Abstract
Background
Tuberous Sclerosis Complex (TSC) is a genetic disorder with high prevalence of associated autism spectrum disorder (ASD). Primary objectives were to determine early predictors of autism risk to identify children with TSC in most need of early interventions. The Autism Observation Scale for Infants (AOSI) was evaluated as a measure of ASD-associated behaviors in infants with TSC at age 12 months and its ability to predict ASD at 24 months.
Methods
Children ages 0 to 36 months with TSC were enrolled in the TSC Autism Center of Excellence Research Network (TACERN), a multicenter, prospective observational study to identify biomarkers of ASD. The AOSI was administered at age 12 months and the Autism Diagnostic Observation Schedule-2 (ADOS-2) and Autism Diagnostic Interview-Revised (ADI-R) at 24 months. Developmental functioning was assessed using the Mullen Scales of Early Learning. Children were classified as ASD or non-ASD according to the ADOS-2.
Results
Analysis included 79 children who had been administered the AOSI at 12 months and ADOS-2 and ADI-R at 24 months. The ASD group had a mean AOSI total score at 12 months significantly higher than the non-ASD group (11.8 ± 7.4 vs. 6.3 ± 4.7; p<0.001). An AOSI total score cut-off of 13 provided a specificity of 0.89 to detect ASD with the ADOS-2. AOSI total score at 12 months was similarly associated with exceeding cut-off scores on the ADI-R.
Conclusions
The AOSI is a useful clinical tool in determining which infants with TSC are at increased risk for developing ASD.
Keywords: Tuberous Sclerosis Complex, autism spectrum disorder, Autism Observational Scale for Infants, Autism Diagnostic Observation Schedule, infants, toddlers
INTRODUCTION
Idiopathic autism spectrum disorder (ASD) affects 1–2% of the general population without a clear understanding of the underlying causes, which presents a major barrier to identifying at-risk infants and developing effective treatments to prevent or alter progression. ASD is typically diagnosed at an average age of 4 years1. However, parental concern has been shown to emerge during the child’s first two years of life with language and communication problems most commonly reported2,3. In children who screened positive on the Modified Checklist for Autism in Toddlers (M-CHAT, M-CHAT-R) between 18–24 months, 93.4% of parents first reported developmental concerns at a mean age of 13.77 months4. These same children were subsequently diagnosed by clinical assessment with ASD or developmental delay 94.4% of the time. Additional studies have demonstrated a high correlation between parental concern and clinical assessments5,6. Despite recognizing concerns regarding ASD-specific behaviors, parents and clinicians often fail to recognize ASD as the diagnosis. This highlights the importance of the clinician’s responsibility to validate parental concerns and perform timely objective assessments to make an appropriate diagnosis of ASD. The earlier this can be accomplished enables appropriate interventions and treatment strategies to be initiated when they offer the greatest potential for change.
Validated, formal assessment tools to identify ASD risk in infants are limited. The Autism Observation Scale for Infants (AOSI) was originally designed as a research tool to evaluate autism-specific behaviors in infants at elevated risk for developing ASD. The AOSI is a semi-structured assessment designed for infants ages 6 to 18 months with 18 individual items meant to evaluate different areas of concern seen in children with ASD, including sensory and motor behaviors, attention, visual tracking, and social emotional behaviors7. Scores for each item are evaluator-judged and range from 0 to 3 with higher numbers indicating elevated ASD risk behaviors. Reliability of the AOSI was tested in high-risk infant siblings (had older siblings with ASD) at ages 6, 12, and 18 months. Inter-rater reliability for individual items and total score were good to excellent, particularly at 12 months and beyond, and test-retest reliability at 12 months was acceptable7. Another study of infant siblings found that using both the AOSI at 18 months and ADOS at 36 months provided complementary information when making the diagnosis of ASD at age 3 years8. More recently, the AOSI was used clinically to evaluate early signs of ASD in high-risk infant siblings to determine the predictive ability of the AOSI to differentiate between high- and low-risk populations, as well as between those high-risk individuals who would eventually be diagnosed with ASD 9. Comparing the AOSI at 7 and 14 months and ADOS at 24 and 36 months, Gammer et al found that children who were diagnosed with ASD scored significantly higher on the AOSI at 14 months than those who were not diagnosed with ASD even when accounting for developmental level9. Collectively, these results indicate that the AOSI may be useful to differentiate concerning features for ASD in high-risk infants, but understanding how coexistent developmental delays impact AOSI scoring for ASD-risk behaviors at younger ages and how those scores may be predictive of later diagnosis of ASD requires additional investigation.
