Abstract
Objective
To evaluate whether a department policy changing the scheduling of the postpartum visit from 6 weeks to 2–3 weeks after delivery is associated with higher long-acting reversible contraception (LARC) initiation at the postpartum visit.
Methods
We conducted a quasi-experimental before–after study to evaluate LARC initiation, specifically an intrauterine device (IUD) or contraceptive implant, at the postpartum visit between women scheduled for follow-up at 6 weeks (before policy change) and 2–3 weeks after delivery (after policy change). Secondary outcomes included postpartum visit completion, overall contraception initiation at the postpartum visit, overall contraceptive use at 6 months after delivery, and repeat pregnancies by 6 months postpartum. We obtained delivery and postpartum information using the electronic medical record and contacted participants 3 and 6 months after delivery to assess contraception use and repeat pregnancies.
Results
We enrolled 586 participants between December 2014 and November 2015, of whom 512 women (256 in each cohort) continued to meet eligibility criteria after delivery. Long-acting reversible contraception initiation rates at the postpartum visit were lower in the 2–3 week (16.5%, 95%CI 12.2–21.8) compared to 6-week group (31.1%, 95%CI 25.2–37.7, p<0.01), primarily due to patient and health care provider preferences for delaying IUD insertion to a later visit. More women completed a scheduled 2–3 week postpartum visit (90.2%, 95%CI 86.0–93.3) compared to 6-week visit (81.6%, 95%CI 76.4–85.9, p<0.01). Deferral of any contraception initiation was higher in the 2–3 week group (27.3%, 95%CI: 21.9–33.4) compared to 6-week group (15.8%, 95%CI: 11.5–21.4, p<0.01), but there were no differences in overall contraceptive use patterns at 6 months postpartum. No IUD perforations or expulsions were observed in women who underwent insertion at 2–3 weeks postpartum. Five pregnancies were reported in each cohort by 6 months after delivery.
Conclusions
Scheduling a visit at 2–3 weeks after delivery was not associated with increased LARC initiation at this visit despite higher postpartum visit attendance.
Introduction
The optimal timing of postpartum long-acting reversible contraception (LARC) initiation, especially intrauterine device (IUD) insertion, remains uncertain. Attendance rates at the 6-week postpartum visit are variable, with as many as 33% of prenatal care recipients not returning for postpartum care (1). Postplacental IUD insertion, which refers to IUD insertion within 10 minutes of placental delivery is one potential way to decrease rapid repeat pregnancies (2). However, expulsion rates are as high as 27% at 6 months postpartum with postplacental IUD insertion, compared to 1–4% with interval insertion at four to 6 weeks after delivery (3–7).
A postpartum visit at 2–3 weeks after delivery presents another potential opportunity for IUD insertion. Recent studies have demonstrated the feasibility and acceptability of IUD insertion at two and three weeks post-delivery with expulsion rates of 3–4% at 6 months postpartum (8,9). Women are more likely to attend an earlier postpartum visit within three weeks after delivery (10), and this increased attendance creates earlier opportunities for contraceptive counseling and LARC provision. Furthermore, an earlier postpartum visit allows for timely assessment of the physical and emotional well-being of mothers, opportunity to address breastfeeding, and time to reschedule missed appointments (11).
Given these potential benefits, the University of California, Davis (UC Davis) Department of Obstetrics and Gynecology planned to implement a policy to change the timing of the scheduled postpartum visit from 6 weeks to 2–3 weeks after delivery. We designed this study to coincide with the planned policy change to assess postpartum health outcomes, primarily LARC initiation, with an earlier visit.
Materials and Methods
In this quasi-experimental before–after study, we evaluated outcomes in women enrolled before and after the department implemented a clinic policy to change the timing of the scheduled postpartum visit from 6 weeks to 2–3 weeks after delivery. We consented women to participate in a prospective study assessing postpartum health outcomes over 6 months after delivery. The UC Davis Institutional Review Board approved this study.
