Abstract
Perivascular adipose tissue (PVAT) is now recognized as an active player in vascular homeostasis. The expansion of PVAT in obesity and its possible role in vascular dysfunction have attracted much interest. In terms of the regulation of vascular tone and blood pressure, PVAT has been shown to release vasoactive mediators, for instance, angiotensin peptides, reactive oxygen species, chemokines and cytokines. The secretory profile of PVAT is altered by obesity, hypertension and other cardiovascular diseases, leading to an imbalance between its pro‐contractile and anti‐contractile effects. PVAT adipocytes represent an important source of the mediators, but infiltrating immune cells may become more important under conditions of hypoxia and inflammation. This review describes recent advances in the effects of PVAT on the regulation of vascular tone, highlighting the evidence for a pro‐contractile action in health and disease. The role of the endothelium, vascular smooth muscle, immune cells and probably perivascular nerves in PVAT function is also discussed.
Linked Articles
This article is part of a themed section on Molecular Mechanisms Regulating Perivascular Adipose Tissue – Potential Pharmacological Targets? To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v174.20/issuetoc
Abbreviations
- ADCF
adipocyte‐derived contractile factor
- ADRF
adipocyte‐derived relaxing factor
- Ang
angiotensin
- DOCA
deoxycorticosterone acetate
- MCP‐1
monocyte chemoattractant protein‐1
- PVAT
perivascular adipose tissue
- RANTES
regulated on activation, normal T cell expressed and secreted
Tables of Links
| TARGETS | |
|---|---|
| GPCRs a | Enzymes d |
| Adrenoceptors | ACE |
| AT1 receptor | Akt |
| Chemerin receptor (ChemR23) | AMPK |
| ETA receptor | COX |
| Voltage‐gated ion channels b | eNOS |
| BKCa (KCa1.1) channel | ERK |
| Kv7 channels | mTOR |
| Nuclear hormone receptors c | PKC |
| PPARγ | Rho kinase |
| LIGANDS | |
|---|---|
| Adiponectin | IL‐10 |
| Ang 1‐7 | Insulin |
| Ang II | Leptin |
| Adrenaline | MCP‐1 |
| Chemerin | NO |
| cGMP | Noradrenaline |
| ET‐1 | PGE2 |
| Hydrogen peroxide | RANTES |
| Hydrogen sulphide | TNFα |
| IL‐6 | TXA2 |
| IL‐8 |
These Tables list key protein targets and ligands in this article that are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Southan et al., 2016), and are permanently archived in the Concise Guide to PHARMACOLOGY 2015/16 (a,b,c,dAlexander et al., 2015a,b,c,d).
Introduction
Perivascular adipose tissue (PVAT) was originally thought to only provide structural support and was thus routinely removed in vessel contractility studies. However, the growing prevalence of obesity, characterized by excessive adipose tissues, and the realization that adipose tissue acts as a complex paracrine and endocrine organ (Ahima and Flier, 2000; Gustafson et al., 2007) have drawn attention to a functional role for PVAT, which might also provide a mechanistic link between obesity and vascular dysfunction. PVAT is now recognized as a specialized fat depot that surrounds most blood vessels, releasing diffusible factors that modulate local vascular reactivity and inflammatory status and, as a result, may contribute to the pathophysiological changes seen in cardiovascular diseases, diabetes and obesity (reviewed in Yudkin et al., 2005; Szasz et al., 2013; Gil‐Ortega et al., 2015; Fernandez Alfonso et al., 2017). Indeed, the Framingham Heart Study shows that a higher volume of PVAT around thoracic aorta is associated with metabolic risk factors and a higher prevalence of cardiovascular disease in volunteers (Lehman et al., 2010; Britton et al., 2012). Numerous mechanisms have been suggested to underlie the crosstalk between PVAT and vascular cells, but the regulation of PVAT function, particularly the balance between its beneficial and deleterious effects, remains poorly understood. In 1991, Soltis and Cassis reported that, in rat aorta, PVAT potentiated contractions to a sympathomimetic, but reduced those to noradrenaline due to its reuptake by adrenergic nerves in PVAT. Subsequently, PVAT was also found to reduce responses to other vasoconstrictors, leading to the suggestion of PVAT‐derived or adipocyte‐derived relaxing factors (ADRFs). Much of the research has since focused on identifying these relaxing factors and establishing their vascular actions (see Gollasch, 2012; Withers et al., 2014, for reviews). However, there is also evidence that PVAT produces contractile factors, which were initially termed perivascular adipocyte‐derived contractor factors (Gao, 2007) and later adipocyte‐derived contractile factors (Meyer et al., 2013). This adds to the complex scenario of anti‐contractile versus pro‐contractile properties of PVAT. In this review, we intend to provide an update on the balance between relaxant and contractile effects of PVAT, highlighting a potential shift from an anti‐contractile action of PVAT in health to a pro‐contractile effect in obesity and related cardiovascular diseases.