Currently, no single factor has been identified as a consistent predictor of ASD; but single-gene disorders with a high prevalence of ASD, such as Tuberous Sclerosis Complex (TSC), provide us with opportunities to investigate the underlying biology and identify potential treatments. TSC is a genetic disorder that affects multiple organ systems and is present in approximately 1 in 6000 individuals10. Retrospective and small pilot prospective studies have identified specific areas of cognitive impairment and autism spectrum behaviors in up to 50% of individuals with TSC11–14. A longitudinal cohort study compared differences between children with ASD with TSC to children with idiopathic ASD and found that syndromic ASD was similar to non-syndromic ASD in terms of their cognitive, behavioral, and social profiles15. Evaluation of the AOSI as an objective assessment tool to identify early ASD-associated behaviors in infants with TSC has been shown in a small prospective longitudinal cohort of infants13, but using the AOSI to predict later ASD risk in this population has not been previously reported. In 2012, we established the TSC Autism Center of Excellence Research Network (TACERN), a large multicenter, prospective observational study to identify clinical, structural, and electrophysiological biomarkers predictive of ASD with the overall goal to establish an infrastructure for early detection of ASD and set the stage for future drug trials in patients with TSC who are at high risk for ASD. Our primary objectives are to determine the earliest age at which autism risk behaviors can be detected in order to identify those children in most need of accessing autism-specific interventions. Here we report on the suitability of using the AOSI as an objective measure of ASD-associated behaviors in infants with TSC at 12 months of age and the ability of the AOSI to predict meeting ASD criteria on the ADOS-2 at 24 months of age.
METHODS
Subject Recruitment
Children ages 0 to 36 months with TSC were enrolled into TACERN (clinical trials.gov, NCT 01780441) at one of five sites across the United States (Cincinnati Children’s Hospital Medical Center, Boston Children’s Hospital, University of Alabama at Birmingham, University of California at Los Angeles, and McGovern Medical School at the University of Texas Health Science Center at Houston). IRB approval was obtained at each of the five sites, and informed consent was acquired from all participating families prior to enrollment.
Study Design
Children were included in the study if they were between the ages of 3 and 12 months at enrollment and met clinical or genetic criteria for definitive diagnosis of TSC16. Potential subjects were excluded if gestational age was <36 weeks at the time of delivery with significant perinatal complications (i.e. respiratory support, confirmed infection, intraventricular hemorrhage, cardiac compromise); they had taken an investigational drug as part of another research study within 30 days prior to study enrollment; were taking an mTOR inhibitor (rapamycin, sirolimus, or everolimus) orally at the time of study enrollment; had a Subependymal Giant Cell Astrocytoma (SEGA) requiring medical or surgical treatment; had a history of epilepsy surgery; or had any contraindications to completion of study procedures such as MRI.
Children were evaluated longitudinally at ages 3, 6, 9, 12, 18, 24 and 36 months. At each age, standardized evaluations, including developmental and adaptive measures, were performed. In addition, clinical information was collected throughout the study, including basic demographics, medical and family history, baseline and interval developmental history, participation in therapies, seizure history, concomitant medications, and medical co-morbidities. A physical examination from which clinical findings were recorded was performed at each visit. A yearly calibration meeting was held to ensure developmental assessment reliability across all sites for the entire study period.
Developmental and Autism-specific Assessments
The AOSI was administered at 12 months of age. The AOSI is an assessment that measures autism risk behaviors in infants ages 6–18 months and consists of 18 items7. From these individual items, a total score is obtained. Higher scores indicate more concerning ASD risk behaviors. At 24 months, the Autism Diagnostic Observation Schedule-2 (ADOS-2) was administered. The ADOS-2 is a semi-structured, interactive observation tool used to assess for ASD17. At 24 months, 78 out of 79 children were administered the Toddler Module, and one child was administered the Module 2. ADOS-2 Modules 1–4 use an algorithm to classify children as autism, ASD, or non-spectrum conditions. The Toddler Module was developed in 2009 as a tool to assess young children for ASD18. An algorithm was developed that classified children according to ‘range of concern’ for ASD (i.e. little-to-no, mild-to-moderate, and moderate-to-severe). Range of concern rather than classification was applied secondary to small patient sample size, as well as the inherent uncertainty that exists with evaluating children this young. The Toddler Module has been shown to have excellent reliability and diagnostic validity for ASD versus non-ASD disorders18,19. The Autism Diagnostic Interview-Revised (ADI-R) was also administered at 24 months. The ADI-R is a parent interview that focuses on a child’s developmental history, current functioning, social skills, communication and behaviors, and interests20. At each visit, developmental functioning was assessed using the Mullen Scales of Early Learning (MSEL)21. The MSEL consists of five domains (gross motor, fine motor, expressive language, receptive language, and visual reception) and also provides an overall composite score (Early Learning Composite). Developmental and autism-specific assessments at each visit were administered by a licensed psychologist, psychological technician/research assistant, and/or speech-language pathologist blinded to child’s clinical and seizure history at the time of testing and who had obtained research reliability on diagnostic assessments (e.g., ADI-R and ADOS-2) and experimental (e.g., AOSI) measures included in this project. Other assessments were performed as part of the larger study but are not included in this paper, as they were not used in our analysis.
