Abstract
Background and Purpose
Perivascular adipose tissue (PVAT) surrounds most blood vessels and secretes numerous active substances, including adiponectin, which produce a net anticontractile effect in healthy individuals. AMPK is a key mediator of cellular energy balance and may mediate the vascular effects of adiponectin. In this study, we investigated the role of AMPK within PVAT in mediating the anticontractile effect of PVAT.
Experimental Approach
Endothelium‐denuded aortic rings from wild‐type (WT; Sv129) and α1AMPK knockout (KO) mice were mounted on a wire myograph. Dose–response curves to the AMPK‐independent vasodilator cromakalim were studied in vessels with and without PVAT, and effect of pre‐incubation with conditioned media and adiponectin on relaxation was also studied. The effect of AMPKα1 KO on the secretory profile of PVAT was assessed by elisa.
Key Results
Thoracic aortic PVAT from KO mice was morphologically indistinct from that of WT and primarily composed of brown adipose tissue. PVAT augmented relaxation to cromakalim in WT but not KO aortic rings. Addition of WT PVAT augmented relaxation in KO aortic rings but KO PVAT had no effect in WT rings. PVAT from KO mice secreted significantly less adiponectin and addition of adiponectin to either KO or WT aortic rings without PVAT augmented relaxation to cromakalim. An adiponectin blocking peptide significantly attenuated relaxation in WT rings with PVAT but not in KO rings.
Conclusions and Implications
AMPKα1 has a critical role in maintaining the anticontractile actions of PVAT; an effect independent of the endothelium but likely mediated through altered adiponectin secretion or sensitivity.
Linked Articles
This article is part of a themed section on Molecular Mechanisms Regulating Perivascular Adipose Tissue – Potential Pharmacological Targets? To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v174.20/issuetoc
Abbreviations
- ACC
Acetyl CoA carboxylase
- ADRF
adventitia‐derived relaxing factor
- AMPK
AMP‐activated protein kinase
- BAT
brown adipose tissue
- CM
conditioned media
- KO
knockout
- PVAT
perivascular adipose tissue
- UCP1
uncoupling protein‐1
- VSMCs
vascular smooth muscle cells
- WAT
white adipose tissue
- WT
wild type
Tables of Links
| TARGETS | |
|---|---|
| Other protein targets a | Enzymes b |
| Adipo1 receptor | ACC |
| Uncoupling protein‐1 (UCP1) | AMPK |
| LIGANDS | |
|---|---|
| Adiponectin | Cromakalim |
| AICAR (acadesine) | U46619 |
These Tables list key protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Southan et al., 2016) and are permanently archived in the Concise Guide to PHARMACOLOGY 2015/16 (a,bAlexander et al., 2015a,b).
Introduction
Perivascular adipose tissue (PVAT) surrounds almost all blood vessels and is increasingly recognized as an important regulator of vascular tone. As an active endocrine organ, the adipocytes and stromal cells within PVAT produce and secrete a range of adipokines, inflammatory cytokines and other factors, which can influence the tone of the underlying blood vessel (Dubrovska et al., 2004; Malinowski et al., 2008; Weston et al., 2013). PVAT can release not only substances with vasodilator activity such as adipocyte‐derived relaxing factor (Lohn et al., 2002; Verlohren et al., 2004; Galvez et al., 2006), leptin (Vecchione et al., 2002), adiponectin (Chen et al., 2003; Fesus et al., 2007), angiotensin 1–7 (Ang 1–7) (Lee et al., 2009), hydrogen peroxide (Gao et al., 2007), NO (Gil‐Ortega et al., 2010) and hydrogen sulphide (H2S) (Fang et al., 2009) but also vasoconstrictor factors such as Ang II (Galvez‐Prieto et al., 2008) and superoxide anions (Gao et al., 2006). Consequently, a balance exists and much research effort has been channelled into discovering what causes the function of PVAT to change in disease states such as obesity and metabolic syndrome where PVAT has a deleterious effect on blood vessel activity.
PVAT is composed of brown adipocytes, white adipocytes or a mixture of both depending on the vascular bed (Gao, 2007; Cinti, 2011; Fitzgibbons et al., 2011). In healthy humans and experimental animals, PVAT has a net anticontractile effect (Lohn et al., 2002; Greenstein et al., 2009), but the precise mechanisms remain elusive. The anti‐contractile effect is probably due to the release of transmissible factors; a process that requires calcium but is not dependent on perivascular nerve activity (Dubrovska et al., 2004). In rat mesenteric vessels, the anticontractile effect of PVAT is dependent on the activation of delayed rectifier potassium channels (KV) in the vascular smooth muscle (Verlohren et al., 2004), while in rat aortic rings, Gao et al. found that a PVAT‐derived transmissible factor induced relaxation by: (i) an endothelium‐dependent effect via NO release and subsequent KCa channel activation, and (ii) an endothelium‐independent mechanism involving H2O2 and subsequent activation of soluble guanylyl cyclase (sGC; Gao et al., 2007). More recent work has identified adiponectin as an abundant adipokine with anticontractile activity. In mouse mesenteric vessels and rat aorta, adiponectin was found to relax vascular smooth muscle cells (VSMCs) via Kv channel opening and membrane hyperpolarisation (Fesus et al., 2007). However, other studies using mouse mesenteric vessels found that adiponectin requires the presence of large conductance calcium‐activated potassium channels (BKCa) in order to hyperpolarise VSMCs and exert an anticontractile effect (Lynch et al., 2013). Interestingly, a recent study by Weston's group has shown that adiponectin‐mediated hyperpolarisation is inhibited by a selective inhibitor of AMP‐activated protein kinase (AMPK) and that this effect of adiponectin is mimicked by the AMPK activator, A769662 (Weston et al., 2013). Activation of AMPK is proposed to trigger the opening of myocyte BKCa channels and the release of NO, which accounts for the anticontractile effect of adiponectin. Indeed, in mice lacking the AMPKα2 catalytic subunit isoform, globular adiponectin failed to induce vascular relaxation (Meijer et al., 2013) suggesting that adiponectin requires AMPK to exert an anticontractile effect.
Although AMPK is often described as a cellular energy gauge and modulator of cellular metabolism (Hardie et al., 2003), it also has important roles in maintaining vascular homeostasis (Ewart and Kennedy, 2011). It is expressed throughout the vessel wall and can respond to changes in cellular energy state (Evans et al., 2005; Fleming et al., 2005), hormonal changes (Nagata et al., 2004; Cheng et al., 2007) and drugs (Levine et al., 2007; Bilodeau‐Goeseels et al., 2011; Ford et al., 2012) to regulate vascular tone. AMPK activation can induce vasodilatation via phosphorylation and activation of endothelial NOS (eNOS) at Ser1177 (Morrow et al., 2003; Davis et al., 2006) and Ser633 (Chen et al., 2009) to increase NO production and vascular relaxation (Morrow et al., 2003; Davis et al., 2006). AMPK can also induce endothelium‐independent relaxation by reducing the sensitivity of myosin light‐chain kinase (MLCK) to intracellular calcium (Horman et al., 2008). Collectively, these findings suggest that AMPK activation can not only induce vasodilatation but may also be involved in the vascular effects of PVAT. However, despite AMPKα1 being the dominant vascular isoform, nothing is known about its role in mediating the anticontractile effect of PVAT.
The aim of this study was to investigate aortic PVAT function in a mouse with a global AMPKα1 isoform knockout (KO). Furthermore, investigated whether a lack of this AMPK isoform in the vascular wall affects the generation and vascular effects of adiponectin.
Methods
Animal model and artery preparation
Mice used in this study were housed at the University of Glasgow and maintained on 12 h cycles of light and dark and at ambient temperature. Wild type (Sv129‐WT) mice were originally purchased from Harlan Laboratories (Oxon, UK). AMPKα1 KO mice were kindly supplied by Benoit Viollet (Institut Cochin, Paris, France), the generation of which has been described previously (Jørgensen et al., 2004). In all experiments, age‐matched male and female WT and KO mice were used since pilot experiments showed no gender difference in vessel contractility and relaxation (data not shown). Procedures conformed to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85‐23, revised 1996) and Directive 2010/63/EU of the European Parliament. Mice were terminally anaesthetized by an i.p. injection of sodium pentobarbital (200 mg·mL−1), and the thoracic and abdominal aorta was removed to ice‐cold oxygenated (95% O2:5% CO2) Krebs solution. In some experiments, mesenteric and other fat depots were also removed for histological analysis. Animal studies are reported in compliance with the ARRIVE guidelines (Kilkenny et al., 2010; McGrath and Lilley, 2015).