Composition of PVAT
Adipose tissue surrounding blood vessels is not physically separated from the vascular wall by a fascial layer, providing access for its paracrine effects. In general, brown adipocytes are larger in size, with smaller oil droplets and larger numbers of mitochondria than white adipocytes, which store triglycerides. Morphological and gene expression analysis indicate that whilst perivascular adipocytes often resemble white adipocytes, they are distinct from visceral and subcutaneous fat and display characteristics of both white and brown adipocytes: sometimes referred to as beige adipocytes. For instance, perivascular adipocytes of human coronary arteries are smaller and irregularly shaped, with fewer differentiation markers but higher expression of some brown adipocyte‐related genes, than subcutaneous adipocytes (Chatterjee et al., 2009). The precise phenotype appears to depend on the vascular region and species (Szasz et al., 2013; Gil‐Ortega et al., 2015). Adipocytes around thoracic aorta are more similar to brown adipocytes, at least in rodents (Gálvez‐Prieto et al., 2008a; Padilla et al., 2013 ). However, adipocytes from the abdominal aorta and mesenteric arteries are closer to white adipocytes in both rodents and humans (Henrichot et al., 2005; Police et al., 2009; Padilla et al., 2013).
Importantly, adipose tissues are dynamically regulated, showing cellular and metabolic plasticity. Sustained obesity is associated with increases in the size and/or number of PVAT white adipocytes (Marchesi et al., 2009; Ketonen et al., 2010; Ma et al., 2010; Greenstein et al., 2009). These are accompanied by functional changes, including an altered secretion pattern of PVAT (Chatterjee et al., 2009; Greenstein et al., 2009; Ketonen et al., 2010). Conversely, an increased proportion of brown to white adipocytes (browning of adipose tissues) promotes thermogenesis and might represent a protective mechanism against metabolic diseases (Pellegrinelli et al., 2016) and perhaps improve vascular function in obesity and atherosclerosis (Fitzgibbons et al., 2011; Chang et al., 2012).
In addition to adipocytes, PVAT contains other important cell types such as macrophages, T‐lymphocytes and fibroblasts, which may also contribute to its function. Indeed, infiltration of immune cells into PVAT is characteristic of disease states associated with vascular inflammation (Omar et al., 2014; Pellegrinelli et al., 2016). The expansion of PVAT is also likely to involve the generation of pre‐adipocytes from resident mesenchymal stem cells and maturation of pre‐adipocytes (Pellegrinelli et al., 2016). Moreover, PVAT is also innervated by sympathetic nerves (Bulloch and Daly, 2014; Darios et al., 2016), which could stimulate the browning of PVAT. However, the interactions among perivascular adipocytes, immune cells and nerves in vascular regulation remain poorly defined. Adipocytes, which are the main cellular component of PVAT, are known to release vasoactive substances (e.g. ADRF and ADCF), but immune cells and sympathetic nerves might serve as additional sources of these molecules (e.g. Gao et al., 2006; Lumeng et al., 2007; Dashwood and Loesch, 2011; Nguyen et al., 2011). Where possible, we will highlight the likely cellular source(s) of vasoactive substances within PVAT.
Evidence for contractile factors from PVAT
Similar to adipocytes in other anatomical locations, increasing evidence suggests that PVAT secretes bioactive molecules, including adipokines and other cytokines that regulate cardiovascular function. A number of these diffusible factors can induce direct vasocontraction and may be referred to as PVAT‐derived or adipocyte‐derived contractor factors (denoted ADCFs herein), which are highlighted in Table 1. Much of the evidence comes from contractility studies using isolated arteries with and without PVAT, combined with isolated PVAT and its conditioned media under physiological conditions.