Classification and Statistical Analysis
Children were grouped according to ASD and non-ASD categories at 24 months based on ADOS-2 classification. Overall scores on the Toddler Module indicating mild-to-moderate or moderate-to-severe level of concern or autism/autism spectrum on Module 2 were used to place subjects into the ASD group. Those children who did not meet criteria for ASD based on the ADOS-2 were placed into the non-ASD group. For this paper, clinical diagnosis of ASD or non-ASD was not assigned, only whether or not they met criteria on the ADOS-2 as described. To determine if the AOSI could be used clinically to distinguish which children were at risk for developing ASD, the AOSI total score obtained at 12 months was used as a predictor for ADOS-2 classification and ADI-R at 24 months using a logistic regression. In a similar manner, individual items on the AOSI were also used as predictors for ASD classification on the ADOS-2 at 24 months. We also wanted to determine how overall development related to AOSI total scores and used the Early Learning Composite on the MSEL as an independent variable.
Remaining analyses consisted of comparing diagnostic groups with respect to discrete responses to assessments at 24 months. For these analyses Fisher’s Exact test was used to see whether a relationship between the variables was present. Lastly cutoffs were examined for the AOSI total score (specifically, 8 through 14 inclusive) and in each case examining the sensitivity and specificity with respect to the ADOS-2 at 24 months.
All analyses were conducted using SAS ® statistical software version 9.3 (SAS Institute, Inc., Cary, NC). Hypothesis tests were conducted at the 0.05 level of statistical significance and no adjustments were made for multiple comparisons.
RESULTS
One hundred and sixty children were enrolled in TACERN. At 24 months, 79 children (36F, 43M) who were administered the AOSI at 12 months were administered the ADOS-2 at 24 months of which 35 (44.3%, 16F, 19M) were classified as ASD according to the ADOS-2. Genetic test results were available on 77 individuals of which nine had mutations in TSC-1, 59 had mutations in TSC-2, and 9 individuals had no mutation identified (NMI). TSC mutational status (TSC1, TSC2, or NMI) did not have significant impact on ASD diagnosis nor presence of ASD-associated behaviors detected by AOSI, ADOS-2, or ADI-R in our cohort.
AOSI total score in predicting ASD classification on the ADOS-2
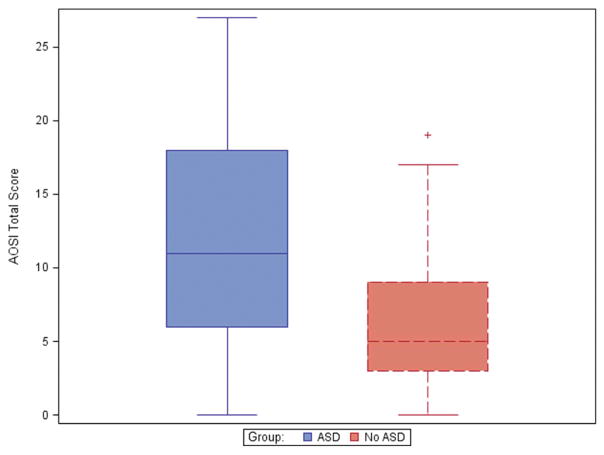
Children in the ASD group had a mean AOSI total score at 12 months significantly higher than the non-ASD group (p<0.001). The mean AOSI total score in the ASD group was 11.8 ± 7.4 vs. 6.3 ± 4.7 in the non-ASD group. Box plot analysis demonstrated normal distribution and clustering of scores (Figure 1), which prompted exploration of sensitivity and specificity using different cut-off scores, which has not previously been reported in the literature (Table 2). Using an AOSI total score cut-off of 13 provided excellent specificity (i.e. scoring less than 13 is a good indication of not meeting ADOS-2 diagnosis cut-off for ASD), but was much less sensitive for identifying at 12 months those who would later meet ASD classification on the ADOS-2 at 24 months.
FIGURE 1.
Normal distribution of AOSI total scores in children with and without ASD based on ADOS-2 classification.
TABLE 2.