We approached women receiving prenatal care at the two Sacramento campus clinics for study participation. We included women if they were 28 weeks gestation or greater at time of enrollment, planning to deliver and return for postpartum care at one of these two clinics, and planning to delay subsequent pregnancy for at least one year. We excluded women who required assisted reproductive technologies to achieve the index pregnancy or planned vasectomy as their postpartum contraceptive method.
Following informed consent, we obtained baseline demographic and medical information. We obtained delivery information through chart review in the electronic medical record. After each participant’s delivery, we excluded women who received sterilization or hysterectomy prior to their postpartum visit, had an IUD or implant placed during the delivery hospitalization, or did not deliver at our institution. We collected postpartum visit information through chart review and called participants at 3 and 6 months after delivery to complete a 10–15 minute telephone questionnaire assessing general health issues, contraception use, satisfaction with the timing of the postpartum visit, breastfeeding status, repeat pregnancies, and pediatrician visit completion. Subjects did not receive any remuneration for participation.
The primary outcome of the overall study was LARC initiation at the postpartum visit. Secondary outcomes included postpartum visit completion, LARC use at 3 and 6 months postpartum, complications with IUD insertion at 2–3 weeks and 6 weeks after delivery, overall contraception initiation at the postpartum visit and contraceptive use over 6 months, satisfaction with timing of the postpartum visit, breastfeeding continuation rates at 3and 6 months postpartum, and pediatrician visit completion. In this manuscript, we focus on findings related to contraceptive use, including the primary outcome of LARC initiation at the postpartum visit and secondary outcomes of postpartum visit follow-up rates by 12 weeks after delivery, overall contraception initiation at the postpartum visit, resumption of intercourse prior to the postpartum visit, participant-reported contraceptive use at 6 months postpartum, and repeat pregnancies by 6 months postpartum.
We estimated the sample size based on the primary outcome of the proportion of women who initiated LARC at the postpartum visit. Institutional data review for the three months preceding study development demonstrated a baseline LARC initiation rate of 15.1% at the postpartum visit. Assuming a 66% increase in LARC initiation to 25% among women scheduled for an earlier postpartum visit, we estimated a sample size of 256 participants per group using an alpha of 0.05 and power of 0.80.
The department implemented the policy change in June 2015 once 256 participants had delivered and did not meet post-delivery exclusion criteria. We continued enrolling women into the study after the policy change until 256 participants met criteria for continued study participation post-delivery. Prior to implementing the policy change, the department held meetings with physicians, nurses, and administrative support staff to provide education on the expected changes associated with an earlier postpartum visit. Department physicians agreed to provide comprehensive postpartum care at 2–3 weeks after delivery, including offering LARC insertions.
We used REDCap electronic data system for data management (12) and SPSS 24 (IBM, Armonk, NY, USA) to perform descriptive statistics and comparisons between groups with Chi-square and Fisher’s exact tests for categorical outcomes and Student’s t-tests for continuous outcomes. We considered a p-value of <0.05 as significant.
Results
We recruited women for study participation between December 2014 and November 2015. We enrolled 586 participants, of whom 74 did not meet all eligibility criteria (Figure 1). There were no differences in the completion of telephone follow-up at 3 months (86.3%, 6-week group; 86.3%, 2–3 week group; p=1.0) and 6 months after delivery (73.8% and 78.5%, respectively, p=0.25). Baseline demographic and obstetric characteristics did not differ between the two cohorts, except for age (Table 1).
Figure 1.
