353 (97.5%) | 15 (100%) | 338 (97.4%) | 196 (90.7%) | 78 (88.6%) | 118 (92.2%) | ||
| R1 | 5 (1.4%) | 0 (0%) | 5 (1.4%) | 11 (5.1%) | 5 (5.7%) | 6 (4.7%) | ||
| R2 | 1 (0.3%) | 0 (0%) | 1 (0.3%) | 3 (1.4%) | 0 (0%) | 3 (2.3%) | ||
| Positive NOS | 3 (0.8%) | 0 (0%) | 3 (0.9%) | 6 (2.8%) | 5 (5.7%) | 1 (0.8%) | ||
| 30-day readmission | 12 (3.4%) | 0 (0%) | 12 (3.5%) | 0.99 | 8 (3.8%) | 4 (4.7%) | 4 (3.1%) | 0.72 |
| 30-day mortality | 0 (0%) | 0 (0%) | 0 (0%) | 0.99 | 0 (0%) | 0 (0%) | 0 (0%) | 0.99 |
| 90-day mortality | 2 (0.6%) | 1 (6.7%) | 1 (0.3%) | 0.15 | 0 (0%) | 0 (0%) | 0 (0%) | 0.99 |
| Hospital LOS (IQR) | 5 (4, 7) | 6 (4, 13) | 5 (4, 7) | 0.046 | 5 (4, 7) | 5 (4, 6) | 5 (4, 7) | 0.032 |
Percentages have been rounded and may not add up to 100 %
NCDB National Cancer Data Base, pN0 pathologically node negative, pN+ pathologically node positive,
LOS Length of stay, IQR Interquartile Range
Propensity Matched Perioperative Outcomes
After 4:1 propensity matching, there were 75 pN0 patients who met criteria: 15 (20%) of whom received adjuvant chemotherapy while 60 (80%) had surgery alone. There were no significant differences between these patients in terms of their demographic, clinical, and facility characteristics. (Table 3) These patients also had similar short-term outcomes including margin status, length of stay, number of lymph nodes examined, readmission, and 30- and 90-day mortality (Table 4). Following propensity matching, pN+ patients receiving adjuvant chemotherapy or surgery alone were well balanced across demographic, clinical, and facility characteristics.
Table 3.
Baseline characteristics for patients with pathological node-negative disease, following 4:1 propensity matching, NCDB 2006–2011
| Variable | Overall | Adjuvant chemo (n = 15) | Surgery alone (n = 60) | P-value |
|---|---|---|---|---|
| Patient characteristics | ||||
| Age, yrs (IQR) | 61 (53, 69.5) | 61 (53.5, 70) | 61 (53, 68.5) | 0.97 |
| Female | 38 (50.7%) | 6 (40%) | 32 (53.3%) | 0.53 |
| Race | 0.74 | |||
| White | 66 (89.2%) | 14 (93.3%) | 52 (88.1%) | |
| Black | 6 (8.1%) | 1 (6.7%) | 5 (8.5%) | |
| Other | 2 (2.7%) | 0 (0%) | 2 (3.4%) | |
| Charlson Comorbidity Score | 0.9 | |||
| 0 | 37 (49.3%) | 8 (53.3%) | 29 (48.3%) | |
| 1 | 31 (41.3%) | 6 (40%) | 25 (41.7%) | |
| ≥2 | 7 (9.3%) | 1 (6.7%) | 6 (10%) | |
| Education census tract | 0.051 | |||
| Bottom | 10 (13.9%) | 2 (13.3%) | 8 (14%) | |
| Second | 12 (16.7%) | 6 (40%) | 6 (10.5%) | |
