Abstract
Thrombus formation is a major adverse event affecting patients implanted with ventricular assist devices (VADs). Despite anti-thrombotic drug administration, thrombotic events remain frequent within the first year post-implantation. Platelet activation (PA) is an essential process underling thrombotic adverse events in VAD systems. Indeed, abnormal shear forces, correlating with specific flow trajectories of VADs, are strong agonists mediating PA. To date, the ability to determine efficacy of anti-platelet (AP) agents under shear stress conditions is limited. Here, we present a novel microfluidic platform designed to replicate shear stress patterns of a clinical VAD, and use it to compare the efficacy of two AP agents in vitro. Gel-filtered platelets were incubated with i) acetylsalicylic acid (ASA) and ii) ticagrelor, at two different concentrations (ASA: 125 and 250μM; ticagrelor: 250 and 500nM) and were circulated in the VAD-emulating microfluidic platform using a peristaltic pump. GFP were collected after 4 and 52 repetitions of exposure to the VAD shear pattern and tested for shear-mediated PA. ASA significantly inhibited PA only at 2-fold higher concentration (250 μM) than therapeutic dose (125 μM). The effect of ticagrelor was not dependent on drug concentration, and did not show significant inhibition with respect to untreated control. This study demonstrates the potential use of microfluidic platforms as means of testing platelet responsiveness and AP drug efficacy under complex and realistic VAD-like shear stress conditions.
Keywords: Shear stress, platelet activation, shear-mediated platelet activation, mechanical circulatory support devices, ventricular assist devices, anti-platelet agents, drug efficacy, microfluidic flow-based assays
1. Introduction
Mechanical circulatory support devices, such as ventricular assist devices (VADs), continue to emerge as effective therapy for patients with advanced and end-stage heart failure [1,2]. Despite significant improvement of patient survival and quality of life associated with VADs versus medical treatment [3], VAD therapy remains plagued by post-implant adverse events including thrombosis, hemolysis, bleeding and infection [4]. Thrombus formation, in particular, is a frequent and serious complication [5] often occurring within the first year post-implant. Thrombotic complications may have catastrophic consequences that might seriously compromise patients’ survival.
To limit the thrombotic risk, VAD patients are routinely administered a combination of anti-thrombotic agents consisting of anti-platelet (AP) agents, e.g. aspirin (ASA), pentoxyfilline and dipyridamole; and anti-coagulants, such as heparin and warfarin; with the aim of inhibiting both platelet activation (PA) and subsequent zymogen coagulation pathways driving thrombotic events [6]. However, despite current anti-thrombotic strategy, the occurrence of post-implant thrombosis persists [7], raising concerns as to the actual efficacy of present drug regimens. Recent studies by our group have in fact demonstrated that at levels of shear stress characteristics of VADs, ASA has limited ability to protect from shear-mediated platelet activation (SMPA) [8].
Current AP agents largely inhibit pathways of platelet activation or aggregation principally triggered by biochemical agonists. Interestingly, limited insight exists as to their efficacy in inhibiting mechanical agonists such as physical stimuli (i.e., shear stress), which are dominant drivers of thrombosis in VADs. Indeed, VADs are characterized by narrow geometries, where flowing blood undergoes rapid accelerations and platelets are subjected to non-physiologic, extreme levels of shear stress and fast dynamics, as reported in different numerical studies in which the fluid dynamics in real devices was simulated [9,10]. In particular, in the study by Chiu and co-authors [10], shear stress patterns along platelet trajectories in two commercial axial VADs were analyzed under normal operating conditions, corresponding to a cardiac output of 4 L/min: such patterns of shear stress are characterized by peaks of shear magnitude in the range 100–300 Pa, significantly higher than physiological values (1 – 5 Pa). Also, exposure time to shear stress in VADs was estimated to be in the range 20–100 ms. Thus, in such a short residence time in the pump, platelets are exposed to highly varying levels of shear stress, due to continuous local accelerations and decelerations of flow. Such mechanical stresses were hypothesized to cause significant platelet membrane alterations, cytoskeleton rearrangements and release of cytoplasmic content, eventually leading to the activation of the coagulation cascade [11–13] which in combination with recirculating flow patterns in specific areas of the device (e.g., in the flow-straightener or between the flow straightener and the impeller) can determine the formation of a thrombus [14,15].
Based on these considerations, studying platelet response under pathological and elevated shear stress generated in VADs would be of utmost importance to understand related pathological behavior inducing thrombotic processes. Also, evaluating the efficacy of pharmacological treatment in altered VAD hemodynamic conditions would be beneficial to develop effective therapeutic strategies.
Recently, different in vitro studies have focused on the effect of non-physiological and elevated shear stress on platelet activation in therapeutic blood recirculating devices [16–19]. As such, our understanding of mechanisms participating in SMPA has only recently begun to be more fully elucidated, involving mechano-destruction, mechano-sensing and mechano-transduction mechanisms [20].
In the clinical setting, monitoring of pro-thrombotic conditions of patients today is predominantly performed by testing platelet function and the formation of coagulation products in response to biochemical agonists, i.e. ADP, collagen, thrombin, and TRAP [21–24] under static or low shear conditions, thus neglecting the contribution of supra-physiological shear stress.
As an alternative, laboratory-based in vitro tests have been developed which allow determination of platelet response under controlled shear stress conditions. Such systems consist of modified viscometers (either parallel plate or cone and plate configurations) in which the platelet sample is exposed to controlled shear conditions [25,26]. In a recent study by Valerio and co-authors [8], the effect of ASA on the shear stress-mediated platelet response was evaluated through use of a viscometer-based system allowing generation of dynamic and complex shear stress patterns. In addition to demonstrating minimal efficacy of ASA as an effective means of limiting SMPA, the study revealed that platelets exhibit differing responses when stimulated with constant versus dynamic shear stress conditions, suggesting that dynamic stresses are relevant drivers of SMPA. Moreover, the authors also suggested that AP efficacy is influenced by the complex nature of hemodynamic shear stress patterns. However, the viscometer-based system limited the maximum levels of shear stress attainable to 10 Pa, which is an order of magnitude lower than actual shear stress estimated in VADs [27]. Additionally, viscometer-based systems are large, bulky and laboratory-based, requiring large volumes of sample – i.e. 5 mL/run, all characteristics that have so far limited their use to pre-clinical research only, with limited translational capability to the clinical setting.
