Abstract
Trehalose is a non-reducing disaccharide sugar that widely exists in a variety of organisms, such as bacteria and eukaryotes except the vertebrates. It plays an important role in a number of critical metabolic functions especially in response to stressful environmental conditions. However, the biosynthetic pathways of trehalose in cold-adapted yeast and its responses to temperature and salinity changes remain little understood. In this study, the genome of Antarctic-isolated Pseudozyma sp. NJ7 was generated from which we identified the gene coding for trehalose phosphate synthase (TPS1) and trehalose phosphate phosphatase (TPS2), the two enzymes most critical for trehalose production. The whole draft genome length of Pseudozyma sp. NJ7 was 18,021,233 bp, and encoded at least 34 rRNA operons and 72 tRNAs. The open reading frame of tps1 contained 1827 nucleotide encoding 608 amino acids with a molecular weight of 67.64 kDa, and an isoelectric point of 5.54, while tps2 contained 3948 nucleotide encoding 1315 amino acids with a molecular weight of 144.47 kDa and an isoelectric point of 6.36. The TPS1 and TPS2 protein sequences were highly homologous to Moesziomyces antarcticus T-34, but TPS2 had obvious specificity and differently with others which suggest species specificity and different evolutionary history. Expression level of tps1 gene was strongly influenced by temperature and high salinity. In addition, addition of 0.5% trehalose preserved yeast cells in the short term but was not effective for cryopreservation for more than 5 days, but still suggesting that exogenous trehalose could indeed significantly improve the survival of yeast cells under freezing conditions. Our results provided new insights on the molecular basis of cold adaptations of Antarctic Pseudozyma sp., and also generated new information on the roles trehalose play in yeast tolerance to extreme conditions in the extreme Antarctic environments.
Keywords: Trehalose, Antarctic, Pseudozyma sp., Genome, Trehalose phosphate synthase
Introduction
Trehalose (α-d-glucopyranosyl-α-d-glucopyranoside) is a naturally occurring non-reducing disaccharide sugar that is made up of two glucose units joined together by an α,α-1,1 linkage (Eastmond and Graham 2003; Paul 2007). It widely exists in bacteria, yeast, filamentous fungi, insects, plants and invertebrates, except in vertebrates (Paul 2007). Recent evidence indicates that trehalose (trehalose-6-phosphate, T6P) is the precursor of an important regulatory molecule that serves as a primary energy source (Argüelles 2000; Avonce et al. 2006; Eastmond and Graham 2003). It is a powerful and indispensable sugar signal that integrates metabolism with development (Paul et al. 2008), and serves as a protectant of bioactive substances and cell structures under adverse environmental stresses, such as high salinity, extreme temperature changes, drought, freezing and oxidation (Argüelles 2000; Avonce et al. 2006; Elbein et al. 2003; Mu et al. 2016).
There are at least six trehalose biosynthetic routes so far reported (Avonce et al. 2006). Many eubacteria possess around two to four pathways, whereas invertebrates as well as fungi and plants only possess one pathway (Avonce et al. 2006). The most widely found in eukaryotes is the trehalose phosphate synthase and trehalose phosphate phosphatase (TPS/TPP) also known as OtsA/OtsB pathway. The others types of pathways are TreS, TreY/TreZ, TreP, TreT and TreH (Argüelles 2000; Avonce et al. 2006; Paul 2007, 2008).
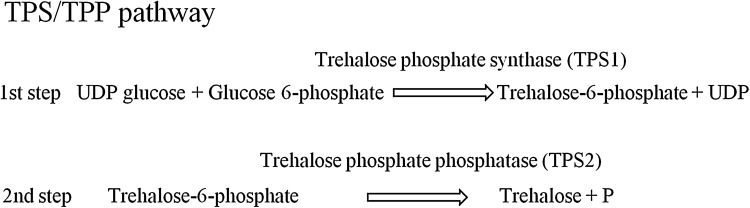
In yeast, synthesis of trehalose is mostly via the TPS/TPP pathway, which is mainly divided into two steps. Generally, trehalose phosphate synthase (TPS or TPS1) is regarded as the crucial enzyme needed in the first step of trehalose synthesis, where it catalyzes the transfer of glucose from UDP glucose (UDPG) to glucose 6-phosphate (G6P), forming T6P and UDP. The second step involves the trehalose phosphate phosphatase (TPP or TPS2) dephosphorylating T6P to trehalose and inorganic phosphate (Fig. 1) (Argüelles 2000; Elbein et al. 2003; Paul 2007; Zhang et al. 2013). To date, knowledge on TPS and TPP have been mostly based on studies in normal and thermophilic yeast such as Saccharomyces cerevisiae (An et al. 2011; Bell et al. 1998; Mahmud et al. 2010; Van Vaeck et al. 2001) and Hansenula polymorpha (Reinders et al. 1999), and investigations on how they are synthesized in cold-adapted yeasts are still limited or even lacking (Zhang et al. 2013). This limits our understanding on the synthesis pathways of trehalose in cold-adapted yeast at low temperature and gene-based responses in extreme environments.
Fig. 1.
Trehalose phosphate synthase pathway (TPS pathway)
Cold-adapted microorganisms are an important part of the biosphere (Margesin and Miteva 2011), and understanding the underlying mechanisms of their acclimation has gained strong interests over many years. Physiological mechanisms that allow of fungi (including yeasts) to tolerate low temperature are complex and not yet fully understood (Maggi et al. 2013), as well as those of other psychrotrophic and psychrophilic microorganisms. The well characterized cold-active enzymes of yeasts in both marine and terrestrial environments of the Polar Regions (Maggi et al. 2013) have widespread applications in various industries (Hamid et al. 2014). The Antarctic yeast Pseudozyma sp. NJ7, isolated and purified from a floating ice near the Zhongshan Station (China) in Antarctica, can be studied as a good subject to investigate low temperature acclimation. In this study, we determined the genes tps1 (TPS1) and tps2 (TPS2) of cold-adapted yeast Pseudozyma sp. NJ7 after sequencing and annotation of its whole genome. We then characterized their amino acid sequences and protein structures, and investigated their gene regulation and expression in extreme conditions such as low temperature and high salinity using real-time quantitative PCR. Eventually, we further extracted the trehalose from P. NJ7 and confirmed its effects on yeast preservation. Understanding of the adaptive and response mechanisms in low temperature and high salinity conditions is helpful in gaining insights on self-protection and acclimation of Pseudozyma sp., and in essence of other organisms, in extreme environments such as the Antarctic.