Histological analysis
Thoracic aortae with intact PVAT were fixed overnight in 10% acetic zinc formalin, dehydrated and embedded in paraffin. Sections (5 μm) of aorta were cut on a rotary microtome and stained with haematoxylin and eosin (H&E). To compare the morphology of aortic PVAT with other fat depots, mesenteric PVAT, subscapular brown adipose tissue (BAT) and epididymal white adipose tissue (WAT) were also fixed, sectioned and stained with H&E.
Immunohistochemistry was used to detect antigens of interest within the PVAT. Briefly, slides were deparaffinised and antigens were retrieved by heating in a microwave oven in sodium citrate buffer [10 mM Na citrate, 0.05% (v v−1) Tween 20, pH 6.0] for 10 min. Slides were cooled at room temperature and endogenous peroxidase activity blocked by immersing in 3% (v v−1) H2O2 in methanol for 20 min. Non‐specific antibody binding was blocked using 2.5% (v v−1) normal horse serum (ImmPRESS Reagent Kit, Vector labs, USA) for 1 h at room temperature and primary antibodies were then added overnight at 4°C. Primary antibodies were diluted in 1% (w v−1) BSA in PBS and used at the following concentrations: anti‐total AMPK 1:100 (Abcam #131 512), anti‐phospho‐AMPK Thr172 1:100 (Cell Signalling Technology #2535), anti‐uncoupling protein‐1 (UCP1) 1:500 (Abcam #10 983). Blanks and negative controls were included in each staining run. Secondary antibodies were incubated for 1 h at room temperature (ImmPRESS anti‐rabbit Ig antibodies; Vector Labs, USA for UCP1 or biotinylated anti‐rabbit IgG antibody from Histostain‐Plus bulk kit, Life technologies, UK for total and phosphor‐AMPK). Antibody binding was visualized using DAB (3,3′ diaminobenzidine) chromogenic substrate (Vector Laboratories) and haematoxylin counter stain. Sections were photographed using AxioVision microscope software (Zeiss, Germany).
Small vessel wire myography
The thoracic and, in some experiments the abdominal aorta, was cut into 2 mm rings. Some rings were cleaned of PVAT, and others were left with the PVAT intact. In all cases, the endothelium on the luminal surface was removed by gently rubbing the interior of the vessel with a piece of fine wire and removal confirmed by lack of (<10%) vasodilator response to 10−6 M acetylcholine. Artery rings were mounted on two stainless steel pins in a four channel wire myograph (Danish Myo Technology), set to an optimum tension of 9.8 mN (Weingartner et al., 2015) and allowed to equilibrate for at least 30 min before use. Vessels were bathed in Krebs buffer of the following composition: 118 mM NaCl, 4.7 mM KCl, 1.2 mM MgSO4, 25 mM NaHCO3, 1.03 mM KH2PO4, 11 mM glucose and 2.5 mM CaCl2 at 37°C and gassed continuously with 95% O2 and 5% CO2. Reproducible responses were obtained to 40 mM KCl and 30 nM 9,11‐dideoxy‐9α,11α‐methanoepoxy PGF2α (U46619, Tocris) before commencing experiments. Cumulative concentration–response curves to the K+ channel opener cromakalim (1 × 10−9 to 1 × 10−6 M; Sigma‐Aldrich, Poole, UK), added at 10 min intervals were constructed. Data are expressed as a percentage loss of U46619‐induced tone.
To produce conditioned media (CM), thoracic aortic PVAT from WT and KO mice was carefully dissected, weighed and incubated in warmed Krebs solution at 37°C for 1 h. The conditioned medium was transferred to the recipient myograph chamber containing an aortic ring at baseline tension. The ring was then contracted and dose–response curves to cromakalim constructed as described previously. In some experiments, PVAT from KO mice was added to WT aortic rings and vice versa (termed a cross‐over experiment).
To study the role of adiponectin in the anticontractile effect of PVAT, two separate experiments were performed. Firstly, 5 μg·mL−1 of an adiponectin blocking peptide against adiponectin receptor 1 (Adipo1 receptor; GeneTex, UK) was added to preconstricted aortic rings with and without PVAT. Secondly, globular adiponectin (1 μg·mL−1 diluted in 1% (w v−1) BSA; Enzo Life Sciences Ltd, UK) was added to arteries prior to contracting with U46619. In both cases, dose–response curves to cromakalim were then constructed in WT and KO aortic rings.
Effect of cromakalim on AMPK activity in cultured cells
Rat aortic smooth muscle cells from Wistar–Kyoto rats were provided by Dr Augusto Montezano (University of Glasgow) and maintained in DMEM supplemented with 10% (v v−1) FBS (Invitrogen, UK), 100 U·mL−1 pencillin and 100 μg·mL−1 streptomycin. At passage 4 to 5, the cells were washed with PBS and incubated in serum‐free medium for 2 h. Cells were incubated with the AMPK‐activating agent 5‐aminoimidazole‐4‐carboxamide ribonucleoside (AICAR; 10−2 to 10−3 M) or cromakalim (10−8 to 10−6 M) for 45 min, the medium was removed, lysis buffer was added [50 mM Tris pH 7.4, 50 mM NaF, 1 mM Na4P2O7, 1 mM EGTA, 1 mM EDTA, 1% (v v−1) Triton X‐100, 1 mM DTT and 1% cocktail of protease inhibitors with 2 mM Na3VO4] and the cells were collected by scraping. Lysates were placed on ice for 30 min, centrifuged at 6.2× g for 10 min, and supernatants were collected and used for immunoblotting.
Protein expression/immunoblotting
The protein content of VSMC lysates (or in some experiments PVAT lysates or CM samples) was determined using Coomassie Plus Protein Assay Reagent (Perbio, USA) against a BSA standard curve. Samples were run on NuPAGE Novex 4–12% Bis‐Tris mini gels (Life Technologies), transferred to nitrocellulose membranes and incubated with specific rabbit anti‐total AMPKα (Cell Signalling Technology #2603), anti‐AMPKα1 (Abcam #ab110036), anti‐AMPKα2 (Generous gift from Prof. Grahame Hardie, University of Dundee, UK), anti‐phospho‐Thr172 AMPK (Cell Signalling Technology #2535) and anti‐phospho‐Ser79 acetyl CoA carboxylase (ACC) (Cell Signalling Technology #3661) antibodies. All primary antibodies were diluted 1:1000 in 50% (v v−1) TBS, 50% (v v−1) Odyssey®‐Block (LI‐COR, USA). Immunolabelled proteins were visualised using infrared dye‐labelled secondary antibodies and an Odyssey Sa Infrared Imaging System (LI‐COR, USA) and expression was normalized to anti‐GAPDH antibody immunoreactivity (Cell Signalling Technology).
Array and adiponectin elisa
Adipokine expression profiling was performed using an adipokine proteome profiler, (R&D systems, UK) following the protocol provided by the manufacturer. Briefly, 1 mL of pooled samples of homogenized PVAT or conditioned medium from WT and KO mice were added to the array membranes and incubated at 4°C overnight. After being washed, membranes were incubated with 2 mL of horseradish peroxidase‐conjugated streptavidin at room temperature for 30 min and the presence of adipokines were detected by chemiluminescence. The resultant film images were scanned with a densitometer and converted to densitometric units using Quantity One software (Bio‐Rad Laboratories, Hercules, CA, USA). Data were normalised against an internal control as recommended by the manufacturer.
To study adiponectin release by PVAT, a mouse adiponectin/Acrp30 Quantikine elisa Kit (MRP300, R&D systems, Minneapolis, MN) was used. Samples of homogenized PVAT and CM from WT and KO mice were prepared and protein concentration determined. Adiponectin was detected as a colourimetric reaction measuring absorbance of the elisa plate at 450 nm with wavelength correction using a FLUOstar OPTIMA microplate reader (BMG Labtech, Germany). The mean absorbance from each sample was measured in duplicate and the adiponectin concentration was determined by comparison with the standard curve.
