Abstract
Background
We examined whether waist circumference (WC) and self-reported abdominal size changes can estimate visceral adipose tissue (VAT) changes for those initiating antiretroviral therapy (ART).
Methods
Prospectively collected data from ACTG A5257 and its metabolic substudy, A5260s, were used for this analysis. ART-naïve HIV-infected participants were randomized to one of three contemporary ART regimens. Changes in abdominal CT-measured VAT and total adipose tissue (TAT) and DXA-measured trunk fat were tested for association with WC changes (by Pearson correlation) and categories of self-reported abdominal size changes (by ANOVA) between entry and week 96. Linear models compared WC and self-reported changes.
Results
The study population (N=328) was predominantly male (90%) and white non-Hispanic (44%) with a baseline median age of 36 years and BMI of 25 kg/m2. At week 96, median WC change was +2.8 cm. Of those reporting at week 96, 53% indicated “No Change/Lost”, 39% “Gained Some/Somewhat Larger”, and 8% “Gained A Lot/Much Larger” as their self-reported changes. Trunk fat, VAT, and TAT changes differed across self-reported groups (ANOVA p<0.0001 for all), and the group ordering was as expected. WC changes were strongly correlated with CT and DXA changes (trunk fat: ρ=0.72, p<0.0001; VAT: ρ=0.52, p<0.0001; TAT: ρ=0.62, p<0.0001). While WC changes explained a greater proportion of VAT, TAT, and trunk fat variation, self-reported changes remained a significant predictor after controlling for WC (p<0.05).
Conclusions
WC and self-reported abdominal changes each correlated directly with imaging-derived abdominal fat measures, and can be used as reliable, affordable tools for central adiposity assessment.
Introduction
Cardiovascular disease (CVD) continues to be an important cause of morbidity and mortality among human immunodeficiency virus (HIV) infected individuals on effective long-term antiretroviral therapy (ART).[1–3] Both HIV infection and ART may play a role in the etiology of CVD.[1] Fat changes during therapy include fat loss or lipoatrophy and fat gain or lipohypertrophy. Studies have found that central fat gain continues to frequently occur in the contemporary ART era, and often includes increases in visceral adipose tissue (VAT), which has been found to be an important risk factor for cardiovascular disease.[4–7]
VAT, the deep adipose tissue depot that surrounds the abdominal organs, has been shown to be metabolically more active than subcutaneous adipose tissue (SAT) and more involved in lipolytic activities and release of pro-inflammatory cytokines.[8] Excess VAT has been associated with insulin resistance, glucose intolerance, dyslipidemia, systemic inflammation, and with elevated CVD risk.[9–11] In HIV-infected individuals, increased VAT has been found to be a predictor of coronary artery calcium (CAC) score, a marker of atherosclerosis.[12,13]
This increase in CVD risk associated with VAT may be higher in HIV-infected individuals compared to uninfected individuals. The Fat Redistribution and Metabolic Change in HIV Infection (FRAM) study found that increased VAT is associated with increased Framingham Risk Scores, and this effect is more pronounced in HIV-infected individuals compared to HIV-negative controls.[14] In addition, increased VAT in HIV-infected individuals is associated with a higher odds of 5-year all-cause mortality.[15] This suggests that VAT accumulation is an important CVD risk factor that should be monitored in HIV-infected individuals.
VAT and other body fat measurements are currently conducted using computed tomography (CT) or dual x-ray absorptiometry (DXA). These techniques, although valid, are expensive and not readily available in clinical practice, making routine monitoring of body fat changes difficult. Self-reported abdominal change perceptions and waist circumference (WC) measurements could potentially prove to be more accessible alternatives for clinicians and for clinical research studies in low resource settings. WC has been found to be strongly associated with visceral and abdominal fat in the general population, and is therefore regarded as a valid measure of regional fat distribution despite its inability to distinguish between VAT and SAT.[16–20] However, the validity of WC changes has not been extensively examined, particularly in the HIV-infected population.
Prior studies have explored whether self-reported fat changes correlate with objective measurements of fat changes. However, such studies are limited, have conflicting results, and have not specifically addressed abdominal fat gain. Several of these studies employed cross-sectional designs, which did not use longitudinal measurements of fat change to predict self-reported lipoatrophy or lipohypertrophy.[21–24] The purpose of this research is to assess whether measured WC and self-reported abdominal changes in patients with HIV prove to be correlated with CT- and DXA-measured changes in central adiposity using prospectively collected clinical trial data, and could therefore be used as valid, cost-effective alternatives to these more labor-intensive and expensive methods of body composition assessment.
Methods
Study Population
This retrospective cohort study was conducted using data from the AIDS Clinical Trials Group (ACTG) A5257 clinical trial and A5260s substudy, and was approved by the UCLA Institutional Review Board (IRB). A5257 was a phase III randomized clinical trial comparing the virologic efficacy and tolerability of three non-nucleoside reverse transcriptase inhibitors (NNRTI) sparing antiretroviral regimens comprised of Tenofovir Disoproxil Fumarate/Emtricitabine (TDF/FTC) plus Atazanavir/Ritonavir (ATV/r), Darunavir/Ritonavir (DRV/r), or Raltegravir (RAL). The A5260s substudy was designed to evaluate the effects of HIV disease and ART on cardiovascular and metabolic outcomes. The design and results of the main study and substudy have been previously reported.[25–30]
In order to be eligible for the A5260s substudy, participants had to be enrolled in A5257. Participants were excluded if they had diabetes mellitus, known CVD, untreated hypothyroidism/hyperthyroidism, or were using statins or other hypolipemic agents. Participants also could not have intention to start pharmacological or surgical intervention for weight loss. Randomization, stratification, and treatment assignments were given as per the A5257 protocol. Participants enrolled between June 2009 and April 2011 at 26 ACTG sites in the United States. The duration of the A5260s substudy was 144 weeks. The substudy required participants to have comprehensive metabolic tests and body composition measurements.