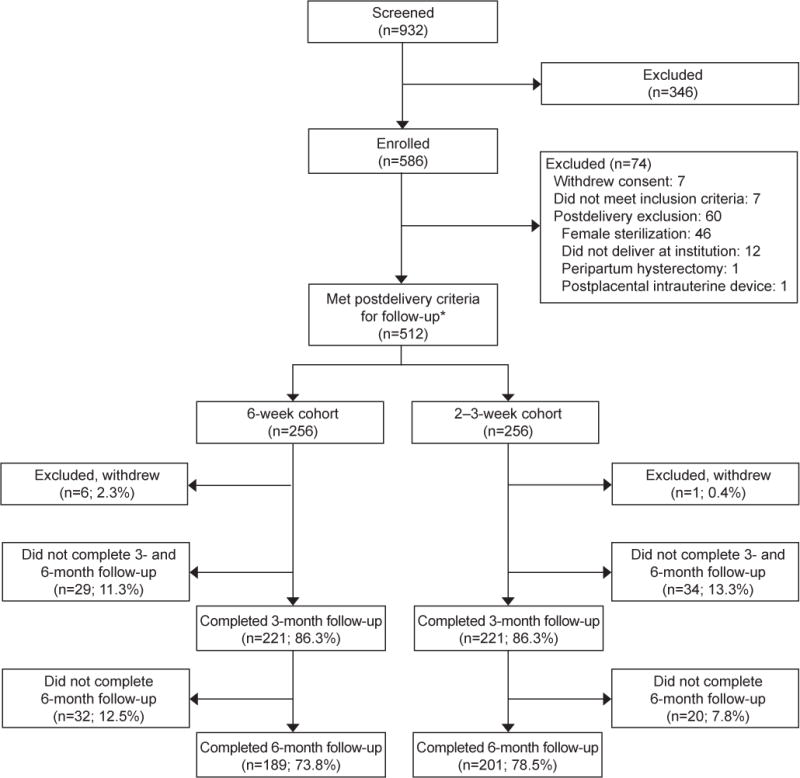
Participant flow diagram for women scheduled for 6-week and 2–3-week postpartum visits. *In this quasi-experimental before–after study, the postpartum scheduling policy change took place once 256 women met postdelivery criteria in the 6-week cohort. Enrollment continued until 256 women met postdelivery criteria in the 2–3-week cohort.
Table 1.
Demographic and obstetric characteristics of women scheduled for a 6-week or 2–3 week postpartum visit
| Characteristics | 6-week (n=256) |
2–3 week (n=256) |
p-value |
|---|---|---|---|
|
| |||
| Age in years | 28.9 ± 5.2 | 30.3 ± 5.5 | <0.01* |
|
| |||
| Hispanic ethnicity | 74 (28.9) | 71 (27.7) | 0.85† |
|
| |||
| Race | 0.58‡ | ||
| White | 171 (66.8) | 169 (66.0) | |
| Black | 24 (9.4) | 27 (10.5) | |
| Asian | 31 (12.1) | 35 (13.7) | |
| Native American and Pacific Islander | 14 (5.5) | 7 (2.7) | |
| Other§ | 16 (6.3) | 18 (7.0) | |
|
| |||
| Education | 0.17‡ | ||
| Did not complete high school | 15 (5.9) | 21 (8.2) | |
| High school graduate | 36 (14.1) | 39 (15.2) | |
| Some college | 84 (32.8) | 71 (27.7) | |
| College graduate | 59 (23.0) | 77 (30.1) | |
| Graduate school | 62 (24.2) | 48 (18.8) | |
|
| |||
| Work status | 0.78‡ | ||
| Full time | 120 (46.9) | 131 (51.2) | |
| Part time | 35 (13.7) | 34 (13.3) | |
| Unemployed | 40 (15.6) | 31 (12.1) | |
| Homemaker | 45 (17.6) | 46 (18.0) | |
| Full time student | 16 (6.3) | 14 (5.5) | |
|
| |||
| Insurance | 0.79‡ | ||
| Public | 70 (27.5) | 77 (30.2) | |
| Private | 171 (67.1) | 165 (64.7) | |
| Military | 14 (5.5) | 13 (5.1) | |
|
| |||
| Relationship status | 0.56‡ | ||
| Single | 20 (7.8) | 26 (10.2) | |
| Partnered, living with partner | 218 (85.5) | 210 (82.0) | |
| Partnered, not living with partner | 17 (6.7) | 20 (7.8) | |
|
| |||
| Gravidity | 0.18‡ | ||
| 1 | 91 (35.5) | 69 (27.0) | |
| 2 | 72 (28.1) | 79 (30.9) | |
| 3 | 44 (17.2) | 48 (18.8) | |
| 4 | 20 (7.8) | 30 (11.7) | |