Statistical analysis
All results are expressed as mean ± SEM. The n number stated in all cases represents the number of mice from which tissue was obtained. Data were analysed with GraphPad Prism 5.0 software. Myography data were analysed using two‐way ANOVA. When comparing three or more data groups, two‐way ANOVA followed by Bonferroni post hoc tests were used. When comparing two or more data groups, a Newman–Keuls post hoc test was used. In all cases, a P value of less than 0.05 was considered statistically significant. The data and statistical analysis comply with the recommendations on experimental design and analysis in pharmacology (Curtis et al., 2015).
Results
PVAT morphology and AMPK expression
In haematoxylin and eosin stained sections, there were no gross differences in PVAT or other fat depots between WT and AMPKα1 KO mice (Figure 1). In both strains, thoracic PVAT had the appearance of BAT with round nuclei, and small, multilocular lipid droplets, whereas mesenteric PVAT was very similar to WAT, with large single lipid vacuoles and marginal nuclei. Abdominal PVAT showed features of both BAT and WAT. Immunohistochemical staining for the BAT marker UCP1 was similar in WT and KO aortic PVAT with very low staining in mesenteric fat and some staining present in abdominal aortic PVAT (Supplementary Figures S1 and S2).
Figure 1.
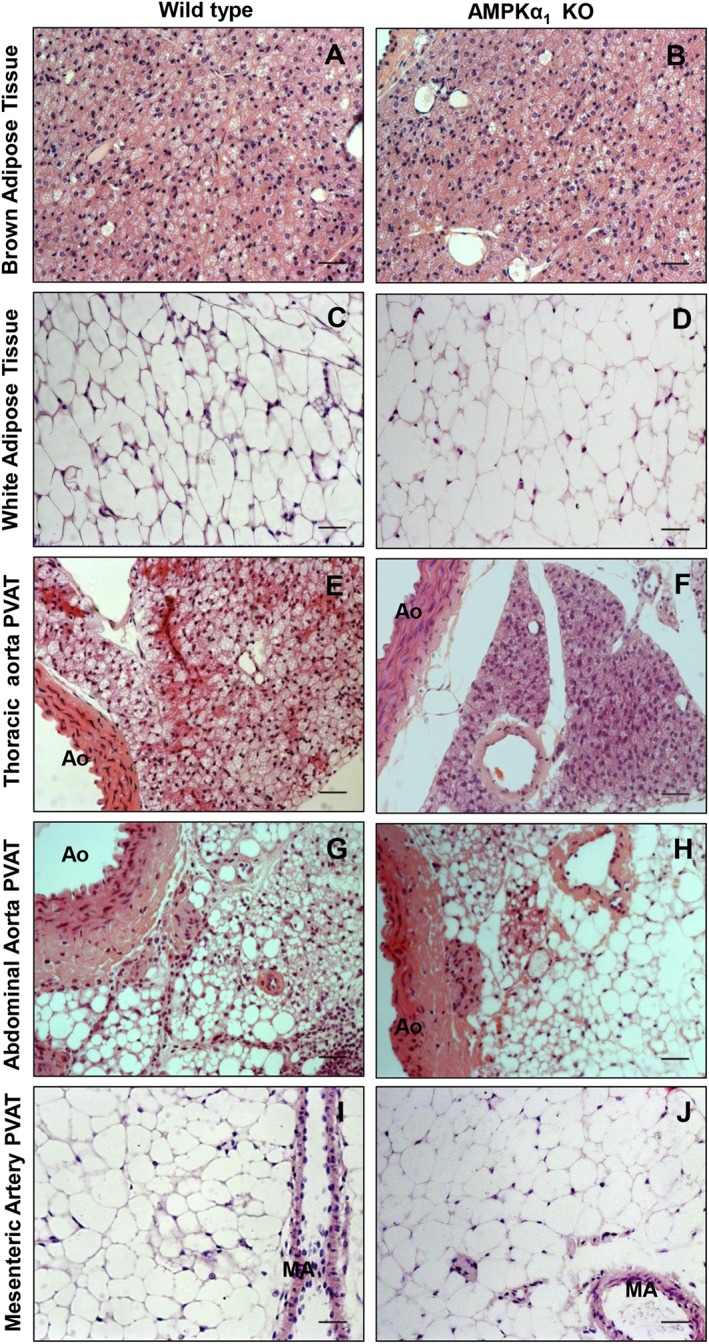
Representative photomicrographs showing the morphology of fat depots in WT and AMPKα1 KO mice. (A and B) Subscapular BAT; (C and D) epididymal WAT; (E and F) thoracic PVAT; (G and H) abdominal PVAT; and (I and J) mesenteric PVAT. Aortic PVAT had the morphology of BAT. AO = aorta, MA = mesenteric artery, scale bar = 20 μm.
AMPKα and phospho‐AMPKα (pAMPKα) were detected immunohistochemically in thoracic PVAT, and there was a marked reduction in both total and phospho‐AMPKα Thr172 in KO mice (data not shown). Western blotting was used to quantify AMPK levels in homogenized PVAT. As expected, there was a marked reduction in total levels of AMPKα, phospho‐AMPKα Thr172 and phospho‐ACC Ser79 (pACC). When expressed relative to total AMPKα, there was a modest reduction in the level of phosphorylated AMPKα in KO mice in comparison with WT (Figure 2). There was no substantial difference in AMPKα2 immunoreactivity between genotypes.
Figure 2.
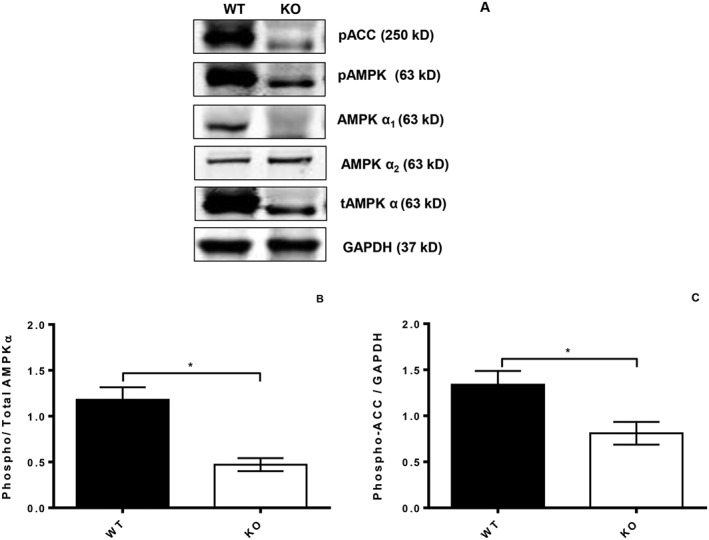
Western blots showing the expression of AMPKα isoforms and expression of the downstream kinase ACC in PVAT from WT (right lanes) and KO mice (left lanes). KO mouse PVAT showed a reduction in expression of AMPKα1 and total AMPKα with no change in AMPKα2. KO PVAT also had significantly reduced phosphoAMPK and ACC. *P < 0.05, KO compared to WT PVAT; n = 5.
Anticontractile effect of PVAT and importance of AMPK
The presence or absence of the vascular endothelium did not affect the contractile response to U46619 in WT or KO aortae (data not shown). In aortic rings without PVAT there was no significant difference in contraction between WT and KO mice (Figure 3A). In rings containing PVAT, contractions to U46619 were significantly reduced in the WT but not the KO mice (Figure 3A), indicating an anticontractile effect of the PVAT which was lost when the PVAT was deficient in AMPKα1.
Figure 3.