Microfluidic-based devices were recently proposed with the aim of studying platelet response under shear flow conditions [28–30]. Microfluidic devices have the potential to overcome the limitations of current bench-top laboratory systems, affording portability and translation to the clinic. They require reduced sample and reagent volumes and are potentially transferrable to the routine clinical laboratory and potential to the bedside. In recent studies, microfluidic platforms were utilized to evaluate anti-thrombotic drug effects on platelets under controlled shear stress conditions. Hosokawa and colleagues [31] proposed a microfluidic device to monitor white thrombus formation on collagen- and tissue thromboplastin-coated surfaces under arterial shear rates. The device was used to evaluate the effects of IIb/IIIa, Ibα inhibitors, heparin and enoxaparin. A similar approach was used by Jain and co-authors [32] who replicated shear rates typical of stenotic vessels in microfluidic channels. Stenotic conditions were also replicated in the system proposed by Li and colleagues [33] who evaluated the effect of ASA and eptifibatide on thrombus formation in collagen-coated microchannels under arterial shear stress conditions.
These studies represent an advance compared to standard in vitro tests of hemostasis, as they allow for monitoring platelet response under shear flow conditions. However, most microfluidic systems to date have been developed to test shear-mediated thrombosis at physiologic or time-constant venous or arterial shear rates. Also, in studies where non-physiological shear conditions were considered, e.g. replicating stenotic vessels [32,33], flow conditions are still far from those occurring in VADs, which is characterized by “hypershear” and complex time-varying shear conditions.
To further advance the use of microfluidic technology in the study of pro-thrombotic processes under shear, here we propose a methodological approach based on microfluidic technology that allowing testing of the platelet response to AP agents under VAD-like supraphysiological shear stress conditions. We make use of a numerical approach that allows the design of microfluidic channels able to generate dynamic and complex shear stress patterns replicating those occurring in VADs [34]. Herein, this approach was applied to a specific case study of a commercial VAD, and two AP agents – aspirin – a conventional agent, and ticagrelor – a newer agent, are tested.
2. Materials and Methods
2.1 Microfluidic platform
The microfluidic device utilized in this study was initially described in a previous work [35]. It features a series of four identical channels (shearing channels) designed to subject platelets to a dynamic shear stress profile that emulates a representative pro-thrombotic flow trajectory of a prototypic VAD – the HeartAssist5 VAD (HA5, MicroMed Technology, USA).
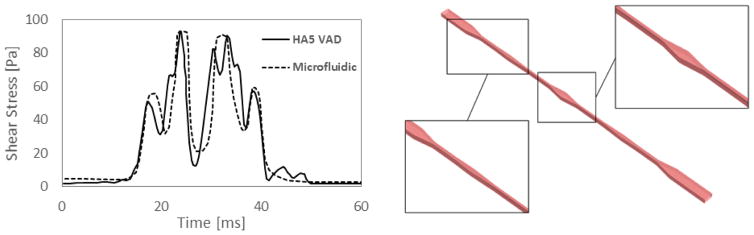
More specifically, the selected flow trajectory was extracted from multiphase CFD analyses of the HA5 [10], in which the VAD operating at a continuous flow rate of 4 L/min (corresponding to 9000 rpm pump speed, as for a typical adult patient) was simulated. The HA5 shear stress pattern derived from the CFD analyses is shown in Fig. 1 (left panel): it is characterized by a highly dynamic pattern with consecutive peaks of elevated shear stress (approaching values of 100 Pa) and a total exposure time of about 25 ms. The corresponding shear stress pattern generated in the microfluidic channel is also shown in Fig. 1 (left panel). The microchannel was designed to replicate the main characteristics of the HA5 shear stress patterns (peak shear stress magnitude and exposure time). The design of the microfluidic channel was obtained through the approach described in [34], that is based on multiphase CFD analyses in which platelet trajectories are simulated through the microchannels.
Figure 1.
Left: pattern of representative shear stress pattern of the HA5 VAD and corresponding pattern of shear stress in the HA5-emulating microfluidic channel. Right: the HA5-emulating microfluidic channel.
The designed HA5-emulating microchannel is shown in Fig. 1 (right panel): it is characterized by a constant height of 50 μm and a width that varies from 15 to 120 μm and is able to expose platelets to the specified shear stress pattern when a constant flow rate of 24 μl/min is applied. Microfluidic platforms featuring a series of four identical HA5-emulating channels were fabricated in polydimethylsiloxane (PDMS) through standard soft-lithography and sealed with glass coverslips after exposure to oxygen plasma treatment.
2.2 Platelet sample preparation
Gel-filtered platelets (GFP) were utilized and SMPA was assayed through the Platelet Activity State (PAS) assay. The PAS assay is a chromogenic assay able to quantify the thrombin production rate of activated platelets, which correlates with the actual level platelet activation [27]. For sample preparation, thirty ml of whole blood was drawn via venipuncture from healthy adult volunteers following informed consent and collected into 10% anticoagulant acid-citrate-dextrose. Blood was centrifuged at 450 g x 15 min to obtain platelet rich plasma (PRP). PRP was then filtered through Sepharose 2B columns (60–200 μm diameter, 2% agarose; Sigma-Aldrich, USA) to separate platelets from plasma proteins yielding GFP. Before experiments, GFP were counted using a Z1 Particle Counter (Coulter, Miami, FL, USA) and diluted to a final concentration of 20,000 platelets/μl in a Hepes-modified Ca2+-free Tyrode’s platelet buffer containing 0.1% fatty-acid-free bovine serum albumin. GFP were subsequently incubated with AP agents, re-calcified with CaCl2 (3 mM final concentration) and evaluated for SMPA in the microfluidic platform. All experiments were conducted within 6 hours of phlebotomy.