| 5 | 17 (6.6) | 12 (4.7) | |
| 6 or more | 12 (4.7) | 18 (7.0) | |
|
| |||
| Parity | 0.63‡ | ||
| 0 | 129 (50.4) | 122 (47.7) | |
| 1 | 84 (32.8) | 83 (32.4) | |
| 2 | 26 (10.2) | 26 (10.2) | |
| 3 or more | 17 (6.6) | 25 (9.8) | |
|
| |||
| Prior miscarriage | 65 (25.4) | 86 (33.6) | 0.05‖ |
|
| |||
| Prior abortion | 40 (15.6) | 56 (21.9) | 0.09† |
|
| |||
| Prior Cesarean delivery | 40 (15.6) | 41 (16.0) | 1.0† |
|
| |||
| Pregnancy planned | 163 (63.7) | 162 (63.3) | 1.0† |
|
| |||
| Planning postpartum LARC | 59 (23.0) | 42 (16.4) | 0.08† |
|
| |||
| Primary obstetric provider | 0.33† | ||
| General faculty obstetrician | 95 (37.1) | 79 (30.9) | |
| Maternal-fetal medicine physician | 68 (26.6) | 74 (28.9) | |
| Resident physician | 93 (36.3) | 103 (40.2) | |
|
| |||
| Index pregnancy considered high risk | 106 (41.4) | 112 (43.8) | 0.66† |
|
| |||
| Index delivery preterm | 23 (9.0) | 17 (6.6) | 0.41† |
|
| |||
| Index delivery vaginal | 183 (71.5) | 177 (69.1) | 0.63† |
LARC = long-acting reversible contraception
Data are presented as n (%) or mean ±standard deviation
Independent samples t-test used for this comparison
Fisher’s exact test used for these comparisons
Chi-square test used for these comparisons
Other includes participants who identified with more than one race
Fisher’s exact test used for this comparison, p>0.05
Both groups demonstrated similar desire for LARC at the postpartum visit, but women in the 2–3 week group had lower rates of LARC initiation at the initial postpartum visit compared to those in the 6-week group (Table 2). Patient and provider preferences accounted for more delays in IUD insertion at 2–3 weeks (84.6%, 95% CI 70.3–92.8) compared to 6-weeks postpartum (33.3%, 95% CI 16.3–56.3, p<0.01) (Table 3). By 6 months postpartum, there were no differences in type of contraceptive method used between both groups, with most women relying on a LARC method (Table 4).
Table 2.