| Third | 32 (44.4%) | 5 (33.3%) | 27 (47.4%) | |
| Top | 18 (25%) | 2 (13.3%) | 16 (28.1%) | |
| Income census tract | 0.68 | |||
| Bottom | 11 (15.3%) | 3 (20%) | 8 (14%) | |
| Second | 19 (26.4%) | 4 (26.7%) | 15 (26.3%) | |
| Third | 14 (19.4%) | 4 (26.7%) | 10 (17.5%) | |
| Top | 28 (38.9%) | 4 (26.7%) | 24 (42.1%) | |
| Insurance status | 0.78 | |||
| Private | 38 (50.7%) | 7 (46.7%) | 31 (51.7%) | |
| Medicare/Medicaid | 31 (41.3%) | 6 (40%) | 25 (41.7%) | |
| Other Government | 1 (1.3%) | 0 (0%) | 1 (1.7%) | |
| Unknown | 2 (2.7%) | 1 (6.7%) | 1 (1.7%) | |
| Uninsured | 3 (4%) | 1 (6.7%) | 2 (3.3%) | |
| Tumor characteristics | ||||
| Tumor size (mm) | 30 (20, 45) | 30 (19, 45) | 30 (23, 45) | 0.87 |
| Pathologic stage | 0.55 | |||
| Stage 1 | 35 (87.5%) | 8 (100%) | 27 (84.4%) | |
| Stage 2 | 5 (12.5%) | 0 (0%) | 5 (15.6%) | |
| Stage 3 | 0 (0%) | 0 (0%) | 0 (0%) | |
| Facility characteristics | ||||
| Distance traveled (miles, IQR) | 11.8 (4.8, 32.6) | 9.8 (4.4, 18.1) | 11.9 (5.5, 33.6) | 0.4 |
| Treatment facility | 0.78 | |||
| Community Program | 4 (5.3%) | 1 (6.7%) | 3 (5%) | |
| Comprehensive Community Program | 31 (41.3%) | 5 (33.3%) | 26 (43.3%) | |
| Academic Program | 40 (53.3%) | 9 (60%) | 31 (51.7%) | |
Table 4.
Outcomes among patients with pathological node-negative disease, following 4:1 propensity matching, NCDB 2006–2011
| Variable | Overall | Adjuvant chemo (n = 15) | Surgery alone (n = 60) | P-value |
|---|---|---|---|---|
| Nodes examined (median, IQR) | 7 (4, 13) | 8 (2.5, 19.5) | 6.5 (4, 13) | 0.72 |
| Margins | 0.99 | |||
| R0 | 74 (98.7%) | 15 (100%) | 59 (98.3%) | |
| R1 | 0 (0%) | 0 (0%) | 0 (0%) | |
| R2 | 1 (1.3%) | 0 (0%) | 1 (1.7%) | |
| 30-day readmission | 4 (5.5%) | 0 (0%) | 4 (6.8%) | 0.99 |
| 30-day mortality | 0 (0%) | 0 (0%) | 0 (0%) | 0.99 |
| 90-day mortality | 2 (2.7%) | 1 (6.7%) | 1 (1.7%) | 0.87 |
| Hospital LOS (IQR) | 6 (4, 7) | 6 (4.2, 12.8) | 5 (4, 6.5) | 0.13 |
Percentages have been rounded and may not add up to 100 %
NCDB National Cancer Data Base
LOS Length of stay, IQR Interquartile Range
Overall Survival
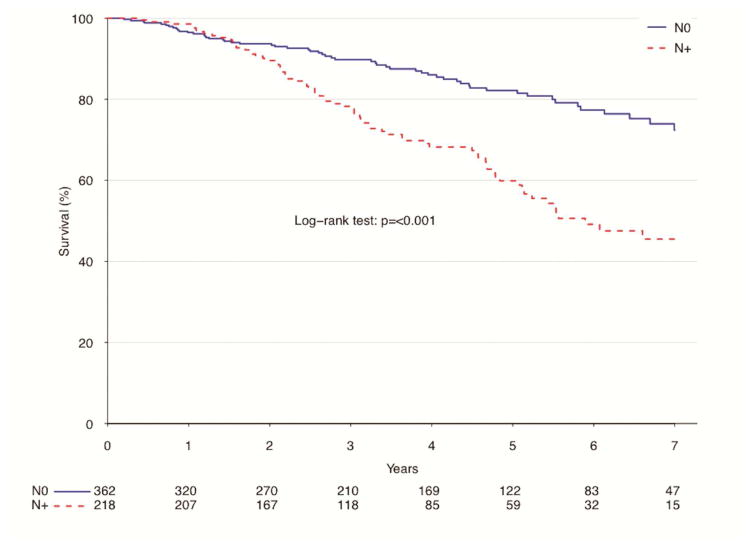
In an unadjusted analysis of patients by nodal status (pN0 vs. pN+) those with node-negative disease had a significantly lower percentage of patients survive after 1-year (96.5 vs. 98.6%, p<0.001), but had a higher survival rate after 5-years (82.2 vs. 59.8%, p<0.001) compared to those with node-positive tumors. (Figure 1).