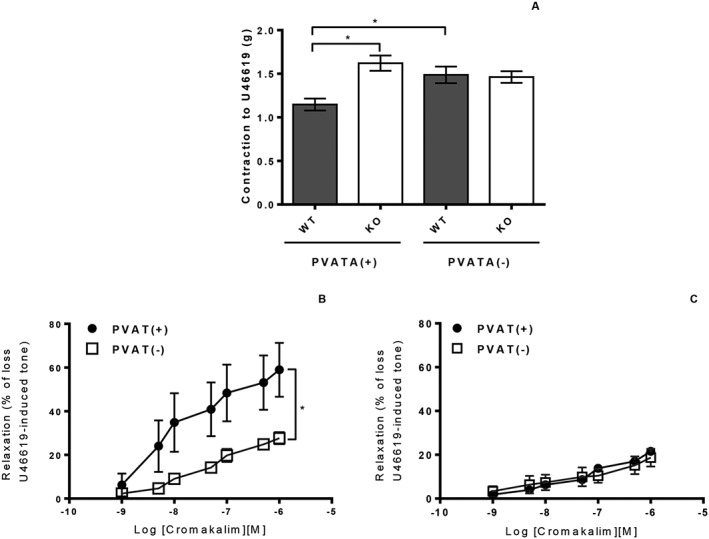
(A) U46619‐induced contraction was not significantly different in WT and KO thoracic aorta rings in the absence of PVAT. In vessels with attached PVAT, there was a significant reduction in contraction in WT but not KO aortic rings. (n = 7; *P < 0.05). (B) The presence of PVAT significantly augmented the relaxation to chromakalim in WT aortic rings [n = 7; *P < 0.05 vs. PVAT(−)]. (C) In aortic rings from AMPKα1 KO mice, the presence of PVAT had no effect on relaxation to cromakalim (n = 7).
The presence of PVAT significantly increased the relaxation to cromakalim in thoracic aortic rings from WT mice, but this effect was completely absent in those from AMPKα1 KO mice (Figure 3B, C). To check that the effect of PVAT was not specific to the thoracic aorta, the experiment was repeated in abdominal aorta and similar results were found: in WT vessels with intact PVAT, maximum relaxation (Emax) to cromakalim was significantly higher, 53.3 ± 5.2%, with PVAT than without PVAT, 24.5 ± 5.7% (n = 6) while in KO mice PVAT had no effect on Emax to cromakalim (22.8 ± 5.8% vs. 17.4 ± 4.1%, n = 6).
In WT mice, PVAT, which had been dissected free of the artery and added back into the organ bath, was able to augment relaxation to cromakalim equally as well as when the PVAT was in contact with the artery (Emax 54.5 ± 8.3% vs. 49.9 ± 6.7%; n = 5), suggesting that the PVAT releases a transmissible factor, which is responsible for increased relaxation. In contrast, addition of PVAT from KO mice to an aortic ring from WT mice did not augment relaxation to cromakalim (Figure 4A, B) whereas adding WT PVAT to a KO aortic ring did augment relaxation. Furthermore, addition of CM produced by WT PVAT was able to augment cromakalim‐induced relaxation in WT aortic rings while CM produced by KO PVAT was unable to augment relaxation in these rings (Figure 4C, D). WT CM also significantly attenuated contraction to U46619 in WT rings without PVAT (1.19 ± 0.1 g vs. 0.86 ± 0.1 g with addition of CM; n = 12). In contrast, KO CM showed a non‐significant trend towards increased contraction (2.33 ± 1.3 g vs. 2.80 ± 1.4 g with addition of CM; n = 12). Transfer of WT PVAT into KO vessels without PVAT and CM experiments indicates that the deficiency in the KO mouse is likely to be at least partly related to differences in the release of adipokines by the PVAT.
Figure 4.
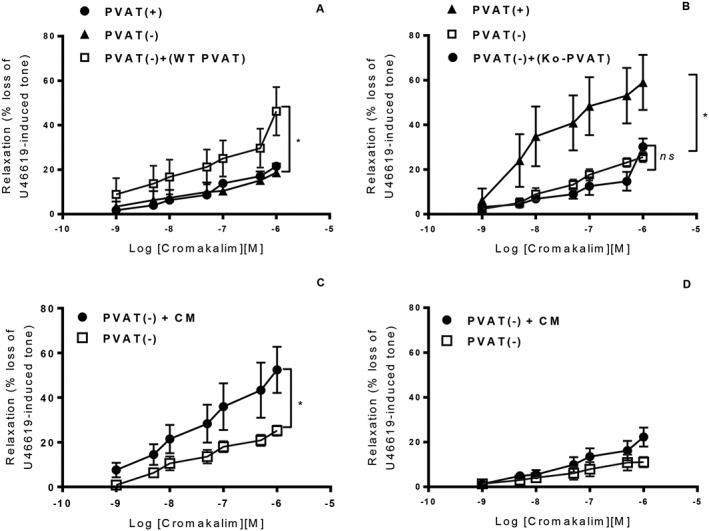
In crossover experiments using aortic rings without PVAT, the addition of WT PVAT enhanced cromakalim‐induced relaxation in KO aortic rings (A). However, the addition of KO PVAT to WT aortic rings (B) did not significantly alter the relaxation to cromakalim [n = 6; *P < 0.05 vs. PVAT(−)]. CM from WT aortic PVAT significantly enhanced relaxation to cromakalim (C) while KO CM had no effect on relaxation to cromakalim (D) [n = 6; *P < 0.05 vs. PVAT(−)].
Cromakalim does not activate AMPK in vascular smooth muscle cells
To rule out the possibility that the vasodilatation to cromakalim is altered in KO mice because cromakalim activates AMPK, cultured rat VSMCs were treated with a range of concentrations of cromakalim and the AMPK activator AICAR as a positive control. Cromakalim did not increase the phosphorylation of AMPK in the vascular smooth muscle and had no effect on the phosphorylation of the downstream substrate ACC. AICAR caused an increase in AMPK activity (Supplementary Figure S3) at a concentration of 10−3 M.
Adipocytokine profile and effect of AMPK KO
Adipocytokine profiling of PVAT‐derived CM showed a striking reduction in the amount of adiponectin released by PVAT from AMPKα1 KO mice (n = 2; data not shown). Further quantification of this difference by elisa in n = 5 samples of conditioned medium from KO and WT mice revealed a significant reduction in the quantity of adiponectin released by KO PVAT (Figure 5).
Figure 5.
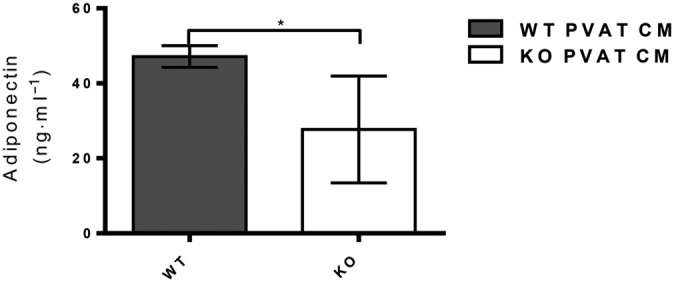
Adiponectin concentration of CM from WT and KO mice determined using an elisa kit. WT PVAT released significantly more adiponectin than KO PVAT (*P˂0.05; n = 5).
Effect of adiponectin on vessel function
Addition of globular adiponectin to WT aortic rings without PVAT significantly increased relaxation to cromakalim (Figure 6A). Interestingly, globular adiponectin enhanced the relaxation to cromakalim in KO vessels both with and without PVAT (Figure 6B, C). Blocking adiponectin receptors in WT aortic rings with intact PVAT significantly attenuated relaxation to cromakalim (Figure 7A), while in KO arteries with intact PVAT, the blocking peptide had no effect (Figure 7B). Taken together, these results suggest that KO PVAT releases less adiponectin but that the medial smooth muscle cells are still able to respond to exogenously added adiponectin.
Figure 6.