Table 1.
PVAT‐derived contractile factors
| Contractile factor | PVAT expression | Effect | Vascular bed | Reference |
|---|---|---|---|---|
| Ang II | Ang II protein; angiotensinogen and ACE mRNA in PVAT adipocytes | ↑ Sympathetic contraction | Rat superior mesenteric artery | Lu et al., 2010 |
| Ang II protein, angiotensinogen, ACE and chymase mRNA | Rat thoracic aorta | Galvez‐Prieto et al., 2008a | ||
| Rat (resistance) mesenteric artery | ||||
| Superoxide | Superoxide; NADPH oxidase protein in PVAT adipocytes | ↑ Sympathetic contraction (via tyrosine kinase and ERK, but independent of NO) | Rat superior mesenteric artery | Gao et al., 2006 |
| Catecholamines | Noradrenaline and adrenaline in PVAT adipocytes | Contraction | Rat thoracic aorta | Ayala‐Lopez et al., 2014 |
| Rat superior mesenteric artery | ||||
| Noradrenaline from sympathetic nerve endings | Contraction | Rat thoracic aorta | Soltis and Cassis, 1991 | |
| Prostanoids | TXA2 in PVAT‐conditioned buffer; COX‐1 and COX‐2 mRNA | ↑ Agonist‐induced contraction (independent of NOS or ETA). | Mouse thoracic aorta (only in monogenic obesity and diet‐induced obesity) | Meyer et al., 2013 |
| PGE2 in PVAT (or PVAT‐conditioned buffer) | Contraction | Rat mesenteric artery | Mendizabal et al., 2013; | |
| TNF‐α | TNF‐α protein in PVAT adipocytes | ↑ Contraction to eNOS inhibition | Human small arteries from visceral fat (enhanced in obese patients) | Virdis et al., 2015 |
| ↑ ET‐1 and NADPH oxidase‐derived superoxide in vascular cells | ||||
| IL‐6 | IL‐6 in PVAT adipocytes | Human coronary artery (enhanced with high‐fat diet) | Chatterjee et al., 2009 | |
| Chemerin | Chemerin protein in PVAT adipocytes |
Contraction ↑ Agonist‐induced contraction (enhanced by endothelial removal or NOS inhibition) |
Rat thoracic aorta Rat superior mesenteric artery (enhanced responses in DOCA‐salt hypertensive but not diet‐induced obese or SHRSP rats) |
Watts et al., 2013 |
| Chemerin protein in PVAT adipocytes |
Contraction ↑ Agonist‐induced contraction |
Human resistance mesenteric artery | Watts et al., 2013 | |
| Chemerin protein in PVAT adipocytes | ↑ Sympathetic contraction | Rat superior mesenteric artery | Darios et al., 2016 | |
| Leptin | PVAT‐conditioned buffer |
↑ Agonist‐ and depolarisation‐induced contraction (via increased voltage‐gated Ca2+ entry) ↑ Smooth muscle proliferation |
Pig coronary artery (enhanced in diet‐induced obesity) |
Owen et al., 2013; Noblet et al., 2016 |
↑, increase; ➔, lead to; eNOS, endothelial NOS; ETA, endothelin ETA receptor; SHRSP, stroke‐prone spontaneously hypertensive rat.
This table shows studies that demonstrate PVAT production of mediators, which either induce direct contraction or potentiate contractions to other vasoconstrictors.