Figure 1.
Survival for patients with atypical lung carcinoids by nodal status, NCDB 2006–2011
| Estimate | Overall | Node Negative | Node Positive | P value |
|---|---|---|---|---|
| 12-month survival | 97.3% (95.9–98.7%) | 96.5% (94.5–98.5%) | 98.6% (97–100%) | |
| 60-month survival | 73.5% (69.2–78.1%) | 82.2% (77.5–87.2%) | 59.8% (52.2–68.6%) | |
| Median survival | 100.2 months | NA months | 70.7 months | <0.001 |
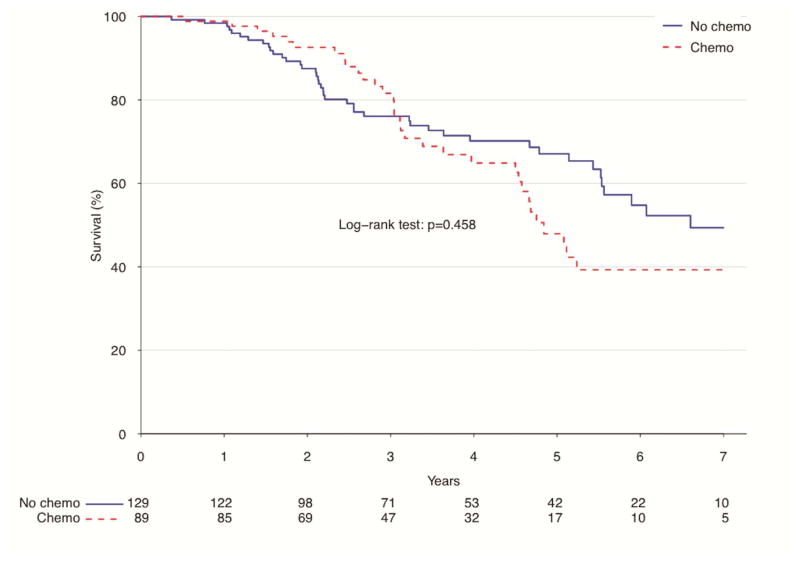
For those patients with pN+ disease, there was no significant difference in long-term survival between those who received adjuvant chemotherapy treatment after resection and those who had surgery alone (Figure 2). Those who had surgery alone did have a lower rate of survival after one year (98.4 vs. 98.9%), but higher survival after 5 years (67.1 vs. 47.9%, p=0.46) compared to those who received adjuvant therapy in the unadjusted analysis.
Figure 2.