AOSI individual items in relation to ASD classification on the ADOS-2
| AOSI Individual Items | ADOS-2 classification |
|---|---|
| Visual tracking | 1.0 |
| Disengagement of attention | 0.15 |
| Orients to name | 0.03* |
| Differential Response to Facial Emotion | 0.050 |
| Imitation of Action | 0.02* |
| Anticipatory Responses | 0.03* |
| Social babbling | 0.10 |
| Eye contact | 0.10 |
| Reciprocal social smile | 0.01* |
| Coordination of eye gaze and action | 0.04* |
| Reactivity | 0.18 |
| Social interest and shared affect | 0.02* |
| Transitions | 1.0 |
| Motor Control and Behavior | <0.001* |
| Atypical motor behaviors | 0.76 |
| Atypical sensory behaviors | 1.0 |
| Engagement of attention | 0.02* |
| Insistence on having or playing with particular objects | 1.0 |
| Sharing interest | 0.01* |
p<0.05
The individual AOSI total score, however, was still useful for identifying risk for ASD at 24 months as measured by the ADOS-2. The odds ratio of the AOSI predicting ASD on the ADOS-2 at 24 months was 1.16 [CI 1.06–1.27] (p<0.001). In other words, for every 1 point increase in the total score on the AOSI, the odds of being classified as ASD increases by 16% (p<0.001). Clinically, we noted that in several instances coexisting developmental delays likely influenced AOSI scores and, thus, later ADOS-2 scores and evaluated MSEL Early Learning Composite (ELC) score as a potential significant covariate. Indeed, when accounting for overall development as measured by ELC, the AOSI total score at 12 months was no longer significant (OR 1.05 [CI 0.95–1.16]) (p=0.33). However, the ELC alone at 12 months was a significant predictor for ASD classification at 24 months (OR=0.94 [CI=0.91–0.97] (p<0.001)). For every 1 point increase on the ELC, the odds of being classified as ASD on the ADOS-2 decreased by 6.4%. Thus overall developmental status appears to be an important independent contributor to ASD risk identified on the ADOS-2 at 24 months and likely secondarily impacts ASD markers identified on the AOSI at 12 months of age in children with TSC.
AOSI total score in predicting ADI-R cut-off scores
When the ADI-R cut-off scores were exceeded children in the ASD group had significantly larger AOSI total scores than the non-ASD group. Areas with highest significance included abnormalities in reciprocal social interaction and communication abnormalities (p<0.001). Restrictive, repetitive, and stereotyped behaviors were also significant (p=0.038), as well as developmental abnormality evident at or prior to 36 months (p=0.005).
Individual AOSI items predictive of ASD on the ADOS-2
Several individual items on the AOSI at 12 months were significantly elevated in children in the ASD group at 24 months (see Table 2). Red flags concerning for social communication (imitation of action, reciprocal social smile, social interest, coordinated eye gaze, sharing interest, and level of attention) and motor behaviors (motor control and behavior) were noted.
The AOSI was able to predict an increased level of concern on both objective assessments (e.g. ADOS-2) and parental measures (e.g. ADI-R). We also looked at childhood developmental concerns as reported by parents at each clinic visit. Out of the 79 individuals, developmental concern was reported in 18 (22.8%) at enrollment (between 0–12 months), with 13 out of 18 (72%) subsequently being classified as ASD on the ADOS-2 at 24 months of age. Conversely, out of the 62 children where no concern was reported, 23 (37%) still were eventually classified as ASD on the ADOS-2. Mean age of concern in the ASD group was 5 months (± 2 months) versus 6.4 months (± 3.5 months) for those expressing concern but not going on to develop ASD. The number of reported developmental concerns for both groups increased at subsequent visits (cumulative total over all visits was 135 out of 421 equaling overall concern at 32%), though this is likely biased since family members may have been notified in many cases if their child was falling behind. It is also expected that the level of concern would increase with age.
DISCUSSION
Cognitive and behavioral difficulties affect the majority of individuals with TSC to varying degrees. These difficulties are collectively referred to as TSC-Associated Neuropsychiatric Disorders (TAND), a term that was coined by the Neuropsychiatry Panel at the 2012 Tuberous Sclerosis Complex International Consensus Conference to help recognize the complex cognitive and behavioral manifestations of TSC and generate screening guidelines22. Approximately 50% of individuals with TSC are affected with ASD, which further contributes to cognitive and behavioral challenges already seen in this population. There is tremendous interest in learning more about the development of ASD in children with TSC at the earliest time points. The TACERN study group was developed to identify ASD biomarkers by utilizing detailed imaging, EEG, and developmental assessments so that early interventions and treatments could be implemented that would have the potential for altering the development of ASD symptoms during critical phases of development.
Our results indicate that the AOSI is useful in determining which individuals will go on to meet criteria for ASD on the ADOS-2. The mean AOSI total score for children who later met criteria for ASD on the ADOS-2 was significantly higher than those in the non-ASD group. Though the range of AOSI total scores was broad for both groups, the scores clustered indicating that the groups were different and that using a specific cut-off score may help determine risk. In our case, we found that 13 gave us a relatively high specificity of 88.6%. In our population that is already at high risk for neurodevelopmental difficulties (and, thus, an already elevated sensitivity), specificity would give us a better indicator of ASD risk. Our study is the first to evaluate a cut-off score in order to use the AOSI clinically. The only other study to look at AOSI scores specifically was Zwaigenbaum et al. who found that children who exhibited 7 or more positive risk markers on the AOSI at 12 months were more likely to meet criteria for ASD via the ADOS at 24 months23. Meeting cut-off scores in 7 out of 18 markers provided a sensitivity and specificity of 84 and 98%, respectively, when the AOSI was administered at 12 months of age.