Adipocytes are known to express a local renin–angiotensin–aldosterone system (RAAS), including angiotensinogen and angiotensin converting enzyme (ACE) for the synthesis of the potent vasoconstrictor, angiotensin II (Ang II; Karlsson et al., 1998; Cassis et al., 2008). The expression of RAAS components can vary depending on the composition and location of adipose tissues (Cassis et al., 1988; Engeli et al., 1999; Galvez‐Prieto et al., 2008a; Riedel et al., 2016). PVAT is thought to express all components of RAAS and that PVAT‐derived Ang II promotes contractions through the activation of AT1 receptors in rat mesenteric arteries (Lu et al., 2010). Gao and co‐workers proposed that Ang II acts indirectly by stimulating superoxide radical production from NADPH oxidase in PVAT adipocytes or the vascular wall itself (Gao et al., 2006; Lu et al., 2008). Ang II has also been shown to play a role in the local inflammation associated with hypertension and obesity, stimulating the infiltration of immune cells, including T‐lymphocytes and macrophages, into PVAT and th eproduction of reactive oxygen species (Police et al., 2009; Guzik et al., 2007; Mikolajczyk et al., 2016). However, the importance of PVAT as a source of Ang II in the control of vascular tone and blood pressure, particular in hypertension and obesity, remains to be established. Moreover, it is likely that the production and function of PVAT‐derived Ang II show regional heterogeneity (Galvez‐Prieto et al., 2008a). Ang II can also further exacerbate PVAT dysfunction, since AT1 receptor activation has been shown to reduce th ebrowning of adipose tissue and promote adipocyte hypertrophy, insulin resistance and weight gain in mice with high fat‐induced obesity (Graus‐Nunes et al., 2017).
In the initial study by Soltis and Cassis (1991), PVAT greatly enhanced contractions to electrical field stimulation or to the indirect sympathomimetic tyramine in rat aorta, suggesting sympathetic nerve activity is involved in this effect of PVAT. The presence of sympathetic nerves has been demonstrated in PVAT of human saphenous veins (Dashwood and Loesch, 2011). Also, the involvement of sympathetic innervation in the regulation of vascular tone and blood pressure is well established. An elevated sympathetic activity is also associated with hypertension, including obesity‐associated hypertension (Thalmann and Meier, 2007); however, the interaction between PVAT and local sympathetic activity in healthy and disease conditions has not been scrutinized. Sympathetic activity and the subsequent release of catecholamines are known to regulate lipolysis and the proliferation and differentiation of adipocytes activation through the stimulation of α‐ and β‐adrenoceptors. Recent evidence has also suggested that adipocytes and alternatively activated macrophages in adipose tissues may synthesize and release noradrenaline and adrenaline (Nguyen et al., 2011; Vargovic et al., 2011). Noradrenaline and the enzymes involved in its synthesis have been detected in PVAT adipoctyes in thoracic aorta and superior mesenteric arteries, where PVAT enhances contraction via α1‐adrenoceptors, (Ayala‐Lopez et al., 2014). PVAT‐dependent contractions to the sympathomimetic tyramine have also been reported in these arteries (Soltis and Cassis, 1991; Ayala‐Lopez et al., 2014). Tyramine is traditionally used to release catecholamines from sympathetic nerve endings, but the possibility that it has an effect on adipocytes or immune cells in PVAT cannot be excluded.
Another factor that may contribute to the contractile effects of PVAT is the adipokine, chemerin (Table 1). Chemerin, in particular chemerin‐9, evokes direct vasocontraction and enhances agonist‐induced contractions via its GPCR, ChemR23, in rat and human arteries. Moreover, these effects are exaggerated in thoracic aorta and mesenteric arteries with reduced endothelium‐dependent relaxation, a phenomenon often found in hypertension and obesity (Watts et al., 2013). A follow‐up study by the same group (Darios et al., 2016) has also shown that PVAT‐derived chemerin potentiates sympathetic contraction through its receptor, which is co‐localized with tyrosine hydrolase in sympathetic nerves of rat superior mesenteric artery. Direct application of chemerin to isolated aorta or mesenteric artery also augments agonist‐induced contraction in a manner dependent on endothelin ETA receptors and ERK activation (Lobato et al., 2012) and increases systolic blood pressure in mice (Kunimoto et al., 2015). Thus, chemerin might play a particularly important role in some forms of hypertension and obesity.
In addition to chemerin, cytokines derived from PVAT might also increase vascular tone. For instance, TNF‐α and IL‐6 are known to enhance contractions, probably via up‐regulation of endothelin signalling or reduced NO production and endothelium‐dependent relaxation, especially in obese patients (Greenberg et al., 1985; Orshal and Khalil, 2004; Virdis et al., 2015). High‐fat diet has also been shown to promote IL‐6 expression in human coronary PVAT (Chatterjee et al., 2009).