2.3 Anti-platelet agent preparation
Two different AP agents were tested, ASA and ticagrelor. Solutions of these agents were prepared by dissolving 113 mg of ASA (Sigma Aldrich, USA) in 1 ml dimethyl sulfoxide (DMSO) and 18 mg of ticagrelor (Thermo Fisher Scientific Inc, USA) in 10 ml DMSO. Actual drug concentrations were obtained via UV spectrometry utilizing a DU® 730 UV/Vis spectrometer (Beckman Coulter®, USA). The presence of drug in solution was confirmed by obtaining the absorbance spectrum in the UV range (190 - 350 nm) and checking the presence of specific peaks representative of the two drugs. Drug concentration was calculated by measuring absorbance at a fixed wavelength (276 nm for ASA and 222 nm for ticagrelor) and using calibration curves [36,37]. Working solutions of AP agents were prepared by dissolving the storage solutions in modified Tyrode’s buffer at final concentrations of 12.5 mM for ASA and 62.5 μM for ticagrelor. Working solutions were prepared and utilized immediately for exposure and incubation of GFP and discarded immediately thereafter to avoid degradation of the active agent in aqueous solutions. For both ASA and ticagrelor, two different final concentrations in GFP were tested: 125 and 250 μM for ASA and 250 and 500 nM for ticagrelor. The lower values were chosen to replicate typical in vivo therapeutic doses, as detected in plasma after administration of commercial tablets of ASA (500 mg) and ticagrelor (90 mg) in humans [38,39]. Higher concentration levels were chosen to replicate conditions tested in previous in vitro studies [40,41], and also to emulate higher doses of drugs in vivo. GFP were incubated 30 minutes with ASA and 10 minutes with ticagrelor at room temperature prior to run the experiments, according to previous studies [40,41]. For all the tested conditions, the final concentration of DMSO in GFP was kept below 0.01% (v/v ratio) to avoid any undesired effect of DMSO on platelet response.
2.4 Static condition and sonication tests
The effects of ASA and ticagrelor were first tested on negative and positive control samples, i.e., resting platelets – platelets in static conditions in the absence of any agonist, and sonicated platelets. Indeed, sonicated platelets yield maximal pro-thrombinase activity as measured by the PAS assay [42]. AP-treated and untreated GFP samples were tested under static conditions and following sonication. For sonication tests, an ultrasonic device (SLPE Branson, MO, USA) characterized by an efficient output frequency of 40 kHz was used. GFP samples were sonicated at 75 W for 10 s before being tested for platelet activation.
2.5 Microfluidic flow loop tests
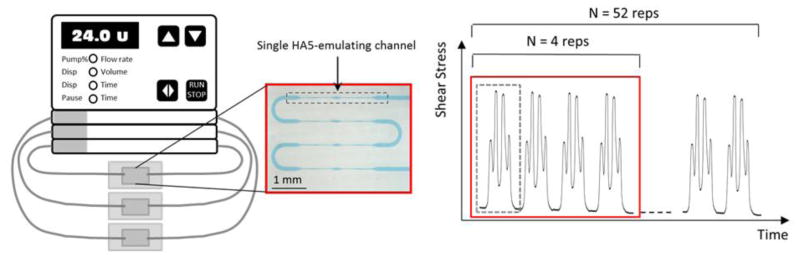
SMPA was assessed in both the presence and absence of AP drugs by recirculating GFP in the microfluidic platform. Three flow loops were setup and tested in parallel using a multichannel peristaltic pump (Ismatec® IPC-N, Cole-Parmer GmbH, Germany). Each hydraulic line comprised a pump tube (PharMed®, 250 μm inner diameter, 20 cm length), two stainless steel couplers (23G, 1 cm length) used to connect the tubing to the access ports of the microfluidic platform, a tygon tube (0.508 inner diameter, 6 cm length), and one HA5-emulating microfluidic platform (Fig. 2, left panel).
Figure 2.
Left: schematic representation of the set-up used for the flow loop tests: three hydraulic lines each one featuring one HA5-emulating microfluidic platform. Each platform (red box) features a series of 4 identical shearing channels (grey dashed box). Right: the collection time points of the flow loop tests are shown: first collection was done at 4 repetitions (N=4 reps) of the HA5 shear stress pattern, while second collection was done at 52 repetitions (N=52 reps) of the shear stress pattern. The red and dashed grey boxes in the plot are used to show correspondence with the microfluidic platform shown in the picture. For a clear representation, only few shear stress patterns are reported in the plot.
Prior to experiments, each hydraulic line comprising the microfluidic platform, connection and pump tubes and the stainless steel couplers, was pre-conditioned by filling with 3% w/v bovine serum albumin in PBS and incubated for 1 hour at room temperature to passivate surfaces and avoid platelet adhesion and activation due to contact with synthetic materials, according to [29]. Lines were then extensively flushed with PBS to remove excess albumin.
Experiments involved all 3 lines in parallel. After filling the hydraulic lines with GFP samples, 30 μl of GFP was collected and tested for PA: this first time point corresponds to platelets exposed to N=4 repetitions of the HA5 shear stress pattern. The hydraulic lines were then closed, the pump operated and GFP recirculated at a flow rate of 24 μl/min for 20 minutes. After 20 min flow loop, GFP in each hydraulic line were collected at the second time point, corresponding to N=52 repetitions of the shear stress pattern (Fig. 2, right panel).
It should be noted that the second time point of the microfluidic flow loops corresponds to 67 minutes of continuous VAD support (if considering a cardiac output of 4 L/min and a total blood volume of 5 L). Although replicating relatively short exposure time to VAD support, this time point (52 repetitions of the shear stress pattern) was chosen based on our previous study in which a stable platelet activation level was achieved in the microfluidic platform above 40 repetitions of shear stress patterns [35].
Preliminary control tests were conducted to assess that negligible platelet activation was obtained following recirculation of platelet samples in the hydraulic lines in the absence of the microfluidic platforms (i.e. in the tubes and stainless steel couplers).
Experiments were replicated with N=6 different blood donors. For each blood donor, 3 lines were used to test different conditions simultaneously: untreated GFP (as negative control), ASA-treated GFP and ticagrelor-treated GFP. Two consecutive tests (one for each drug concentration) were performed using the same hydraulic lines. Between consecutive tests the hydraulic lines were extensively flushed with Hepes-modifed platelet buffer, while for different blood donors new hydraulic lines (including the microfluidic platforms) were used.
2.6 Platelet activity test
Samples from resting, sonicated and GFP recirculated in the microfluidic channels was assayed for platelet activation using the modified-prothrombinase Platelet Activity State (PAS) assay [27]. This method was specifically developed to quantify SMPA in vitro. According to the PAS assay, after static incubation, sonication or shearing, GFP are incubated for 10 minutes at 37°C in the presence of acetylated prothrombin, CaCl2 and factor Xa. During incubation, prothrombin is converted into thrombin depending on the pro-thrombotic activity of platelets, i.e., proportionally to the actual level of platelet activation. The use of acetylated prothrombin as a substrate for thrombin prevents the positive thrombin-mediated feedback on platelet activation, allowing to uniquely correlate the thrombin production rate to the shear-mediated activation of platelets. For the measurements, a VersaMax™ ELISA Microplate Reader (Molecular Devices LLC, CA, USA) in kinetic mode (8-min kinetic reading). Chromozym-TH (Roche Diagnostics, USA) was used as the thrombin specific chromogenic substrate. Measurements obtained from sheared GFP samples were normalized against activation measured for sonicated GFP, i.e., against maximal platelet pro-thrombinase. Thus, PAS values on sheared samples are reported as a percentage of activation where 100% is the activation measured on sonicated GFP.