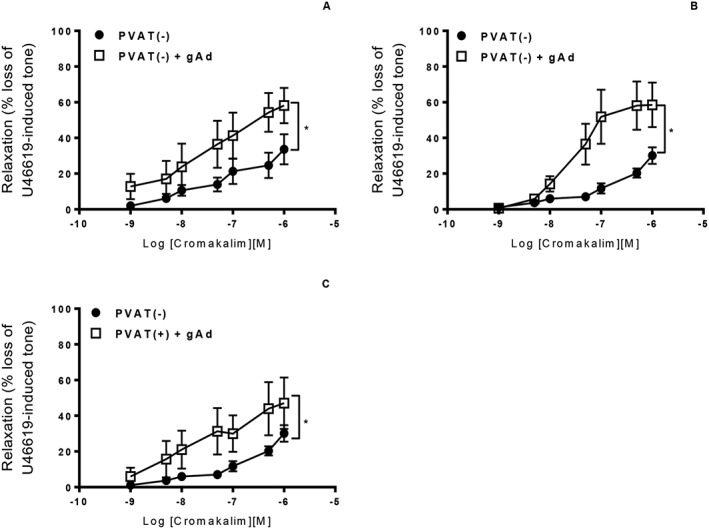
(A) Incubation of WT aortic rings without PVAT with 1 μg·mL−1 globular adiponectin (gAd) significantly augmented relaxation to cromakalim. In KO mice, incubation with globular adiponectin significantly enhanced relaxation to cromakalim in aortic rings without PVAT (B) and also in rings where the PVAT was intact (C). [n = 5, *P < 0.05 vs. PVAT(−) alone].
Figure 7.
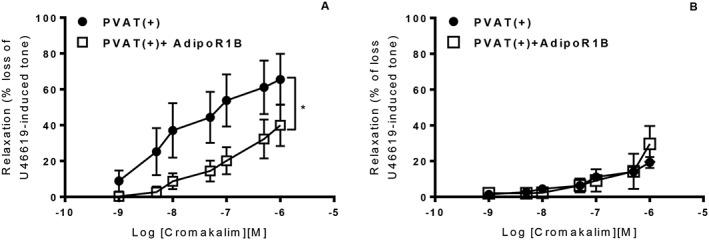
In aortic rings with intact PVAT, addition of 5 μg·mL−1 of the Adipo1 receptor blocking peptide significantly attenuated the relaxation to cromakalim in WT mice (A) but had no effect in rings from KO mice (B). [n = 6, *P < 0.05 vs PVAT(+)].
Discussion
The main findings of this study are that the anticontractile effect of aortic PVAT is lost in mice that lack AMPKα1, and this is due to the reduced generation of a transmissible factor, probably adiponectin, by the PVAT. The results also demonstrate that the lack of an anticontractile effect in the KO mouse is not due to alterations in PVAT morphology or the ratio of BAT to WAT or to the absence of AMPK expression in the medial smooth muscle cells of the aorta. Since incubation with globular adiponectin augmented relaxation to cromakalim in KO arteries without PVAT, it is also unlikely to be due to a reduced sensitivity of the medial VSMCs to adiponectin. In summary, AMPKα1 plays a critical role in maintaining the anticontractile actions of PVAT; an effect independent of the endothelium but likely to be mediated through altered adiponectin secretion.
Morphological studies revealed no difference in PVAT from WT and KO mice. The thoracic aorta was similar in appearance to depots of BAT from the subscapsular region while abdominal aorta showed a mixture of WAT and BAT. This is in agreement with previous studies using Sv129 mice (Frontini and Cinti, 2010; Cinti, 2011) and also from other mouse strains (Cannon and Nedergaard, 2004; Fitzgibbons et al., 2011; Padilla et al., 2013). It has been speculated that differences in PVAT phenotype could contribute to disease susceptibility of certain regions of the arterial tree (Jeong et al., 2007; Greif et al., 2009), possibly through the release of pro‐inflammatory cytokines (Zhao et al., 2003; Chatterjee et al., 2009; Payne et al., 2010). Certainly, in animal models of obesity, there is evidence for a detrimental effect of PVAT on vascular function in aortic (Ma et al., 2010) and mesenteric arteries (Marchesi et al., 2009; Ketonen et al., 2010), and in animals fed a high fat diet, there was reduced phosphorylation of AMPK in thoracic aortic PVAT, an increased adipocyte size and increased intimal thickness (Ma et al., 2010). Taken together, these studies suggest that AMPK in the PVAT is likely to be protective and this agrees with the data presented here showing that the anticontractile effect of thoracic (and abdominal) PVAT is lost in mice lacking AMPKα1. We also demonstrated that in the KO mouse, there was reduced phosphorylated and total AMPK in PVAT as well as reduced phosphorylation of the downstream kinase ACC. There was also no obvious compensatory up‐regulation in AMPKα2 isoforms. The dramatic effect of AMPK α1 KO on PVAT function is in agreement with other studies showing that in adipose tissue the catalytic α1 subunit is the major isoform expressed and is also responsible for the major part of AMPK's activity (Lihn et al., 2004; Daval et al., 2005).
The lack of AMPKα1 caused the PVAT to lose its anticontractile effect and its presence no longer augmented relaxation to cromakalim. To rule out any differences being due to cromakalim acting through AMPK, we added cromakalim to cultured VSMCs and found it had no effect on AMPK expression or phosphorylation. Many other studies have found an anticontractile effect of PVAT in a variety of vascular beds (Gao et al., 2005b; Greenstein et al., 2009) and in vessels contracted with several different vasoconstrictor agents including phenylephrine, 5‐HT, Ang II and U46619 (Lohn et al., 2002; Verlohren et al., 2004; Gao et al., 2005a). The mechanism has been proposed as release of transferable relaxation factor(s), termed adventitia‐derived relaxation factor (ADRF). The nature of ADRF is largely unknown. However, it has been shown to act in part via activation of K+ channels and tyrosine kinase and independently of NO and sympathetic nerve stimulation (Lohn et al., 2002). Our data agree with this in that transfer of CM from WT mice augmented relaxation of aortic rings with PVAT. In addition, it was not necessary for the PVAT to be in contact with the vessel to exert an anticontractile effect. In our protocol, we added the CM prior to contraction of the vessel ring with U46619 and found that WT but not KO CM attenuated aortic contraction. This strongly suggests a transmissible factor is responsible for the attenuation of the contraction and increased relaxation induced by aortic PVAT, and in the KO mice, this factor is reduced or absent. The deficiency in the KO mouse is unlikely to be due to a lack of AMPK in the medial smooth muscle cells since in crossover studies, WT PVAT was able to augment the relaxation response to cromakalim in KO vessels. However, it is worth noting that relaxations to cromakalim tended to be lower in KO rings without PVAT and while exogenously added WT PVAT or WT CM significantly augmented relaxation to cromakalim in KO aortic rings, it still did not reach levels seen in WT vessels with intact PVAT. This suggests there may be some deficit in the function of the VSMCs in the KO mice and future experiments should use an adipocyte‐specific AMPK KO to address this.
The release of vasorelaxing factors (ADRF) has been reported to be dependent on calcium and is regulated by intracellular signalling pathways involving tyrosine kinase and protein kinase A, and is not dependent on perivascular nerve endings (Dubrovska et al., 2004). There are very few studies where the role of AMPK in the release of ADRFs has been investigated. A study by Lihn et al. indicated that the AMPK activator AICAR stimulated adipose tissue AMPKα1 activity and adiponectin gene expression and reduced the release of TNF‐α and IL‐6 (Lihn et al., 2004). These cytokines have been shown to have inhibitory effects on adiponectin gene expression and release (Fasshauer et al., 2002; Maeda et al., 2002; Lihn et al., 2003), indicating that the activity of AMPK in the PVAT could regulate adiponectin expression (Lihn et al., 2004). Similarly, the PPARγ agonist troglitazone, which also activates AMPK, has a positive effect on adiponectin expression in mature adipocytes (Phillips et al., 2003). However, other studies using cultured 3T3‐L1 adipocytes found that prolonged exposure to AMPK activating agents actually causes a significant reduction in the adiponectin protein content of the adipocytes (Huypens et al., 2005).