Materials and methods
Materials and culture media
The Antarctic yeast Pseudozyma sp. NJ7 was isolated and purified from a floating ice near the Chinese Zhongshan Station in Antarctica located at 69°S, 77°E during the 18th Chinese Antarctic Science Exploration from 2001 to 2002. It could be grown from 0 to 30 °C but optimal 20 to 25 °C (based on our preliminary date, unpublished). Cultures were maintained in a liquid medium made up of 2 g of yeast extract, 8 g of glucose, 1 g of MgSO4, K2HPO4 and 1 L of distilled water, with a final pH 7.0–7.5. Inocula were then incubated in a shaker-flask at 150 rpm in a temperature-controlled environment maintained at 20 °C.
Preparation, sequencing and analysis of Pseudozyma sp. NJ7 genomic DNA
For DNA extraction, cultures of P. NJ7 were grown in the same media and conditions for 14 days before being collected by centrifugation at 6000 rpm for 5 min. Samples were sent to Shanghai Personal Biotechnology Co., Ltd. (Shanghai, China) for both DNA extraction and whole genome sequencing. Briefly, genomic DNA extraction was carried out using a commercial kit (OMEGA, bacterial DNA extraction kit, USA). The DNA was then suspended in distilled water and stored at − 80 °C. Complete genome sequencing was performed using the whole shotgun method in an Illumina NextSeq 500 Sequencing platform. After sequencing, sequence base quality was evaluated, and the splice sequences were reassembled by in Newbler (version 2.8, 20110517_1502). The integrity and continuity of the genome sequence was assessed using CEGMA (Parra et al. 2007). Blastx searches against the non-redundant (NR) database (from NCBI database) were performed to determine significant homology. TMHMM was used to identify the transmembrane domains, while Signal P was used to predict signal peptides (Petersen et al. 2011). All trRNA were determined using the tRNA scan-SE and RNAmmer for the rRNAs (Lowe and Eddy 1997).
The draft genome of P. NJ7 was annotated using GO (Conesa and Götz 2008), eggNOG (Powell et al. 2014) and KEGG (Moriya et al. 2007) databases to identify, predict and analyze the protein coding genes. Subsequently, we specifically identified TPS1 and TPS2 genes (tps1 and tps2).
Gene cloning and bioinformatics analysis of tps1 and tps2
The total RNA was extracted using TRIzol reagent (TransGen Biotech) following the manufacturer’s protocol. The quality and concentration of the extracted nucleic acid samples were analyzed in NanoVue (GE Healthcare). First strand of the complimentary DNA (cDNA) was synthesized from the high-quality RNA by PrimerScript 1st cDNA Synthesis Kit (Takara) following the suggestions of the manufacturer. Generated cDNA was stored at − 20 °C until use.
Primers for the target genes TPS1 and TPS2 (TPS-R: GGGGCGGGACGAGAGGCTGGAGGT, TPS-F: ATGGCGGCCGCTTCATCCTCGCAG) were designed from the consensus sequences generated from the whole genome sequence of P. NJ7. The PCR amplification was performed as follows: 94 °C for 5 min, 35 cycles of 94 °C for 30 s, 55 °C for 30 s, 72 °C for 45 s, at last 10 min at 72 °C. Then, 2 μL of the amplicons were checked and visualized in 1% agarose gel, and the remaining product was directly sent for sequencing to BGI (Shanghai).
The open reading frames (ORFs) were predicted in NCBI Open Reading Frame Finder (http://www.ncbi.nlm.nih.gov/gorf/gorf.html), and unknown sequences were searched for most similar sequences against the NCBI GenBank using BlastN function (http://blast.ncbi.nlm.nih.gov/Blast) and were downloaded. A multiple-sequence alignment was then carried out in Clustal X program (Thompson et al. 1997). Similarity matrix based on multiple-sequence alignments of different translated TPS1 and TPS2 amino acid sequences were generated by DNAMAN software. After the amino acid-like sequences (Table 1) of the TPS1and TPS2 were downloaded from NCBI, MEGA 6.6 software was used to construct the 1000 replications phylogenetic trees of TPS1 and TPS2 using the neighbor-joining method (Tamura et al. 2013). Protein translation and its predicted physicochemical properties, as well as gene sequence analysis were carried out in ExPASy (http://expasy.org/tolls/protparam.html). Secondary structure and protein folding were analyzed by predictProtein (http://www.predictprotein.org), while the membrane-spanning domain was completed using TMPRED (http://embnet.vital-it.ch/software/TMPRED_form.html) and TMHMM (http://www.cbs.dtu.dk/services/TMHMM-2.0). The tertiary structure of the targeted protein was established using Swiss model (http://swissmodel.expasy.org/).
Table 1.
TPS1 and TPS2-like protein species and their GenBank accession numbers
| Similar sequences | GenBank accession No. | |
|---|---|---|
| TPS1 | Moesziomyces antarctica T-34 | GAC73660 |
| Ustilago hordei | CCF54177 | |
| Ceraceosorus bombacis | CEH18602 | |
| Moniliella megachiliensis | BAO72964 | |
| Wallemia mellicola CBS 633.66 | EIM19889 | |
| Auricularia subglabra TFB-10046 SS5 | XP-007336253 | |
| Flammulina velutipes | AJW82040 | |
| Gymnopus luxurians FD-317 M1 | KIK62399 | |
| TPS2 | Moesziomyces antarctica T-34 | GAC72506.1 |
| Saccharomyces cerevisiae | CAA50025.1 | |
| Mycobacterium tuberculosis H37Rv | NP 217889.1 | |
| Archangium gephyra | AKI99051.1 | |
| Cryptococcus neoformans var. grubii H99 | DAA05785.1 | |
| Xanthophyllomyces dendrorhous | CED83290.1 | |
| Rhizoctonia solani AG-1 IA | ELU41761.1 | |
| Malassezia pachydermatis | KOS15544.1 | |
| Rhodococcus sp. B7740 | AJW42450.1 | |
| Alloactinosynnema sp. L-07 | CRK61194.1 |
Expression of tps1 gene by quantitative real-time PCR
Responses of the tps1 gene of the Pseudozyma sp. NJ7 to three different stress gradients were investigated by looking at the changes in their expression levels. In the first group, the temperature gradients tested were 0, 5, 10, 15, and 20 °C was used as the control condition. Samples were collected periodically to monitor changes in expression with time, specifically at (0, 3, 6, 12, 24, 36, 48, 72 and 96 h). The cultures were grown in the same liquid media and incubated with shaking at 200 rpm. In the second group, effects of salinity were also investigated by growing the cultures in the same agitated and temperature conditions (i.e., 200 rpm and 20 °C) but different salinity, namely at 32‰ (normal seawater), 48‰ (1.5 fold), 64‰ (twofold) and 96‰ (threefold) NaCl at different incubation time (0, 3, 6, 12, 24, 36, 48, 72 and 96 h). Each treatment was carried out separately and in triplicates.