LARC use intentions and outcomes by 8 weeks postpartum for women scheduled for and attended a 6-week or 2–3 week postpartum visit
| Characteristics | 6-week (n=209) |
2–3 week (n=231) |
p-value* |
|---|---|---|---|
|
| |||
| Planned LARC method for postpartum contraception before delivery | 45 (21.5, 16.5–27.6) | 39 (16.9, 12.6–22.2) | 0.23 |
|
| |||
| Desired LARC at postpartum visit | 87 (41.6, 35.2–48.4) | 86 (37.2, 31.3–43.6) | 0.38 |
| IUD | 65 (31.1, 25.2–37.7) | 66 (28.6, 23.1–34.7) | |
| Implant | 22 (10.5, 7.1–15.4) | 20 (8.7, 5.7–13.0) | |
|
| |||
| Initiated LARC at postpartum visit | 65 (31.1, 25.2–37.7) | 38 (16.5, 12.2–21.8) | <0.01 |
| IUD | 47 (22.5, 17.4–28.6) | 27 (11.7, 8.2–16.5) | |
| Levonorgestrel IUD | 39 (83.0, 69.9–91.1) | 20 (74.1, 55.3–86.8) | |
| Copper IUD | 8 (17.0, 8.9–30.1) | 7 (25.9, 13.2–44.7) | |
| Implant | 18 (8.6, 5.5–13.2) | 11 (4.8, 2.7–8.3) | |
|
| |||
| Initiated LARC by 8 weeks postpartum | 65 (31.1, 25.2–37.7) | 62 (26.8, 21.5–32.9) | 0.34 |
| IUD | 47 (22.5, 17.4–28.6) | 47 (20.4, 15.7–26.0) | |
| Levonorgestrel IUD | 39 (83.0, 69.9–91.1) | 35 (74.5, 60.5–84.8) | |
| Copper IUD | 8 (17.0, 8.9–30.1) | 12 (25.5, 15.3–39.5) | |
| Implant | 18 (8.6, 5.5–13.2) | 15 (6.5, 4.0–10.4) | |
LARC = long-acting reversible contraception; IUD = intrauterine device
Data are presented as n (%, 95% CI)
Fisher’s exact test used for the overall LARC comparisons
Table 3.
Reasons for delay in LARC insertion at the initial 6-week and 2–3 week postpartum visits
| Reason for delay | Timing of scheduled postpartum visit
|
|
|---|---|---|
| 6-week | 2–3 week | |
|
| ||
| IUD | n=18 | n=39 |
| Insurance authorization* | 11 (61.1) | 6 (15.4) |
| Patient preference | 2 (11.1) | 19 (48.7) |
| Provider preference | 4 (22.2) | 14 (35.9) |
| Concern for pregnancy | 1 (5.6) | 0 |
|
| ||
| Implant | n=4 | n=9 |
| Insurance authorization* | 2 (50.0) | 4 (44.4) |
| Provider preference | 2 (50.0) | 2 (22.2) |
| Patient preference | 0 | 3 (33.3) |
LARC =long-acting reversible contraception
IUD = intrauterine device
Data are presented as n (%)
Insurance authorization refers to situations in which insurance coverage for LARC was not verified prior to the postpartum visit
Table 4.
Contraception initiated at the 6-week or 2–3 week postpartum visits and reported contraception use at 6 months postpartum
| Contraceptive method | Initiated at postpartum visit | Reported use at 6 months postpartum | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| 6-week (n=209) |
2–3 week (n=231) |
p value* | 6-week† (n=182) |
2–3 week‡ (n=195) |
p value* | |
|
| ||||||
| Tier 1 | ||||||
| Levonorgestrel IUD | 39 (18.7, 14.0–24.5) | 20 (8.7, 5.7–13.0) | <0.01 | 43 (23.6, 18.0–30.3) | 37 (19.0, 14.1–25.1) | 0.31 |
| Copper IUD | 8 (3.8, 2.0–7.7) | 7 (3.0, 1.5–6.1) | 0.79 | 8 (4.4, 2.3–8.4) | 11 (5.6, 3.2–9.8) | 0.64 |
| Implant | 18 (8.6, 5.3–13.5) | 11 (4.8, 2.7–8.3) | 0.12 | 13 (7.1, 4.2–11.8) | 17 (8.7, 5.5–13.5) | 0.70 |
| Sterilization | 0 | 0 | ND | 3 (1.7, 0.1–4.7) § | 3 (1.5, 0.1–4.4)‖ | 1.0 |
|
| ||||||
| Tier 2 | ||||||
| DMPA | 9 (4.3, 2.3–8.0) | 8 (3.5, 1.8–6.7) | 0.81 | 8 (4.4, 2.3–8.4) | 5 (2.6, 1.1–5.9) | 0.40 |
| Pills, patch, ring | 58 (27.8, 22.1–34.2) | 53 (22.9, 18.0–28.8) | 0.27 | 33 (18.1, 13.2–24.4) | 34 (17.4, 12.8–23.4) | 0.89 |
|
| ||||||
| Tier 3 | ||||||
| Condoms | 42 (20.1, 15.2–26.1) | 65 (28.1, 22.7–34.3) | 0.06 | 38 (20.9, 15.6–27.4) | 45 (23.1, 17.7–29.5) | 0.62 |
| Fertility awareness | 2 (1.0, 0.3–3.4) | 4 (1.7, 0.7–4.4) | 0.69 | 3 (1.7, 0.1–4.7) | 1 (0.5, 0.1–2.8) | 0.36 |
|
| ||||||
