Abstract
Perivascular adipose tissue (PVAT) releases numerous factors and adipokines with paracrine effects on both vascular structure and function. These effects are variable as they depend on regional differences in PVAT among blood vessels and vary with changes in adiposity. There is considerable evidence demonstrating an association between coronary PVAT and the development and progression of coronary artery disease, which is associated with inflammation, oxidative stress, angiogenesis, vascular remodelling and blood clotting. However, PVAT also has a protective role in vascular grafts, especially the no‐touch saphenous vein, in patients undergoing coronary artery bypass. This beneficial influence of PVAT involves factors such as adipocyte‐derived relaxing factor, nitric oxide (NO), leptin, adiponectin, prostanoids, hydrogen sulphide and neurotransmitters, as well as mechanical protection. This article aims to highlight and compare the dual role of PVAT in the development and progression of coronary atherosclerosis, as well as in increased graft patency. Different deleterious and protective mechanisms of PVAT are also discussed and the inside‐outside signalling paradigm of atherosclerosis development re‐evaluated. The bidirectional communication between the arterial and venous wall and their surrounding PVAT, where signals originating from the vascular wall or lumen can affect PVAT phenotype, has been shown to be very complex. Moreover, signals from PVAT also influence the structure and function of the vascular wall in a paracrine manner.
Linked Articles
This article is part of a themed section on Molecular Mechanisms Regulating Perivascular Adipose Tissue – Potential Pharmacological Targets? To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v174.20/issuetoc
Abbreviations
- ADRF
adipocyte‐derived relaxing factor
- AT
adipose tissue
- BH4
tetrahydrobiopterin
- CABG
coronary artery bypass graft
- IL
interleukin
- ITA
internal thoracic artery
- LDL
low‐density lipoprotein
- MCP‐1
monocyte chemoattractant protein‐1
- NO
nitric oxide
- PCAT
pericoronary adipose tissue
- PPAR
peroxisome proliferator‐activated receptors
- PVAT
perivascular adipose tissue
- SV
saphenous vein
- TNF
tumor necrosis factor
- 4‐HNE
4‐hydroxi nonenal
- VSMC
vascular smooth muscle cells
Tables of Links
| TARGETS | |
|---|---|
| Voltage‐gated ion channels a | Enzymes c |
| Calcium‐activated potassium channels | Akt (PKB) |
| Cav1.2 channel | eNOS |
| Nuclear hormone receptors b | |
| PPAR‐γ |
| LIGANDS | ||
|---|---|---|
| 4‐HNE | BH4 | NO |
| 5‐HT | IL‐1β | Noradrenaline |
| Adiponectin | IL‐6 | TNFα |
| Angiotensin II | Leptin |
These Tables list key protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Southan et al., 2016), and are permanently archived in the Concise Guide to PHARMACOLOGY 2015/16 (a,b,cAlexander et al., 2015a,b,c).
Perivascular adipose tissue (PVAT) is a local adipose tissue (AT) depot surrounding blood vessels. It was previously thought to act only as a passive structural support for the vasculature and was routinely removed in experiments on isolated blood vessel function and bypass graft preparation (Fernandez‐Alfonso, 2004). There is now a large body of evidence suggesting that PVAT is phenotypically different in adipocytes from different origin, morphology or stage of differentiation, as well as in cell composition from other AT depots, such as subcutaneous or visceral AT (Gil‐Ortega et al., 2015). It releases an important number of vasoactive factors that elicit a paracrine signalling effect on the vascular wall, influencing structure and function. This paracrine effect is an additional, distinctive, feature compared to the endocrine signalling associated with subcutaneous and visceral AT. There are regional phenotypic differences among PVATs, depending on the specific vascular bed, which differentially affect both the physiology of the blood vessel wall as well as the pathogenesis of vascular disease (Gil‐Ortega et al., 2015). Moreover, the influence of PVAT differs with changes in adiposity (Gálvez‐Prieto et al., 2012; Fernández‐Alfonso et al., 2013). Therefore, PVAT exhibits either a deleterious or a protective role depending on the vascular bed location, its magnitude and on the physiological or pathophysiological situation.
PVAT has been suggested to be associated with the development of atherosclerosis in the aorta (Fitzgibbons et al., 2011; Chang et al., 2012; Kawahito et al., 2013; Shields et al., 2013). However, most evidence, covered previously in several excellent recent reviews (Fitzgibbons and Czech, 2014; Iacobellis, 2015: Mazurek and Opolski, 2015), also demonstrates an association between pericoronary AT and the development of coronary artery disease. Coronary artery disease, due to atherosclerosis and/or coronary vasospasm, is a leading cause of mortality worldwide (World Health Organization factsheet, 2016). A mainstay in the treatment of symptomatic coronary artery disease patients, which reduces ischaemia to a greater extent than medical treatment, is revascularization. Coronary artery bypass grafting (CABG) is the most commonly performed revascularization procedure alongside percutaneous coronary intervention (Farooq and Serruys, 2015). The preservation of PVAT in blood vessel segments used for CABG, mainly saphenous vein (SV), significantly increases graft patency (Souza et al., 2002; 2006; Samano et al., 2015).
This article aims to highlight and compare the dual role of PVAT in the development and progression of coronary atherosclerosis, as well as on increased graft patency. It also discusses different deleterious and protective mechanisms of PVAT, and re‐evaluates the inside‐outside signalling paradigm of atherosclerosis development.
Pericoronary adipose tissue and coronary atherosclerosis development and progression
The AT of the heart, known as pericardial AT, consists of paracardial and epicardial fat. Both depots are different in embryological origin, anatomy and function (Sacks and Fain, 2007; Iacobellis, 2014). Paracardial fat is located within the mediastinum on the external surface of the parietal pericardium. Epicardial AT is contained beneath the visceral layer of the serous pericardium, mainly concentrated in the atrioventricular and interventricular grooves, covering the major branches of the coronary arteries [pericoronary adipose tissue (PCAT)] and to a lesser extent, the atria, the right ventricle and the apex of the left ventricle (myocardial AT) (Sacks and Fain, 2007; Iacobellis, 2014). Since PCAT constitutes part of the epicardial AT with no distinct anatomical barrier, there has been some confusion and both terms have been treated synonymously (Iacobellis, 2015).
PCAT volume increases with age, is higher in men than in women (Rabkin, 2007) and correlates with coronary artery disease risk factors (Fitzgibbons and Czech, 2014). Even though epicardial AT shows a strong correlation with anthropometric and imaging measurements of visceral AT with increasing body mass index (Iacobellis et al., 2003), the amount by which the PCAT is increased is not necessarily related to obesity and seems to be mostly regulated locally, and is independent of increases in systemic AT (Ohyama et al., 2016). Greater volumes of epicardial AT are not only associated with the severity of coronary artery stenosis in patients with known coronary artery disease (Jeong et al., 2007) but also with high‐risk plaques independently of risk factors, coronary artery calcification score and obstructive coronary artery disease (Lu et al., 2016). In addition, atherosclerotic plaques and regions of coronary artery calcification co‐localize with PCAT (Prati et al., 2003; Mahabadi et al., 2013), whereas they are absent in segments underneath myocardial bridges (Ishii et al., 1998; Ishikawa et al., 2006) (Figure 1A). However, this is not always the case. PCAT and coronary artery calcification are not always present together, and plaques are not completely absent in myocardial bridges, suggesting that other factors, such as differences in haemodynamic parameters might contribute to these effects.
Figure 1.