2.7 Statistical analyses
Data were analyzed with GraphPad Prism 7.2 (GraphPad Software, Inc., CA, USA). One-way ANOVA or Kruskal-Wallis tests were performed. When significant difference was found with ANOVA, Dunnett’s test was used for multiple comparisons of drug-treated vs untreated groups. Kruskal-Wallis was utilized when non-normality was found in at least one group. In this case, for multiple comparisons, the Dunn’s test was used to test difference of treated vs untreated groups.
3. Results
3.1 Static conditions and sonication
Results of the PAS assay performed on non-stimulated and sonicated GFP, in the absence and presence of AP drugs, are shown in Figure 3. Exposure of GFP to either ASA or ticagrelor under static conditions did not affect platelet response as measured by the PAS assay (p>0.05). Significant activation of sonicated platelets was detected, with no inhibition provided by either ASA or ticagrelor with respect to untreated control (p>0.05). As expected, regardless of AP agent concentration, high PAS values were obtained via sonication, while low values were observed for resting platelets.
Figure 3.
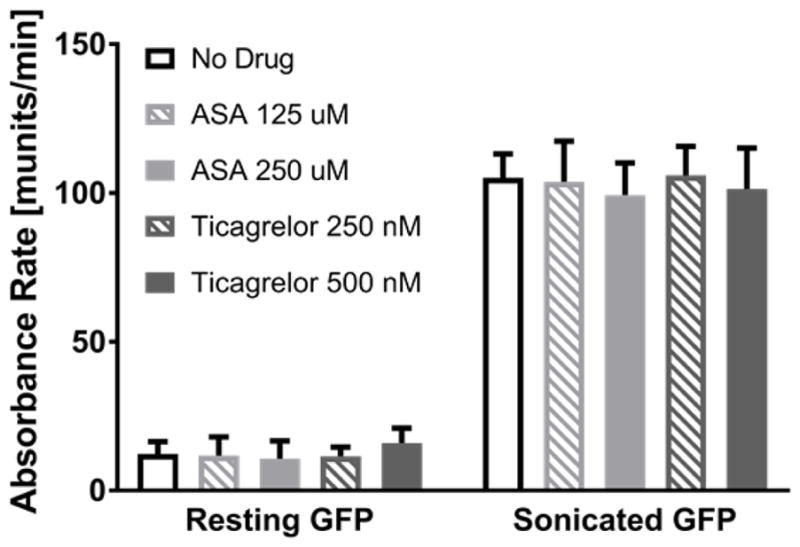
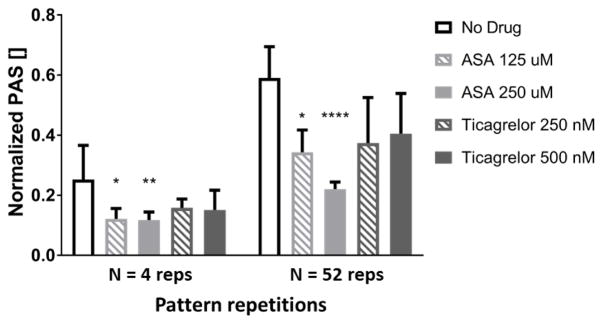
Results of the PAS assay for non stimulated (resting) and sonicated GFP in the absence and presence of ASA and ticagrelor at two different concentrations. Data are shown as mean ± standard deviation.
3.2 Microfluidic flow loop tests
Results of flow loop testing utilizing the HA5-emulating microfluidic platforms are reported in Figure 4. PAS values of sheared platelets following exposure to N=4 and N=52 repetitions of the HA5 emulating shear stress pattern both in the presence and absence of AP agents were normalized against PAS of sonicated GFP. For both treated and untreated GFP, a significant increase of platelet activation was obtained following 52 repetitions of the shear stress pattern. In the absence of drugs a high level of activation was reached, equal to 25 ± 9 % and 57 ± 11 % of sonication activation at N=4 and N=52 repetitions, respectively (compared to PAS level of 11 ± 0.3 % for non stimulated untreated GFP). In contrast, incubation of GFP with drugs resulted in decreased levels of platelet activation, indicating that under the conditions tested both AP agents were able to somewhat limit SMPA. However, inhibition was statistically significant only in the case of ASA (p<0.5 and p<0.01 for ASA 125 and 250 μM at N=4 repetitions, respectively). In addition, with the increase in ASA concentration from 125 to 250 μM SMPA was further inhibited following 52 repetitions of sear stress (from 40 to 60% inhibition compared to control, p<0.05 for ASA 125 μM and p<0.0001 for ASA 250 μM). On the other hand, ticagrelor showed lower inhibitory efficacy (22% at 500 nM and longer shearing time), which was not statistically significant with respect to untreated control (p =0.16).
Figure 4.
PAS results normalized to sonicated GFP obtained from flow loop tests in the HA5 emulating platform in the presence and absence of ASA and ticagrelor. Results obtained at the two time points of collection (corresponding to N=4 and N=52 repetitions of the shear stress pattern) are reported. Data are shown as mean ± standard deviation. Significant difference with respect to untreated GFP (no drug condition) is reported (* p<0.05, ** p<0.01, *** p<0.001, **** p<0.0001).
4. Discussion
In this study, we present a new approach for evaluating AP agent efficacy in vitro based on the use of a microfluidic platform specifically designed to replicate shear stress patterns of commercial clinical VADs. Both ASA, a traditional AP drug utilized to prevent VAD thrombosis and ticagrelor, a newer agent in widespread use for coronary artery disease with promising experience with VADs, were tested. Our results, demonstrate that the microfluidic platforms and recirculation regimes employed effectively activated platelets mechanically, leading to shear-mediated platelet activation. Further, the platforms and methodology utilized allowed for the discrimination of inhibitory efficacy mediated by different drugs and drug concentrations under emulated VAD-like shear stress conditions.
While previous studies have evaluated efficacy of AP drugs in vitro [41,43–46] few have examined efficacy under shear flow conditions [33,47]. The present study provides an advance in both device and method, affording the possibility of testing platelet responsiveness following AP drug treatment in vitro under realistic and VAD-specific shear stress conditions. Indeed, to the best of our knowledge, no prior studies utilizing microfluidics allow for assessment of platelet activity under such complex and elevated shear flow conditions.