Similar to the approach of Gammer et al. attempting to utilize the AOSI to predict ASD diagnosis using the ADOS in high-risk infants9, we found the AOSI most useful to differentiate those who are least likely to have ASD at 24 months. A higher AOSI total score increased individual risk for ASD as well, although this effect size was much more modest. Just as in their study, interquartile ranges for both groups overlap, indicating there is a broad autism phenotype in our TSC cohort despite the unifying genetic diagnosis. We suspect that individual variability is at least in part due to coexisting developmental impairments at 12 months that cause AOSI scores to be higher, even though they may not be directly attributable to ASD. We also found that when accounting for overall development, differences seen in the AOSI were not as robust. Although the ADOS combined with the ADI-R is currently the gold standard in diagnosing ASD when combined with expert clinical assessment, this highlights the continued importance for the latter especially when developmental delays are highly prevalent, such as in TSC, in which the delays can indirectly contribute to higher AOSI and/or ADOS scores. Clinician expertise may also be important for differentiating between social language impairments and cognitive language impairments, which at 12 months using the AOSI is difficult but more easily accomplished using the ADOS-2 at 24 months and beyond. In addition, findings from multiple high-risk infant sibling studies indicate the need for monitoring at multiple time points to account for highly variable developmental trajectories. The same appears to be true for TSC.
A previous ASD sibling study showed that multiple individual items on the AOSI differentiated high-risk from low-risk groups at 6 and 18 months8. Another earlier study demonstrated that several items on the AOSI indicated increased risk for meeting ASD classification on the ADOS at 24 months23. Similar to these non-syndromic ASD populations, our data indicate that multiple individual items on the AOSI are predictive of later ASD classification on the ADOS-2 at 24 months, including orienting to name, imitation of action, anticipatory response, social smile, coordination of eye gaze and action, social interest, motor control and behavior, engagement of attention and shared interest. Jeste et al. also reported abnormalities in visual tracking, disengagement of attention, and anticipatory responses at 6 months of age and additional red flags in the areas of eye contact, orienting to name, and motor control and behavior in young children with ASD in the setting of TSC13.
It is important to note that the ASD rate in our cohort was 40–50% with no sex difference versus a 4:1 ratio of M:F in the idiopathic ASD population. This equal gender distribution has also been reported in previous studies assessing the prevalence of ASD in TSC24,25.
Most of our children at 24 months received the Toddler Module of the ADOS-2, which was administered regardless of developmental level (criteria for administration include age 12 to 30 months with a nonverbal mental age of at least 12 months and walking independently). As TACERN continues to assess these children when they become 36 months of age, we expect to be able to determine if the relationship between the AOSI and later ASD diagnosis continues to be present using the ADOS-2. However, if this proves not to be the case, this could suggest that the ASD phenotype in TSC is much more dynamic than previously appreciated, with comorbid conditions such as degree of seizure control or exposure to specific treatments (molecular and interventional) impacting long-term outcomes. It may be that both the AOSI and ADOS-2 Toddler Module are tapping into a broader range of concern for ASD that may be better clarified at 36 months. A major goal of future analysis includes comparison of ADOS-2 classification to ASD clinical diagnosis and evaluating the impact of coexisting conditions and interventional therapies and treatments.
Epilepsy is once such coexisting condition that has been shown to be associated with poor neurodevelopmental outcomes in TSC, including poor cognitive and behavioral outcomes as well as increased risk for developing ASD12,26–34. We recently published a study of the TACERN cohort that specifically evaluated the temporal relationship between seizures and early development35. Seizures were seen in approximately 73% of the children with the majority of the children developing seizures prior to 12 months of age (average age of seizure onset 5.6 ± 3.9 months). We were able to show that earlier seizure onset, particularly prior to 12 months of age, along with higher seizure frequency negatively affected developmental outcomes. This effect was not only immediate but persisted through 24 months of age. Children with early seizure onset prior to 12 months of age were also noted to exhibit increased autism risk behaviors on the AOSI administered at 12 months (p<0.001) as well as elevated scores on the ADOS-2 at 24 months (p< 0.01). In addition, children with a history of seizures were more likely to meet cut-off scores for ASD-associated abnormalities in communication (p=0.03) on the ADI-R compared to children without seizures. Therefore, it suspected that ASD diagnosis and ASD-associated behaviors demonstrated by the ADOS-2 and ADI-R at 24 months and predicted by the AOSI at 12 months seen in the current study are largely influenced by overall developmental functioning and comorbid epilepsy.