Quantitative real-time PCR (Stratagene Mx3005p, US) was used to reveal changes in levels of mRNA expression of tps1 in different conditions. Primers for qRT-PCR were TPS1-F (AACCTGCGTGCTACGGTCAA) and TPS1-R (GCCCTTGATGTAGTCGAGTCTGTC). PCR amplification cycles were as follows: 95 °C for 30 s, followed by 40 cycles of 95 °C for 5 s, 58 °C for 15 s, and 72 °C for 10 s; 95 °C for 30 s, 40 cycles of 95 °C for 5 s, 58 °C for 15 s. Generated data were processed by the 2−ΔΔCT method (Livak and Schmittgen 2001) using SPSS 17.0 software (SPSS Inc. Chicago, USA). Student’s t test and analysis of variance (ANOVA) were used to analyze significant differences in relative expressions under different conditions. Each sample was processed in triplicate QRT-PCRs.
Separation and extraction of trehalose
Cultures were prepared as described previously for 4 days, after which the yeast was collected by centrifugation, and trehalose extracted using trichloroacetic acid method (Zhao et al. 2013). Based on our preliminary date (unpublished), the optimal conditions for response surface analysis were using 15.16 mL of 0.6 mol/L trichloroacetic acid with the extraction time of 29.76 min. The theoretical extracted amount of trehalose was 17.9005 mg/g under such conditions. The same method was used to extract the trehalose of P. NJ7 to verify the reliability and accuracy of the optimal conditions (i.e., 0.6 mol/L, volume 15.2 mL and 29 min). Measurements were repeated three times.
Verification of low temperature preservation of trehalose
We added exogenous trehalose extracted from P. NJ7 to the yeast solution and revived the yeast to verify the cryoprotective function of trehalose. The yeast was first inoculated in a shaker-flask containing in a liquid medium with 100 mL and pH 7 at 150 rpm and 20 °C for 12 h, to obtain the required preservation of yeast liquid. We then tested different cryoprotectants including glycerol (20% v/v), dimethyl sulphoxide (DMSO, 10% m/v) and trehalose (0.5%, 1%, 2% m/v) as cryopreservation reagents. Blank groups were used as controls. Samples were stored at − 80 °C for 5, 10, 20 and 40 days, respectively, and the survival rate of these samples was calculated. After three-time replicate experiments, Excel 2007 was used to analyze the significance of data difference.
Results
Draft genome of Pseudozyma sp. NJ7
The whole draft genome length of Pseudozyma sp. NJ7 was 18,021,233 bp, with a G + C content of 61.05%. The accumulated length of gene sequences (without introns and exons) was 10,914,032 bp, making up 60.54% of the entire genome. The genome was also shown to encode at least 34 rRNA operons and 72 tRNAs. Based on KEGG annotation, two steps of trehalose synthesis pathways were identified with the corresponding enzymes trehalose phosphate synthase (EC2.4.1.15) and trehalose phosphate phosphatase (EC3.1.3.12). To the best of our knowledge, only four draft genomes of four different strains of Pseudozyma sp. have been deposited in GenBank to date (Table 2), these include Pseudozyma hubeiensis SY62 (NW_012133827), Pseudozyma aphidis DSM 70725 (AWNI01000008), Pseudozyma antarctica ASM74776v1 (NW_014639009) and Pseudozyma brasiliensis GHG001 (KI545873). We then compared and analyzed the P. NJ7 genome against these references (Tab.1) and found that their total draft genome lengths were between 17.33 and 18.44 Mbp, and G + C contents were 56.5 to 60.9%, which were very similar to ours.
Table 2.
The genome information of Pseudozyma sp. in GenBank
| Strains and registration number | Total draft genome length (Mbp) | G + C (%) | Gene number | Protein number | TRNA |
|---|---|---|---|---|---|
| Pseudozyma sp. NJ7 (this paper) | 18.02 | 61.05 | 6041 | 6031 | 72 |
| Pseudozyma hubeiensis SY62 (NW_012133827) | 18.44 | 56.5 | 7619 | 7472 | 121 |
| Pseudozyma aphidis DSM 70725 (AWNI01000008) | 17.92 | 60.9 | 6011 | 6011 | – |
| Pseudozyma antarctica ASM74776 (NW_014639009) | 18.11 | 60.9 | 6966 | 6766 | 121 |
| Pseudozyma brasiliensis GHG001 (KI545873) | 17.33 | 58.1 | 5889 | 5765 | 119 |
Bioinformatics analysis of tps1 and tps2
After databases identify, predict and analyze the protein coding genes by GO, eggNOG and KEGG, we specifically identified tps1 and tps2 genes. Assembly of the whole genome yielded a full-length tps1 (GenBank No. KY407563), which is 1827 bp, encoding 608 amino acid polypeptides. The molecular weight of the TPS1 protein was 67.64 kDa with a theoretical isoelectric point (pI) of 5.54. Meanwhile, tps2 has a full length of 3948 bp (GenBank No. KY407564), encoding 1315 amino acid polypeptides with the predicted molecular weight of 144.47 kDa and a theoretical isoelectric point of 6.36. After BLAST search against NCBI, tps1 was defined to glycosyltransferase family 20 protein partial mRNA of Moesziomyces antarcticus (XM_014804051). These coding proteins were confirmed to belong to the glycosyltransferase GTB-type superfamily (Zhang et al. 2013).