Unadjusted survival for patients with pN+ atypical lung carcinoids by use of adjuvant chemotherapy, NCDB 2006–2011
| Estimate | Overall | Surgery Alone | Adjuvant Chemo | P value |
|---|---|---|---|---|
| 12-month survival | 98.6% (97–100%) | 98.4% (96.2–100%) | 98.9% (96.6–100%) | |
| 60-month survival | 59.8% (52.2–68.6%) | 67.1% (58.3–77.2%) | 47.9% (35.7–64.3%) | |
| Median survival | 70.7 months | 79.2 months | 58.1 months | 0.458 |
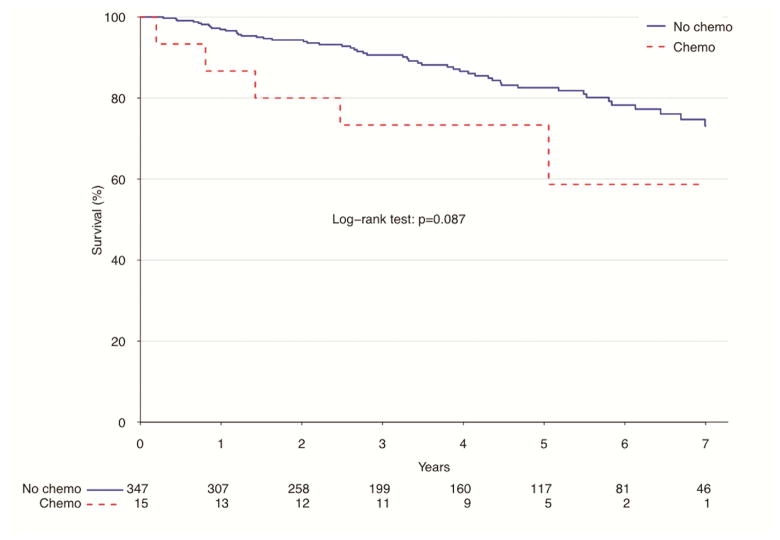
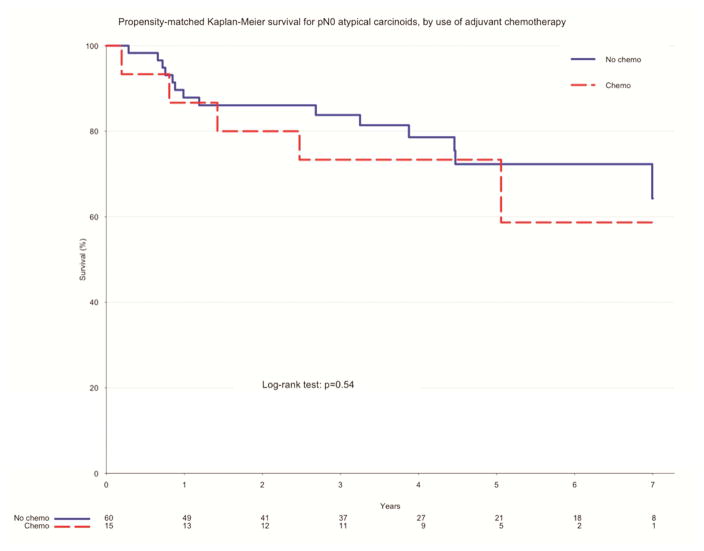
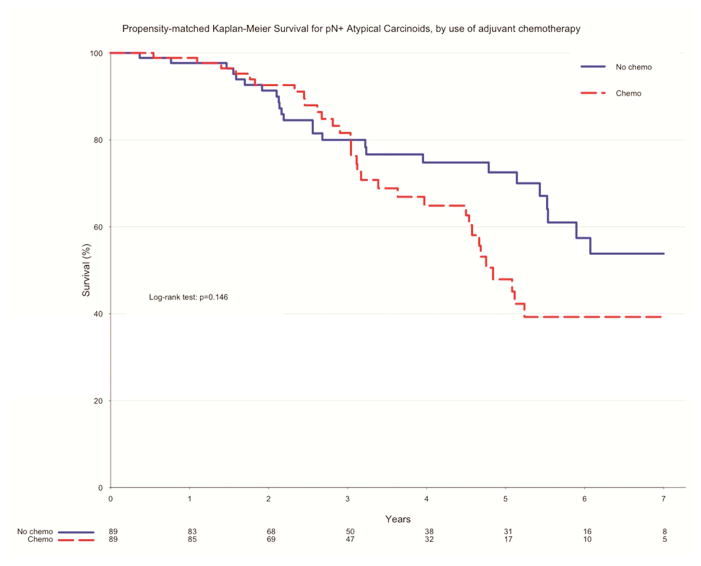
In the unadjusted measure of overall survival of pN0 patients who had surgery alone and those who received adjuvant chemotherapy, there was no significant difference between the two groups. Those who underwent surgery alone had higher rates of survival at 1 year (96.9 vs. 86.7%, p=0.09) and 5 years (82.6 vs. 73.3%, p=0.09) (Figure 3). After propensity matching, there remained no difference in overall survival between the two groups. Patients who had surgery alone had a higher rate of survival to 1 year (87.9 vs. 86.7%, p=0.54), while those who received adjuvant therapy had a higher rate of survival to 5 years (73.3 vs. 72.3%, p=0.54) (Figure 4). Of propensity-matched pN+ patients, those receiving chemotherapy and surgery alone were equally likely to survive to one year (97.7% v. 98.9% for surgery alone and adjuvant chemotherapy, respectively, p=0.15). Five-year survival favored surgery alone (72.5% v. 47.9%, p =0.15), though this finding was not determined to be significant (Figure 5).
Figure 3.
Survival for pN0 atypical carcinoids by use of adjuvant chemotherapy, NCDB 2006–2011
| Estimate | Overall | Surgery Alone | Adjuvant Chemo | P value |
|---|---|---|---|---|