Using the AOSI as a clinical tool, combined with expert clinician assessment, may help clinicians have a better sense of which children to monitor more closely. It also might provide an effective means to identify individuals at early ages with the highest potential to benefit from early treatments and interventions aimed at preventing or ameliorating the severity of ASD. For example, a previous study using the mTOR inhibitor everolimus to treat epilepsy in TSC identified improvement in multiple areas of behavior 36, as did a pilot study using early preventative treatment with vigabatrin37.
CONCLUSIONS
Developmental trajectories in children with ASD are complex and highly variable, which presents a major barrier to identifying at-risk infants and developing effective treatments to prevent or alter progression. Single-gene syndromes with a high prevalence of neurodevelopmental disorders such as TSC provide us with unique opportunities to investigate the underlying biology and identify potential treatments for ASD by providing highly enriched populations in which ASD symptoms can be identified and measured before the formal diagnosis of ASD is made. However, evaluation of existing tools such as the AOSI and continued development of new assessment approaches with improved sensitivity and specificity are essential for this progress to take place, not only for TSC but the broad spectrum of ASD causes, including idiopathic ASD.
TABLE 1.
Sensitivity and Specificity of the AOSI Total Score Predicting ASD via the ADOS-2
| AOSI Total Score | Sensitivity (95% CI) | Specificity (95% CI) |
|---|---|---|
| 8 | 66.7 (48.2 – 82.0) | 70.5 (54.8 – 83.2) |
| 9 | 66.7 (48.2 – 82.0) | 72.7 (57.2 – 85.0) |
| 10 | 57.6 (39.2 – 74.5) | 77.3 (62.2 – 88.5) |
| 11 | 51.5 (33.5 – 69.2) | 81.8 (67.3 – 91.8) |
| 12 | 48.5 (30.8 – 66.5) | 81.8 (67.3 – 91.8) |
| 13 | 39.4 (22.9 – 57.9) | 88.6 (75.4 – 96.2) |
| 14 | 36.4 (20.4 – 54.9) | 90.9 (78.3 – 97.5) |
Acknowledgments
This research was supported by the National Institute of Neurological Disorders and Stroke (NINDS) of the NIH (U01-NS082320, P20-NS080199), the Tuberous Sclerosis Alliance, the Developmental Synaptopathies Consortium (U54NS092090), which is a part of the NCATS Rare Diseases Clinical Research Network (RDCRN). RDCRN is an initiative of the Office of Rare Diseases Research (ORDR), National Center for Advancing Translational Sciences (NCATS), funded through collaboration between NCATS, National Institute of Mental Health, NINDS and National Institute of Child Health and Human Development (NICHD). The study utilized clinical research facilities and resources supported by the NCATS of the National Institutes of Health Grant (UL1-TR000077 and UL1-TR000124).
Footnotes
DISCLOSURE OF CONFLICTS OF INTEREST
JYW serves on the professional advisory board for the Tuberous Sclerosis Alliance; has received honoraria from and serves on the scientific advisory board and the speakers’ bureau for Novartis Pharmaceuticals Inc. and Lundbeck; and has received research support from the Tuberous Sclerosis Alliance, Novartis Pharmaceuticals Inc., Today’s and Tomorrow’s Children Fund, Department of Defense/Congressionally Directed Medical Research Program, and the NIH (U01NS082320, P20NS080199, R01NS082649, U54NS092090, and U01NS092595).
MS is supported by Developmental Synaptopathies Consortium (U54 NS092090), which is part of the NCATS Rare Diseases Clinical Research Network (RDCRN). His lab receives research funding from Roche, Novartis, Pfizer, and he has served on the Scientific Advisory Board of Sage Therapeutics. In addition, he serves on the Professional Advisory Board of the Tuberous Sclerosis Alliance and is an Associate Editor of Pediatric Neurology.
DAK has received consulting and speaking fees and travel expenses from Novartis and additional research support from the National Institute of Neurological Disorders and Stroke of the NIH (U01-NS082320, U54-NS092090, P20-NS080199), the Tuberous Sclerosis Alliance, the Van Andel Research Institute, Novartis, and Upsher-Smith Pharmaceuticals. In addition he serves on the professional advisory board and international relations committee for the Tuberous Sclerosis Alliance and the editorial board of Pediatric Neurology.
DAP has received research support from NIH (U01-NS082320; U54-NS092090; U01-NS092595); research support, consulting fees, and travel reimbursement from Curemark, LLC; and research support from Biomarin and Novartis.
HN currently has a project funded by BioMarin testing efficacy of an enzyme substitution drug for phenylketonuria (PKU).