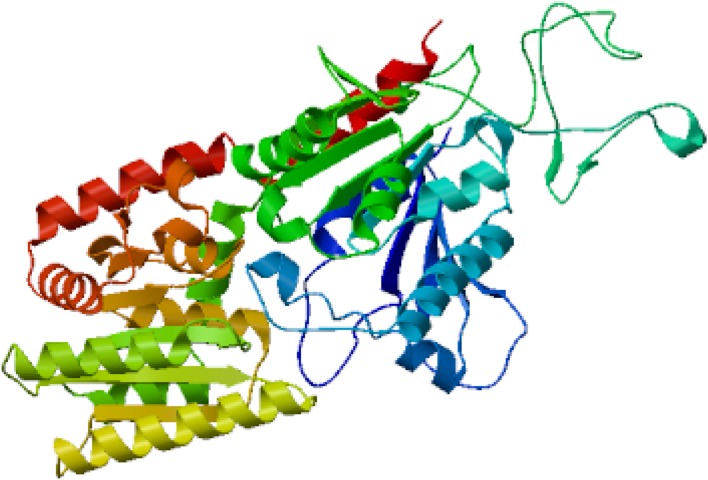
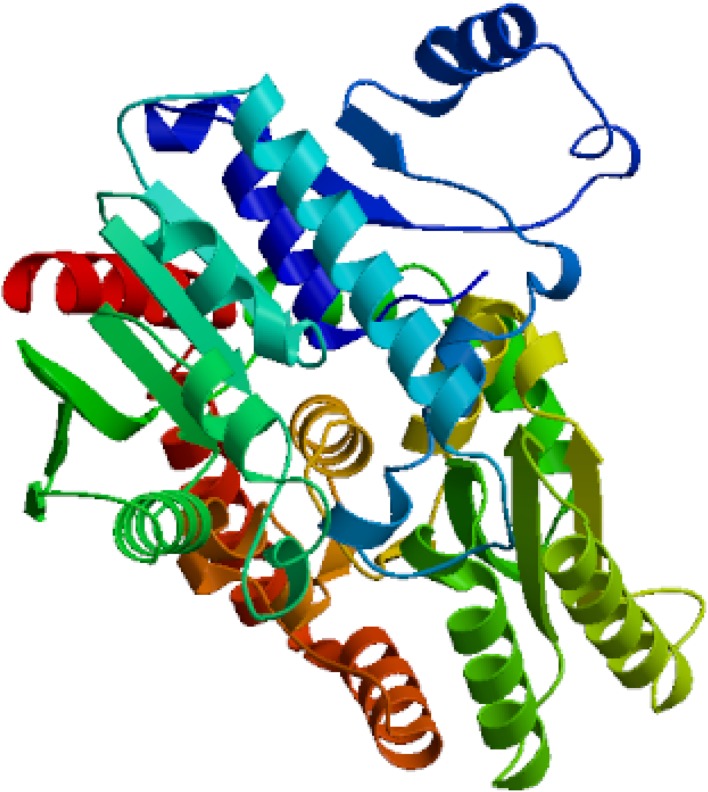
The predicted translated protein of TPS1 revealed that it coded 608 amino acids and TPS2 with 1315 amino acids. Tertiary structure of TPS1 and TPS2 were modeled with Burkholderia xenovorans (Mayclin et al. 2016) and Candida albicans (Miao et al. 2016), reconstructed as shown in Figs. 2 and 3. Tertiary structure analysis further showed that the TPS1 of NJ7 was similar to the alpha, alpha-trehalose-phosphate synthase of 5hxa.1 (Mayclin et al. 2016), while that of the TPS2 was trehalose-6-phosphate phosphatase, which closely resembled the template 5dxf.2 (Miao et al. 2016). These verifying that the gene fragments were indeed tps1and tps2 gene.
Fig. 2.
Tertiary structure fabrication of TPS1 in P. NJ7
Fig. 3.
Tertiary structure fabrication of TPS2 in P. NJ7
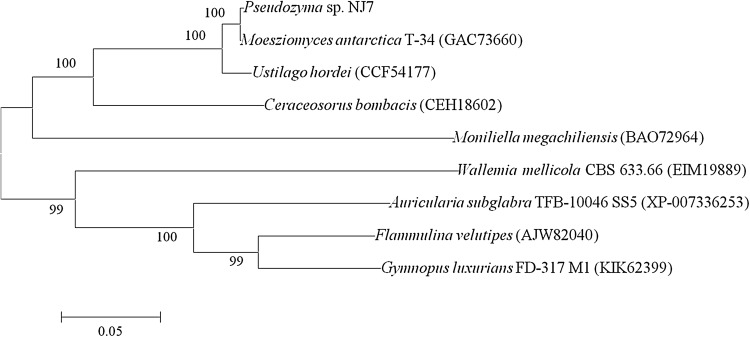
The obtained amino acid sequence of P. NJ7 TPS1 protein was compared with other sequences available in the GenBank (Fig. 4). TPS1 particularly, significantly matched the conserved regions and was homologous to other closely related sequences (M. antarcticus T-34, Ustilago hordei, Ceraceosorus bombacis and Moniliella megachiliensis), with similarities ranging from 52.75 to 99.51%, the highest of which was with M. antarcticus T-34(GAC73660). The conserved amino acid domains were drawn into ten frames, which played important roles in maintaining the structure and function of TPS1. Phylogenetic analysis also clustered our TPS1 sequences with their related reference sequences with strong bootstrap support (Fig. 5). It was divided into two separate clades where P. NJ7 and M. antarcticus T-34 (GAC73660) clustered closest with similarity identity of 100%, followed by U. hordei (CCF54177) and C. bombacis (CEH18602).
Fig. 4.

The similarity analysis and multisequencing alignment of different TPS1 amino acid sequences. Pse_NJ7: Pseudozyma sp. NJ7; Moe_ant: Moesziomyces antarcticus T-34(GAC73660); Ust_hor: Ustilago hordei (CCF54177); Cer_bom: Ceraceosorus bombacis (CEH18602); Mon_meg: Moniliella megachiliensis (BAO72964)
Fig. 5.
Phylogenetic analysis of TPS1-like proteins
Compared to TPS1, the sequence similarity of TPS2 with other available sequences was very low except M. antarcticus T-34 (Fig. 6). The TPS2 protein sequence significantly matched conserved regions and was homologous to other closely related sequences but with very low similarities ranging from 16.39 to 21.84%. The most similar sequences belonged to M. antarcticus (99.54%), suggesting that tps2 genes were highly specific and unique in other species.
Fig. 6.