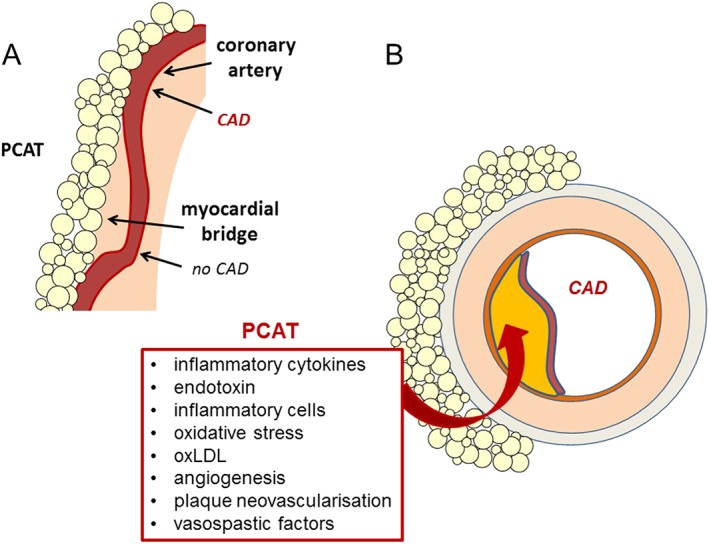
Influence of PCAT on the development and progression of coronary artery disease (CAD). (A) Coronary artery segments in contact with PCAT often exhibit atherosclerotic plaques, whereas coronary artery segments under myocardial bridges do usually not develop CAD. (B) Paracrine mechanisms through which PCAT influences the development of CAD. PCAT‐derived adipokines, cytokines, reactive oxygen species and inflammatory cells diffuse through the adventitia and media or enter through the vasa vasorum to reach the coronary endothelium and contribute to atherosclerotic plaque development and progression.
Following the original proposal of Russell Ross (1999), the development of atherosclerotic lesions is initiated by signalling originating from the vessel lumen or at the endothelial/intimal interface. However, due to its close contact with the coronary arteries, PCAT might also play a role in the development and progression of coronary artery disease. Several observations support the local paracrine influence of PCAT on the development of coronary artery disease; PCAT‐derived adipokines and cytokines diffuse through the adventitia and media (Iacobellis, 2015) or pass through the vasa vasorum (Yudkin et al., 2005) to reach the coronary endothelium and lumen (Figure 1).
Pathophysiological mechanisms involved in plaque development and progression are inflammation, oxidative stress, angiogenesis, vascular remodelling and blood clotting. Inflammation plays a central role in atherosclerosis (Ross, 1999; Libby et al., 2002). Patients with coronary artery disease exhibit an increased expression of inflammatory genes in epicardial AT compared to subcutaneous AT independently of several clinical variables (Mazurek et al., 2003). Gene expression analysis of in vitro differentiated pericoronary adipocytes shows an increased expression of genes associated with inflammation (Chatterjee et al., 2013). In addition, pericoronary adipocytes exhibit a pro‐inflammatory phenotype (high levels of MCP‐1, IL‐6, IL‐8 and pro‐angiogenic mediators and reduced adiponectin) thus playing a key role in both the recruitment of inflammatory cells and the development of vascular disease (for review, Omar et al., 2014). Moreover, inflammatory cells involved in the atherosclerotic process, in particular activated monocytes, macrophages and T‐lymphocytes, are also resident in epicardial AT and PCAT (Mazurek et al., 2003; Iacobellis, 2015). Oxidized LDL (oxLDL) is stored in PCAT and conveyed by CD68(+)‐macrophages into the coronary intima (Uchida et al., 2016). Oxidative stress is higher in epicardial AT than in subcutaneous AT from coronary artery disease patients with lower catalase levels (Salgado‐Somoza et al., 2010).
Another consequence of PCAT‐derived inflammation is the stimulation of an angiogenic response and the development of a collateral circulation in patients with coronary artery disease (Okamoto et al., 2001). Plaque neovascularization is composed of a network of capillaries that arise from the adventitial vasa vasorum and extend into the intimal layer of atherosclerotic lesions. These plaque capillaries have been proposed to be important regulators of plaque growth and lesion instability and may thus play an important role in modulating the disease process (Okamoto et al., 2001). In this context, in vitro differentiated pericoronary adipocytes exhibit an increased expression of angiogenesis‐related genes (Chatterjee et al., 2013). In addition, pericoronary adipocytes also show an increased expression of genes related to vascular remodelling and blood clotting (Chatterjee et al., 2013).
Whereas inflammation is most often associated with atherosclerosis, vasospastic angina is a more rare condition. The underlying causes of coronary artery spasm are endothelial dysfunction, primary vascular smooth muscle cell hyperreactivity to a variety of vasoconstrictors and adventitial abnormalities (for review see Lanza et al., 2011). In fact, the degree of adventitial coronary stimulation is associated with inflammatory cytokine‐induced spasmogenic changes of vascular smooth muscle cells in animal models (Shimokawa et al., 1996). More recently, a role for PCAT in coronary artery spasm has also been suggested. In Ossabaw swine, PCAT releases factors that initiate and/or potentiate contraction of coronary arteries independently of their effects on the coronary endothelium. The augmented contractile effects of PCAT from obese individuals are related to alterations in calpastatin, rho‐dependent signalling, increased functional expression of CaV1.2 channels or altered activity of potassium channels in coronary arteries (Owen et al., 2013). These signalling pathways may contribute to the initiation or progression of coronary artery disease in obesity (Noblet et al., 2015)
Vasa vasorum formation is also enhanced at the spastic coronary segment in patients with vasospastic angina, which correlates with the extent of the coronary contractions (Nishimiya et al., 2016). Moreover, PCAT volume is increased at the spastic segments, suggesting it contributes to coronary spasm (Nishimiya et al., 2016). Interestingly, these are patients with normal weight (BMI < 25 kg·m−2) suggesting that the increased volume of PCAT is not necessarily related to obesity (Ohyama et al., 2016).
Altogether, these observations led to the outside‐to‐inside signalling paradigm, in which changes in the adventitia and/or PCAT could also induce changes in the inner layer of the intima thus having a deleterious influence on the development and progression of coronary artery disease (Figure 1). Indeed, periadventitial application of endotoxin, MCP‐1, IL‐1β or oxLDL induces an influx of inflammatory cells into the arterial wall, coronary vasospasm or intimal lesions, suggesting that bioactive molecules from the pericoronary tissues may alter arterial homeostasis (Prescott et al., 1989; Shimokawa et al., 1996; Miyata et al., 2000).
The protective role of PVAT in vessels used for CABG
There is evidence that PVAT has a protective role in blood vessels commonly used as grafts in patients undergoing CABG. The first CABG performed was connecting the right internal thoracic artery (ITA) to the atherosclerotic coronary artery, an operation carried out at the Bronx Municipal Hospital Centre in New York by Robert H. Goetz in 1960 (Konstantinov, 2000). Within a decade, Favaloro (1969) introduced the use of the SV as a conduit in patients undergoing CABG. Over subsequent years, a variety of other blood vessels have been used as bypass grafts, including the radial artery and the right gastroepiploic artery. At present the ITA, the radial artery and the SV are the three most commonly used vessels in patients undergoing CABG (Lytle et al., 1985; Mehta et al., 1997).
Regarding the methods of harvesting these vessels, the ITA is used as an in situ graft that remains connected at its proximal end, whereas both the SV and radial artery are excised and harvested as free grafts. It is noteworthy that both arteries are generally harvested with the surrounding pedicle of PVAT intact, whereas this pedicle is removed from the SV, following the original harvesting technique described by Favaloro (1969), ‘Care must be taken to dissect only the vein, avoiding as much as possible the adventitia that surrounds it’. Thus the surrounding PVAT was routinely removed. One reason for SV graft stripping is that it is easier to detect side branches, which need to be carefully ligated to prevent bleeding or a tamponade and death in the worst case scenario (Sabik, 2011). However, stripping vessels of their outer layers leads to considerable vascular damage and structural changes in the graft (Tsui and Dashwood, 2002; 2013). In using the term ‘conduit’ (a ‘pipe’ or ‘channel’), it seems that when preparing the vessel by established methods the vascular damage caused may produce a graft of poor quality that may require early replacement.
The ITA is accepted as the ‘gold standard’ for CABG since the patency rate at 10 years for this vessel is reported to be 95% compared with ~60% for the SV (Mehta et al., 1997). Nevertheless, the SV is most commonly used as a graft because: (i) it is technically easy to use due to its relatively large diameter and wall characteristics; (ii) it is long and can be used to perform multiple grafts and reach any coronary artery; and (iii) it is easily harvested (Sabik, 2011). However, the performance of SV grafts is poor compared with the ITA; thrombotic occlusion being the main cause of early SV graft failure (for review see de Vries and Quax, 2016). Certainly, there are publications suggesting that the SV is inferior to the ITA (Izzat et al., 1994; George et al., 2006) and perhaps also the radial artery (Habib et al., 2013). However, most, if not all reviews and multi‐centre studies, are based on data from patients receiving SV grafts that are damaged due to using the harvesting technique originally described by Favaloro (1969).