| None | 33 (15.8, 11.5–21.4) | 63 (27.3, 21.9–33.4) | <0.01 | 33 (18.1, 13.2–24.4) | 42 (21.5, 16.4–27.8) | 0.44 |
IUD = intrauterine device; DMPA = depot medroxyprogesterone acetate; ND = not done
Data are presented as n (%, 95% CI)
Tier levels are adapted from World Health Organization rating of contraceptive efficacy (13)
Fisher’s exact test used for comparisons
Seven participants not included in table: one who received a hysterectomy for fibroids after the postpartum visit, one who reported using spermicide, and five who became pregnant by 6 months postpartum
Six participants not included in table: one who reported using lactational amenorrhea method and five who became pregnant by 6 months postpartum
Two participants using vasectomy and one participant who underwent a female sterilization procedure
One participant using vasectomy and two participants who underwent a female sterilization procedure
More women attended their postpartum visit when scheduled at 2–3 weeks after delivery (90.2%, 95% CI 86.0–93.3) compared to 6 weeks after delivery (81.6%, 95% CI 76.4–85.9, p<0.01). The median number of days between delivery and postpartum visit attendance was 43 days (range 16–63) in women scheduled for a 6-week visit and 18 days (range 8–70) in those scheduled for 2–3 weeks postpartum. More couples resumed intercourse prior to the postpartum visit in the 6-week group (16.1%, 95% CI 11.3–22.3) compared to 2–3 week group (3.3%, 95% CI 1.4–7.2, p<0.01).
No IUD perforations occurred based on participant report and chart abstraction. Among 38 women in the 6-week cohort who had an IUD inserted within 56 days after delivery with follow-up through 6 months postpartum, one participant requested removal of her copper IUD after 81 days for side effects. Of 21 women in the 2–3 week cohort who had an IUD inserted within 28 days after delivery with follow-up through 6 months postpartum, four participants requested IUD removal for side effects. Two levonorgestrel IUDs were removed after 7 and 84 days, and two copper IUDs were removed after 60 and 100 days. No expulsions occurred in these 59 women.
Five participants in each group became pregnant within 6 months after delivery. Among women in the 6-week group, three planned to continue their pregnancies, one received treatment for an ectopic pregnancy, and one underwent termination. In the 2–3 week group, three women planned to continue their pregnancies and two experienced early pregnancy loss. One woman became pregnant after her copper IUD was removed for heavy bleeding, one participant was using progestin-only pills, two women were using condoms, and the rest were not using contraception when they became pregnant.
Discussion
We found that scheduling a 2–3 week visit after delivery was associated with lower LARC initiation rates at the initial postpartum visit. We had hypothesized that an earlier visit would be associated with higher postpartum visit attendance, increased opportunity for contraception counseling, and resultant increase in LARC initiation. Instead, LARC initiation rates were lower, primarily due to delays in levonorgestrel IUD insertion, despite higher postpartum visit completion in women scheduled for an earlier visit. Overall contraceptive initiation at the 2–3 week postpartum visit was also lower, with more women choosing not to initiate a method. Contraceptive use patterns were similar in both groups by 6 months postpartum, indicating that women who initially deferred IUD insertion at 2–3 weeks after delivery did return for IUD initiation at a later visit.