The similarity analysis and multisequencing alignment of different TPS2 amino acid sequences. Pse_NJ7: Pseudozyma sp. NJ7; Moe_ant: Moesziomyces antarcticus (GAC72506.1); Sac_cer: Saccharomyces cerevisiae (CAA50025.1); Rhi_sol: Rhizoctonia solani AG-1 IA (ELU41761.1); Mal_pac: Malassezia pachydermatis (KOS15544.1)
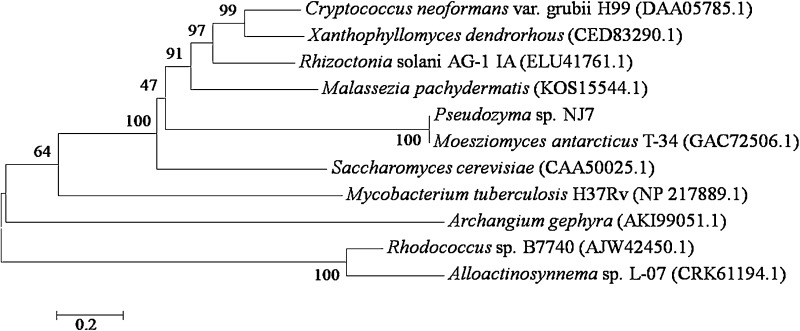
Phylogeny (Fig. 7) revealed that P. NJ7 was indeed evolutionarily related to M. antarcticus T-34 (GAC72506.1), Malassezia pachydermatis (KOS15544.1) and Xanthophyllomyces dendrorhous (CED83290.1).
Fig. 7.
Phylogenetic analysis of TPS2-like proteins
Real-time PCR analysis of tps1 gene expression
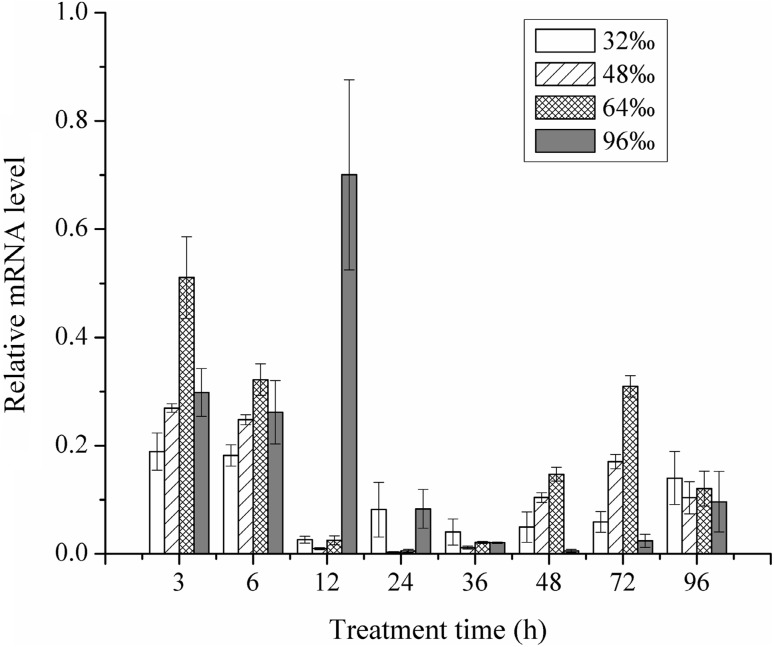
Figures 8 and 9 showed that different salinity and temperature conditions had different effects on mRNA expression. The relative mRNA expression first decreased in the first few hours followed by drastic increase (Fig. 8), and gradual decrease towards the end of the incubation in each salinity condition. The relative expression of tps1 reached the maximum level in 96‰ salinity at 12 h. In the first 6 h, tps1 had a higher expression level in 64‰ than the other salinity conditions, and the next was at 96‰ salinity. This suggests that high salinity stimulated the expression of tps1, which rapidly increased in a short time. After 12 h, tps1 expression was relatively lower in all other four salinity treatments indicating acclimatization after a rapid but short high expression. Highest expression level was observed in 64‰ after 72 h, followed by 48‰, but those expression levels were significantly lower than that of in the first 12 h.
Fig. 8.
Relative mRNA expression of tps1 in response to different salinity at different times (standard error bars are shown)
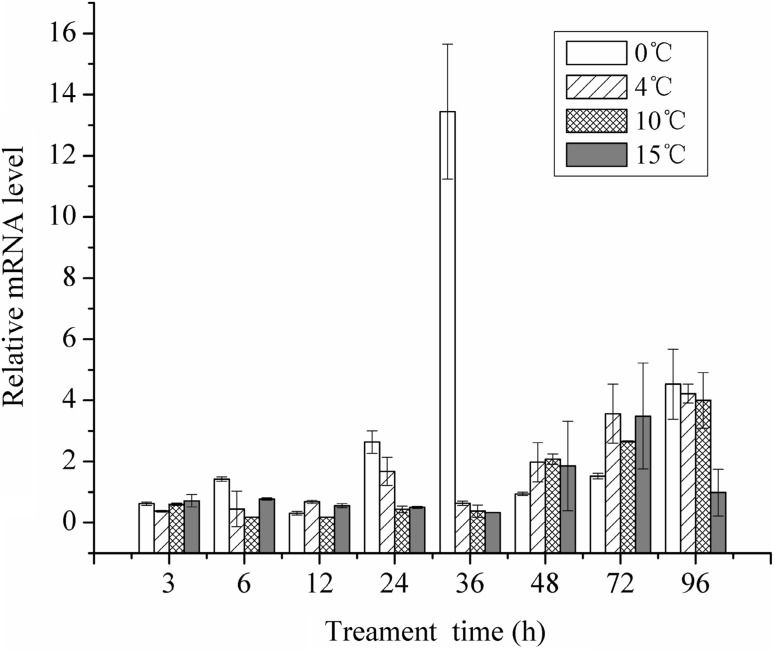
Fig. 9.
Relative mRNA expression of tps1 in response to different temperature at different times (standard error bars are shown)
Meanwhile, Fig. 9 showed that different temperature conditions also had different impacts on the mRNA expression. In the first 12 h, tps1 had a relatively sustained expression levels at 0, 4, 10, and 15 °C. After this period, however, the expression level increased at 24 h and reached the highest level at 36 h in 0 °C condition, while expression levels in other conditions did not change. Relative to the lower temperatures, expression of tps1 in 4 and 10 °C were delayed. After 36 h, all expression levels generally increased with time in all conditions. These results indicate that low temperature seemed to have the strongest influence on P. NJ7 and significantly affecting the expression of cold-active tps1 gene.