Utilizing the microfluidic VAD-emulating device, we successfully achieved SMPA and were able to discriminate between varying degrees of inhibition mediated by AP agents. Total exposure time to shear was in the order of 13 s (following 52 repetitions of the shear stress patterns), which resulted in a significant level of platelet activation for untreated platelets. This result is in agreement with the platelet activation response expected from Hellum’s plot [48], in which a few seconds of exposure time to shear stress of about 100 Pa overcome the threshold for mechanical shear activation. However, Hellum’s plot specifically refers to constant shear stress conditions: conversely, in this study dynamic shear stress patterns were considered and tested, whose effects on platelet response have not been fully described in the literature.
We observed that ASA somewhat limited SMPA, with an elevated dose (250 μM) providing additional inhibition of SMPA. Importantly, with the proposed methodology we could discriminate differential levels of inhibition of SMPA between different agents and different doses (concentrations) of drugs. Our results suggest that microfluidic platforms could set the basis for a promising system for clinical testing of AP drugs under controlled, and well-defined shear conditions mimicking those encountered in mechanical circulatory support devices.
AP agents are the mainstay of clinical pharmacological therapy to limit SMPA associated with VADs [49]. Currently, ASA is the dominant and often sole agent utilized, at doses of 81 – 325 mg/day, for patients implanted with continuous flow VADs, which are comparable to those we have emulated in the present study [50]. Despite ASA management, VAD thrombosis continues to occur to varying degrees in patients and recently has been on the rise [4]. Our findings of only partial inhibition of SMPA by ASA are consistent with and supported by recent studies from our group which demonstrated that under elevated shear conditions associated with VADs ASA has limited ability to limit SMPA [8]. In the present study, we extend this observation with the finding that at ASA levels above conventional therapeutic dose, i.e. 250 μM, further inhibition of SMPA can be achieved under dynamic VAD-like shear stress conditions. The translational implication of this finding raises the potential of utility of higher dose ASA in cases in which exacerbated hypershear is suspected associated with imminent VAD thrombosis. A note of caution though as high dose aspirin is associated with significant risk and toxicity including tinnitus, abdominal pain, metabolic acidosis and bleeding risk. Of note, the clinical trend today is in fact to limit ASA use due to concomitant bleeding observed in VAD patients associated with hypershear-mediated acquired von Willebrand disease [51], thus simultaneous monitoring of bleeding risk would be necessary.
In this study, shear stress patterns of the HA5 VAD resulting from CFD analyses [10] in which complex fluid dynamic effects (such as swirling, secondary and turbulent flows) are accounted for were considered. According to our approach the VAD-specific shear pattern extracted from CFD is mapped onto a microfluidic channel geometry such that platelets flowing in the channel are exposed to the same shear pattern [34]. It is worth noting that differently from the VAD, the flow regime in microfluidic devices is dominated by viscous forces: thus, they allow emulation of VAD-like shear stress levels and exposure time to shear without replicating the actual complex flow conditions of a VAD. The latter would be more accurately recreated in vitro by means of flow loops in the presence of the real VAD. However, as opposed to these methodologies, the microfluidic-based approach has the advantage of being potentially advanced to routine laboratory or bedside-based tests of the VAD patients’ pro-thrombotic risk. Devices may be adopted for point-of care use to aid in screening differing AP drug regimens and doses in patients prior to VAD implantation. The approach described in the present study specifically evaluated the platelet response following AP drug treatment under HA5-specific shear stress conditions. While it is our intent for this approach to be generally applicable broadly to VAD therapy, the specific findings reported are reflective of the VAD tested. Indeed, similar studies could be extended to different VADs and devices (i.e., axial vs. centrifugal pumps), since different shear stress dynamics have proven to distinctly modulate platelet response [8]. This can be readily achieved with the approach and devices described by designing ad hoc microfluidic channels, as previously proposed in our numerical study [34].
Nonetheless, our approach presents some limitations that must be overcome in order to further translate the microfluidic system for clinical use. First, GFP were used as the platelet test sample. Preparing GFP requires gel-column filtration, which makes the preparation of the platelet sample time consuming and thus less suitable to translation to the clinic. Further, even if a filtration system might be incorporated within the microfluidic platform, assessing SMPA with platelets free of plasma coagulation factors provides a partial story – that of the impact of flow conditions on platelets – which is valuable and unique information on the one hand but does not take into account other aspects of Virchows triad, which also contribute to overall net thrombus formation. It is worth clarifying that in this study, GFP was selected to properly conduct platelet activation tests by means of the PAS assay [52], which is recognized as a valuable and sensitive tool to quantify platelet activation associated to the effect of shear stress conditions in vitro [16,42]. A recent study also introduced the PAS assay in the clinical setting for the assessment of prothrombotic conditions and monitoring of drug regimen of hospitalized patients implanted with continuous flow left VADs [53]. However, more extensive studies are needed to support the use of the PAS assay as a sensitive tool for predicting the development of thrombotic processes in vivo.
As an alternative, the use of whole blood samples and inclusion of sub-endothelial extracellular matrix protein substrates for studying platelet adhesion and aggregation following exposure to shear could be integrated in the microfluidic platform. This will allow accounting for different elements involved in thrombotic processes in vivo, such as: i) the effect of hemoglobin, ADP and LDH release from hemolyzed red blood cells [54], ii) the presence of prothrombinase complex elements in plasma that once converted in their active form, strongly influence thrombosis; and iii) the effect of other factors determining hemostatic processes under shear flow conditions, such as von Willebrand factor.
In addition, collection and manipulation of the platelet sample was necessary to perform the PAS assay. This two-step procedure is not optimal as manipulation of the sample may introduce experimental variability, and the delay between the end of the stimulus (shear stress in the microfluidic channels) and the assay may also alter platelet response.
To overcome these limitations, integrated on chip tests of platelet activation should be developed, allowing real-time monitoring of platelet response without the need for sample collection and limiting operator-dependent procedures. A fluorescence-based approach could be utilized, such as monitoring of platelet Ca2+ influx by using Ca2+ fluorescent probe incorporated in the platelet sample and performing fluorescence acquisition on chip using fluorescence microscopy. Similar approaches have been utilized in previous studies [55–57] and allowed to discriminate different levels of platelet response. Fluorescence-based assays are one of the main approaches for microfluidic on chip tests as they allow for targeting specific molecules or biological products and due to their non-destructive characteristic, which allows acquisitions at different time points of the experimental procedure. However, staining of the biological sample may require expensive antibodies and preliminary manipulation. In addition, adequate equipment and high magnification objectives are required for proper acquisitions.