In contrast to prior studies, we uncovered patient and provider barriers to IUD insertion with implementation of an earlier postpartum visit in clinical practice. Although IUD insertion at two (8) and three weeks (9) was acceptable to clinical trial participants, patient preferences accounted for almost half of the delays in IUD insertion among women scheduled for a 2–3 week postpartum visit. Provider barriers were the second most common reason for delay. Prior to policy implementation, department physicians agreed to offer LARC insertion at 2–3 weeks postpartum, including IUDs; still, many physicians recommended deferring IUD initiation. The exact reasons for provider deferral of IUD initiation are unknown, which is a limitation of this study, but possible factors could include appointment time limitations. Previous studies have also demonstrated provider attitudes regarding safety as a limitation to both interval and immediate postpartum IUD initiation (14,15).
A limitation of this study is the potential for baseline differences between the two groups to influence outcomes that would have been avoided in a randomized trial. While not statistically significant, fewer women in the 2–3 week cohort planned for postpartum LARC at enrollment compared to women in the 6-week group. Furthermore, this study design introduces the possibility that external factors, other than the policy change itself, influenced the primary outcome of LARC initiation. The policy changed in June 2015, and the combination of new resident trainees and introduction of a new levonorgestrel IUD inserter device into the clinics could have contributed to the decrease in IUD insertion post-policy implementation.
Despite these limitations, our study highlights the advantages of scheduling an earlier postpartum visit. Postpartum visit attendance rates were higher at 2–3 weeks after delivery, which presents more opportunities for clinicians to address postpartum concerns. Most couples had not resumed intercourse prior to the 2–3 week visit, allowing women to initiate effective contraception initiation before they are at risk of pregnancy (16). Our experience, albeit limited, also supports the present data on the safety of IUD insertion at 2–3 weeks postpartum (8,9).
The higher postpartum visit attendance with implementation of an earlier visit was not enough to increase LARC or overall contraception initiation; rather, more clinician training and anticipatory guidance during the antenatal period is needed to maximize the visit (11). The optimal timing for LARC initiation differs based on several factors, including a woman’s insurance coverage, ability to attend follow-up visits, and contraceptive preferences. With these considerations in mind, obstetric care providers should also present the option of LARC initiation at 2–3 weeks after delivery in addition to discussing immediate postpartum and interval insertion.
Acknowledgments
The authors thank Aubrey Blanton, MPH, for her assistance with recruiting participants, performing chart review, and reaching participants for 3- and 6-month follow-up phone calls.
Supported by the Society of Family Planning
Supported by the National Center for Advancing Translational Sciences (NCATS), National Institutes of Health (NIH), through grant UL1 TR001860 for use of REDCap.
Footnotes
Presented as a poster at the North American Forum on Family Planning, Denver, CO, November 5–7, 2016.
Financial Disclosure
Dr. Melo is currently a Merck Nexplanon trainer and has received compensation for leading the Nexplanon Clinical Training Program in the past. She has also been a consultant to Ipas for clinical review of Mozambique Standards and Guidelines for Safe Abortion Care. Dr. Creinin receives speaking honoraria from Allergan and Merck & Co., serves on an Advisory Board for Merck & Co., and is a consultant for Estetra, Health Decisions, and Medicines360. The Department of Obstetrics and Gynecology, University of California, Davis, receives research funding for contraceptive clinical trials from Contramed, Medicines360, Merck & Co., NIH/NICHD, and the Society of Family Planning.
The other authors did not report any potential conflicts of interest.
The authors have indicated that he or she has met the journal’s requirements for authorship.