An atraumatic, no‐touch, technique of harvesting the SV with surrounding PVAT intact was introduced 20 years ago by Souza (1996) (Figure 2). This technique provides a superior graft to those prepared conventionally, with a patency superior to the radial artery (Dreifaldt et al., 2013) and comparable to the ITA both in the short‐ (Souza et al., 2002) and long‐term (Souza et al., 2006; Samano et al., 2015). Could the cushion of surrounding PVAT, that remains intact in no‐touch SV grafts, play a role in its improved performance? The most likely answer to this question is affirmative, as this distinct outermost layer of fat may ‘protect’ the SV in a number of ways once it has been implanted as a bypass graft in patients undergoing coronary revascularization.
Figure 2.
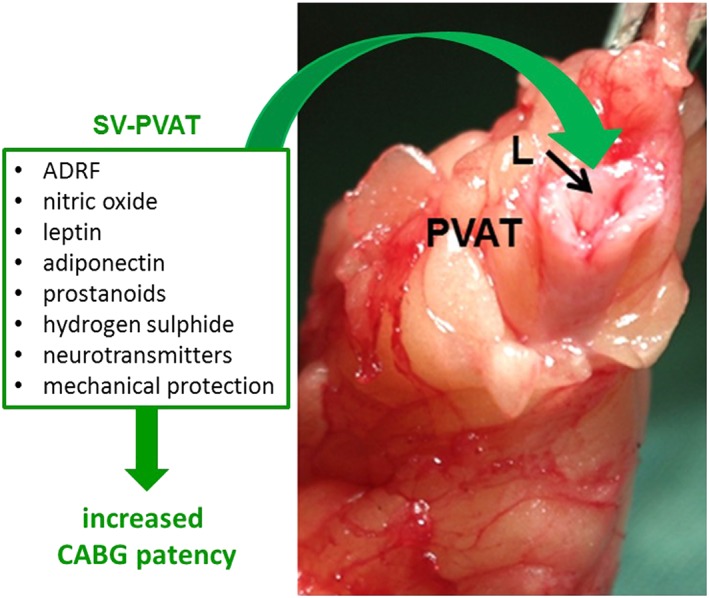
No‐touch saphenous vein (SV) used as coronary artery bypass graft (CABG). The SV is surrounded by a cushion of PVAT. The vein lumen (L) represents a site where several of the protective PVAT‐derived factors listed may confer beneficial effects that contribute to the superior performance of SV grafts in patients undergoing CABG.
Mechanical protection of PVAT
Firstly, there is evidence that PVAT has mechanical properties that protect other vascular structures from the various traumatic conditions inflicted on the vein during harvesting. For example, a high proportion of veins go into spasm while being harvested due to their manipulation by surgical instruments, a condition that can be overcome using high‐pressure intraluminal distension (Baron et al., 2010). Since no‐touch SV are handled via their surrounding tissue (Figure 2), spasm does not occur and the need for distension and resulting tissue damage is obviated. The cushion of surrounding fat also protects against pressure‐induced disruption of the luminal endothelium and the harmful consequences of reduced local NO levels, including vasospasm, increased platelet activation and neointimal hyperplasia (Tsui and Dashwood, 2002; Baron et al., 2010; Dashwood and Tsui, 2013). Once implanted, the surrounding fat acts as a buffer against arterial haemodynamics and prevents grafts of excessive length from kinking, a situation that reduces myocardial blood supply with serious consequences for the patient (Souza et al., 2008).
Histologically, no‐touch SVs have a dramatically different appearance to those prepared by conventional surgery. The no‐touch technique minimizes trauma and damage, with the SV maintaining its normal architecture and, in so doing, provides useful control samples for comparative studies (Dashwood et al., 2009). Since the introduction of this technique, a number of studies have described the considerable damage that occurs to a variety of important vascular structures likely to affect vein graft patency. At the vein's innermost layer, the endothelium, regions of denudation and ultrastructural shape changes in conventionally compared with no‐touch SVs have been described (Tsui et al., 2001, Tsui and Dashwood, 2002, Ahmed et al., 2004, Vasilakis et al., 2004, Dashwood et al., 2009). Within the media, ultrastructural changes in vascular smooth muscle cells occur in conventional versus no‐touch veins where dramatic shape changes and signs of nuclear mitotic activity are observed using transmission electron microscopy (Vasilakis et al., 2004). Stripping of the outer layers of the SV damages the adventitia and the vasa vasorum (Ahmed et al., 2004; Vasilakis et al., 2004; Dreifaldt et al., 2011). Disruption of the vasa vasorum is most likely to have a detrimental effect on veins, since these microvessels are more pronounced than in arteries where circulating blood is oxygenated. In addition, there is experimental evidence that removal of the adventitia or occlusion of the vasa vasorum by surrounding them with a close fitting collar reduces transmural blood supply and promotes neointimal and atheroma formation, events involved in graft occlusion (Barker et al., 1993; 1994).
Protective role of PVAT‐derived vasoactive factors
A number of factors have been identified in the PVAT of human vessels that may play a role in their performance as bypass conduits (Figure 2). In a recent review Szasz and colleagues, discuss adipocytes and stromal cells contained within PVAT as a source of molecules affecting the underlying smooth muscle and endothelial cells that possess ‘anti‐contractile’ rather than ‘relaxant properties’ (Szasz et al., 2013). This terminology may be important when considering the beneficial role of PVAT‐derived factors.
Adipocyte‐derived relaxing factor
The first functional studies on PVAT surrounding human ITA showed that it elicits an anti‐contractile effect due to the release of a transferable factor named adipocyte‐derived relaxing factor (ADRF) (Gao et al., 2005). This finding suggests that PVAT may play a role in the superior patency rate reported for this vessel when used as a conduit in patients undergoing CABG (Lytle et al., 1985: Mehta et al., 1997). The earlier evidence that PVAT affected the human ITA (Gao et al., 2005) is supported by other groups. In vitro studies using segments of both animal and human vessels indicate that the effects of PVAT are mediated via ADRF (Aghamohammadzadeh et al., 2012; Gollasch, 2012). For example, Malinowski et al. (2013) used isolated rings of human ITA in vitro to examine the possibility that potassium channels may be involved in the action of ADRF. Segments of ITA were precontracted with 5‐HT and relaxed by adding PVAT to the tissue bath, first without and then in the presence of an appropriate potassium channel blocker. It was finally concluded that PVAT of human ITA releases relaxing factor(s) and that Ca2+‐dependent potassium channels are involved in this relaxant effect. In a previous study, these authors reported data relating to the use of ‘skeletonized’ ITA. By comparing 5‐HT‐induced contractions of human ITA with and without pedicle intact in the presence of various potassium channel blockers, it was concluded that PVAT of human ITA releases a relaxing factor that appears to act through Ca2+‐dependent potassium channels (Malinowski et al., 2008). Although, to date, ADRF has not been identified several candidates have been suggested including adiponectin, hydrogen sulphide and palmitic acid methyl ester (Tano et al., 2014).
NO
Endothelial NOS (eNOS), the main enzyme responsible for the conversion of L‐arginine to NO, is present within the PVAT of no‐touch SV and also localized to the endothelial cells lining the main vessel lumen, vasa vasorum and capillaries. High protein levels of eNOS protein were found in tissue extracts of PVAT and NO production was shown to occur using the citrulline assay (Dashwood et al., 2007). Given that NO is a potent relaxant, these results suggest that PVAT‐derived NO may play an important role in the improved patency of no‐touch SVs via a local action on the vessel's outermost surface.
Leptin and adiponectin
Using similar in vitro methodology, the adipokine, leptin, was demonstrated to have a relaxant effect that was neither NO nor endothelial‐dependent (Momin et al., 2006). High protein expression of leptin was detected in PVAT surrounding no‐touch SV grafts using immunohistochemistry and western blot analysis (Dashwood et al., 2011). Also, the leptin levels assessed by elisa were within the active range in in vitro blood vessel preparations (Momin et al., 2006).
More recently, adiponectin, an adipokine known to be anti‐inflammatory, that promotes insulin sensitivity and provides cardiometabolic protection has also been identified in PVAT of bypass vessels (Shen et al., 2016), a finding of potential importance. Interestingly, in this study, positive adiponectin immunostaining was identified that was associated, not only with the endothelium of the main vessel lumen but also with the vasa vasorum and capillary network within the PVAT (Shen et al., 2016).