Separation, extraction and verification of low temperature preservation of trehalose
The highest concentration of extracted trehalose was 17.723 mg/g, which was very close to the expected concentration based on theoretical extraction, and was used to cryopreserve yeast. Revival rate was determined using plate count test, as summarized in Table 3. We observed that 0.5% trehalose yielded the highest number of colonies just after 5 days in − 80 °C. However, 20% glycerin had the best effect allowing survival of colonies after preservation of 10–40 days at − 80 °C. Different concentrations of trehalose had varied effects on preservation. Generally, preservation using 0.5% trehalose had better results than 20% glycerin. However, its effects decreased rapidly with prolonged incubation, suggesting that 20% glycerin had more lasting effects.
Table 3.
Number of colonies in different preserving reagents
| Preserving reagent | Colony numbers after treatment | |||
|---|---|---|---|---|
| 5 days | 10 days | 20 days | 40 days | |
| Control | 10 ± 2 | 9 ± 2 | 2 ± 1 | 2 ± 1 |
| 20% glycerin | 341 ± 14** | 327 ± 9** | 296 ± 20** | 288 ± 17** |
| 10% DMSO | 79 ± 6** | 73 ± 6** | 56 ± 3* | 33 ± 3** |
| 0.5% trehalose | 396 ± 18** | 58 ± 12* | 30 ± 13* | 27 ± 2** |
| 1% trehalose | 50 ± 7* | 47 ± 5** | 10 ± 2* | 3 ± 1 |
| 2% trehalose | 15 ± 5 | 11 ± 2 | 11 ± 2* | 2 ± 1 |
*0.01 < p < 0.05
**p < 0.01
Discussions
Trehalose is able to preserve cell integrity against a variety of environmental stresses and nutritional limitations in many organisms except in mammalian cells. Exogenous trehalose can even be used as the sole source of carbon and energy in bacteria, single-cell eukaryotes such as yeast cells (Argüelles 2000; Elbein et al. 2003). Recent studies have shown that trehalose stabilizes and protects proteins and cellular membranes from inactivation or denaturation caused by a variety of environmental stressful conditions (Avonce et al. 2006; Elbein et al. 2003; Mu et al. 2016). It can also act as a sensing compound, growth regulator and structural component of cell wall in mycobacteria and corynebacteria (Elbein et al. 2003).
Pseudozyma sp. NJ7 has unique mechanisms that allow it to resist the extreme environment of the Antarctic, and could well represent other fungal species in the Antarctic. Based on our results, it is then possible that trehalose played a vital role in the resistance of P. NJ7 against extreme environmental changes, and that tps1 gene was directly involved in the synthesis of trehalose. TPS1 and TPS2 play an important role in the survival of cold temperatures. Because TPS1 is regarded as the crucial enzyme needed in the first step of trehalose synthesis; it is also the most important step. Therefore, this paper focuses on the role of TPS1 at cold and high salinity conditions. Analysis of the P. NJ7 revealed that it was highly similar with the other several Pseudozyma strains found in NCBI. Further, the two proteins found related to the synthesis of trehalose were also consistent with previous reports that eukaryotes are the only group that have the most conserved TPS/TPP pathway (Avonce et al. 2006).
In Saccharomyces cerevisiae, TPS is present as part of a complex that is made up of four subunits, TPS1, TPS2, TPS3 and TPL1 (Bell et al. 1998). Research indicates that 6-phosphotrehalose strongly inhibited the activity of hexokinase, making it possible to synthesize trehalose in the form of an enzyme complex to strictly control the 6-phosphotrehalose level in cells of S. cerevisiae (Bell et al. 1998). Trehalose 6-phosphate, a trehalose intermediate, is a subunit of trehalose synthase complex in fungi (Chi et al. 2009), and has been shown to regulate glucose metabolic flux during glycolysis in yeast (Avonce et al. 2006; Gancedo and Flores 2004). The TPS2 in Candida albicans on the other hand was suggested to regulate the concentration of free T6P and increase efficiency in trehalose production during biosynthesis (Miao et al. 2016).
Petitjean et al. (2015) identified the TPS1 protein to be crucial in maintaining cell integrity, which is a key factor in yeast survival in response to temperature, oxidative, and desiccation stress, but not of trehalose. TPS1 could improve cotton (Mu et al. 2016) and rice-seed (Li et al. 2011) tolerance to low temperature, salinity, and drought. In yeast, TPS2 increased dramatically below 10 °C and even at 0 °C, and declined dramatically within minutes upon return to 30 °C (Kandror et al. 2004). It has been shown that the TPS1 gene was expressed at very low levels in S.cerevisiae, but the transcript levels increased dramatically when temperature downshifted to 10, 4 and 0 °C, increasing by 20-fold at 0 °C (Bell et al. 1992). The IbTPS gene cloned from sweet potato in transgenic tobacco plants may also enhance salt tolerance of plants by increasing the amount of treahalose and proline and regulating the expression of stress tolerance-related genes (Jiang et al. 2014). The RT-PCR results in this study showed that low temperature and high salinity seemed to have the strongest influences on tps1 mRNA expression of P. NJ7, which was consistent with the conclusion of previous studies.
The TPS sequences in S. cerevisiae, Arabidopsis thaliana and algae contained at least a TPS domain, and most microorganisms also included a C-terminal TPP domain (Deng et al. 2014). Our newly generated TPS1 apparently shared more similarity of about 99.51% with M. antarcticus T-34 (GAC73660) and exhibited distinct structural characteristics with other strains. It is similar that the TPS2 protein we sequenced shared more similarity of about 99.54% with M. antarcticus T-34, but had obvious specificity and differently with others, maybe the tps2 genes that were species specific.
The TPS gene from yeast was over-expressed and improved tolerance against abiotic stresses in tomato (Cortina and Culianezmacia 2005). In baker’s yeast, overexpressing TPS1 gene and deleting trehalose genes were sufficient to improve the freeze tolerance in frozen dough (Tan et al. 2014), and adding exogenous trehalose could increase the intracellular trehalose content and enhance freeze tolerance (Hirasawa et al. 2001). The results of preservation experiment in this paper also demonstrated that exogenous trehalose could indeed significantly improve the survival of yeast cells under freezing conditions. Relative to the 20% glycerin with long-term preservation effect, 0.5% trehalose was a good additive for preservation in a short term, specifically for less than 5 days. It still, however, confirmed the effect of trehalose on yeast preservation, and could indicate that it was beneficial to Antarctic yeast in response to extreme freezing conditions.