To clarify how AMPKα1 KO affects the secretion of adipocytokines by aortic PVAT, we performed an array and the most striking difference was a reduction in adiponectin in KO PVAT and CM. Quantitative studies using elisa further confirmed a significant reduction in adiponectin in KO CM. Since adiponectin is a vasodilator (Fesus et al., 2007), it could account for the lack of an anticontractile effect in KO PVAT. Indeed, in WT mice, the anticontractile effect of PVAT was abolished using a adiponectin receptor blocking peptide, an effect demonstrated previously in human gluteal arteries (Fesus et al., 2007) and mouse mesenteric arteries (Lynch et al., 2013). We also demonstrated that the relaxation response to cromakalim was increased in aortic rings of both KO and WT without PVAT by pretreating the rings with globular adiponectin. Since all rings were denuded of endothelium, this suggests adiponectin has direct access to VSMCs (Weston et al., 2013). The adiponectin‐enhanced vascular relaxation was also observed in KO vessels with intact PVAT, indicating that there was no perfusion barrier between the PVAT and vascular smooth muscle layer. Additionally, vascular relaxation was seen in both WT and AMPKα1 KO vessels suggesting that adiponectin can act directly on VSMCs and that the AMPKα1 isoform is not involved.
Only one vasodilator was used in the current study; the KATP channel activator cromakalim. KATP channels in VSMCs may be involved in mediating the anticontractile effects of PVAT since a previous study showed glibenclamide inhibits these effects (Lohn et al., 2002). KATP channels appear to regulate basal tone in a number of vascular beds, such as the coronary circulation (Samaha et al., 1992) and mesenteric vessels (Nelson and Quayle, 1995), and inhibition of this channel by glibenclamide has been found to attenuate the autoregulation of coronary and cerebral vessels (Narishige et al., 1993; Hong et al., 1994). In our study, since PVAT enhanced the relaxation induced by cromakalim, adiponectin released by the PVAT may act in part through modulating KATP channels. However, the role and the mechanism of activation need to be investigated further.
In conclusion, we have shown that PVAT has a profound anticontractile effect on mouse aortic rings. This effect may be due to the release of adiponectin by the PVAT and this release is regulated by the activity of AMPKα1. In mouse aortic rings, adiponectin augments the relaxation to cromakalim in an endothelium‐independent manner, although other effects of adiponectin on the endothelium cannot be ruled out. Indeed, although aortic rings in this study were mechanically denuded of the endothelium, endothelial cells in vessels within the vasa vasorum will remain and adiponectin may have effects here. Clinically, there is evidence that PVAT becomes dysfunctional in obese humans and plasma adiponectin levels are reduced (Aghamohammadzadeh et al., 2015). Alterations in AMPK activity in the PVAT may be behind this effect.
Author contributions
T.A.M., A.B.U. and O.J.K. performed the experiments, acquired and analysed the data and prepared the figures. T.A.M. drafted sections of the paper. I.S. and S.K. conceived and planned the experiments, S.K. prepared the final version of the manuscript and S.K. and I.S. proof read the final version of the manuscript.
Conflict of interest
The authors declare no conflicts of interest.
Declaration of transparency and scientific rigour
This Declaration acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research recommended by funding agencies, publishers and other organisations engaged with supporting research.
Supporting information
Figure S1 Brown adipose tissue marker (UCP1) present in different depots of adipose tissue from both wild type and AMPKα1 knockout mice. Representative histological sections of brown adipose tissue (BAT) (A&B), white adipose tissue (WAT)(C&D), thoracic PVAT (E&F), abdominal PVAT (G&H) and mesenteric PVAT (I&J) stained with anti UCP1 and counterstained with haematoxylin. Positive immunoreactivity for UCP1 is indicated by brown colour. (AO = aorta, MA = mesenteric artery, scale bar 20 μm, magnification ×20).
Figure S2 Brown adipose tissue marker (UCP1) expression in different PVAT depots. UCP1 expression was divided by GAPDH to adjust for protein loading. Western blots were performed in BAT (brown adipose tissue); WAT (white adipose tissue); TA (thoracic artery PVAT); AA (abdominal aorta PVAT); MES (mesenteric artery PVAT) from WT and KO homogenates. Blot shown are representative n = 3 for all groups.
Figure S3 AMPK expression and activity in cultured rat aortic vascular smooth muscle cells (VSMCs). VSMCs samples were treated with AICAR and cromakalim, lysed and immunoblotting was performed. Graphs are expressed as the fold‐change of the phosphorylated form of each enzyme divided by the total AMPKα (B) or total ACC (C) to measure the activation of the enzyme. Blots shown are representative; n = 3 for all groups.
Supporting info item
Supporting info item
Supporting info item
Acknowledgements
The authors gratefully acknowledge support from Zawia University (Libya) and the Ministry of Higher Education (Libya) in the form of a PhD Studentship awarded to Dr Almabrouk.
Almabrouk, T. A. M. , Ugusman, A. B. , Katwan, O. J. , Salt, I. P. , and Kennedy, S. (2017) Deletion of AMPKα1 attenuates the anticontractile effect of perivascular adipose tissue (PVAT) and reduces adiponectin release. British Journal of Pharmacology, 174: 3398–3410. doi: 10.1111/bph.13633.
References
- Aghamohammadzadeh R, Unwin RD, Greenstein AS, Heagerty AM (2015). Effects of obesity on perivascular adipose tissue vasorelaxant function: nitric oxide, inflammation and elevated systemic blood pressure. J Vasc Res 52: 299–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SP, Kelly E, Marrion N, Peters JA, Benson HE, Faccenda E et al. (2015a). The Concise Guide to PHARMACOLOGY 2015/16: Overview. Br J Pharmacol 172: 5729–5743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Fabbro D, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015b). The Concise Guide to PHARMACOLOGY 2015/16: Enzymes. Br J Pharmacol 172: 6024–6109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilodeau‐Goeseels S, Panich PL, Kastelic JP (2011). Activation of AMP‐activated protein kinase may not be involved in AICAR‐ and metformin‐mediated meiotic arrest in bovine denuded and cumulus‐enclosed oocytes in vitro. Zygote 19: 97–106. [DOI] [PubMed] [Google Scholar]
- Cannon B, Nedergaard J (2004). Brown adipose tissue: function and physiological significance. Physiol Rev 84: 277–359. [DOI] [PubMed] [Google Scholar]
- Chatterjee TK, Stoll LL, Denning GM, Harrelson A, Blomkalns AL, Idelman G et al. (2009). Proinflammatory phenotype of perivascular adipocytes: influence of high‐fat feeding. Circ Res 104: 541–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Montagnani M, Funahashi T, Shimomura I, Quon MJ (2003). Adiponectin stimulates production of nitric oxide in vascular endothelial cells. J Biol Chem 278: 45021–45026. [DOI] [PubMed] [Google Scholar]
- Chen Z, Peng IC, Sun W, Su MI, Hsu PH, Fu Y et al. (2009). AMP‐activated protein kinase functionally phosphorylates endothelial nitric oxide synthase Ser633. Circ Res 104: 496–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng KK, Lam KS, Wang Y, Huang Y, Carling D, Wu D et al. (2007). Adiponectin‐induced endothelial nitric oxide synthase activation and nitric oxide production are mediated by APPL1 in endothelial cells. Diabetes 56: 1387–1394. [DOI] [PubMed] [Google Scholar]