| 12-month survival | 96.5% (94.5–98.5%) | 96.9% (95.1–98.8%) | 86.7% (71.1–100%) | |
| 60-month survival | 82.2% (77.5–87.2%) | 82.6% (77.7–87.7%) | 73.3% (54–99.5%) | |
| Median survival | NA months | NA months | NA months | 0.087 |
Figure 4.
Propensity-matched survival for pN0 atypical carcinoids by use of adjuvant chemotherapy, NCDB 2006–2011
| Estimate | Overall | Surgery Alone | Adjuvant Chemo | P value |
|---|---|---|---|---|
| 12-month survival | 87.6% (80.4–95.5%) | 87.9% (79.8–96.7%) | 86.7% (71.1–100%) | |
| 60-month survival | 72.8% (62.2–85.2%) | 72.3% (60–87.2%) | 73.3% (54–99.5%) | |
| Median survival | 95.7 months | 95.7 months | NA months | 0.54 |
Figure 5.
Propensity-Matched survival for pN+ atypical carcinoids by use of adjuvant chemotherapy, NCDB 2006–2011.
| Estimate | Overall | Surgery Alone | Adjuvant Chemo | P value |
|---|---|---|---|---|
| 12-month survival | 98.3% (96.4–100%) | 97.7% (94.5–100%) | 98.9% (96.6–100%) | |
| 60-month survival | 60.9% (52.4–70.7%) | 72.5% (62.4–84.3%) | 47.9% (35.7–64.3%) | |
| Median survival | 78.3 months | 80.8 months | 72 months | 0.146 |
COMMENT
This nationwide analysis aimed to determine the impact of adjuvant chemotherapy on survival of patients with pathologically node-negative atypical carcinoma. In both the unadjusted and propensity-matched analysis, we saw no significant difference in the demographic, clinical, and treatment characteristics between those patients who had surgery alone and those who received chemotherapy postoperatively. Survival, however, was similar between the two groups in both the unadjusted and propensity-matched cohorts, which illustrates that utilization of adjuvant therapy did not confer a survival advantage in our patient population.