In summary, trehalose plays an important role in enhancing tolerance and adaptation of Pseudozyma sp. NJ7 to extreme changes in the Antarctic environment. In this study, we determined the genome of P. NJ7 and cloned the TPS1 and TPS2 genes, which are the key enzymes in the biosynthesis of trehalose. Genetic structural and phylogenetic analyses further confirmed the identities of the genes we isolated. We also determined the expression levels of tps1 gene in response to change in conditions, particularly low temperature and high salinity analyzed by real-time quantitative PCR. Finally, we confirmed the effects of trehalose on yeast preservation, and could indicate that its production was beneficial to Antarctic yeast in response to the freezing conditions. Antarctic Pseudozyma sp. NJ7 produces trehalose, to resist extreme environments and ensure normal physiological activities of cells through the production of trehalose. Our study provided the foundation for further studies on TPS functions and the researches on Antarctic yeast. These are helpful in understanding self-protection mechanisms and in revealing the molecular mechanisms of adaptation of yeasts in extreme environments such as the Antarctic.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (No. 41576187), the Natural Science Foundation of China-Shandong Joint Fund (No. U1606403), the Polar Strategic Foundation of China (No. 20150303), the Shandong Provincial Natural Science Foundation, China (No. ZR2017QD008), the Public Science and Technology Research Funds Projects of Ocean (No. 201405015), the Key Research and Development Program of Shandong Province (No. 2016ZDJS06A03), the Scientific and Technological Innovation Project Financially Supported by Qingdao National Laboratory for Marine Science and Technology and the Science (No. 2015ASKJ02), the Open Research Fund of State Key Laboratory of Biological Fermentation Engineering of Beer (No. K2014002), the Technology Planning Project of Shandong Province (No. 2014GHY115003), and Qingdao Entrepreneurship and Innovation Pioneers Program (No. 15-10-3-15-(44)-zch). We thank MogoEdit Co. for its linguistic assistance during the preparation of this manuscript.
Compliance with ethical standards
Conflict of interest
All the authors declare that they have no conflicts of interest regarding this paper.
Footnotes
Hua Yin and Yibin Wang contributed equally to this work.
References
- An MZ, Tang YQ, Mitsumasu K, Liu ZS, Shigeru M, Kenji K. Enhanced thermotolerance for ethanol fermentation of Saccharomyces cerevisiae strain by overexpression of the gene coding for trehalose-6-phosphate synthase. Biotechnol Lett. 2011;33:1367–1374. doi: 10.1007/s10529-011-0576-x. [DOI] [PubMed] [Google Scholar]
- Argüelles JC. Physiological roles of trehalose in bacteria and yeasts: a comparative analysis. Arch Microbiol. 2000;174:217–224. doi: 10.1007/s002030000192. [DOI] [PubMed] [Google Scholar]
- Avonce N, Mendoza-Vargas A, Morett E, Iturriaga G. Insights on the evolution of trehalose biosynthesis. BMC Evol Biol. 2006;6:109. doi: 10.1186/1471-2148-6-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell W, Klaassen P, Ohnacker M, Boller T, Herweijer M, Schoppink P, Van der Zee P, Wiemken A. Characterization of the 56-kDa subunit of yeast trehalose-6-phosphate synthase and cloning of its gene reveal its identity with the product of CIF1, a regulator of carbon catabolite inactivation. Eur J Biochem. 1992;209:951–959. doi: 10.1111/j.1432-1033.1992.tb17368.x. [DOI] [PubMed] [Google Scholar]
- Bell W, Sun W, Hohmann S, Wera S, Reinders A, De Virgilio C, Wiemken A, Thevelein JM. Composition and functional analysis of the Saccharomyces cerevisiae trehalose synthase complex. J Biol Chem. 1998;273:33311–33319. doi: 10.1074/jbc.273.50.33311. [DOI] [PubMed] [Google Scholar]
- Chi ZM, Chi Z, Liu GL, Wang F, Ju L, Zhang T. Saccharomycopsis fibuligera and its applications in biotechnology. Biotechnol Adv. 2009;27:423–431. doi: 10.1016/j.biotechadv.2009.03.003. [DOI] [PubMed] [Google Scholar]
- Conesa A, Götz S. Blast2GO: a comprehensive suite for functional analysis in plant genomics. Int J Plant Genom. 2008;2008:619832. doi: 10.1155/2008/619832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortina C, Culianezmacia FA. Tomato abiotic stress enhanced tolerance by trehalose biosynthesis. Plant Sci. 2005;169:75–82. doi: 10.1016/j.plantsci.2005.02.026. [DOI] [Google Scholar]
- Deng Y, Wang X, Guo H, Duan D. A trehalose-6-phosphate synthase gene from Saccharina japonica (Laminariales, Phaeophyceae) Mol Biol Rep. 2014;41:529–536. doi: 10.1007/s11033-013-2888-5. [DOI] [PubMed] [Google Scholar]
- Eastmond PJ, Graham IA. Trehalose metabolism: a regulatory role for trehalose-6-phosphate? Curr Opin Plant Biol. 2003;6:231–235. doi: 10.1016/S1369-5266(03)00037-2. [DOI] [PubMed] [Google Scholar]
- Elbein AD, Pan YT, Pastuszak I, Carroll D. New insights on trehalose: a multifunctional molecule. Glycobiology. 2003;13:17r. doi: 10.1093/glycob/cwg047. [DOI] [PubMed] [Google Scholar]
- Gancedo C, Flores CL. The importance of a functional trehalose biosynthetic pathway for the life of yeast and fungi. FEMS Yeast Res. 2004;4:351–359. doi: 10.1016/S1567-1356(03)00222-8. [DOI] [PubMed] [Google Scholar]
- Hamid B, Rana RS, Chauhan D, Singh P, Mohiddin FA, Sahay S, Abidi I. Psychrophilic yeasts and their biotechnological applications-a review. Afr J Biotech. 2014;13:2188–2197. doi: 10.5897/AJB2014.13739. [DOI] [Google Scholar]
- Hirasawa R, Yokoigawa K, Isobe Y, Kawai H. Improving the freeze tolerance of bakers’ yeast by loading with trehalose. Biosci Biotechnol Biochem. 2001;65:522–526. doi: 10.1271/bbb.65.522. [DOI] [PubMed] [Google Scholar]