- Cinti S (2011). Between brown and white: novel aspects of adipocyte differentiation. Ann Med 43: 104–115. [DOI] [PubMed] [Google Scholar]
- Curtis MJ, Bond RA, Spina D, Ahluwalia A, Alexander SP, Giembycz MA et al. (2015). Experimental design and analysis and their reporting: new guidance for publication in BJP. Br J Pharmacol 172: 3461–3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daval M, Diot‐Dupuy F, Bazin R, Hainault I, Viollet B, Vaulont S et al. (2005). Anti‐lipolytic action of AMP‐activated protein kinase in rodent adipocytes. J Biol Chem 280: 25250–25257. [DOI] [PubMed] [Google Scholar]
- Davis BJ, Xie Z, Viollet B, Zou MH (2006). Activation of the AMP‐activated kinase by antidiabetes drug metformin stimulates nitric oxide synthesis in vivo by promoting the association of heat shock protein 90 and endothelial nitric oxide synthase. Diabetes 55: 496–505. [DOI] [PubMed] [Google Scholar]
- Dubrovska G, Verlohren S, Luft FC, Gollasch M (2004). Mechanisms of ADRF release from rat aortic adventitial adipose tissue. Am J Physiol Heart Circ Physiol 286: H1107–H1113. [DOI] [PubMed] [Google Scholar]
- Evans AM, Mustard KJ, Wyatt CN, Peers C, Dipp M, Kumar P et al. (2005). Does AMP‐activated protein kinase couple inhibition of mitochondrial oxidative phosphorylation by hypoxia to calcium signaling in O2‐sensing cells? J Biol Chem 280: 41504–41511. [DOI] [PubMed] [Google Scholar]
- Ewart MA, Kennedy S (2011). AMPK and vasculoprotection. Pharmacol Ther 131: 242–253. [DOI] [PubMed] [Google Scholar]
- Fang L, Zhao J, Chen Y, Ma T, Xu G, Tang C et al. (2009). Hydrogen sulfide derived from periadventitial adipose tissue is a vasodilator. J Hypertens 27: 2174–2185. [DOI] [PubMed] [Google Scholar]
- Fasshauer M, Klein J, Neumann S, Eszlinger M, Paschke R (2002). Hormonal regulation of adiponectin gene expression in 3T3‐L1 adipocytes. Biochem Biophys Res Commun 290: 1084–1089. [DOI] [PubMed] [Google Scholar]
- Fesus G, Dubrovska G, Gorzelniak K, Kluge R, Huang Y, Luft FC et al. (2007). Adiponectin is a novel humoral vasodilator. Cardiovasc Res 75: 719–727. [DOI] [PubMed] [Google Scholar]
- Fitzgibbons TP, Kogan S, Aouadi M, Hendricks GM, Straubhaar J, Czech MP (2011). Similarity of mouse perivascular and brown adipose tissues and their resistance to diet‐induced inflammation. Am J Physiol Heart Circ Physiol 301: H1425–H1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming I, Fisslthaler B, Dixit M, Busse R (2005). Role of PECAM‐1 in the shear‐stress‐induced activation of Akt and the endothelial nitric oxide synthase (eNOS) in endothelial cells. J Cell Sci 118 (Pt 18): 4103–4111. [DOI] [PubMed] [Google Scholar]
- Ford RJ, Teschke SR, Reid EB, Durham KK, Kroetsch JT, Rush JW (2012). AMP‐activated protein kinase activator AICAR acutely lowers blood pressure and relaxes isolated resistance arteries of hypertensive rats. J Hypertens 30: 725–733. [DOI] [PubMed] [Google Scholar]
- Frontini A, Cinti S (2010). Distribution and development of brown adipocytes in the murine and human adipose organ. Cell Metab 11: 253–256. [DOI] [PubMed] [Google Scholar]
- Galvez B, de Castro J, Herold D, Dubrovska G, Arribas S, Gonzalez MC et al. (2006). Perivascular adipose tissue and mesenteric vascular function in spontaneously hypertensive rats. Arterioscler Thromb Vasc Biol 26: 1297–1302. [DOI] [PubMed] [Google Scholar]
- Galvez‐Prieto B, Bolbrinker J, Stucchi P, de Las Heras AI, Merino B, Arribas S et al. (2008). Comparative expression analysis of the renin‐angiotensin system components between white and brown perivascular adipose tissue. J Endocrinol 197: 55–64. [DOI] [PubMed] [Google Scholar]
- Gao YJ (2007). Dual modulation of vascular function by perivascular adipose tissue and its potential correlation with adiposity/lipoatrophy‐related vascular dysfunction. Curr Pharm Des 13: 2185–2192. [DOI] [PubMed] [Google Scholar]
- Gao YJ, Holloway AC, Zeng ZH, Lim GE, Petrik JJ, Foster WG et al. (2005a). Prenatal exposure to nicotine causes postnatal obesity and altered perivascular adipose tissue function. Obes Res 13: 687–692. [DOI] [PubMed] [Google Scholar]
- Gao YJ, Lu C, Su LY, Sharma AM, Lee RM (2007). Modulation of vascular function by perivascular adipose tissue: the role of endothelium and hydrogen peroxide. Br J Pharmacol 151: 323–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao YJ, Takemori K, Su LY, An WS, Lu C, Sharma AM et al. (2006). Perivascular adipose tissue promotes vasoconstriction: the role of superoxide anion. Cardiovasc Res 71: 363–373. [DOI] [PubMed] [Google Scholar]
- Gao YJ, Zeng ZH, Teoh K, Sharma AM, Abouzahr L, Cybulsky I et al. (2005b). Perivascular adipose tissue modulates vascular function in the human internal thoracic artery. J Thorac Cardiovasc Surg 130: 1130–1136. [DOI] [PubMed] [Google Scholar]
- Gil‐Ortega M, Stucchi P, Guzman‐Ruiz R, Cano V, Arribas S, Gonzalez MC et al. (2010). Adaptative nitric oxide overproduction in perivascular adipose tissue during early diet‐induced obesity. Endocrinology 151: 3299–3306. [DOI] [PubMed] [Google Scholar]
- Greenstein AS, Khavandi K, Withers SB, Sonoyama K, Clancy O, Jeziorska M et al. (2009). Local inflammation and hypoxia abolish the protective anticontractile properties of perivascular fat in obese patients. Circulation 119: 1661–1670. [DOI] [PubMed] [Google Scholar]
- Greif M, Becker A, von Ziegler F, Lebherz C, Lehrke M, Broedl UC et al. (2009). Pericardial adipose tissue determined by dual source CT is a risk factor for coronary atherosclerosis. Arterioscler Thromb Vasc Biol 29: 781–786. [DOI] [PubMed] [Google Scholar]
- Hardie DG, Scott JW, Pan DA, Hudson ER (2003). Management of cellular energy by the AMP‐activated protein kinase system. FEBS Lett 546: 113–120. [DOI] [PubMed] [Google Scholar]
- Hong KW, Pyo KM, Lee WS, Yu SS, Rhim BY (1994). Pharmacological evidence that calcitonin gene‐related peptide is implicated in cerebral autoregulation. Am J Physiol Heart Circ Physiol 266: H11–H16. [DOI] [PubMed] [Google Scholar]
- Horman S, Morel N, Vertommen D, Hussain N, Neumann D, Beauloye C et al. (2008). AMP‐activated protein kinase phosphorylates and desensitizes smooth muscle myosin light chain kinase. J Biol Chem 283: 18505–18512. [DOI] [PubMed] [Google Scholar]
- Huypens P, Quartier E, Pipeleers D, Van de Casteele M (2005). Metformin reduces adiponectin protein expression and release in 3T3‐L1 adipocytes involving activation of AMP activated protein kinase. Eur J Pharmacol 518: 90–95. [DOI] [PubMed] [Google Scholar]
- Jeong JW, Jeong MH, Yun KH, Oh SK, Park EM, Kim YK et al. (2007). Echocardiographic epicardial fat thickness and coronary artery disease. Circ J 71: 536–539. [DOI] [PubMed] [Google Scholar]
- Jørgensen SB, Viollet B, Andreelli F, Frøsig C, Birk JB, Schjerling P et al. (2004). Knockout of the α2 but Not α1 5′‐AMP‐activated protein kinase isoform abolishes 5‐aminoimidazole‐4‐carboxamide‐1‐β‐4‐ribofuranosidebut not contraction‐induced glucose uptake in skeletal muscle. J Biol Chem 279: 1070–1079. [DOI] [PubMed] [Google Scholar]
- Ketonen J, Shi J, Martonen E, Mervaala E (2010). Periadventitial adipose tissue promotes endothelial dysfunction via oxidative stress in diet‐induced obese C57Bl/6 mice. Circ J 74: 1479–1487. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG (2010). Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol 160: 1577–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RM, Lu C, Su LY, Gao YJ (2009). Endothelium‐dependent relaxation factor released by perivascular adipose tissue. J Hypertens 27: 782–790. [DOI] [PubMed] [Google Scholar]