Aortic and small mesenteric PVAT also releases contractile COX products, including TXA2 and PGE2 (Meyer et al., 2013; Mendizabal et al., 2013). In the same vascular regions, contractile responses to prostanoids and the expression of their receptors are enhanced in obese mice (Traupe et al., 2002) and diabetic rats (Ishida et al., 2012). Interestingly, however, significant amounts of PVAT‐derived TXA2 and PGE2 are also detected in healthy controls, suggesting a possible physiological role for PVAT (Meyer et al., 2013). Prostanoids might also mediate PVAT‐induced endothelial dysfunction in both normotensive and hypertensive rats (Mendizabal et al., 2013), contributing to its pro‐contractile effects. It remains to be clarified how the secretory pattern of various prostanoids is altered in pathophysiological conditions.
Taken together, these findings indicate that PVAT is capable of releasing various contractile factors, which elicit direct vasocontraction or enhance nerve‐ or agonist‐mediated contractions by acting on the vascular smooth muscle. These factors appear, at least partly, to be active even in normal, healthy circumstances especially in larger arteries. Previous studies have reported elevated systemic levels of angiotensin II, superoxide, catecholamines, contractile prostanoids, TNF‐α, chemerin and leptin in hypertension, diabetes and obesity (Brunner et al., 2005; Gu et al., 2015), but PVAT is yet to be established as a major source of these mediators. There is, however, evidence pointing to an increased responsiveness to chemerin, TNF‐α and prostanoids in aorta and resistance arteries (Traupe et al., 2002; Ishida et al., 2012; Meyer et al., 2013; Watts et al., 2013; Virdis et al., 2015). In addition to acute vasocontraction, sustained elevation of some of the ADCF, such as superoxide, Ang II and TNF‐α, might stimulate vascular smooth muscle growth and arterial stiffness (Almabrouk et al., 2014; Fleenor et al., 2014; Kunimoto et al., 2015; Noblet et al., 2016; also reviewed by Miao and Li, 2012, Aroor et al., 2013 and Villacorta and Chang, 2015), commonly found in atherosclerosis, hypertension and ageing. In line with this, the expression of chemerin in PVAT is positively correlated with atherosclerosis in human aorta and coronary artery (Spiroglou et al., 2010). The vascular remodelling effect of PVAT is also associated with endothelial dysfunction, a hallmark of cardiovascular diseases. A reduction in the endothelium‐dependent relaxation of vascular smooth muscle would exaggerate the pro‐contractile effects of PVAT and this will be explored further in the following section.
Evidence for PVAT‐induced endothelial dysfunction
The vascular endothelium is critical for maintaining cardiovascular homeostasis, and its dysfunction is considered an early sign or predictor of cardiovascular diseases, including those associated with obesity and diabetes (Brunner et al., 2005). Endothelial dysfunction can manifest, for example, as reduced endothelium‐dependent relaxation, endothelium‐dependent contraction, leukocyte adhesion and reduced anti‐coagulation properties. In Table 2, we highlight some of the studies demonstrating the inhibitory effect of PVAT on responses to endothelium‐dependent relaxants, which could enhance vasocontraction and might be particularly relevant for hypertension linked to obesity and diabetes. Where possible, the specific PVAT‐derived mediators and disease conditions involved are also indicated in Table 2.
Table 2.