5. Conclusion
Microfluidic platforms, designed to emulate and reproduce dynamic shear stress patterns occurring within VADs, effectively activate platelets as a small format surrogate. Use of these platforms has been shown herein to allow determination and discrimination of AP agent efficacy as to limiting SMPA. Our results further corroborate that ASA, the mainstay of VAD AP therapy, has limited efficacy in modulating SMPA, though at very high dose may provide additional inhibition. Further development of the microfluidic approach has the potential to result in simple, portable, easy-to-use, small footprint systems with applicability to the laboratory and the field for point-of care setting. Utilization of such systems may further assist clinicians in the complex management of patients with VADs in an effort to personalize use of AP agents to limit thrombotic complications associated with VAD use.
Highlights.
A microfluidic platform is proposed to test anti-platelet agents under shear
High and dynamic “VAD-like” shear patterns are replicated in the microfluidic device
Platelet activity state assay is utilized to evaluate inhibitory effects of two anti-platelet agents
Acknowledgments
This work was funded by Fondazione Cariplo and Regione Lombardia [grant no. 2016-0901], by support from the University of Arizona Center for Accelerated Biomedical Innovation (ACABI) and Tech Launch Arizona [grant no. UA 15-035] and funds from National Institute of Biomedical Imaging and Bioengineering Quantum Grant Award No. 5U01EB012487-00.
The authors thank Yana Roka Moiia PhD for her technical assistance with drug studies described.
Competing interests: None declared.
Ethical approval: Not required.
Abbreviations
- ASA
acetylsalicylic acid
- AP
anti-platelet
- DMSO
dimethyl sulfoxide
- GFP
gel-filtered platelets
- HA5
HeartAssist5
- PAS
platelet activity state
- SMPA
shear-mediated platelet activation
- VAD
ventricular assist device
Footnotes
Disclosures
Authors have no conflict of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stewart GC, Givertz MM. Mechanical circulatory support for advanced heart failure: Patients and technology in evolution. Circulation. 2012;125:1304–15. doi: 10.1161/CIRCULATIONAHA.111.060830. [DOI] [PubMed] [Google Scholar]
- 2.Bonacchi M, Harmelin G, Bugetti M, Sani G. Mechanical Ventricular Assistance as Destination Therapy for End-Stage Heart Failure: Has it Become a First Line Therapy? Front Surg. 2015;2:35. doi: 10.3389/fsurg.2015.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kirklin JK, Naftel DC, Pagani FD, Kormos RL, Stevenson LW, Blume ED, et al. Sixth INTERMACS annual report: A 10,000-patient database. J Hear Lung Transplant. 2014;33:555–64. doi: 10.1016/j.healun.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 4.Starling RC, Moazami N, Silvestry SC, Ewald G, Rogers JG, Milano Ca, et al. Unexpected abrupt increase in left ventricular assist device thrombosis. N Engl J Med. 2014;370:33–40. doi: 10.1056/NEJMoa1313385. [DOI] [PubMed] [Google Scholar]
- 5.Bartoli CR, Ailawadi G, Kern JA. Diagnosis, nonsurgical management, and prevention of LVAD thrombosis. J Card Surg. 2014;29:83–94. doi: 10.1111/jocs.12238. [DOI] [PubMed] [Google Scholar]
- 6.Kalantzi KI, Tsoumani ME, Goudevenos IA, Tselepis AD. Pharmacodynamic properties of antiplatelet agents: current knowledge and future perspectives. Expert Rev Clin Pharmacol. 2012;5:319–36. doi: 10.1586/ecp.12.19. [DOI] [PubMed] [Google Scholar]
- 7.Gladding P, Webster M, Ormiston J, Olsen S, White H. Antiplatelet drug nonresponsiveness. Am Heart J. 2008;155:591–9. doi: 10.1016/j.ahj.2007.12.034. [DOI] [PubMed] [Google Scholar]
- 8.Valerio L, Tran PL, Sheriff J, Brengle W, Ghosh R, Chiu WC, et al. Aspirin has limited ability to modulate shear-mediated platelet activation associated with elevated shear stress of ventricular assist devices. Thromb Res. 2016;140:110–7. doi: 10.1016/j.thromres.2016.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thamsen B, Blümel B, Schaller J, Paschereit CO, Affeld K, Goubergrits L, et al. Numerical Analysis of Blood Damage Potential of the HeartMate II and HeartWare HVAD Rotary Blood Pumps. Artif Organs. 2015;39:651–9. doi: 10.1111/aor.12542. [DOI] [PubMed] [Google Scholar]
- 10.Chiu W-C, Girdhar G, Xenos M, Alemu Y, Soares JS, Einav S, et al. Thromboresistance Comparison of the HeartMate II Ventricular Assist Device With the Device Thrombogenicity Emulation-Optimized HeartAssist 5 VAD. J Biomech Eng. 2014;136:21014. doi: 10.1115/1.4026254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ruggeri ZM. Platelet Adhesion under Flow. Microcirculation. 2009;16:58–83. doi: 10.1080/10739680802651477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hargett La, Bauer NN. On the origin of microparticles: From “platelet dust” to mediators of intercellular communication. Pulm Circ. 2013;3:329–40. doi: 10.4103/2045-8932.114760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maxwell MJ, Dopheide SM, Turner SJ, Jackson SP. Shear induces a unique series of morphological changes in translocating platelets: Effects of morphology on translocation dynamics. Arterioscler Thromb Vasc Biol. 2006;26:663–9. doi: 10.1161/01.ATV.0000201931.16535.e1. [DOI] [PubMed] [Google Scholar]
- 14.Capoccia M, Bowles CT, Sabashnikov A, Simon A. Recurrent Early Thrombus Formation in HeartMate II Left Ventricular Assist Device. J Investig Med High Impact Case Reports. 2013;1:2324709613490676. doi: 10.1177/2324709613490676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meyer AL, Kuehn C, Weidemann J, Malehsa D, Bara C, Fischer S, et al. Thrombus formation in a HeartMate II left ventricular assist device. J Thorac Cardiovasc Surg. 2008;135:203–4. doi: 10.1016/j.jtcvs.2007.08.048. [DOI] [PubMed] [Google Scholar]