- Levine YC, Li GK, Michel T (2007). Agonist‐modulated regulation of AMP‐activated protein kinase (AMPK) in endothelial cells. Evidence for an AMPK ‐ > Rac1 ‐ > Akt ‐ > endothelial nitric‐oxide synthase pathway. J Biol Chem 282: 20351–20364. [DOI] [PubMed] [Google Scholar]
- Lihn AS, Jessen N, Pedersen SB, Lund S, Richelsen B (2004). AICAR stimulates adiponectin and inhibits cytokines in adipose tissue. Biochem Biophys Res Commun 316: 853–858. [DOI] [PubMed] [Google Scholar]
- Lihn AS, Richelsen B, Pedersen SB, Haugaard SB, Rathje GS, Madsbad S et al. (2003). Increased expression of TNF‐alpha, IL‐6, and IL‐8 in HALS: implications for reduced adiponectin expression and plasma levels. Am J Physiol Endocrinol Metab 285: E1072–E1080. [DOI] [PubMed] [Google Scholar]
- Lohn M, Dubrovska G, Lauterbach B, Luft FC, Gollasch M, Sharma AM (2002). Periadventitial fat releases a vascular relaxing factor. FASEB J 16: 1057–1063. [DOI] [PubMed] [Google Scholar]
- Lynch FM, Withers SB, Yao Z, Werner ME, Edwards G, Weston AH et al. (2013). Perivascular adipose tissue‐derived adiponectin activates BK(Ca) channels to induce anticontractile responses. Am J Physiol Heart Circ Physiol 304: H786–H795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Ma S, He H, Yang D, Chen X, Luo Z et al. (2010). Perivascular fat‐mediated vascular dysfunction and remodeling through the AMPK/mTOR pathway in high‐fat diet‐induced obese rats. Hypertens Res 33: 446–453. [DOI] [PubMed] [Google Scholar]
- Maeda N, Shimomura I, Kishida K, Nishizawa H, Matsuda M, Nagaretani H et al. (2002). Diet‐induced insulin resistance in mice lacking adiponectin/ACRP30. Nat Med 8: 731–737. [DOI] [PubMed] [Google Scholar]
- Malinowski M, Deja MA, Golba KS, Roleder T, Biernat J, Wos S (2008). Perivascular tissue of internal thoracic artery releases potent nitric oxide and prostacyclin‐independent anticontractile factor. Eur J Cardiothorac Surg 33: 225–231. [DOI] [PubMed] [Google Scholar]
- Marchesi C, Ebrahimian T, Angulo O, Paradis P, Schiffrin EL (2009). Endothelial nitric oxide synthase uncoupling and perivascular adipose oxidative stress and inflammation contribute to vascular dysfunction in a rodent model of metabolic syndrome. Hypertension 54: 1384–1392. [DOI] [PubMed] [Google Scholar]
- McGrath JC, Lilley E (2015). Implementing guidelines on reporting research using animals (ARRIVE etc.): new requirements for publication in BJP. Br J Pharmacol 172: 3189–3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer RI, Bakker W, Alta CL, Sipkema P, Yudkin JS, Viollet B et al. (2013). Perivascular adipose tissue control of insulin‐induced vasoreactivity in muscle is impaired in db/db mice. Diabetes 62: 590–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow VA, Foufelle F, Connell JM, Petrie JR, Gould GW, Salt IP (2003). Direct activation of AMP‐activated protein kinase stimulates nitric‐oxide synthesis in human aortic endothelial cells. J Biol Chem 278: 31629–31639. [DOI] [PubMed] [Google Scholar]
- Nagata D, Takeda R, Sata M, Satonaka H, Suzuki E, Nagano T et al. (2004). AMP‐activated protein kinase inhibits angiotensin II‐stimulated vascular smooth muscle cell proliferation. Circulation 110: 444–451. [DOI] [PubMed] [Google Scholar]
- Narishige T, Egashira K, Akatsuka Y, Katsuda Y, Numaguchi K, Sakata M et al. (1993). Glibenclamide, a putative ATP‐sensitive K+ channel blocker, inhibits coronary autoregulation in anesthetized dogs. Circ Res 73: 771–776. [DOI] [PubMed] [Google Scholar]
- Nelson MT, Quayle JM (1995). Physiological roles and properties of potassium channels in arterial smooth muscle. Am J Physiol 268 (4 Pt 1): C799–C822. [DOI] [PubMed] [Google Scholar]
- Padilla J, Jenkins NT, Vieira‐Potter VJ, Laughlin MH (2013). Divergent phenotype of rat thoracic and abdominal perivascular adipose tissues. Am J Physiol Regul Integr Comp Physiol 304: R543–R552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne GA, Borbouse L, Kumar S, Neeb Z, Alloosh M, Sturek M et al. (2010). Epicardial perivascular adipose‐derived leptin exacerbates coronary endothelial dysfunction in metabolic syndrome via a protein kinase C‐β pathway. Arterioscler Thromb Vasc Biol 30: 1711–1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips SA, Ciaraldi TP, Kong AP, Bandukwala R, Aroda V, Carter L et al. (2003). Modulation of circulating and adipose tissue adiponectin levels by antidiabetic therapy. Diabetes 52: 667–674. [DOI] [PubMed] [Google Scholar]
- Samaha FF, Heineman FW, Ince C, Fleming J, Balaban RS (1992). ATP‐sensitive potassium channel is essential to maintain basal coronary vascular tone in vivo. Am J Physiol Cell Physiol 262: C1220–C1227. [DOI] [PubMed] [Google Scholar]
- Southan C, Sharman JL, Benson HE, Faccenda E, Pawson AJ, Alexander SP et al. (2016). The IUPHAR/BPS guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucleic Acids Res 44: D1054–D1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vecchione C, Maffei A, Colella S, Aretini A, Poulet R, Frati G et al. (2002). Leptin effect on endothelial nitric oxide is mediated through Akt‐endothelial nitric oxide synthase phosphorylation pathway. Diabetes 51: 168–173. [DOI] [PubMed] [Google Scholar]
- Verlohren S, Dubrovska G, Tsang SY, Essin K, Luft FC, Huang Y et al. (2004). Visceral periadventitial adipose tissue regulates arterial tone of mesenteric arteries. Hypertension 44: 271–276. [DOI] [PubMed] [Google Scholar]
- Weingartner O, Husche C, Schott HF, Speer T, Bohm M, Miller CM et al. (2015). Vascular effects of oxysterols and oxyphytosterols in apoE −/− mice. Atherosclerosis 240: 73–79. [DOI] [PubMed] [Google Scholar]
- Weston AH, Egner I, Dong Y, Porter EL, Heagerty AM, Edwards G (2013). Stimulated release of a hyperpolarizing factor (ADHF) from mesenteric artery perivascular adipose tissue: involvement of myocyte BKCa channels and adiponectin. Br J Pharmacol 169: 1500–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Kalivendi S, Zhang H, Joseph J, Nithipatikom K, Vásquez‐Vivar J et al. (2003). Superoxide reacts with hydroethidine but forms a fluorescent product that is distinctly different from ethidium: potential implications in intracellular fluorescence detection of superoxide. Free Radic Biol Med 34: 1359–1368. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Brown adipose tissue marker (UCP1) present in different depots of adipose tissue from both wild type and AMPKα1 knockout mice. Representative histological sections of brown adipose tissue (BAT) (A&B), white adipose tissue (WAT)(C&D), thoracic PVAT (E&F), abdominal PVAT (G&H) and mesenteric PVAT (I&J) stained with anti UCP1 and counterstained with haematoxylin. Positive immunoreactivity for UCP1 is indicated by brown colour. (AO = aorta, MA = mesenteric artery, scale bar 20 μm, magnification ×20).
Figure S2 Brown adipose tissue marker (UCP1) expression in different PVAT depots. UCP1 expression was divided by GAPDH to adjust for protein loading. Western blots were performed in BAT (brown adipose tissue); WAT (white adipose tissue); TA (thoracic artery PVAT); AA (abdominal aorta PVAT); MES (mesenteric artery PVAT) from WT and KO homogenates. Blot shown are representative n = 3 for all groups.
Figure S3 AMPK expression and activity in cultured rat aortic vascular smooth muscle cells (VSMCs). VSMCs samples were treated with AICAR and cromakalim, lysed and immunoblotting was performed. Graphs are expressed as the fold‐change of the phosphorylated form of each enzyme divided by the total AMPKα (B) or total ACC (C) to measure the activation of the enzyme. Blots shown are representative; n = 3 for all groups.
Supporting info item
Supporting info item
Supporting info item