Other PVAT‐derived factors that influence the reactivity of human vessels used as grafts include prostanoids (Ozen et al., 2013) and hydrogen sulphide (Bełtowski, 2013).
Role of perivascular nerves
Sympathetic control of ‘body fat’ is well established, with noradrenaline being the predominant neurotransmitter involved. However, the interaction between the vascular nerves and PVAT is not well documented. Certainly, given the distribution of perivascular nerves within, or in close proximity to PVAT, the activity of adipokines may be influenced via vascular nerve activity or they may affect transmitter action, uptake or stability (Dashwood and Loesch, 2011; Bulloch and Daly, 2014). Apart from their transmitter action, many neurotransmitters possess mitogenic activity and, in this way, may play a role in the vascular remodelling associated with graft failure. This possibility was corroborated using a porcine vein graft model, where dramatic neural reorganization was demonstrated. Here, in the short‐term, there was an almost complete depletion of perivascular nerves located in the media whereas, in the long‐term, there was an obvious appearance of large paravascular nerve bundles and a marked increase in small paravascular nerves in the neoadventitia, changes that are associated with the VSMC proliferation and neointimal thickening that occurs in graft failure (Dashwood et al., 2000; 2002). Data regarding ‘nerve/fat’ interactions are sparse; however, using rat mesenteric arteries, there is evidence for an interaction between adipocyte‐derived angiotensin II and contractile responses mediated by neuronal excitation (Lu et al., 2010).
Differences in patency between arteries and veins used for CABG
A number of groups remove the pedicle when preparing the ITA as a graft, a technique termed ‘skeletonization’ (Gaudiani et al., 1988). This method is mainly used for practical purposes whereby ‘elongation of the internal mammary artery during balloon calibration aids in the performance of sequential grafts’ (Gaudiani et al., 1988). There are, however, conflicting reports regarding the patency of pedicled versus skeletonized ITA (Del Campo, 2003) and some concerns regarding the use of skeletonization. Recent studies (Benedetto et al., 2016) have shown no increased risk for skeletonized single ITAs, not even in diabetic patients. Skeletonization of the radial artery has also been used, although not often (Hirose et al., 2003).
Therefore, the preservation of PVAT on arteries may not play as important a role as in the veins used in bypass procedures. There also appears to be regional differences between arterial and venous PVAT. In fact, adipocytes in PVAT surrounding the ITA are smaller than those in the PVAT of the SV and, apart from adipocytes, the artery is encapsulated in connective tissue, muscle and brown AT (Shen et al., 2016). From studies cited in this review, it seems that many of the factors derived from PVAT have similar anti‐contractile activity and have been identified in both arteries and veins. However, there might be differences in the expression pattern and relative amount of these factors leading to variations in the protective effects of PVAT in arterial and venous grafts. Moreover, differences in the anatomical site, rather than between arteries and veins should not be excluded. Further studies need to be performed to elucidate these possibilities.
Vasa vasorum and transmural blood flow
Apart from a local, adventitial, effect, there is the potential for PVAT‐derived factors to be transported from the outermost to the innermost layers of the SV (Fernandez‐Alfonso et al., 2016). The in vivo blood supply of the vasa vasorum of human SV (Figure 3) is via the external iliac artery (Lescalié et al., 1986), and this route may convey a variety of factors that act on the outer vein layers and the media. However, retrograde transport of blood through the vasa vasorum from the lumen to the adventitia in no‐touch SVs has been observed after removal of vascular clamps in CABG patients (Souza et al., 2006), and this is supported by results from studies where the adventitial vasa vasorum have been filled with blood perfused in vitro via the lumen of isolated human SV segments (Souza et al., 2008; Dreifaldt et al., 2011). This microvessel network extends further than the adventitial surface, since India ink staining appears not only in medial and adventitial vasa vasorum, but also in capillaries within the PVAT of no‐touch SV segments perfused in vitro. These observations are taken as evidence for a novel system of transporting factors from outer to inner layers of the vessel, as well as to the lumen. In this way, ADRFs may play a role in maintaining a healthy graft after bypass surgery by preserving transmural flow through the vasa vasorum (Fernandez‐Alfonso et al., 2016). This may be of particular relevance to the SV, as this microvascular network is more pronounced and penetrates deeper into the media than in the ITA or the radial artery (Dreifaldt et al., 2011).
Figure 3.
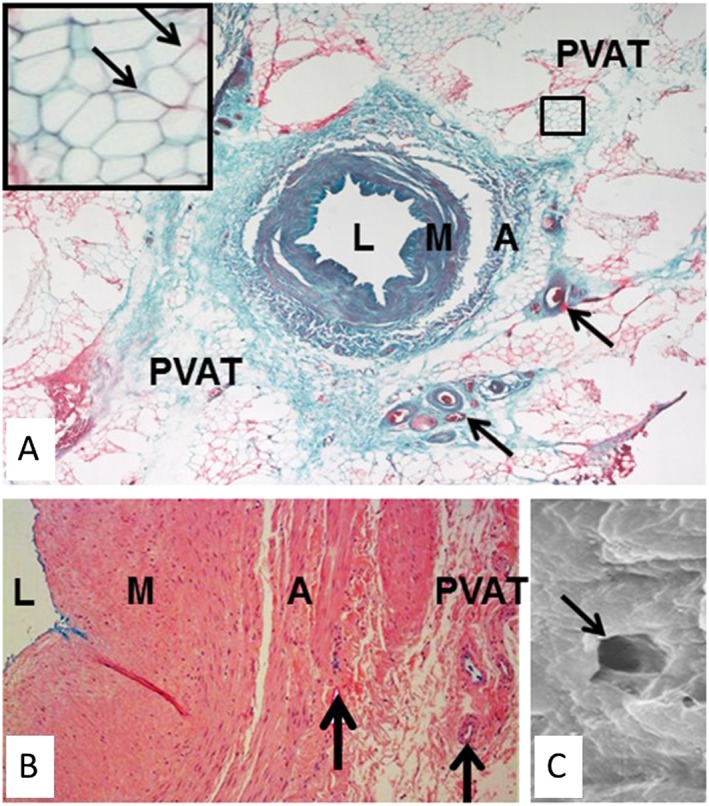
Inside‐out/outside‐in connections in human no‐touch saphenous vein (SV). (A) Transverse section of a no‐touch SV. Arrows indicate vasa vasorum. Inset, higher magnification of area in (B) showing adipocytes with embedded capillaries (arrows). (B) Part of a transverse section of no‐touch SV perfused with Indian ink (blue stain). There is staining of the lumen and vasa vasorum within the adventitia and PVAT. (C) Scanning electron micrograph of the endothelial surface of the lumen of a no‐touch SV showing the termination of a vasa vasorum (arrow). L, lumen; M, media; A, adventitia. Stained section in (B) provided by Dr Craig Daly (www.cardiovascular.org). Indian ink perfused vein in (C) is from Dr Mats Dreifaldt (unpublished). Scanning electron micrograph in (C) is from Vasilakis et al., 2004.
Although this ‘transmural transport system’ has only been shown experimentally, and in isolated SV segments, it is possible that under certain conditions, pathological factors circulating in the vessel lumen or released in the vessel wall may undergo retrograde transport to affect medial cells or cells within PVAT that are involved in inflammatory or other processes associated with coronary artery disease. Interestingly, a ‘corrupted feedback hypothesis’ has been suggested for noradrenaline, which, although conventionally classified as a venoconstrictor, dilates the SV when it diffuses from the vein's vasa venarum into the media from the vessel lumen (Crotty, 2003). In vitro, this process is involved in the retrograde blood flow from the lumen of the canine SV to its vasa vasorum (Crotty, 1989). Such a process might occur in no‐touch SV grafts and, if so, these inside‐to‐outside connections are possibly involved in the superior results obtained using this technique.
Bidirectional crosstalk between the vascular wall and PVAT
Based on the preceding data, the inside‐to‐outside signalling paradigm of Russel Ross might be re‐evaluated and improved. An attractive hypothesis is that PVAT dysfunction might originate in the lumen and at the endothelial/luminal surface, starting a vicious cycle of bidirectional crosstalk between the vessel wall and PVAT (Figure 4). There is increasing evidence for this in several scenarios.
Figure 4.