- 16.Piatti F, Sturla F, Marom G, Sheriff J, Claiborne TE, Slepian MJ, et al. Hemodynamic and thrombogenic analysis of a trileaflet polymeric valve using a fluid-structure interaction approach. J Biomech. 2015;48:3650–8. doi: 10.1016/j.jbiomech.2015.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pelosi A, Sheriff J, Stevanella M, Fiore GB, Bluestein D, Redaelli A. Computational evaluation of the thrombogenic potential of a hollow-fiber oxygenator with integrated heat exchanger during extracorporeal circulation. Biomech Model Mechanobiol. 2014;13:349–61. doi: 10.1007/s10237-012-0445-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Consolo F, Valerio L, Brizzola S, Rota P, Marazzato G, Vincoli V, et al. On the Use of the Platelet Activity State Assay for the In Vitro Quantification of Platelet Activation in Blood Recirculating Devices for Extracorporeal Circulation. Artif Organs. 2016;40:971–80. doi: 10.1111/aor.12672. [DOI] [PubMed] [Google Scholar]
- 19.Consolo F, Sheriff J, Gorla S, Magri N, Bluestein D, Pappalardo F, et al. High Frequency Components of Hemodynamic Shear Stress Profiles are a Major Determinant of Shear-Mediated Platelet Activation in Therapeutic Blood Recirculating Devices. Sci Rep. 2017;7:4994. doi: 10.1038/s41598-017-05130-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Slepian MJ, Sheriff J, Hutchinson M, Tran P, Bajaj N, Garcia JGN, et al. Shear-mediated platelet activation in the free flow: Perspectives on the emerging spectrum of cell mechanobiological mechanisms mediating cardiovascular implant thrombosis. J Biomech. 2017;50:20–5. doi: 10.1016/j.jbiomech.2016.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frontroth JP. Light transmission aggregometry. Methods Mol Biol. 2013;992:227–40. doi: 10.1007/978-1-62703-339-8_17. [DOI] [PubMed] [Google Scholar]
- 22.Schimmer C, Hamouda K, Sommer SP, Ozkur M, Hain J, Leyh R. The predictive value of multiple electrode platelet aggregometry (multiplate) in adult cardiac surgery. Thorac Cardiovasc Surg. 2013;61:733–43. doi: 10.1055/s-0033-1333659. [DOI] [PubMed] [Google Scholar]
- 23.Luddington RJ. Thrombelastography/thromboelastometry. Clin Lab Haematol. 2005;27:81–90. doi: 10.1111/j.1365-2257.2005.00681.x. [DOI] [PubMed] [Google Scholar]
- 24.Favaloro EJ. Clinical utility of the combin. Semin Therombosis Hemost. 2008;34:709–33. doi: 10.1055/s-0029-1145254. [DOI] [Google Scholar]
- 25.Bluestein D, Girdhar G, Einav S, Slepian MJ. Device thrombogenicity emulation: a novel methodology for optimizing the thromboresistance of cardiovascular devices. J Biomech. 2013;46:338–44. doi: 10.1016/j.jbiomech.2012.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Giorgio TD, Hellums JD. A cone and plate viscometer for the continuous measurement of blood platelet activation. Biorheology. 1988;25:605–24. doi: 10.3233/bir-1988-25402. [DOI] [PubMed] [Google Scholar]
- 27.Girdhar G, Xenos M, Alemu Y, Chiu WC, Lynch BE, Jesty J, et al. Device thrombogenicity emulation: A novel method for optimizing mechanical circulatory support device thromboresistance. PLoS One. 2012;7:1–10. doi: 10.1371/journal.pone.0032463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gutierrez E, Petrich BG, Shattil SJ, Ginsberg MH, Groisman A, Kasirer-Friede A. Microfluidic devices for studies of shear-dependent platelet adhesion. Lab Chip. 2008;8:1486–95. doi: 10.1039/b804795b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Neeves KB, Onasoga AA, Hansen RR, Lilly JJ, Venckunaite D, Sumner MB, et al. Sources of variability in platelet accumulation on type 1 fibrillar collagen in microfluidic flow assays. PLoS One. 2013;8:e54680. doi: 10.1371/journal.pone.0054680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hansen RR, Wufsus AR, Barton ST, Onasoga AA, Johnson-Paben RM, Neeves KB. High content evaluation of shear dependent platelet function in a microfluidic flow assay. Ann Biomed Eng. 2013;41:250–62. doi: 10.1007/s10439-012-0658-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hosokawa K, Ohnishi T, Kondo T, Fukasawa M, Koide T, Maruyama I, et al. A novel automated microchip flow-chamber system to quantitatively evaluate thrombus formation and antithrombotic agents under blood flow conditions. J Thromb Haemost. 2011;9:2029–37. doi: 10.1111/j.1538-7836.2011.04464.x. [DOI] [PubMed] [Google Scholar]
- 32.Jain A, Graveline A, Waterhouse A, Vernet A, Flaumenhaft R, Ingber DE. A shear gradient-activated microfluidic device for automated monitoring of whole blood haemostasis and platelet function. Nat Commun. 2016;7:10176. doi: 10.1038/ncomms10176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li M, Hotaling NA, Ku DN, Forest CR. Microfluidic thrombosis under multiple shear rates and antiplatelet therapy doses. PLoS One. 2014:9. doi: 10.1371/journal.pone.0082493. [DOI] [PMC free article] [PubMed]
- 34.Dimasi A, Rasponi M, Sheriff J, Chiu W-C, Bluestein D, Tran PL, et al. Microfluidic emulation of mechanical circulatory support device shear-mediated platelet activation. Biomed Microdevices. 2015:17. doi: 10.1007/s10544-015-0015-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Consolo F, Dimasi A, Rasponi M, Valerio L, Pappalardo F, Bluestein D, et al. Microfluidic approaches for the assessment of blood cell trauma: A focus on thrombotic risk in mechanical circulatory support devices. Int J Artif Organs. 2016;39:184–93. doi: 10.5301/ijao.5000485. [DOI] [PubMed] [Google Scholar]
- 36.Pandya D, Patel M, Ghediya R, Shah A, Khunt R. UV-Vis spectrophotometric assay determination of oral antiplatelet ticagrelor drug in pharmaceutical formulation3: Application to content uniformity. J Chem Pharm Res. 2016;8:316–21. [Google Scholar]