Data Collection
Demographic and Anthropometric Data
The demographic information, including race/ethnicity, age, and sex, collected at baseline as part of the parent study, was used for these analyses. Baseline BMI (in kg/m2) was categorized according to the CDC definitions: underweight as a BMI below 18.5 kg/m2, normal weight between 18.5 kg/m2 – 24.9 kg/m2, overweight between 25.0 kg/m2 – 29.9 kg/m2, and obese at 30 kg/m2 and over.[31]
Self-Reported Abdominal Change
The A5257 body image questionnaire was adopted from the FRAM study (NIH Grants: R01DK57508, R01HL74814, and R01HL53359)[32], and included self-reported measures on perception of current body weight, and assessment about gain or loss of size in specific regions of the body. This analysis focused on self-reported belly size changes only. While the questionnaire was self-administered, participants could request help from the clinical staff for assistance in reading or understanding the questions. The questionnaire responses from week 96 were used to examine self-reported belly size changes from baseline, which were scored as “No Change/Lost”, “Gained Some/Somewhat Larger”, and “Gained A Lot/Much Larger”.
Waist Circumference Measurements
WC (in cm) was measured during study visits by trained clinical staff at week 0 and week 96. Participants were told to stand erect, relaxed, and to not hold in their stomach during measurement. A mid-waist circumference measurement was taken at the level of the upper border of the right ilium. Each measurement was conducted post exhalation with the tape measure parallel to the floor. The WC was required to be measured in triplicate for each participant. The average of the provided readings was used as the final WC value.
DXA Trunk Fat Measurements
Whole body DXA measurements were conducted in the A5260s substudy at entry and at week 96. DXA measurements of regional body fat content were performed in an anteroposterior view using either a Lunar or Hologic scanner at the local site. The DXA scan was used to measure regional trunk fat mass (in kg). All scans for each participant were performed on the same machine model throughout the study. DXA scans were centrally read at the Body Composition Analysis Center at Tufts University (Boston, MA, USA) by staff blinded to treatment assignment and clinical characteristics.
CT Abdominal Fat Measurements
Non-contrast single slice abdominal CT scans at the L4–L5 level were also conducted at baseline and at week 96. The CT scan was used to measure VAT and TAT by taking cross-sectional images of the abdominal area (in cm2). All scans for this study were performed on an approved scanner, using the same software version and same type of instrument. CT scans were centrally read at the LA Biomed CT Reading Center (Torrance, CA, USA) by staff blinded to treatment assignment and clinical characteristics.
In absence of a week 96 visit, self-report responses or waist circumference, DXA or CT measurements closest to this time within an 8 week window before and after week 96 were used.
Data Analysis and Statistical Methods
ANOVA was used to determine whether the self-reported perception of body image at week 96 was associated with absolute measurements of body fat change, by examining whether group means of CT- and DXA-measured fat change were not equal across the self-reported categories. Specifically, self-reported change was used as a categorical predictor variable and trunk fat, VAT, and TAT changes between week 0 and week 96 were used as continuous outcome variables. Pairwise contrasts were used to analyze differences in measured fat between specific self-reported categories, using the Tukey-Kramer method to adjust for multiple comparisons. Stratified analyses were also performed to look at the association in subgroups defined by sex, race, baseline BMI, and age.
Pearson coefficients were used to examine the strength of the correlation between the absolute changes in WC and CT- and DXA-measured fat between baseline and week 96. Changes in WC, trunk fat, VAT, and TAT between week 0 and week 96 were continuous variables used in this analysis. Correlations between WC and trunk fat, VAT, and TAT changes were also examined for sex, race, baseline BMI, and age subgroups.
Separate models with WC and self-reported abdominal changes as single predictors were compared to joint models that included both variables together in order to examine the relative importance of WC and self-reported changes in explaining the variation in trunk fat, VAT, and TAT changes between week 0 and week 96. The Tukey-Kramer multiple comparisons adjustment method was used for pairwise contrasts between self-reported categories. All analyses were performed using SAS Software, Version 9.4 of the SAS System for Windows (© SAS Institute Inc, Cary, NC).
Results
Study Participants
Of the 334 participants initially enrolled in A5260s, 3 did not meet the study eligibility criteria and 3 were lost to follow-up immediately following enrollment. Details about participant disposition can be found in McComsey et al. (2016).[30]
Our resulting analysis population consists of the 328 HIV-infected adults in A5260s whose age ranged from 19 to 72 years and averaged 37 years (Table 1). Participants were 89.6% male (N=294) and 10.4% female (N=34). The diverse substudy population consisted of 43.9% white (N=144), 32.0% black (N=105), and 19.8% Hispanic (N=65) participants. The average baseline weight was 79 kg, with the majority of participants having a normal BMI (N=166, 50.6%) or overweight BMI (N=103, 31.4%) and fewer individuals being obese (N=53, 16.2%) or underweight (N=6, 1.8%) at baseline. The underweight and normal BMI categories were combined for the purposes of the subgroup analyses.
Table 1.
Demographic and baseline information about A5260s study population (N=328).
| Demographics | A5260s (N = 328) |
|---|---|
| Sex [N (%)] | |
| Male | 294 (89.6%) |
| Female | 34 (10.4%) |
| Race [N (%)] | |
| White non-Hispanic | 144 (43.9%) |
| Black non-Hispanic | 105 (32.0%) |
| Hispanic | 65 (19.8%) |
| Other | 13 (4.0%) |
| Unknown | 1 (0.3%) |
| Baseline BMI, kg/m2 [N (%)] | |
| <18.5 | 6 (1.8%) |
| 18.5–24.9 | 166 (50.6%) |
| 25–29.9 | 103 (31.4%) |
| ≥30.0 | 53 (16.2%) |
| Weight, kg [Mean (SD, range)] | 79.0 (16.6, 46.1–138.3) |
| Height, cm [Mean (SD, range] | 175.4 (8.9, 142.2–196.0) |
| Age, years [Mean (SD, range)] | 37 (11, 19–72) |
SD = Standard Deviation
Those who did not have week 96 follow-up measurements for each abdominal fat type, self-report, or waist circumference showed baseline and demographic characteristics that were representative of the overall substudy population of 328 individuals.