To the best of our knowledge, this is the largest study to evaluate pN0 atypical pulmonary carcinoids and the first to assess the difference in outcomes between patients who receive adjuvant therapy and those who receive surgery alone. Currently, there are consensus-based guidelines from the North American Neuroendocrine Tumor Society (NANETs) that make note of insufficient data to recommend the use of adjuvant therapy after complete resection of locoregional disease for any subgroup, including atypical carcinoid [14]. This practice pattern was evident in our cohort with 96% of pN0 patients receiving surgery alone. The literature demonstrates that nodal involvement clearly impacts survival with the 5- and 10-year survival for pN0 atypical carcinoid at 85% and 70% respectively compared to 60% and 50% respectively for pN1 and pN2 patients [5, 12, 15]. Although the sample size is small, the use of adjuvant chemotherapy in pN0 patients conferred no survival advantage in this national retrospective cohort study; therefore, the use of chemotherapy should be carefully considered in patients with node-negative disease. The advantage of adjuvant chemotherapy in the pN+ population was also examined using both unadjusted and adjusted methodologies. We were unable to establish a benefit to adjuvant chemotherapy in the pN+ population. It is clear by the balanced rates of surgery-alone and adjuvant chemotherapy strategies that there exists equipoise in this regard, or that the decision to proceed with adjuvant chemotherapy is based on variables unmeasured in NCDB. Adequately-powered prospective studies might better ascertain the role of adjuvant chemotherapy in the pN+ population, but will likely never be completed due to the uncommon nature of the disease and logistical challenges. At this time, current NCCN guidelines provide a category 2A recommendation for the use of platinum-based adjuvant therapy for pathologically stage II or III atypical pulmonary carcinoids.
Bronchopulmonary carcinoids are rare tumors that have been increasing in prevalence at an estimated rate of 6% over the last 30 years after accounting for confounding factors such as age, gender, race, and stage distribution [16–18]. The use of chemotherapeutic agents in the treatment of pulmonary carcinoids has provided limited benefits, most of which are short-term [19]. Because of minimal response rates to chemotherapy in carcinoid tumors along with the associated comorbidities of such a therapy - including nephrotoxicity and cytopenia - the indications to use chemotherapy are limited [20].
Some experts, based on clinical experience and personal preference, recommend further treatment postoperatively for patients with an atypical carcinoid that has spread to lymph nodes. However, it is not clear if the added treatments lower the chance of the cancer recurring, or if they prolong survival. In many situations the determination of whether to utilize adjuvant therapy is based due to the presence of positive margins, local invasion, and/or lymph node involvement. All of the patients who received adjuvant therapy in this study underwent complete resection Due to the lack of prospective randomized clinical trials, the role of adjuvant therapy in node-negative atypical carcinoid is undefined. However, the benefits of adjuvant chemotherapy have been analyzed in small retrospective cohort series [1, 9, 21–23]. In a single institutional study by Perkins et al, in a study of 73 patients with both typical and atypical lung carcinoids, seven of whom received adjuvant chemotherapy and/or radiation therapy due to margin status, lymph node status, or invasion into surrounding structures. Only one of these patients who underwent chemotherapy and/or radiation had no evidence of disease and was alive after ten years [24]. In a series of 42 patients with pulmonary carcinoids (26 typical, 16 atypical) by Divisi et al, chemotherapy and/or radiation therapy was administered to seven patients with stage III disease. Despite utilization of adjuvant therapy, five of those patients recurred and died of their disease, all of whom had atypical histology [21]. In a national level analysis, by Nussbaum et al., of 629 patients with typical carcinoid, 37 (6%) of whom received adjuvant therapy, there was no significant difference in survival compared to those who underwent surgery alone in the propensity-matched cohort. Both the Perkins et al and Divisi et al analyses are limited in their generalizability and sample size as single institutional studies. They also don’t reflect the advancement of surgical and chemotherapeutic techniques since the data was collected between 1959 and 2003. While Nussbaum et al does capture an updated national representation of clinical practice and outcomes, the studies focus is that of typical carcinoids, which are considered to be less responsive to medical therapies.