- 37.Iwunze MO. Absorptiometric Determination of Acetylsalicylic Acid in Aqueous Ethanolic Solution. Anal Lett. 2008;41:2944–53. doi: 10.1080/00032710802440574. [DOI] [Google Scholar]
- 38.Nagelschmitz J, Blunck M, Kraetzschmar J, Ludwig M, Wensing G, Hohlfeld T. Pharmacokinetics and pharmacodynamics of acetylsalicylic acid after intravenous and oral administration to healthy volunteers. Clin Pharmacol Adv Appl. 2014;6:51–9. doi: 10.2147/CPAA.S47895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Teng R, Oliver S, Hayes M, Butler K. Absorption, distribution, metabolism, and excretion of ticagrelor in healthy subjects. Drug Metab Dispos. 2010;38:1514–21. doi: 10.1124/dmd.110.032250.and. [DOI] [PubMed] [Google Scholar]
- 40.Mangalpally KKR, Siqueiros-Garcia A, Vaduganathan M, Dong JF, Kleiman NS, Guthikonda S. Platelet activation patterns in platelet size sub-populations: Differential responses to aspirin in vitro. J Thromb Thrombolysis. 2010;30:251–62. doi: 10.1007/s11239-010-0489-x. [DOI] [PubMed] [Google Scholar]
- 41.van Giezen JJJ, Nilsson L, Berntsson P, Wissing BM, Giordanetto F, Tomlinson W, et al. Ticagrelor binds to human P2Y12 independently from ADP but antagonizes ADP-induced receptor signaling and platelet aggregation. J Thromb Haemost. 2009;7:1556–65. doi: 10.1111/j.1538-7836.2009.03527.x. [DOI] [PubMed] [Google Scholar]
- 42.Sheriff J, Soares JS, Xenos M, Jesty J, Bluestein D. Evaluation of shear-induced platelet activation models under constant and dynamic shear stress loading conditions relevant to devices. Ann Biomed Eng. 2013;41:1279–96. doi: 10.1007/s10439-013-0758-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Husted S, Van Giezen JJJ. Ticagrelor: The first reversibly binding oral p2y12 receptor antagonist. Cardiovasc Ther. 2009;27:259–74. doi: 10.1111/j.1755-5922.2009.00096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Myat A, Tantry US, Kubica J, Gurbel PA. Current controversies in the use of aspirin and ticagrelor for the treatment of thrombotic events. Expert Rev Cardiovasc Ther. 2016;14:1361–70. doi: 10.1080/14779072.2016.1247693. [DOI] [PubMed] [Google Scholar]
- 45.Nylander S, Femia EA, Scavone M, Berntsson P, Asztély AK, Nelander K, et al. Ticagrelor inhibits human platelet aggregation via adenosine in addition to P2Y12 antagonism. J Thromb Haemost. 2013;11:1867–76. doi: 10.1111/jth.12360. [DOI] [PubMed] [Google Scholar]
- 46.Jeong YH, Bliden KP, Antonino MJ, Park KS, Tantry US, Gurbel PA. Usefulness of the VerifyNow P2Y12 assay to evaluate the antiplatelet effects of ticagrelor and clopidogrel therapies. Am Heart J. 2012;164:35–42. doi: 10.1016/j.ahj.2012.03.022. [DOI] [PubMed] [Google Scholar]
- 47.Li R, Diamond SL. Detection of platelet sensitivity to inhibitors of COX-1, P2Y1, and P2Y12 using a whole blood microfluidic flow assay. Thromb Res. 2014;133:203–10. doi: 10.1016/j.thromres.2013.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hellums JD. 1993 Whitaker lecture: Biorheology in thrombosis research. Ann Biomed Eng. 1994;22:445–55. doi: 10.1007/BF02367081. [DOI] [PubMed] [Google Scholar]
- 49.Mehra MR, Stewart GC, Uber PA. The vexing problem of thrombosis in long-term mechanical circulatory support. J Hear Lung Transplant. 2014;33:1–11. doi: 10.1016/j.healun.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 50.Jennings DL, Weeks PA. Thrombosis in continuous-flow left ventricular assist devices: Pathophysiology, prevention, and pharmacologic management. Pharmacotherapy. 2015;35:79–98. doi: 10.1002/phar.1501. [DOI] [PubMed] [Google Scholar]
- 51.Suarez J, Patel CB, Felker GM, Becker R, Hernandez AF, Rogers JG. Mechanisms of bleeding and approach to patients with axial-flow left ventricular assist devices. Circ Hear Fail. 2011;4:779–84. doi: 10.1161/CIRCHEARTFAILURE.111.962613. [DOI] [PubMed] [Google Scholar]
- 52.Jesty J, Bluestein D. Acetylated prothrombin as a substrate in the measurement of the procoagulant activity of platelets: elimination of the feedback activation of platelets by thrombin. Anal Biochem. 1999;272:64–70. doi: 10.1006/abio.1999.4148. [DOI] [PubMed] [Google Scholar]
- 53.Valerio L, Consolo F, Bluestein D, Tran P, Slepian M, Redaelli A, et al. Shear-mediated platelet activation in patients implanted with continuous flow LVADs: A preliminary study utilizing the platelet activity state (PAS) assay. Proc Annu Int Conf IEEE Eng Med Biol Soc EMBS; 2015–Novem; pp. 1255–8. [DOI] [PubMed] [Google Scholar]
- 54.Helms CC, Marvel M, Zhao W, Stahle M, Vest R, Kato GJ, et al. Mechanisms of hemolysis-associated platelet activation. J Thromb Haemost. 2013;11:2148–54. doi: 10.1111/jth.12422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Van Zijp HM, Barendrecht AD, Riegman J, Goudsmits JMH, De Jong AM, Kress H, et al. Quantification of platelet-surface interactions in real-time using intracellular calcium signaling. Biomed Microdevices. 2014;16:217–27. doi: 10.1007/s10544-013-9825-1. [DOI] [PubMed] [Google Scholar]
- 56.Kuwahara M, Sugimoto M, Tsuji S, Matsui H, Mizuno T, Miyata S, et al. Platelet shape changes and adhesion under high shear flow. Arterioscler Thromb Vasc Biol. 2002;22:329–34. doi: 10.1161/hq0202.104122. [DOI] [PubMed] [Google Scholar]
- 57.Roberts DE, McNicol A, Bose R. Mechanism of Collagen Activation in Human Platelets. J Biol Chem. 2004;279:19421–30. doi: 10.1074/jbc.M308864200. [DOI] [PubMed] [Google Scholar]
- 58.Evander M, Ricco AJ, Morser J, Kovacs GTa, Leung LLK, Giovangrandi L. Microfluidic impedance cytometer for platelet analysis. Lab Chip. 2013;13:722–9. doi: 10.1039/c2lc40896a. [DOI] [PubMed] [Google Scholar]