Self-Reported Abdominal Size Changes
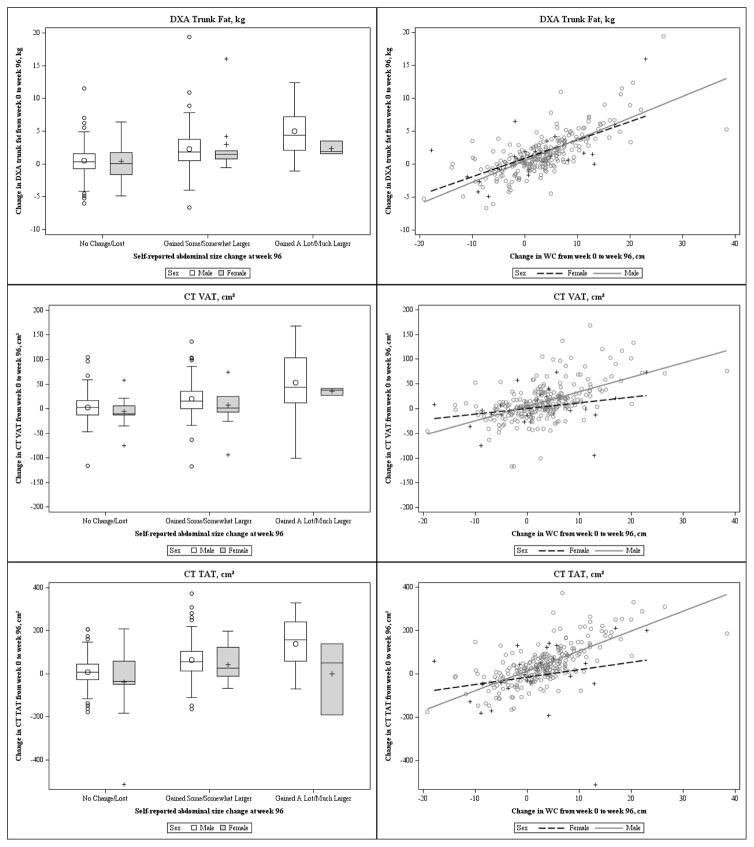
The overall and sex specific average trunk fat, VAT, and TAT measured changes from week 0 to week 96 across self-reported categories can be found in Table 2. As participants reported gaining more abdominal size, the measured changes also increased accordingly (Table 2, Figure 1). This trend was consistent for males, however, it was not seen for trunk fat and TAT in the small female group.
Table 2.
Overall and sex-specific means of trunk fat, VAT, and TAT across self-reported abdominal size change categories in A5260s study population (N=328).
| No Change/Lost | Gained Some/ Somewhat Larger | Gained A Lot/ Much Larger | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| N | Mean (SD) | N | Mean (SD) | N | Mean (SD) | |
| Overall | ||||||
| Trunk fat, kg | 152 | 0.5 (2.5) | 110 | 2.3 (3.4) | 20 | 4.6 (3.5) |
| VAT, cm2 | 148 | 1.7 (28.5) | 112 | 18.7 (37.0) | 21 | 50.1 (58.8) |
| TAT, cm2 | 148 | 3.1 (85.2) | 112 | 62.4 (88.1) | 21 | 120.5 (129.2) |
| Males | ||||||
| Trunk fat, kg | 134 | 0.5 (2.4) | 101 | 2.3 (3.2) | 17 | 5.0 (3.7) |
| VAT, cm2 | 131 | 2.7 (28.5) | 103 | 19.8 (35.6) | 18 | 52.5 (63.4) |
| TAT, cm2 | 131 | 8.4 (69.7) | 103 | 64.0 (88.3) | 18 | 140.6 (114.7) |
| Females | ||||||
| Trunk fat, kg | 18 | 0.4 (3.2) | 9 | 3.0 (5.0) | 3 | 2.3 (1.0) |
| VAT, cm2 | 17 | −5.8 (27.6) | 9 | 6.7 (51.3) | 3 | 35.3 (6.9) |
| TAT, cm2 | 17 | −37.5 (159.0) | 9 | 43.7 (89.1) | 3 | −0.5 (171.7) |
VAT = Visceral Adipose Tissue; TAT = Total Adipose Tissue; SD = Standard Deviation
Figure 1.
Sex-specific change in trunk fat, VAT, and TAT between week 0 and week 96 across self-reported abdominal size change categories and WC changes in A5260s study population (N=328).
aVAT = Visceral Adipose Tissue; TAT = Total Adipose Tissue; WC = Waist Circumference
ANOVA models revealed that measured average changes in trunk fat, VAT, and TAT were not the same across self-reported groups in the overall substudy population (Overall p<0.0001). For trunk fat, when compared to the reference “No Change/Lost” group, the “Gained A Lot/Much Larger” group had greater mean gains in fat (differential mean change of 4.1 kg, 95% CI: 2.8 to 5.5) than the “Gained Some/Somewhat Larger” group (differential mean change of 1.9 kg, 95% CI: 1.1 to 2.6). A similar pattern was observed for VAT (48.3 cm2, 95% CI: 32.3 to 64.4 vs. 17.0 cm2, 95% CI: 8.4 to 25.6) and for TAT (117.3 cm2, 95% CI: 75.9 to 158.8 vs. 59.3 cm2, 95% CI: 37.1 to 81.5). Pairwise contrasts between each of the self-reported abdominal change categories revealed a significant difference between each of the groups after adjustment for multiple comparisons (p<0.05). See Tables A1 and A2 in Additional File 1 for these results.