References
- 1.Wilcox A, Levi EE, Garrett JM. Predictors of non-attendance to the postpartum follow-up visit. Matern Child Health J. 2016;20:22–7. doi: 10.1007/s10995-016-2184-9. [DOI] [PubMed] [Google Scholar]
- 2.Sonalkar S, Kapp N. Intrauterine device insertion in the postpartum period: a systematic review. Eur J Contracept Reprod Health Care. 2015;20:4–18. doi: 10.3109/13625187.2014.971454. [DOI] [PubMed] [Google Scholar]
- 3.Chen BA, Reeves MF, Hayes JL, Hohmann HL, Perriera LK, Creinin MD. Postplacental or delayed insertion of the levonorgestrel intrauterine device after vaginal delivery: a randomized controlled trial. Obstet Gynecol. 2010;116:1079–87. doi: 10.1097/AOG.0b013e3181f73fac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dahlke JD, Terpstra ER, Ramseyer AM, Busch JM, Rieg T, Magann EF. Postpartum insertion of levonorgestrel intrauterine system at three time periods: a prospective randomized pilot study. Contraception. 2011;84:244–8. doi: 10.1016/j.contraception.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 5.Jatlaoui TC, Marcus M, Jamieson DJ, Goedken P, Cwiak C. Postplacental intrauterine device insertion at a teaching hospital. Contraception. 2014;89:528–33. doi: 10.1016/j.contraception.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 6.Woo I, Seifert S, Hendricks D, Jamshidi RM, Burke AE, Fox MC. Six-month and 1-year continuation rates following postpartum insertion of implants and intrauterine devices. Contraception. 2015;92:532–5. doi: 10.1016/j.contraception.2015.09.007. [DOI] [PubMed] [Google Scholar]
- 7.Eroglu K, Akkuzu G, Vural G, Dilbaz B, Akin A, Taskin L, et al. Comparison of efficacy and complications of IUD insertion in immediate postplacental/early postpartum period with interval period: 1 year follow-up. Contraception. 2006;74:376–81. doi: 10.1016/j.contraception.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 8.Zerden ML, Stuart GS, Charm S, Bryant A, Garrett J, Morse J. Two-week postpartum intrauterine contraception insertion: a study of feasibility, patient acceptability and short-term outcomes. Contraception. 2017;95:65–70. doi: 10.1016/j.contraception.2016.08.005. [DOI] [PubMed] [Google Scholar]
- 9.Baldwin MK, Edelman AB, Lim JY, Nichols MD, Bednarek PH, Jensen JT. Intrauterine device placement at 3 versus 6 weeks postpartum: a randomized trial. Contraception. 2016;93:356–63. doi: 10.1016/j.contraception.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 10.Tsai PJ, Nakashima L, Yamamoto J, Ngo L, Kaneshiro B. Postpartum follow-up rates before and after the postpartum follow-up initiative at Queen Emma Clinic. Hawaii Med J. 2011;70:56–9. [PMC free article] [PubMed] [Google Scholar]
- 11.American College of Obstetricians and Gynecologists. Optimizing postpartum care. Committee Opinion No. 666. Obstet Gynecol. 2016;127:e187–92. doi: 10.1097/AOG.0000000000001487. [DOI] [PubMed] [Google Scholar]
- 12.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap) – a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–81. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Health Organization Department of Reproductive Health and Research (WHO/RHR) and Johns Hopkins Bloomberg School of Public Health/Center for Communication Programs (CCP) Knowledge for Health Project. Family planning: A global handbook for providers (2011 update) Baltimore and Geneva: CCP and WHO; 2011. [Google Scholar]
- 14.Rauh-Benoit LA, Tepper NK, Zapata LB, Whiteman MK, Curtis KM, Mandel MG, et al. Healthcare provider attitudes of safety of intrauterine devices in the postpartum period. J Womens Health. 2016 Dec 19; doi: 10.1089/jwh.2016.5985. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ogburn JA, Espey E, Stonehocker J. Barriers to intrauterine device insertion in postpartum women. Contraception. 2005;72:426–9. doi: 10.1016/j.contraception.2005.05.016. [DOI] [PubMed] [Google Scholar]
- 16.Speroff L, Mishell DR., Jr The postpartum visit: it’s time for a change in order to optimally initiate contraception. Contraception. 2008;78:90–8. doi: 10.1016/j.contraception.2008.04.005. [DOI] [PubMed] [Google Scholar]