This population-level analysis should be considered in light of limitations that are inherent to all large national databases. Despite the standardized and highly analyzed data reporting, there is always the potential for coding errors in this database. This study is also retrospective in nature, which makes it susceptible to selection bias of patients treated with adjuvant chemotherapy who may have received additional therapy on account of concerning features not captured in the standard NCDB variables. Our study is also limited by the small number of pN0 patients who received adjuvant chemotherapy and in its ability to ascertain whether specific mediastinal nodal stations were sampled which has a possibility of type II error. Due to the limited sample size of pN0 adjuvant patients, the strength of the conclusions are limited in scope and generalizability. However, even in the more numerous pN+ analysis, which in theory would contain patients more likely to benefit from chemotherapy, there was no detectable difference in survival whether adjuvant chemotherapy was provided.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Marty-Ane CH, Costes V, Pujol JL, Alauzen M, Baldet P, Mary H. Carcinoid tumors of the lung: Do atypical features require aggressive management? Ann Thorac Surg. 1995;59:78–83. doi: 10.1016/0003-4975(94)00630-P. [DOI] [PubMed] [Google Scholar]
- 2.Wirth LJ, Carter MR, Janne PA, Johnson BE. Outcome of patients with pulmonary carcinoid tumors receiving chemotherapy or chemoradiotherapy. Lung cancer. 2004;44:213–220. doi: 10.1016/j.lungcan.2003.11.016. [DOI] [PubMed] [Google Scholar]
- 3.Kulke MH, Mayer RJ. Carcinoid tumors. NEJM. 1999;340:858–868. doi: 10.1056/NEJM199903183401107. [DOI] [PubMed] [Google Scholar]
- 4.Travis WD, Rush W, Flieder DB, et al. Survival analysis of 200 pulmonary neuroendocrine tumors with clarification of criteria for atypical carcinoid and its separation from typical carcinoid. Am J Surg Pathol. 1998;22:934–944. doi: 10.1097/00000478-199808000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Garcia-Yuste M, Matilla JM, Cueto A, et al. Typical and atypical carcinoid tumours: Analysis of the experience of the spanish multi-centric study of neuroendocrine tumours of the lung. Eur J Cardiothorac Surg. 2007;31:192–197. doi: 10.1016/j.ejcts.2006.11.031. [DOI] [PubMed] [Google Scholar]
- 6.Oberg K, Hellman P, Ferolla P, Papotti M. Neuroendocrine bronchial and thymic tumors: Esmo clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23:120–123. doi: 10.1093/annonc/mds267. [DOI] [PubMed] [Google Scholar]
- 7.Steuer CE, Behera M, Kim S, et al. Atypical carcinoid tumor of the lung: A surveillance, epidemiology, and end results database analysis. J Thorac Oncol. 2015;10:479–485. doi: 10.1097/JTO.0000000000000419. [DOI] [PubMed] [Google Scholar]
- 8.Filosso PL, Ferolla P, Guerrera F, et al. Multidisciplinary management of advanced lung neuroendocrine tumors. J Thorac Dis. 2015;7:S163–171. doi: 10.3978/j.issn.2072-1439.2015.04.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Filosso PL, Oliaro A, Ruffini E, et al. Outcome and prognostic factors in bronchial carcinoids: A single-center experience. J Thorac Oncol. 2013;8:1282–1288. doi: 10.1097/JTO.0b013e31829f097a. [DOI] [PubMed] [Google Scholar]