Subgroup results for males showed similar trends to the overall cohort results for differential mean changes of trunk fat, VAT, and TAT relative to the reference category “No Change/Lost”. Analyses for females were not sufficiently powered to draw conclusions about differences in self-reported categories. A sensitivity analysis was conducted for the females by pooling the two self-reported size gain categories together. While those that reported gaining size actually gained more abdominal fat than those reporting “No Change/Lost” for trunk fat, VAT, and TAT, the results indicated that the two groups were not statistically different.
Stratified analyses for trunk fat, VAT, and TAT also failed to show trends in the obese BMI, Hispanic, and age 18–30 subgroups, which similarly had lower sample sizes. From the sensitivity analyses grouping the two self-report gain categories together, the Hispanic and age 18–30 subgroups additionally showed a significant difference between self-reported groups for each of the measured fat types, with the pooled self-reported gain groups showing higher gains in abdominal fat compared to the “No Change/Lost” group.
Waist Circumference
The median change in WC between entry and week 96 was 2.83 cm (N=287; Q1 25%: −1.50 cm; Q3 75%: 6.47 cm). Pearson correlation tests showed strong correlations between changes in WC and changes in trunk fat, VAT, and TAT (trunk fat: ρ=0.72, p<0.0001; VAT: ρ=0.52, p<0.0001; TAT: ρ=0.62, p<0.0001) (Table 3). Correlations of similar size between WC and each type of abdominal fat change were also observed in all subgroups, except for VAT and TAT in females and VAT in the obese. Figure 1 shows changes in WC plotted against trunk fat, VAT, and TAT changes for males and females. The regression results (Table 4) indicate that a change of 1 cm in WC corresponds to a 0.32 kg trunk fat change (95% CI: 0.28 to 0.35), 2.68 cm2 VAT change (95% CI: 2.15 to 3.22), and 8.43 cm2 TAT change (95% CI: 7.15 to 9.71).
Table 3.
Overall and subgroup-specific Pearson correlations between WC and trunk fat, VAT, and TAT changes between week 0 and week 96 in A5260s study population (N=328).
| Subgroup | Trunk Fat, kg | VAT, cm2 | TAT, cm2 | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| N | ρ | p-value | N | ρ | p-value | N | ρ | p-value | |
| Overall | 276 | 0.72 | <.0001 | 274 | 0.52 | <.0001 | 274 | 0.62 | <.0001 |
| Sex | |||||||||
| Male | 246 | 0.74 | <.0001 | 246 | 0.55 | <.0001 | 246 | 0.71 | <.0001 |
| Female | 30 | 0.64 | 0.0001 | 28 | 0.27 | 0.162 | 28 | 0.21 | 0.282 |
| Baseline BMI, kg/m2 | |||||||||
| Underweight/Normal: ≤24.9 | 149 | 0.69 | <.0001 | 148 | 0.61 | <.0001 | 148 | 0.68 | <.0001 |
| Overweight: 25–29.9 | 82 | 0.83 | <.0001 | 80 | 0.62 | <.0001 | 80 | 0.82 | <.0001 |
| Obese: ≥30.0 | 45 | 0.70 | <.0001 | 46 | 0.26 | 0.082 | 46 | 0.34 | 0.021 |
| Race/Ethnicity | |||||||||
| White Non-Hispanic | 125 | 0.73 | <.0001 | 123 | 0.58 | <.0001 | 123 | 0.60 | <.0001 |
| Black Non-Hispanic | 83 | 0.61 | <.0001 | 83 | 0.32 | 0.003 | 83 | 0.53 | <.0001 |
| Hispanic | 55 | 0.86 | <.0001 | 55 | 0.64 | <.0001 | 55 | 0.86 | <.0001 |
| Other | 12 | 0.86 | 0.0004 | 12 | 0.45 | 0.146 | 12 | 0.55 | 0.067 |
| Age, years | |||||||||
| 18–30 | 79 | 0.81 | <.0001 | 81 | 0.46 | <.0001 | 81 | 0.69 | <.0001 |
| 31–50 | 166 | 0.69 | <.0001 | 163 | 0.55 | <.0001 | 163 | 0.59 | <.0001 |
| 51–76 | 31 | 0.67 | <.0001 | 30 | 0.51 | 0.004 | 30 | 0.60 | 0.0004 |
VAT = Visceral Adipose Tissue; TAT = Total Adipose Tissue; WC = Waist Circumference
Table 4.
Individual predictor and joint models for change in trunk fat, VAT, and TAT between week 0 and week 96 in A5260s study population (N=328).