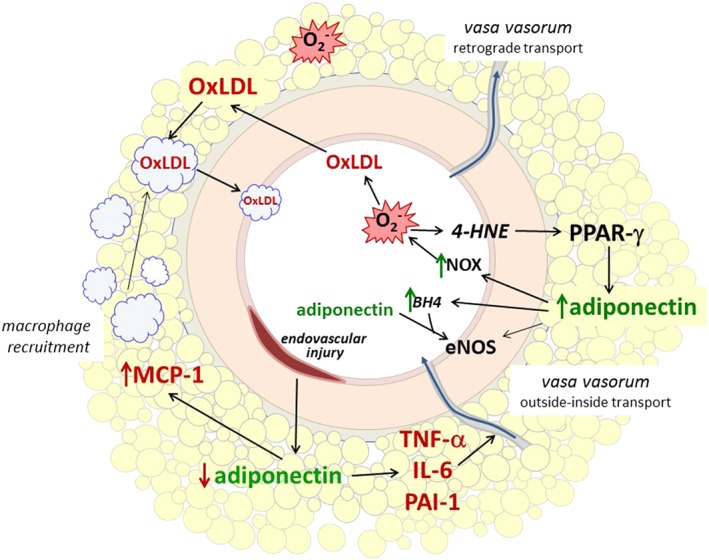
Bidirectional paradigm. Signals from the lumen and from the endothelial/luminal surface modulate PVAT function/dysfunction by the inside‐outside paradigm described by Russel Ross. Alterations in PVAT then have a paracrine beneficial/deleterious influence on the endothelium. 4‐HNE, 4‐hydroxynonenal; BH4, tetrahydrobiopterin; MCP‐1, monocyte chemoattractant protein; NOX, NADPH oxidase; O2 −, superoxide anion; PAI‐1, plasminogen activator inhibitor type 1.
Uchida et al. (2016) have suggested that macrophages from the systemic circulation enter the myocardium and the PCAT through neovascularized vasa vasorum, obtain oxLDL from the PCAT and transport it to the intima. After endothelial denudation of mice femoral and rat iliac arteries there is a significant down‐regulation in adiponectin and an up‐regulated expression of pro‐inflammatory cytokines in PVAT (Takaoka et al., 2009). This is followed by PPAR‐γ‐mediated phenotypic changes and the infiltration of inflammatory cells into PVAT that is dependent on vascular TNF‐α expression, which influences neointimal formation following vascular injury (Takaoka et al., 2010). This suggests that PVAT may protect against neointimal formation after angioplasty under physiological conditions and that inflammatory changes in the PVAT may have a direct role in the pathogenesis of vascular disease (Takaoka et al., 2009).
Two studies by Antonopoulos et al. (2015, 2016) further support bidirectional signalling in human vessels surrounded by PVAT. In SV and ITA, peroxidation products produced in the vascular wall [4‐hydroxynonenal (4‐HNE)] up‐regulate adiponectin gene expression in PVAT via PPAR‐γ. PVAT‐derived adiponectin improves NO production and reduces superoxide anions through a combined effect on eNOS activation (via PI3/Akt phosphorylation) and coupling (by increasing BH4 bioavailability) (Margaritis et al., 2013). Moreover, these authors showed that an increased level of NADPH oxidase‐derived superoxide anion in the arterial wall is correlated with increased adiponectin gene (ADIPOQ) expression in the PVAT surrounding it. Activation of NADPH oxidase in the ITA wall leads to the local production of oxidation products, that is, 4‐HNE up‐regulates PPAR‐γ‐mediated ADIPOQ expression in the neighbouring PVAT (Antonopoulos et al., 2015). These findings indicate that oxidation products released from the arterial wall may represent ‘rescue signals’ towards PVAT to increase the expression of adiponectin as a local mechanism for controling vascular NADPH oxidase activity.
In summary, there appears to be a more complex bidirectional communication between the arterial and venous wall and their surrounding PVAT than first realized. Signals originating from the vascular wall or lumen can affect PVAT structure and function, whereas signals from PVAT also influence structure and function of the vascular wall in a paracrine manner.
Conclusions and future perspectives
Taken together, the data presented in this review suggest that PVAT has a dual role depending on the blood vessel it surrounds and on the physiological/pathophysiological situation. Coronary PVAT has a deleterious influence in the development and progression of coronary artery disease, associated with inflammation, oxidative stress, angiogenesis, vascular remodelling and blood clotting. However, a protective role of PVAT is observed in vascular grafts, especially the no‐touch SV, increasing graft patency in patients undergoing CABG. These influences involve a complex bidirectional communication between the vascular wall and its surrounding PVAT, observations that require further analysis in different vessels and physiological/pathophysiological situations.
The characterization of deleterious PVAT‐derived factors and mechanisms associated with vascular disease development and progression provides a list of potential therapeutic targets (i.e. inflammatory cytokines and oxidant molecules) for designing novel therapeutic strategies. The most logical approach might be to shift the local imbalance of PVAT‐derived factors towards an increase in protective factors, such as ADRF, leptin and adiponectin, thus reducing oxidative stress and inflammation. However, the finding that oxidation products from the vascular wall may be ‘rescue signals’ targeted towards PVAT to increase adiponectin as a control mechanism of vascular NADPH oxidase (Antonopoulos et al., 2015) suggests any such treatment may be more complex. Indeed, in certain cases antioxidant therapy has been ineffective (Myung et al., 2013) or, in some situations, even detrimental (Gale et al., 1995).
It should be noted that most of these findings show an association between PVAT and both beneficial and deleterious influences on the vascular wall, although there is no current proof of causality. Therefore, there is no doubt that further experimental research is needed to mechanistically link PCAT as a cause of coronary artery disease, as well as further evidence that preservation of PVAT plays an important role in increased graft patency.
Without doubt, many questions regarding the bidirectional pathways and PVAT‐vascular wall interactions in health and disease remain unanswered. A deeper understanding of the pathological and physiological conditions is required in order to confirm whether these fat depots confer beneficial or detrimental effects on the vessel in question and whether therapeutic targeting should be luminal or abluminal.
Author contributions
M.G.‐O., I.A. and B.S. wrote the PCAT section and contributed to Figures 1 and 4. D.S. and M.D. wrote the SV section and contributed to Figures 2 and 3. M.S.F.‐A. and M.R.D. organized and supervised text and figures; wrote the final version of the manuscript.
Conflict of interest
The authors declare no conflicts of interest.
Acknowledgements
The authors' work is supported by BFU2011‐25303, GR921645‐Santander, Fundación Mutua Madrileña, Fundación Eugenio Rodriguez Pascual, SESCAMET. Early studies on the no‐touch technique were supported by a British Heart Foundation Project Grant to M R D and Dr J Tsui.
Fernández‐Alfonso, M. S. , Gil‐Ortega, M. , Aranguez, I. , Souza, D. , Dreifaldt, M. , Somoza, B. , and Dashwood, M. R. (2017) Role of PVAT in coronary atherosclerosis and vein graft patency: friend or foe?. British Journal of Pharmacology, 174: 3561–3572. doi: 10.1111/bph.13734.
Contributor Information
M S Fernández‐Alfonso, Email: marisolf@farm.ucm.es.
M R Dashwood, Email: m.dashwood@ucl.ac.uk.