- Jiang T, Zhai H, Wang FB, Zhou HN, Si ZZ, He SZ, Liu QC. Cloning and characterization of a salt tolerance-associated gene encoding trehalose-6-phosphate synthase in sweetpotato. J Integr Agric. 2014;13:1651–1661. doi: 10.1016/S2095-3119(13)60534-1. [DOI] [Google Scholar]
- Kandror O, Bretschneider N, Kreydin E, Cavalieri D, Goldberg AL. Yeast adapt to near-freezing temperatures by STRE/Msn2,4-dependent induction of trehalose synthesis and certain molecular chaperones. Mol Cell. 2004;13:771–781. doi: 10.1016/S1097-2765(04)00148-0. [DOI] [PubMed] [Google Scholar]
- Li HW, Zang BS, Deng XW, Wang XP. Overexpression of the trehalose-6-phosphate synthase gene OsTPS1 enhances abiotic stress tolerance in rice. Planta. 2011;234:1007–1018. doi: 10.1007/s00425-011-1458-0. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lowe TM, Eddy SR. tRNAscan-SE: a program for improved detection of transfer RNA genes in genomic sequence. Nucleic Acids Res. 1997;25(5):955–964. doi: 10.1093/nar/25.5.0955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggi O, Tosi S, Angelova M, Lagostina E, Fabbri AA, Pecoraro L, Altobelli E, Picco AM, Savino E, Branda E, Turchetti B. Adaptation of fungi, including yeasts, to cold environments. Plant Biosyst. 2013;147:247–258. doi: 10.1080/11263504.2012.753135. [DOI] [Google Scholar]
- Mahmud SA, Hirasawa T, Shimizu H. Differential importance of trehalose accumulation in Saccharomyces cerevisiae in response to various environmental stresses. J Biosci Bioeng. 2010;109:262–266. doi: 10.1016/j.jbiosc.2009.08.500. [DOI] [PubMed] [Google Scholar]
- Margesin R, Miteva V. Diversity and ecology of psychrophilic microorganisms. Res Microbiol. 2011;162:346–361. doi: 10.1016/j.resmic.2010.12.004. [DOI] [PubMed] [Google Scholar]
- Mayclin SJ, Dranow DM, Lorimer DD, Edwards TE (2016) Crystal structure of an UDP-forming alpha, alpha-terhalose-phosphate synthase from Burkholderia xenovorans. doi:10.2210/pdb5hxa/pdb. http://www.rcsb.org/pdb/explore.do?structureId=5HXA
- Miao Y, Tenor JL, Toffaletti DL, Washington EJ, Liu J, Shadrick WR, Schumachera MA, Leec RE, Perfectb JR, Brennan RG. Structures of trehalose-6-phosphate phosphatase from pathogenic fungi reveal the mechanisms of substrate recognition and catalysis. Proc Natl Acad Sci. 2016;113:201601774. doi: 10.1073/pnas.1601774113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriya Y, Itoh M, Okuda S, Yoshizawa AC, Kanehisa M. KAAS: an automatic genome annotation and pathway reconstruction server. Nucleic Acids Res. 2007;35:W182–W185. doi: 10.1093/nar/gkm321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu M, Lu XK, Wang JJ, Wang DL, Yin ZJ, Wang S, Fan WL, Ye WW. Genome-wide Identification and analysis of the stress-resistance function of the TPS (trehalose-6-phosphate synthase) gene family in cotton. BMC Genet. 2016;17:1–11. doi: 10.1186/s12881-015-0265-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parra G, Bradnam K, Korf I. CEGMA: a pipeline to accurately annotate core genes in eukaryotic genomes. Bioinformatics. 2007;23:1061–1067. doi: 10.1093/bioinformatics/btm071. [DOI] [PubMed] [Google Scholar]
- Paul M. Trehalose 6-phosphate. Curr Opin Plant Biol. 2007;10:303–309. doi: 10.1016/j.pbi.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Paul MJ, Primavesi LF, Jhurreea D, Zhang Y. Trehalose metabolism and signaling. Annu Rev Plant Biol. 2008;59:417–441. doi: 10.1146/annurev.arplant.59.032607.092945. [DOI] [PubMed] [Google Scholar]
- Petersen TN, Brunak S, von Heijne G, Nielsen H. Signal P 4.0: discriminating signal peptides from transmembrane regions. Nat Methods. 2011;8:785–786. doi: 10.1038/nmeth.1701. [DOI] [PubMed] [Google Scholar]
- Petitjean M, Teste MA, François JM, Parrou JL. Yeast tolerance to various stresses relies on the trehalose-6P synthase (Tps1) protein, not on trehalose. J Biol Chem. 2015;290:16177–16190. doi: 10.1074/jbc.M115.653899. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Powell S, Forslund K, Szklarczyk D, Trachana K, Roth A, Huerta-Cepas J, Gabaldón T, Rattei T, Creevey C, Kuhn M, Jensen LJ, von Mering C, Bork P. eggNOG v4.0: nested orthology inference across 3686 organisms. Nucleic Acids Res. 2014;42:D231–D239. doi: 10.1093/nar/gkt1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinders A, Romano I, Wiemken A, De Virgilio C. The thermophilic yeast Hansenula polymorpha does not require trehalose synthesis for growth at high temperatures but does for normal acquisition of thermotolerance. J Bacteriol. 1999;181:4665–4668. doi: 10.1128/jb.181.15.4665-4668.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan H, Dong J, Wang G, Xu H, Zhang C, Xiao D. Enhanced freeze tolerance of baker’s yeast by overexpressed trehalose-6-phosphate synthase gene (TPS1) and deleted trehalase genes in frozen dough. J Ind Microbiol Biotechnol. 2014;41:1275–1285. doi: 10.1007/s10295-014-1467-7. [DOI] [PubMed] [Google Scholar]
- Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997;25:4876–4882. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Vaeck C, Stefaan W, Van Dijck P, Thevelein JM. Analysis and modification of trehalose 6-phosphate levels in the yeast Saccharomyces cerevisiae with the use of Bacillus subtilis phosphotrehalase. Biochem J. 2001;353:157–162. doi: 10.1042/bj3530157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Wang ZP, Chi Z, Paoufi Z, Abdollahi S, Chi ZM. The changes in Tps1 activity, trehalose content and expression of gene in the psychrotolerant yeast 17-1 grown at different temperatures. Extremophiles. 2013;17:241–249. doi: 10.1007/s00792-013-0511-2. [DOI] [PubMed] [Google Scholar]
- Zhao YQ, Du YJ, Wang W, Zhao Q. Study on extraction methods of trehalose in marine yeast. Food Res Dev. 2013;34:34–37. [Google Scholar]