| Trunk Fat, kg | ||||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Individual Predictor Models | Joint Model | |||||||
|
| ||||||||
| N | R2 | Prob > F | Differential Mean Change (95% CI) | N | R2 | Prob > F | Differential Mean Change (95% CI) | |
| Waist Circumference | 276 | 0.53 | <.0001 | 0.32 (0.28, 0.35) | 273 | 0.54 | <.0001 | 0.30 (0.26, 0.34) |
| Self-Reported Abdominal Size Change | 282 | 0.15 | <.0001 | -- | -- | 0.0114 | ||
| No Change/Lost | ref | ref | ||||||
| Gained Some/ Somewhat Larger | 1.85 (1.13. 2.58) | 0.42 (-0.16, 1.00) | ||||||
| Gained A Lot/Much Larger | 4.13 (2.76, 5.51) | 1.62 (0.54, 2.69) | ||||||
|
| ||||||||
| VAT, cm2 | ||||||||
|
| ||||||||
| Individual Predictor Models | Joint Model | |||||||
|
| ||||||||
| N | R2 | Prob > F | Differential Mean Change (95% CI) | N | R2 | Prob > F | Differential Mean Change (95% CI) | |
|
| ||||||||
| Waist Circumference | 274 | 0.27 | <.0001 | 2.68 (2.15, 3.22) | 272 | 0.30 | <.0001 | 2.35 (1.78, 2.92) |
| Self-Reported Abdominal Size Change | 281 | 0.13 | <.0001 | -- | -- | 0.0014 | ||
| No Change/Lost | ref | ref | ||||||
| Gained Some/ Somewhat Larger | 17.00 (8.37, 25.63) | 5.15 (−3.27, 13.56) | ||||||
| Gained A Lot/Much Larger | 48.32 (32.26, 64.39) | 28.57 (13.21, 43.92) | ||||||
|
| ||||||||
| TAT, cm2 | ||||||||
|
| ||||||||
| Individual Predictor Models | Joint Model | |||||||
|
| ||||||||
| N | R2 | Prob > F | Differential Mean Change (95% CI) | N | R2 | Prob > F | Differential Mean Change (95% CI) | |
|
| ||||||||
| Waist Circumference | 274 | 0.38 | <.0001 | 8.43 (7.15, 9.71) | 272 | 0.40 | <.0001 | 7.55(6.17, 8.93) |
| Self-Reported Abdominal Size Change | 281 | 0.15 | <.0001 | -- | -- | 0.0068 | ||
| No Change/Lost | ref | ref | ||||||
| Gained Some/ Somewhat Larger | 59.30 (37.05, 81.55) | 22.96 (2.61, 43.31) | ||||||
| Gained A Lot/Much Larger | 117.34 (75.92, 158.76) | 54.08 (16.96, 91.21) | ||||||
VAT = Visceral Adipose Tissue; TAT = Total Adipose Tissue; CI = Confidence Interval
Self-Reported Abdominal Size and Waist Circumference Joint Models
Individual predictor models of self-reported size change and WC change separately were compared to joint models including both measurements with each of the abdominal fat measures as outcome variables (Table 4). The individual predictor models show that self-reported abdominal change and WC change were each associated with the measured abdominal fat changes separately (p<0.0001). The R2 values for each of the joint models were modest (trunk fat: R2=0.54; VAT: R2=0.30; TAT: R2=0.40). When included together in the model, both WC (p<0.0001) and self-report (p<0.05) continued to remain significant predictors of trunk fat, VAT, and TAT changes, even though the R2 value did not change dramatically from the WC-only model. When examining the differential mean changes of trunk fat, VAT, and TAT compared to the reference group “No Change/Lost”, the coefficient for the “Gained A Lot/Much Larger” group was significant (p<0.05), while the coefficient for “Gained Some/Somewhat Larger” was no longer significant for trunk fat and VAT (Table 4). When adjusting for multiple comparisons in the joint model, the pairwise contrast between the “Gained A Lot/Much Larger” group and the “No Change/Lost group” remained statistically significant (p<0.05) for the trunk fat, VAT, and TAT models.
Discussion
Findings from this longitudinal study indicate that both WC and self-reported abdominal size changes appear to be correlated with standard measurements of abdominal fat change, including visceral adipose tissue accumulation. Both WC and self-reported abdominal changes were correlated with CT and DXA measurements and explained a moderate proportion of the variation in these objective measurements. Previous studies have shown a range of results concerning the validity of self-reported fat changes. A cross-sectional study of HIV-infected individuals in the thymidine nucleoside reverse transcriptase inhibitor era found a significant correlation between DXA measurements of limb fat and lipoatrophy scores independently reported by both physicians and participants.[21] In addition, it has been found that in HIV-infected women, triceps and thigh skinfold thicknesses as well as DXA measured lower limb fat were predictive of self-reported lipoatrophy, and that waist-to-hip ratio and DXA trunk fat/percentage limb fat were predictive of self-reported lipohypertrophy.[22] Another cross-sectional study of HIV-infected individuals found that self-assessment of both central fat adiposity and peripheral lipoatrophy did not agree with clinical assessment.[23] By using longitudinal measurements over 96 weeks after ART initiation, our study specifically addressed whether self-reported abdominal size changes relative to treatment initiation correlated with centrally read and standardized imaging of central fat using CT and DXA scans. Results from a previous longitudinal study suggested that self-reported lipoatrophy of extremities is not related to DXA measurements in HIV-infected individuals initiating treatment. However, this study did not specifically examine increases in abdominal visceral adipose tissue.[24] It is important to note that these research studies were also conducted in different HIV patient populations, which may have contributed to variation of results.
In our study, subgroup analyses revealed that the usefulness of self-reported abdominal changes may vary by sex, age, and BMI subgroup. The limited female enrollment in the A5260s study resulted in insufficient power to detect differences across self-reported categories. For the female subgroup, our results cannot confirm that self-reported abdominal change is directionally consistent with measured trunk fat and TAT increases. Previous research has shown that women tend to misreport weight more than men, indicating that self-reported size gains may not be an ideal measurement for this demographic.[33,34] Further research is needed to validate this form of measurement in the female population. It has also been found that being older or overweight/obese was also associated with misreporting.[33] Our study also had smaller sample sizes resulting in lower power for the obese and older age subgroups.
Results from this study show that WC measurements are correlated with abdominal fat changes, specifically CT-measured VAT, both overall and by sex, race, age, and BMI subgroups. Previous analyses have shown that CT-measured VAT has been highly associated with WC in cross-sectional settings.[18–20] A recent cross-sectional study of HIV-infected men reported a substantial correlation (ρ=0.613) between WC and CT-measured VAT, and found a limited proportion of variation in VAT explained by WC (R2=0.35). While the authors of this study concluded that WC was not a reliable predictor of VAT, it is clear that WC does appear to be indicative of VAT levels and may be useful for tracking changes in body composition.[35] In contrast to these studies, our research addressed changes in both WC and abdominal fat over time and showed that WC increases are also a good indicator of changes in abdominal fat.