References
- Aghamohammadzadeh R, Withers S, Lynch F, Greenstein A, Malik R, Heagerty A (2012). Perivascular adipose tissue from human systemic and coronary vessels: the emergence of a new pharmacotherapeutic target. Br J Pharmacol 165670–165682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed SR, Johansson BL, Karlsson MG, Souza DS, Dashwood MR, Loesch A (2004). Human saphenous vein and coronary bypass surgery: ultrastructural aspects of conventional and “no‐touch” vein graft preparations. Histol Histopathol 19: 421–433. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Catterall WA, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015a). The Concise Guide to PHARMACOLOGY 2015/16: Voltage‐gated ion channels. Br J Pharmacol 172: 5904–5941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Cidlowski JA, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015b). The Concise Guide to PHARMACOLOGY 2015/16: Nuclear hormone receptors. Br J Pharmacol 172: 5956–5978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Fabbro D, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015c). The Concise Guide to PHARMACOLOGY 2015/16: Enzymes. Br J Pharmacol 172: 6024–6109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonopoulos AS, Margaritis M, Coutinho P, Shirodaria C, Psarros C, Herdman L et al. (2015). Adiponectin as a link between type 2 diabetes and vascular NADPH oxidase activity in the human arterial wall: the regulatory role of perivascular adipose tissue. Diabetes 64: 2207–2219. [DOI] [PubMed] [Google Scholar]
- Antonopoulos AS, Margaritis M, Verheule S, Recalde A, Sanna F, Herdman L et al. (2016). Mutual regulation of epicardial adipose tissue and myocardial redox state by PPAR‐γ/adiponectin signalling. Circ Res 118: 842–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker SG, Talbert A, Cottam S, Baskerville PA, Martin JF (1993). Arterial intimal hyperplasia after occlusion of the adventitial vasa vasorum in the pig. Arterioscler Thromb 13: 70–77. [DOI] [PubMed] [Google Scholar]
- Barker SG, Tilling LC, Miller GC, Beesley JE, Fleetwood G, Stavri GT et al. (1994). The adventitia and atherogenesis: removal initiates intimal proliferation in the rabbit which regresses on generation of a ‘neoadventitia’. Atherosclerosis 105: 131–144. [DOI] [PubMed] [Google Scholar]
- Baron R, Dashwood MR, Arbeus M, Filbey D, Souza DSR (2010). Recent strategies to improve graft performance in patients undergoing coronary artery bypass surgery. Are best results achieved by improved surgical techniques of graft preparation? Advances in Vascular Medicine Springer, Chapter 21: 371–398. [Google Scholar]
- Bełtowski J (2013). Endogenous hydrogen sulfide in perivascular adipose tissue: role in the regulation of vascular tone in physiology and pathology. Can J Physiol Pharmacol 91: 889–898. [DOI] [PubMed] [Google Scholar]
- Benedetto U, Altman DG, Gerry S, Gray A, Lees B, Pawlaczyk R et al. (2016). Pedicled and skeletonized single and bilateral internal thoracic artery grafts and the incidence of sternal wound complications: Insights from the Arterial Revascularization Trial. J Thorac Cardiovasc Surg 152: 270–276. [DOI] [PubMed] [Google Scholar]
- Bulloch JM, Daly CJ (2014). Autonomic nerves and perivascular fat: interactive mechanisms. Pharmacol Ther 143: 61–73. [DOI] [PubMed] [Google Scholar]
- Chang L, Villacorta L, Li R, Hamblin M, Xu W, Dou C et al. (2012). Loss of perivascular adipose tissue on peroxisome proliferator‐activated receptor‐gamma deletion in smooth muscle cells impairs intravascular thermoregulation and enhances atherosclerosis. Circulation 126: 1067–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee TK, Aronow BJ, Tong WS, Manka D, Tang Y, Bogdanov VY et al. (2013). Human coronary artery perivascular adipocytes overexpress genes responsible for regulating vascular morphology, inflammation, and hemostasis. Physiol Genomics 45: 697–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crotty TP (1989). The path of retrograde flow from the lumen of the lateral saphenous vein of the dog to its vasa vasorum. Microvasc Res 37: 119–122. [DOI] [PubMed] [Google Scholar]
- Crotty TP (2003). The corrupted feedback hypothesis. Med Hypotheses 61: 605–616. [DOI] [PubMed] [Google Scholar]
- Dashwood MR, Angelini GD, Wan S, Yim A, Mehta D, Izzat MB et al. (2002). Does external stenting reduce porcine vein‐graft occlusion via an action on vascular nerves? J Card Surg 17: 556–560. [DOI] [PubMed] [Google Scholar]
- Dashwood MR, Dooley A, Shi‐Wen X, Abraham DJ, Dreifaldt M, Souza DS (2011). Perivascular fat‐derived leptin: a potential role in improved vein graft performance in coronary artery bypass surgery. Interact Cardiovasc Thorac Surg 12: 170–173. [DOI] [PubMed] [Google Scholar]
- Dashwood MR, Dooley A, Shi‐Wen X, Abraham DJ, Souza DS (2007). Does periadventitial fat‐derived nitric oxide play a role in improved saphenous vein graft patency in patients undergoing coronary artery bypass surgery? J Vasc Res 44: 175–181. [DOI] [PubMed] [Google Scholar]
- Dashwood MR, Gibbins R, Mehta D, Bashar Izzat M, Angelini GD, Jeremy JY (2000). Neural reorganisation in porcine vein grafts: a potential role for endothelin‐1. Atherosclerosis 150: 43–53. [DOI] [PubMed] [Google Scholar]
- Dashwood MR, Loesch A (2011). Does perivascular fat influence neural control of the saphenous vein? Implications in coronary artery bypass surgery. Current Neurobiology 2: 71–74. [Google Scholar]
- Dashwood MR, Savage K, Tsui JC, Dooley A, Shaw SG, Fernández Alfonso MS et al. (2009). Retaining perivascular tissue of human saphenous vein grafts protects against surgical and distension‐induced damage and preserves endothelial nitric oxide synthase and nitric oxide synthase activity. J Thorac Cardiovasc Surg 138: 334–340. [DOI] [PubMed] [Google Scholar]
- Dashwood MR, Tsui JC (2013). No‐touch' saphenous vein harvesting improves graft performance in patients undergoing coronary artery bypass surgery: a journey from bedside to bench. Vascul Pharmacol 58: 240–250. [DOI] [PubMed] [Google Scholar]
- de Vries MR, Quax PH (2016). Plaque angiogenesis and its relation to inflammation and atherosclerotic plaque destabilization (2016). Curr Opin Lipidol 27: 499–506. [DOI] [PubMed] [Google Scholar]
- Del Campo C (2003). Pedicled or skeletonized? A review of the ITA graft. Tex Heart Inst J 30: 170–175. [PMC free article] [PubMed] [Google Scholar]
- Dreifaldt M, Mannion JD, Bodin L, Olsson H, Zagozdzon L, Souza D (2013). The no‐touch saphenous vein as the preferred second conduit for coronary artery bypass grafting. Ann Thorac Surg 96: 105–111. [DOI] [PubMed] [Google Scholar]
- Dreifaldt M, Souza DS, Loesch A, Muddle JR, Karlsson MG, Filbey D et al. (2011). The “no‐touch” harvesting technique for vein grafts in coronary artery bypass surgery preserves an intact vasa vasorum. J Thorac Cardiovasc Surg 141: 145–150. [DOI] [PubMed] [Google Scholar]
- Farooq V, Serruys PW (2015). Bypass grafting versus percutaneous intervention‐which is better in multivessel coronary disease: lessons from SYNTAX and beyond. Prog Cardiovasc Dis 58: 316–334. [DOI] [PubMed] [Google Scholar]
- Favaloro RG (1969). Saphenous vein graft in the surgical treatment of coronary artery disease. Operative technique J Thorac Cardiovasc Surg 58: 178–185. [PubMed] [Google Scholar]
- Fernandez‐Alfonso MS (2004). Regulation of vascular tone: the fat connection. Hypertension 44: 255–256. [DOI] [PubMed] [Google Scholar]
- Fernández‐Alfonso MS, Gil‐Ortega M, García‐Prieto CF, Aranguez I, Ruiz‐Gayo M, Somoza B (2013). Mechanisms of perivascular adipose tissue dysfunction in obesity. Int J Endocrinol 402053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez‐Alfonso MS, Souza DS, Dreifaldt M, Dashwood MR (2016). Commentary: perivascular fat and improved vein graft patency in patients undergoing coronary artery bypass surgery (2016). Curr Vasc Pharmacol 14: 308–312. [DOI] [PubMed] [Google Scholar]