The remaining authors have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Christensen DL, Baio J, Van Naarden Braun K, et al. Prevalence and Characteristics of Autism Spectrum Disorder Among Children Aged 8 Years--Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2012. Morbidity and mortality weekly report. Surveillance summaries (Washington, D.C.: 2002) 2016;65(3):1–23. doi: 10.15585/mmwr.ss6503a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Giacomo A, Fombonne E. Parental recognition of developmental abnormalities in autism. European child & adolescent psychiatry. 1998;7(3):131–136. doi: 10.1007/s007870050058. [DOI] [PubMed] [Google Scholar]
- 3.Ozonoff S, Young GS, Steinfeld MB, et al. How early do parent concerns predict later autism diagnosis? Journal of developmental and behavioral pediatrics: JDBP. 2009;30(5):367–375. doi: 10.1097/dbp.0b013e3181ba0fcf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Richards M, Mossey J, Robins DL. Parents’ Concerns as They Relate to Their Child’s Development and Later Diagnosis of Autism Spectrum Disorder. Journal of developmental and behavioral pediatrics: JDBP. 2016;37(7):532–540. doi: 10.1097/DBP.0000000000000339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dale PS, Bates E, Reznick JS, Morisset C. The validity of a parent report instrument of child language at twenty months. Journal of child language. 1989;16(2):239–249. doi: 10.1017/s0305000900010394. [DOI] [PubMed] [Google Scholar]
- 6.Pulsifer MB, Hoon AH, Palmer FB, Gopalan R, Capute AJ. Maternal estimates of developmental age in preschool children. The Journal of pediatrics. 1994;125(1):S18–24. doi: 10.1016/s0022-3476(94)70171-7. [DOI] [PubMed] [Google Scholar]
- 7.Bryson SE, Zwaigenbaum L, McDermott C, Rombough V, Brian J. The Autism Observation Scale for Infants: scale development and reliability data. J Autism Dev Disord. 2008;38(4):731–738. doi: 10.1007/s10803-007-0440-y. [DOI] [PubMed] [Google Scholar]
- 8.Brian J, Bryson SE, Garon N, et al. Clinical assessment of autism in high-risk 18-month-olds. Autism. 2008;12(5):433–456. doi: 10.1177/1362361308094500. [DOI] [PubMed] [Google Scholar]
- 9.Gammer I, Bedford R, Elsabbagh M, et al. Behavioural markers for autism in infancy: scores on the Autism Observational Scale for Infants in a prospective study of at-risk siblings. Infant Behav Dev. 2015;38:107–115. doi: 10.1016/j.infbeh.2014.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Osborne JP, Fryer A, Webb D. Epidemiology of tuberous sclerosis. Annals of the New York Academy of Sciences. 1991;615:125–127. doi: 10.1111/j.1749-6632.1991.tb37754.x. [DOI] [PubMed] [Google Scholar]
- 11.Humphrey A, Williams J, Pinto E, Bolton PF. A prospective longitudinal study of early cognitive development in tuberous sclerosis - a clinic based study. European child & adolescent psychiatry. 2004;13(3):159–165. doi: 10.1007/s00787-004-0383-1. [DOI] [PubMed] [Google Scholar]
- 12.Jeste S, Sahin M, Bolton P, Ploubidis G, Humphrey A. Characterization of autism in young children with tuberous sclerosis complex. J Child Neurol. 2008;23(5):520–525. doi: 10.1177/0883073807309788. [DOI] [PubMed] [Google Scholar]
- 13.Jeste SS, Wu JY, Senturk D, et al. Early developmental trajectories associated with ASD in infants with tuberous sclerosis complex. Neurology. 2014;83(2):160–168. doi: 10.1212/WNL.0000000000000568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Eeghen AM, Chu-Shore CJ, Pulsifer MB, Camposano SE, Thiele EA. Cognitive and adaptive development of patients with tuberous sclerosis complex: a retrospective, longitudinal investigation. Epilepsy & behavior: E&B. 2012;23(1):10–15. doi: 10.1016/j.yebeh.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 15.Jeste SS, Varcin KJ, Hellemann GS, et al. Symptom profiles of autism spectrum disorder in tuberous sclerosis complex. Neurology. 2016;87(8):766–772. doi: 10.1212/WNL.0000000000003002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Northrup H, Krueger DA. Tuberous sclerosis complex diagnostic criteria update: recommendations of the 2012 international tuberous sclerosis complex consensus conference. Pediatric neurology. 2013;49(4):243–254. doi: 10.1016/j.pediatrneurol.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lord C, Risi S, Lambrecht L, et al. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord. 2000;30(3):205–223. [PubMed] [Google Scholar]