WC has been shown to be highly associated with cardiometabolic risk.[10,36] Although WC is not a widely utilized clinical measure, its usefulness in practice as a predictor of cardiometabolic risk has been recognized.[37] Showing that changes in WC reflect adipose accumulation, including VAT increases, further supports its usefulness as a monitoring tool for HIV-infected individuals, who are more prone to abdominal lipohypertrophy.
We show that both WC and self-reported abdominal changes can be utilized to monitor abdominal fat gain in HIV-infected individuals. Between WC and self-reported changes, WC accounts for most of the variability in predicting abdominal fat changes, however, self-reported abdominal size changes may give additional information about those with extreme changes in abdominal fat after treatment initiation, as seen in the joint models with WC.
One limitation of this study is that the results do not indicate whether WC is more strongly correlated with CT-measured VAT or TAT, or DXA-measured trunk fat. Other limitations include the small sample size for the female and extreme BMI subgroups, which reduced the power for those analyses. In addition, our results may not be generalizable to the broader HIV-infected population due to the restrictive inclusion and exclusion criteria of the randomized controlled trial. An important strength of our study is that it was conducted using prospectively collected clinical trial data that specifically addressed the association between WC and self-reported abdominal size changes with changes in standard measures of abdominal fat over 96 weeks. To our knowledge, such proxy measurements for changes in abdominal fat have not been widely examined.
HIV-associated abdominal lipohypertrophy remains a highly prevalent medical issue for HIV patients on antiretroviral therapy.[38–41] Monitoring visceral adipose accumulation is especially important for HIV-infected patients, who are at a higher risk of metabolic abnormalities and cardiovascular disease. WC and self-reported abdominal size changes are more accessible and affordable forms of body fat assessment that can be adopted by clinicians as valid measures of fat gain for HIV-infected patients undergoing ART. These measures will be especially valuable in resource-limited settings that do not have access to extensive tests, as well as clinical trial settings for ease of monitoring fat changes in larger study populations.
Supplementary Material
Acknowledgments
Research reported in this publication was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award Number UM1 AI068634, UM1 AI068636 and UM1 AI106701. This research was also supported by NIH grants HL095132, HL095126, AI069501, AI69471, and AI56933. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
The following AIDS Clinical Trials Units participated in this study: 103 - Beth Israel Deaconess Medical Center ACTG CRS 6; 107 - Brigham and Women’s Hospital ACTG CRS 5; 201 - Johns Hopkins Adult AIDS CRS 11; 401 - NY University HIV/AIDS CRS 11; 601 - UCLA CARE Center CRS 8; 603 - Harbor-UCLA Med. Ctr. CRS 24; 801 - UCSF AIDS CRS 4; 1001 - University of Pittsburgh CRS 4; 1108 - AIDS Care CRS 8; 1201 - USC CRS 30; 1401 - University of Washington AIDS CRS 18; 1601 - Duke University Medical Cener Adult CRS 3; 2101 - Washington U CRS 23; 2301 - Ohio State University AIDS CRS 9; 2401 - Univ. of Cincinnati CRS 28; 2501 - Case Western Reserve CRS 12; 2503 - MetroHealth CRS 1; 2701 - Northwestern University CRS 23; 2702 - Rush University Medical Center ACTG CRS 8; 3201 - UNC AIDS CRS 15; 3652 - Vanderbilt Therapeutics CRS 17; 5802 - Ponce de Leon Center CRS 3; 6101 - University of Colorado Hospital CRS 40; 31473 - Houston AIDS Research Team CRS 10; 31786 - New Jersey Medical School - Adult Clinical Research Ctr. CRS 9; 31787 - University of Rochester ACTG CRS 4. Please refer to the A5260s Acknowledgement Appendix for site specific grant numbers and acknowledgements (see Additional File 2).
An earlier version of this manuscript was presented as a poster at the 2016 Conference on Retroviruses and Opportunistic Infections (CROI) in Boston, Massachussetts, USA.
Disclosure Statement
Dr. McComsey has served as a scientific advisor for Bristol-Myers Squibb, GlaxoSmithKline/ViiV, Pfizer, ICON, and Gilead Sciences and has received research grants from Bristol-Myers Squibb, GlaxoSmithKline, and Gilead Sciences. Dr. Brown has served as a consultant to Gilead Sciences, Merck, AbbVie, EMD-Serono, and Theratechnologies, and his research is supported in part by NIH/NIAID K24AI120834. Dr. Bhagwat, Dr. Ofotokun, Dr. Moser, Dr. Sugar, and Dr. Currier have no Conflict of Interest disclosures.