- Fitzgibbons TP, Czech MP (2014). Epicardial and perivascular adipose tissues and their influence on cardiovascular disease: basic mechanisms and clinical associations. J Am Heart Assoc 3 e000582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgibbons TP, Kogan S, Aouadi M, Hendricks GM, Straubhaar J, Czech MP (2011). Similarity of mouse perivascular and brown adipose tissues and their resistance to diet‐induced inflammation. Am J Physiol 301: H1425–H1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale CR, Martyn CN, Winter PD, Cooper C (1995). Vitamin C and risk of death from stroke and coronary heart disease in cohort of elderly people. BMJ 310: 1563–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gálvez‐Prieto B, Somoza B, Gil‐Ortega M, García‐Prieto CF, de Las Heras AI, González MC et al. (2012). Anticontractile effect of perivascular adipose tissue and leptin are reduced in hypertension. Front Pharmacol 3: 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao YJ, Zeng ZH, Teoh K, Sharma AM, Abouzahr L, Cybulsky I et al. (2005). Perivascular adipose tissue modulates vascular function in the human internal thoracic artery. J Thorac Cardiovasc Surg 130: 1130–1136. [DOI] [PubMed] [Google Scholar]
- Gaudiani VA, Buch WS, Chin AK, Ayres LJ, Fogarty TJ (1988). An improved technique for the internal mammary artery coronary bypass graft procedure. J Card Surg 3: 467–473. [DOI] [PubMed] [Google Scholar]
- George SJ, Channon KM, Baker AH (2006). Gene therapy and coronary artery bypass grafting: current perspectives. Curr Opin Mol Ther 8: 288–294. [PubMed] [Google Scholar]
- Gil‐Ortega M, Somoza B, Huang Y, Gollasch M, Fernández‐Alfonso MS (2015). Regional differences in perivascular adipose tissue impacting vascular homeostasis. Trends Endocrinol Metab 26: 367–375. [DOI] [PubMed] [Google Scholar]
- Gollasch M (2012). Vasodilator signals from perivascular adipose tissue. Br J Pharmacol 65: 633–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habib RH, Abou‐Arraj NE, Schwann TA (2013). Radial artery as a second arterial graft in the elderly and both sexes. Ann Cardiothorac Surg 2: 453–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirose H, Amano A, Takahashi A, Takanashi S (2003). Skeletonization of the radial artery with the ultrasonic scalpel: clinical and angiographic results. Heart Surg Forum 6: E42–E47. [DOI] [PubMed] [Google Scholar]
- Iacobellis G (2014). Epicardial adipose tissue in endocrine and metabolic diseases. Endocrine 46: 8–15. [DOI] [PubMed] [Google Scholar]
- Iacobellis G (2015). Local and systemic effects of the multifaceted epicardial adipose tissue depot. Nat Rev Endocrinol 11: 363–371. [DOI] [PubMed] [Google Scholar]
- Iacobellis G, Assael F, Ribaudo MC et al. (2003). Epicardial fat from echocardiography: a new method for visceral adipose tissue prediction. Obes Res 11: 304–310. [DOI] [PubMed] [Google Scholar]
- Ishii T, Asuwa N, Masuda S, Ishikawa Y (1998). The effects of a myocardial bridge on coronary atherosclerosis and ischaemia. J Pathol 185: 4–9. [DOI] [PubMed] [Google Scholar]
- Ishikawa Y, Akasaka Y, Ito K et al. (2006). Significance of anatomical properties of myocardial bridge on atherosclerosis evolution in the left anterior descending coronary artery. Atherosclerosis 186: 380–389. [DOI] [PubMed] [Google Scholar]
- Izzat MB, West RR, Bryan AJ, Angelini GD (1994). Coronary artery bypass surgery: current practice in the United Kingdom. Br Heart J 71: 382–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong JW, Jeong MH, Yun KH, Oh SK, Park EM, Kim YK et al. (2007). Echocardiographic epicardial fat thickness and coronary artery disease. Circ J 71: 536–539. [DOI] [PubMed] [Google Scholar]
- Kawahito H, Yamada H, Irie D, Kato T, Akakabe Y, Kishida S et al. (2013). Periaortic adipose tissue‐specific activation of the renin‐angiotensin system contributes to atherosclerosis development in uninephrectomized apoE−/− mice. Am J Physiol Heart Circ Physiol 305: H667–H675. [DOI] [PubMed] [Google Scholar]
- Konstantinov IE (2000). Robert H. Goetz: the surgeon who performed the first successful clinical coronary bypass operation. Ann Thorac Surg 69: 1966–1972. [DOI] [PubMed] [Google Scholar]
- Lanza GA, Careri G, Crea F (2011). Mechanisms of coronary artery spasm. Circulation 124: 1774–1782. [DOI] [PubMed] [Google Scholar]
- Lescalié F, Germouty I, Chevalier JM, Moreau P, Pillet J (1986). Extrinsic arterial supply of the great saphenous vein: an anatomic study. Ann Vasc Surg 2: 273–277. [DOI] [PubMed] [Google Scholar]
- Libby P, Ridker PM, Maseri A (2002). Inflammation and atherosclerosis. Circulation 105: 1135–1143. [DOI] [PubMed] [Google Scholar]
- Lu C, Su LY, Lee RM, Gao YJ (2010). Mechanisms for perivascular adipose tissue‐mediated potentiation of vascular contraction to perivascular neuronal stimulation: the role of adipocyte‐derived angiotensin II. Eur J Pharmacol 634: 107–112. [DOI] [PubMed] [Google Scholar]
- Lu MT, Park J, Ghemigian K, Mayrhofer T, Puchner SB, Liu T et al. (2016). Epicardial and paracardial adipose tissue volume and attenuation – Association with high‐risk coronary plaque on computed tomographic angiography in the ROMICAT II trial. Atherosclerosis 251: 47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lytle BW, Loop FD, Cosgrove DM, Ratliff NB, Easley K, Taylor PC (1985). Long‐term (5 to 12 years) serial studies of internal mammary artery and saphenous vein coronary bypass grafts. J Thorac Cardiovasc Surg 89: 248–258. [PubMed] [Google Scholar]
- Mahabadi AA, Berg MH, Lehmann N, Kälsch H, Bauer M, Kara K et al. (2013). Association of epicardial fat with cardiovascular risk factors and incident myocardial infarction in the general population: the Heinz Nixdorf recall study. Am Coll Cardiol 61: 1388–1395. [DOI] [PubMed] [Google Scholar]
- Malinowski M, Deja MA, Gołba KS, Roleder T, Biernat J, Woś S (2008). Perivascular tissue of internal thoracic artery releases potent nitric oxide and prostacyclin‐independent anticontractile factor. Eur J Cardiothorac Surg 33: 225–231. [DOI] [PubMed] [Google Scholar]
- Malinowski M, Deja MA, Janusiewicz P, Golba KS, Roleder T, Wos S (2013). Mechanisms of vasodilatatory effect of perivascular tissue of human internal thoracic artery. J Physiol Pharmacol 64: 309–316. [PubMed] [Google Scholar]
- Margaritis M, Antonopoulos AS, Digby J, Lee R, Reilly S, Coutinho P et al. (2013). Interactions between vascular wall and perivascular adipose tissue reveal novel roles for adiponectin in the regulation of endothelial nitric oxide synthase function in human vessels. Circulation 127: 2209–2221. [DOI] [PubMed] [Google Scholar]
- Mazurek T, Zhang L, Zalewski A, Mannion JD, Diehl JT, Arafat H et al. (2003). Human epicardial adipose tissue is a source of inflammatory mediators. Circulation 108: 2460–2466. [DOI] [PubMed] [Google Scholar]
- Mazurek T, Opolski G (2015). Pericoronary adipose tissue: a novel therapeutic target in obesity‐related coronary atherosclerosis. J Am Coll Nutr 34: 244–254. [DOI] [PubMed] [Google Scholar]
- Mehta D, Izzat MB, Bryan AJ, Angelini GD (1997). Towards the prevention of vein graft failure. Int J Cardiol 62: S55–S63. [DOI] [PubMed] [Google Scholar]
- Miyata K, Shimokawa H, Kandabashi T et al. (2000). Rho‐kinase is involved in macrophage‐mediated formation of coronary vascular lesions in pigs in vivo. Arterioscler Thromb Vasc Biol 20: 2351–2358. [DOI] [PubMed] [Google Scholar]
- Momin AU, Melikian N, Shah AM, Grieve DJ, Wheatcroft SB, John L et al. (2006). Leptin is an endothelial‐independent vasodilator in humans with coronary artery disease: evidence for tissue specificity of leptin resistance. Eur Heart J 27: 2294–2299. [DOI] [PubMed] [Google Scholar]