- 18.Luyster R, Gotham K, Guthrie W, et al. The Autism Diagnostic Observation Schedule-toddler module: a new module of a standardized diagnostic measure for autism spectrum disorders. J Autism Dev Disord. 2009;39(9):1305–1320. doi: 10.1007/s10803-009-0746-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guthrie W, Swineford LB, Nottke C, Wetherby AM. Early diagnosis of autism spectrum disorder: stability and change in clinical diagnosis and symptom presentation. Journal of child psychology and psychiatry, and allied disciplines. 2013;54(5):582–590. doi: 10.1111/jcpp.12008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 1994;24(5):659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- 21.Mullen E. Mullen Scales of Early Learning. Circle Pines, MN: American Guidance Service; 1995. [Google Scholar]
- 22.de Vries PJ, Whittemore VH, Leclezio L, et al. Tuberous Sclerosis Associated Neuropsychiatric Disorders (TAND) and the TAND Checklist. Pediatric neurology. 2015;52(1):25–35. doi: 10.1016/j.pediatrneurol.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zwaigenbaum L, Bryson S, Rogers T, Roberts W, Brian J, Szatmari P. Behavioral manifestations of autism in the first year of life. International journal of developmental neuroscience: the official journal of the International Society for Developmental Neuroscience. 2005;23(2–3):143–152. doi: 10.1016/j.ijdevneu.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 24.Vignoli A, La Briola F, Peron A, et al. Autism spectrum disorder in tuberous sclerosis complex: searching for risk markers. Orphanet journal of rare diseases. 2015;10:154. doi: 10.1186/s13023-015-0371-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Curatolo P, Porfirio MC, Manzi B, Seri S. Autism in tuberous sclerosis. European Journal of Paediatric Neurology. 2004;8(6):327–332. doi: 10.1016/j.ejpn.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 26.Bolton PF. Neuroepileptic correlates of autistic symptomatology in tuberous sclerosis. Ment Retard Dev Disabil Res Rev. 2004;10(2):126–131. doi: 10.1002/mrdd.20024. [DOI] [PubMed] [Google Scholar]
- 27.Bolton PF, Clifford M, Tye C, et al. Intellectual abilities in tuberous sclerosis complex: risk factors and correlates from the Tuberous Sclerosis 2000 Study. Psychological medicine. 2015;45(11):2321–2331. doi: 10.1017/S0033291715000264. [DOI] [PubMed] [Google Scholar]
- 28.Cusmai R, Moavero R, Bombardieri R, Vigevano F, Curatolo P. Long-term neurological outcome in children with early-onset epilepsy associated with tuberous sclerosis. Epilepsy and Behavior. 2011;22(4):735–739. doi: 10.1016/j.yebeh.2011.08.037. [DOI] [PubMed] [Google Scholar]
- 29.Curatolo P, Aronica E, Jansen A, et al. Early onset epileptic encephalopathy or genetically determined encephalopathy with early onset epilepsy? Lessons learned from TSC. Eur J Paediatr Neurol. 2016;20(2):203–211. doi: 10.1016/j.ejpn.2015.12.005. [DOI] [PubMed] [Google Scholar]
- 30.Jansen FE, Vincken KL, Algra A, et al. Cognitive impairment in tuberous sclerosis complex is a multifactorial condition. Neurology. 2008;70(12):916–923. doi: 10.1212/01.wnl.0000280579.04974.c0. [DOI] [PubMed] [Google Scholar]
- 31.Numis AL, Major P, Montenegro MA, Muzykewicz DA, Pulsifer MB, Thiele EA. Identification of risk factors for autism spectrum disorders in tuberous sclerosis complex. Neurology. 2011;76(11):981–987. doi: 10.1212/WNL.0b013e3182104347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vignoli A, La Briola F, Turner K, et al. Epilepsy in TSC: certain etiology does not mean certain prognosis. Epilepsia. 2013;54(12):2134–2142. doi: 10.1111/epi.12430. [DOI] [PubMed] [Google Scholar]
- 33.Humphrey A, MacLean C, Ploubidis GB, et al. Intellectual development before and after the onset of infantile spasms: a controlled prospective longitudinal study in tuberous sclerosis. Epilepsia. 2014;55(1):108–116. doi: 10.1111/epi.12484. [DOI] [PubMed] [Google Scholar]
- 34.Bolton PF, Park RJ, Higgins JNP, Griffiths PD, Pickles A. Neuro-epileptic determinants of autism spectrum disorders in tuberous sclerosis complex. Brain. 2002;125(6):1247–1255. doi: 10.1093/brain/awf124. [DOI] [PubMed] [Google Scholar]
- 35.Capal JK, Bernardino-Cuesta B, Horn PS, et al. Influence of seizures on early development in tuberous sclerosis complex. Epilepsy & behavior: E&B. 2017;70(Pt A):245–252. doi: 10.1016/j.yebeh.2017.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Krueger DA, Wilfong AA, Talley CM, et al. Long-term treatment of epilepsy with everolimus in tuberous sclerosis. Neurology. 2016;87(23):2408–2415. doi: 10.1212/WNL.0000000000003400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.JóŸwiak S, Kotulska K, Domańska-Pakieła D, et al. Antiepileptic treatment before the onset of seizures reduces epilepsy severity and risk of mental retardation in infants with tuberous sclerosis complex. European Journal of Paediatric Neurology. 2011;15(5):424–431. doi: 10.1016/j.ejpn.2011.03.010. [DOI] [PubMed] [Google Scholar]