References
- 1.Cheruvu S, Holloway CJ. Cardiovascular disease in human immunodeficiency virus Internal Medicine Journal Volume 44, Issue 4. Internal Medicine Journal. 44:315–24. doi: 10.1111/imj.12381. [DOI] [PubMed] [Google Scholar]
- 2.Currier JS, Lundgren JD, Carr A, et al. Epidemiological Evidence for Cardiovascular Disease in HIV-Infected Patients and Relationship to Highly Active Antiretroviral Therapy. Circulation. 2008;118:e29–35. doi: 10.1161/CIRCULATIONAHA.107.189624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Esser S, Gelbrich G, Brockmeyer N, et al. Prevalence of cardiovascular diseases in HIV-infected outpatients: results from a prospective, multicenter cohort study. Clin Res Cardiol. 2012;102:203–13. doi: 10.1007/s00392-012-0519-0. [DOI] [PubMed] [Google Scholar]
- 4.Domingo P, Estrada V, López-Aldeguer J, Villaroya F, Martínez E. Fat redistribution syndromes associated with HIV-1 infection and combination antiretroviral therapy. AIDS Rev. 2012;14:112–23. [PubMed] [Google Scholar]
- 5.Hughes-Austin J, Larsen B, Allison M. Visceral Adipose Tissue and Cardiovascular Disease Risk. Curr Cardiovasc Risk Rep. 2013;7:95–101. [Google Scholar]
- 6.McComsey GA, Kitch D, Sax PE, et al. Peripheral and Central Fat Changes in Subjects Randomized to Abacavir-Lamivudine or Tenofovir-Emtricitabine With Atazanavir-Ritonavir or Efavirenz: ACTG Study A5224s. Clin Infect Dis. 2011;53:185–96. doi: 10.1093/cid/cir324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martinez E, Gonzalez-Cordon A, Ferrer E, et al. Differential Body Composition Effects of Protease Inhibitors Recommended for Initial Treatment of HIV Infection: A Randomized Clinical Trial. Clin Infect Dis. 2014:ciu898. doi: 10.1093/cid/ciu898. [DOI] [PubMed] [Google Scholar]
- 8.Wronska A, Kmiec Z. Structural and biochemical characteristics of various white adipose tissue depots Acta Physiologica Volume 205, Issue 2. Acta Physiologica. 205:194–208. doi: 10.1111/j.1748-1716.2012.02409.x. [DOI] [PubMed] [Google Scholar]
- 9.Després JP. Body fat distribution and risk of cardiovascular disease: an update. Circulation. 2012;126:1301–13. doi: 10.1161/CIRCULATIONAHA.111.067264. [DOI] [PubMed] [Google Scholar]
- 10.Katzmarzyk PT, Heymsfield SB, Bouchard C. Clinical utility of visceral adipose tissue for the identification of cardiometabolic risk in white and African American adults123. Am J Clin Nutr. 2013;97:480–6. doi: 10.3945/ajcn.112.047787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fox CS, Massaro JM, Hoffmann U, et al. Abdominal Visceral and Subcutaneous Adipose Tissue Compartments Association With Metabolic Risk Factors in the Framingham Heart Study. Circulation. 2007;116:39–48. doi: 10.1161/CIRCULATIONAHA.106.675355. [DOI] [PubMed] [Google Scholar]
- 12.Guaraldi G, Stentarelli C, Zona S, et al. Lipodystrophy and anti-retroviral therapy as predictors of sub-clinical atherosclerosis in human immunodeficiency virus infected subjects. Atherosclerosis. 2010;208:222–7. doi: 10.1016/j.atherosclerosis.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 13.Guaraldi G, Zona S, Orlando G, et al. Progression of coronary artery calcium in men affected by human immunodeficiency virus infection. Int J Cardiovasc Imaging. 2011;28:935–41. doi: 10.1007/s10554-011-9898-y. [DOI] [PubMed] [Google Scholar]
- 14.Lake JE, Wohl D, Scherzer R, et al. Regional fat deposition and cardiovascular risk in HIV infection: the FRAM study. AIDS Care. 2011;23:929–38. doi: 10.1080/09540121.2010.543885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scherzer R, Heymsfield SB, Lee D, et al. Decreased limb muscle and increased central adiposity are associated with 5-year all-cause mortality in HIV infection. AIDS. 2011;25:1405–14. doi: 10.1097/QAD.0b013e32834884e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Amato MC, Guarnotta V, Giordano C. Body composition assessment for the definition of cardiometabolic risk. J Endocrinol Invest. 2013;36:537–43. doi: 10.3275/8943. [DOI] [PubMed] [Google Scholar]
- 17.Després JP, Prud’homme D, Pouliot MC, Tremblay A, Bouchard C. Estimation of deep abdominal adipose-tissue accumulation from simple anthropometric measurements in men. Am J Clin Nutr. 1991;54:471–7. doi: 10.1093/ajcn/54.3.471. [DOI] [PubMed] [Google Scholar]
- 18.Direk K, Cecelja M, Astle W, et al. The relationship between DXA-based and anthropometric measures of visceral fat and morbidity in women. BMC Cardiovasc Disord. 2013;13:25. doi: 10.1186/1471-2261-13-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rankinen T, Kim SY, Pérusse L, Després JP, Bouchard C. The prediction of abdominal visceral fat level from body composition and anthropometry: ROC analysis. Int J Obes Relat Metab Disord. 1999;23:801–9. doi: 10.1038/sj.ijo.0800929. [DOI] [PubMed] [Google Scholar]
- 20.Pouliot MC, Després JP, Lemieux S, et al. Waist circumference and abdominal sagittal diameter: best simple anthropometric indexes of abdominal visceral adipose tissue accumulation and related cardiovascular risk in men and women. Am J Cardiol. 1994;73:460–8. doi: 10.1016/0002-9149(94)90676-9. [DOI] [PubMed] [Google Scholar]
- 21.Tungsiripat M, O’Riordan MA, Storer N, et al. Subjective clinical lipoatrophy assessment correlates with DEXA-measured limb fat. HIV Clin Trials. 2009;10:314–9. doi: 10.1310/hct1005-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abrahams Z, Dave JA, Maartens G, Lesosky M, Levitt NS. The development of simple anthropometric measures to diagnose antiretroviral therapy-associated lipodystrophy in resource limited settings. AIDS Res Ther. 2014;11:26. doi: 10.1186/1742-6405-11-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Belloso WH, Quirós RE, Ivalo SA, et al. Agreement analysis of variables involved in lipodystrophy syndrome definition in HIV-infected patients. J Acquir Immune Defic Syndr. 2003;32:104–11. doi: 10.1097/00126334-200301010-00015. [DOI] [PubMed] [Google Scholar]