- Myung SK, Ju W, Cho B, Oh SW, Park SM, Koo BK et al. (2013). Efficacy of vitamin and antioxidant supplements in prevention of cardiovascular disease: systematic review and meta‐analysis of randomised controlled trials. BMJ 346: f10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimiya K, Matsumoto Y, Takahashi J, Uzuka H, Hongxin W, Tsuburaya R et al. (2016). Enhanced adventitial vasa vasorum formation in patients with vasospastic angina: assessment with OFDI. J Am Coll Cardiol 67: 598–600. [DOI] [PubMed] [Google Scholar]
- Noblet JN, Owen MK, Goodwill AG, Sassoon DJ, Tune JD (2015). Lean and obese coronary perivascular adipose tissue impairs vasodilation via differential inhibition of vascular smooth muscle K+ channels. Arterioscler Thromb Vasc Biol 35: 1393–1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohyama K, Matsumoto Y, Nishimiya K, Hao K, Tsuburaya R, Ota H et al. (2016). Increased coronary perivascular adipose tissue volume in patients with vasospastic angina. Circ J 80: 1653–1656. [DOI] [PubMed] [Google Scholar]
- Okamoto E, Couse T, De Leon H, Vinten‐Johansen J, Goodman RB, Scott NA et al. (2001). Perivascular inflammation after balloon angioplasty of porcine coronary arteries. Circulation 104: 2228–2235. [DOI] [PubMed] [Google Scholar]
- Omar A, Chatterjee TK, Tang Y, Hui DY, Weintraub NL (2014). Proinflammatory phenotype of perivascular adipocytes. Arterioscler Thromb Vasc Biol 34: 1631–1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen MK, Witzmann FA, McKenney ML, Lai X, Berwick ZC, Moberly SP et al. (2013). Perivascular adipose tissue potentiates contraction of coronary vascular smooth muscle: influence of obesity. Circulation 128: 9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozen G, Topal G, Gomez I, Ghorreshi A, Boukais K, Benyahia C et al. (2013). Control of human vascular tone by prostanoids derived from perivascular adipose tissue. Prostaglandins Other Lipid Mediat 107: 13–17. [DOI] [PubMed] [Google Scholar]
- Prati F, Arbustini E, Labellarte A, Sommariva L, Pawlowski T, Manzoli A et al. (2003). Eccentric atherosclerotic plaques with positive remodelling have a pericardial distribution: a permissive role of epicardial fat? A three‐dimensional intravascular ultrasound study of left anterior descending artery lesions. Eur Heart J 24: 329–336. [DOI] [PubMed] [Google Scholar]
- Prescott MF, McBride CK, Court M (1989). Development of intimal lesions after leukocyte migration into the vessel wall. Am J Pathol 135: 835–846. [PMC free article] [PubMed] [Google Scholar]
- Rabkin SW (2007). Epicardial fat: properties, function and relationship to obesity. Obes Rev 8: 253–261. [DOI] [PubMed] [Google Scholar]
- Ross R (1999). Atherosclerosis—an inflammatory disease. N Engl J Med 340: 115–126. [DOI] [PubMed] [Google Scholar]
- Sabik JF III (2011). Understanding saphenous vein graft patency. Circulation 124: 273–275. [DOI] [PubMed] [Google Scholar]
- Sacks HS, Fain JN (2007). Human epicardial adipose tissue: a review. Am Heart J 153: 907–917. [DOI] [PubMed] [Google Scholar]
- Salgado‐Somoza A, Teijeira‐Fernández E, Fernández AL, González‐Juanatey JR, Eiras S (2010). Proteomic analysis of epicardial and subcutaneous adipose tissue reveals differences in proteins involved in oxidative stress. Am J Physiol Heart Circ Physiol 299: H202–H209. [DOI] [PubMed] [Google Scholar]
- Samano N, Geijer H, Liden M, Fremes S, Bodin L, Souza D (2015). The no‐touch saphenous vein for coronary artery bypass grafting maintains a patency, after 16 years, comparable to the left internal thoracic artery: a randomized trial. J Thorac Cardiovasc Surg 150: 880–888. [DOI] [PubMed] [Google Scholar]
- Shen L, Evans IM, Souza D, Dreifaldt M, Dashwood MR, Vidya MA (2016). Adiponectin: an endothelium‐derived vasoprotective factor? Curr Vasc Pharmacol 14: 168–174. [DOI] [PubMed] [Google Scholar]
- Shields KJ, Barinas‐Mitchell E, Gingo MR, Tepper P, Goodpaster BH, Kao AH et al. (2013). Perivascular adipose tissue of the descending thoracic aorta is associated with systemic lupus erythematosus and vascular calcification in women. Atherosclerosis 231: 129–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimokawa H, Ito A, Fukumoto Y, Kadokami T, Nakaike R, Sakata M et al. (1996). Chronic treatment with interleukin‐1β induces coronary intimal lesions and vasospastic responses in pigs in vivo: the role of platelet‐derived growth factor. J Clin Invest 97: 769–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southan C, Sharman JL, Benson HE, Faccenda E, Pawson AJ, Alexander SP et al. (2016). The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucl Acids Res 44: D1054–D1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza DS, Dashwood MR, Tsui JC, Filbey D, Bodin L, Johansson B et al. (2002). Improved patency in vein grafts harvested with surrounding tissue: results of a randomized study using three harvesting techniques. Ann Thorac Surg 73: 1189–1195. [DOI] [PubMed] [Google Scholar]
- Souza DS, Johansson B, Bojö L, Karlsson R, Geijer H, Filbey D et al. (2006). Harvesting the saphenous vein with surrounding tissue for CABG provides long‐term graft patency comparable to the left internal thoracic artery: results of a randomized longitudinal trial. J Thorac Cardiovasc Surg 132: 373–378. [DOI] [PubMed] [Google Scholar]
- Souza DSR (1996). A new no‐touch preparation technique. Technical notes. Scand J Thor Cardiovasc Surg 30: 41–44. [DOI] [PubMed] [Google Scholar]
- Souza DSR, Arbeus M, Botelho Pinheiro B, Filbey D (2008). The no‐touch technique of harvesting the saphenous vein for coronary artery bypass grafting surgery. Multimedia Manual Cardiothor Surg 731:mmcts.2008.003624. [DOI] [PubMed] [Google Scholar]
- Szasz T, Bomfim GF, Webb RC (2013). The influence of perivascular adipose tissue on vascular homeostasis. Vasc Health Risk Manag 9: 105–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takaoka M, Nagata D, Kihara S, Shimomura I, Kimura Y, Tabata Y et al. (2009). Periadventitial adipose tissue plays a critical role in vascular remodeling. Circ Res 105: 906–911. [DOI] [PubMed] [Google Scholar]
- Takaoka M, Suzuki H, Shioda S, Sekikawa K, Saito Y, Nagai R et al. (2010). Endovascular injury induces rapid phenotypic changes in perivascular adipose tissue. Arterioscler Thromb Vasc Biol 30: 1576–1582. [DOI] [PubMed] [Google Scholar]
- Tano JY, Schleifenbaum J, Gollasch M (2014). Perivascular adipose tissue, potassium channels, and vascular dysfunction. Arterioscler Thromb Vasc Biol 34: 1827–1830. [DOI] [PubMed] [Google Scholar]
- Tsui JC, Dashwood MR (2002). Recent strategies to reduce vein graft occlusion: a need to limit the effect of vascular damage. Eur J Vasc Endovasc Surg 23: 202–208. [DOI] [PubMed] [Google Scholar]
- Tsui JC, Souza DS, Filbey D, Bomfim V, Dashwood MR (2001). Preserved endothelial integrity and nitric oxide synthase in saphenous vein grafts harvested by a ‘no‐touch’ technique. Br J Surg 88: 1209–1215. [DOI] [PubMed] [Google Scholar]
- Uchida Y, Uchida Y, Shimoyama E, Hiruta N, Kishimoto T, Watanabe S (2016). Human pericoronary adipose tissue as storage and possible supply site for oxidized low‐density lipoprotein and high‐density lipoprotein in coronary artery. J Cardiol pii: S0914‐5087(16)30042‐9. [DOI] [PubMed] [Google Scholar]
- Vasilakis V, Dashwood MR, Souza DS, Loesch A (2004). Human saphenous vein and coronary bypass surgery: scanning electron microscopy of conventional and “no‐touch” vein grafts. Vasc Dis Prev 1: 133–139. [Google Scholar]
- World Health Organization factsheet , (2016). http://www.who.int/mediacentre/factsheets/fs317/en/
- Yudkin JS, Eringa E, Stehouwer CD (2005). “Vasocrine” signalling from perivascular fat: a mechanism linking insulin resistance to vascular disease. Lancet 365: 1817–1820. [DOI] [PubMed] [Google Scholar]