- 10.Han B, Sun JM, Ahn JS, Park K, Ahn MJ. Clinical outcomes of atypical carcinoid tumors of the lung and thymus: 7-year experience of a rare malignancy at single institute. Med Oncol. 2013;30:479. doi: 10.1007/s12032-013-0479-x. [DOI] [PubMed] [Google Scholar]
- 11.Pavel ME, Hainsworth JD, Baudin E, et al. Everolimus plus octreotide long-acting repeatable for the treatment of advanced neuroendocrine tumours associated with carcinoid syndrome (radiant-2): A randomised, placebo-controlled, phase 3 study. Lancet. 2011;378:2005–2012. doi: 10.1016/S0140-6736(11)61742-X. [DOI] [PubMed] [Google Scholar]
- 12.Detterbeck FC. Management of carcinoid tumors. Ann Thorac Surg. 2010;89:998–1005. doi: 10.1016/j.athoracsur.2009.07.097. [DOI] [PubMed] [Google Scholar]
- 13.Raval MV, Bilimoria KY, Stewart AK, Bentrem DJ, Ko CY. Using the ncdb for cancer care improvement: An introduction to available quality assessment tools. J Surg Oncol. 2009;99:488–490. doi: 10.1002/jso.21173. [DOI] [PubMed] [Google Scholar]
- 14.Phan AT, Oberg K, Choi J, et al. Nanets consensus guideline for the diagnosis and management of neuroendocrine tumors: Well-differentiated neuroendocrine tumors of the thorax (includes lung and thymus) Pancreas. 2010;39:784–798. doi: 10.1097/MPA.0b013e3181ec1380. [DOI] [PubMed] [Google Scholar]
- 15.Travis WD, Giroux DJ, Chansky K, et al. The IASLC lung cancer staging project: Proposals for the inclusion of broncho-pulmonary carcinoid tumors in the forthcoming (seventh) edition of the TNM classification for lung cancer. J Thorac Oncol. 2008;3:1213–1223. doi: 10.1097/JTO.0b013e31818b06e3. [DOI] [PubMed] [Google Scholar]
- 16.Caplin ME, Baudin E, Ferolla P, et al. Pulmonary neuroendocrine (carcinoid) tumors: European neuroendocrine tumor society expert consensus and recommendations for best practice for typical and atypical pulmonary carcinoids. Ann Oncol. 2015;26:1604–1620. doi: 10.1093/annonc/mdv041. [DOI] [PubMed] [Google Scholar]
- 17.Travis WD, Travis LB, Devesa SS. Lung cancer. Cancer. 1995;75:191–202. doi: 10.1002/1097-0142(19950101)75:1+<191::aid-cncr2820751307>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 18.Modlin IM, Lye KD, Kidd M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer. 2003;97:934–959. doi: 10.1002/cncr.11105. [DOI] [PubMed] [Google Scholar]
- 19.Beasley MB, Thunnissen FB, Brambilla E, et al. Pulmonary atypical carcinoid: Predictors of survival in 106 cases. Human pathology. 2000;31:1255–1265. doi: 10.1053/hupa.2000.19294. [DOI] [PubMed] [Google Scholar]
- 20.Gustafsson BI, Kidd M, Chan A, Malfertheiner MV, Modlin IM. Bronchopulmonary neuroendocrine tumors. Cancer. 2008;113:5–21. doi: 10.1002/cncr.23542. [DOI] [PubMed] [Google Scholar]
- 21.Divisi D, Crisci R. Carcinoid tumors of the lung and multimodal therapy. The Thoracic and cardiovascular surgeon. 2005;53:168–172. doi: 10.1055/s-2005-837539. [DOI] [PubMed] [Google Scholar]
- 22.Mackley HB, Videtic GM. Primary carcinoid tumors of the lung: A role for radiotherapy. Oncology. 2006;20:1537–1543. [PubMed] [Google Scholar]
- 23.Cooper WA, Thourani VH, Gal AA, Lee RB, Mansour KA, Miller JI. The surgical spectrum of pulmonary neuroendocrine neoplasms. Chest. 2001;119:14–18. doi: 10.1378/chest.119.1.14. [DOI] [PubMed] [Google Scholar]
- 24.Perkins P, Kemp BL, Putnam JB, Jr, Cox JD. Pretreatment characteristics of carcinoid tumors of the lung which predict aggressive behavior. Am J Clin Oncol. 1997;20:285–288. doi: 10.1097/00000421-199706000-00016. [DOI] [PubMed] [Google Scholar]