- 24.Haubrich Richard H, Riddler SA, DiRienzo G, et al. Clinical Associations of Extremity Fat Loss from ACTG 5142: A Prospective, Randomized, Phase III Trial of NRTI-, PI-, and NNRTI-sparing Regimens for Antiretroviral Therapy (ART) of Naive, HIV-1 infected Subjects [Internet] Boston, United States: 2008. [cited 2014 Aug 27]. Available from: http://www.natap.org/2008/CROI/croi_61.htm. [Google Scholar]
- 25.Lennox JL, Landovitz RJ, Ribaudo HJ, et al. A Phase III Comparative Study of the Efficacy and Tolerability of Three Non-Nucleoside Reverse Transcriptase Inhibitor-Sparing Antiretroviral Regimens for Treatment-Naïve HIV-1-Infected Volunteers: A Randomized, Controlled Trial. Ann Intern Med. 2014;161:461–71. doi: 10.7326/M14-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ofotokun I, Na LH, Landovitz RJ, et al. Comparison of the Metabolic Effects of Ritonavir-Boosted Darunavir or Atazanavir Versus Raltegravir, and the Impact of Ritonavir Plasma Exposure: ACTG 5257. Clin Infect Dis. 2015;60:1842–51. doi: 10.1093/cid/civ193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stein JH, Brown TT, Ribaudo HJ, et al. Ultrasonographic measures of cardiovascular disease risk in antiretroviral treatment-naïve individuals with HIV infection. AIDS. 2013;27:929–37. doi: 10.1097/QAD.0b013e32835ce27e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kelesidis T, Tran TTT, Stein JH, et al. Changes in Inflammation and Immune Activation With Atazanavir-, Raltegravir-, Darunavir-Based Initial Antiviral Therapy: ACTG 5260s. Clin Infect Dis. 2015;61:651–60. doi: 10.1093/cid/civ327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brown TT, Chen Y, Currier JS, et al. Body Composition, Soluble Markers of Inflammation, and Bone Mineral Density in Antiretroviral Therapy-Naïve HIV-1 Infected Individuals. J Acquir Immune Defic Syndr. 2013;63:323–30. doi: 10.1097/QAI.0b013e318295eb1d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McComsey GA, Moser C, Currier J, et al. Body Composition Changes after Initiation of Raltegravir or Protease Inhibitors: ACTG A5260s. Clin Infect Dis. 2016 doi: 10.1093/cid/ciw017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.About Adult BMI | Assessing Your Weight | Healthy Weight | DNPAO | CDC [Internet] [cited 2015 Jul 14];Available from: http://www.cdc.gov/healthyweight/assessing/bmi/adult_bmi/
- 32.Tien PC, Benson C, Zolopa AR, Sidney S, Osmond D, Grunfeld C. The study of fat redistribution and metabolic change in HIV infection (FRAM): methods, design, and sample characteristics. Am J Epidemiol. 2006;163:860–9. doi: 10.1093/aje/kwj111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wen M, Kowaleski-Jones L. Sex and Ethnic Differences in Validity of Self-reported Adult Height, Weight and Body Mass Index. Ethn Dis. 2012;22:72–8. [PMC free article] [PubMed] [Google Scholar]
- 34.Krul AJ, Daanen HAM, Choi H. Self-reported and measured weight, height and body mass index (BMI) in Italy, the Netherlands and North America. The European Journal of Public Health. 2011;21:414–9. doi: 10.1093/eurpub/ckp228. [DOI] [PubMed] [Google Scholar]
- 35.Falutz J, Rosenthall L, Kotler D, Zona S, Guaraldi G. Surrogate markers of visceral adipose tissue in treated HIV-infected patients: accuracy of waist circumference determination. HIV Med. 2014;15:98–107. doi: 10.1111/hiv.12085. [DOI] [PubMed] [Google Scholar]
- 36.Janiszewski PM, Ross R, Despres J-P, et al. Hypertriglyceridemia and Waist Circumference Predict Cardiovascular Risk among HIV Patients: A Cross-Sectional Study. PLoS One [Internet] 2011 doi: 10.1371/journal.pone.0025032. [cited 2015 Nov 6];6. Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3178598/ [DOI] [PMC free article] [PubMed]
- 37.Klein S, Allison DB, Heymsfield SB, et al. Waist circumference and cardiometabolic risk: a consensus statement from Shaping America’s Health: Association for Weight Management and Obesity Prevention; NAASO, The Obesity Society; the American Society for Nutrition; and the American Diabetes Association. Am J Clin Nutr. 2007;85:1197–202. doi: 10.1093/ajcn/85.5.1197. [DOI] [PubMed] [Google Scholar]
- 38.Jaime PC, Florindo AA, Latorre M, do RD, de O, Segurado AAC. Central obesity and dietary intake in HIV/AIDS patients. Revista de Saúde Pública. 2006;40:634–40. doi: 10.1590/s0034-89102006000500012. [DOI] [PubMed] [Google Scholar]
- 39.Leclercq P, Goujard C, Duracinsky M, et al. High Prevalence and Impact on the Quality of Life of Facial Lipoatrophy and Other Abnormalities in Fat Tissue Distribution in HIV-Infected Patients Treated with Antiretroviral Therapy. AIDS Research and Human Retroviruses. 2012;29:761–8. doi: 10.1089/aid.2012.0214. [DOI] [PubMed] [Google Scholar]
- 40.Moyle G, Moutschen M, Martínez E, et al. Epidemiology, assessment, and management of excess abdominal fat in persons with HIV infection. AIDS Rev. 2010;12:3–14. [PubMed] [Google Scholar]
- 41.Cabrero E, Griffa L, Burgos A. Prevalence and Impact of Body Physical Changes in HIV Patients Treated with Highly Active Antiretroviral Therapy: Results from a Study on Patient and Physician Perceptions. AIDS Patient Care and STDs. 2010;24:5–13. doi: 10.1089/apc.2009.0191. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.