Inhibitory effect of PVAT on endothelium‐dependent relaxation
| PVAT‐derived factor | Proposed mechanism of inhibition | Endothelium‐dependent relaxant affected | Vascular bed | Reference |
|---|---|---|---|---|
| Resistin | ↓ IRS‐1 and PI3K activity in endothelium ➔ ↓ eNOS activity | Insulin |
Mouse aorta Mouse mesenteric artery |
Gentile et al., 2008 |
| ↑ Endothelial superoxide ➔ ↓ eNOS expression | Bradykinin | Pig coronary artery | Kougias et al., 2005 | |
| Adiponectin | ↓ Adiponectin in PVAT ➔ ↓ AMPK and Akt phosphorylation | Insulin | Mouse resistance artery from skeletal muscle (only in genetic model of obesity and type 2 diabetes) | Meijer et al., 2013 |
| Unknown | ↓ AMPK phosphorylation ➔ ↑ mTOR phosphorylation ➔ ↓ eNOS expression | Acetylcholine | Rat thoracic aorta and mesenteric artery (only in diet‐induced obesity) | Ma et al., 2010 |
| Leptin | ↑ Leptin in PVAT and ↑ leptin receptor expression ➔ ↑ PKCβ activity in vascular cells | Bradykinin |
Pig coronary artery (only in obesity with metabolic syndrome) |
Payne et al., 2010 |
| Unknown | ↑ PKCβ‐mediated eNOS phosphorylation ➔ ↓ endothelial NO | Bradykinin | Dog coronary artery | Payne et al., 2008; Payne et al., 2009 |
| Unknown | ↓ Endothelial Ca2+ signal |
Acetylcholine Methacholine |
Rat coronary septal artery | Aalbaek et al., 2015 |
| Unknown (but independent of superoxide, prostanoids, ET‐1 and AT1) | ↑ Endothelial caveolin‐1 ➔ ↓ NO production | Acetylcholine | Rat thoracic aorta | Lee et al., 2014 |
|
Superoxide Hydrogen peroxide Leptin MCP‐1 |
↑ Superoxide, hydrogen peroxide, leptin and MCP‐1 in PVAT | Acetylcholine |
Mouse abdominal aorta (only in diet‐induced obesity) |
Ketonen et al., 2010 |
| Visfatin | ↑ NADPH oxidase activity in vascular cells ➔ ↓ endothelial NO | Bradykinin or acetylcholine | Rat and human resistance mesenteric arteries | Vallejo et al., 2011 |
|
TNF‐α Adiponectin |
↑ TNF‐α and ↓ adiponectin in PVAT ➔ NADPH oxidase activation ➔ ↓ eNOS expression ➔ ↓ basal endothelial NO ↑ superoxide and ET‐1 in vascular cells |
Human small arteries from visceral fat (enhanced effects in obesity) |
Virdis et al., 2015 | |
| RANTES |
↑ RANTES ➔ ↑ T‐lymphocytes in PVAT but not visceral fat ↑ Ang II‐induced superoxide production in vascular cells |
Acetylcholine | Mouse thoracic and abdominal aorta (enhanced in Ang II‐induced hypertension) | Mikolajczyk et al., 2016 |
↑, increase; ↓, decrease; →, lead to; AT1, Ang II receptor type 1; eNOS, endothelial NOS; ET‐1, endothelin‐1; IRS‐1, insulin receptor substrate‐1; mTOR, mechanistic target of rapamycin; PI3K, phosphoinositide 3‐kinase.
An inhibitory effect of PVAT is often demonstrated by studying the effects of PVAT on responses to endothelium‐dependent relaxants that are applied to isolated arteries or isolated vascular cells. The up‐ or down‐regulation of PVAT‐derived factors are thought to exaggerate the reduction in endothelium‐dependent relaxation in disease states. However, in some studies, the diffusible factors responsible for the inhibitory effects of PVAT on endothelial function are not identified
A primary mechanism of action for PVAT is to reduce NO production or bioavailability, although NO‐independent signalling pathways may also be compromised. Given the physical distance between PVAT and the endothelium particularly in conduit arteries, it is thought that mediators released by PVAT are involved. They include NADPH oxidase‐derived reactive oxygen species (superoxide and hydrogen peroxide) and pro‐inflammatory cytokines (leptin, TNF‐α, IL‐6, resistin and visfatin) (Greenstein et al., 2009; Marchesi et al., 2009; Ketonen et al., 2010; Payne et al., 2010; Vallejo et al., 2011; Aghamohammadzadeh et al., 2016). Importantly, targeting dysregulation of these PVAT factors, which accompanies adipocyte hypertrophy in obesity and metabolic syndrome can improve endothelial function (Marchesi et al., 2009; Aghamohammadzadeh et al., 2016). Circulating visfatin levels may also predict the extent of endothelium‐dependent, flow‐mediated dilation in patients with atherosclerosis and diabetes (Romacho et al., 2013). These findings support the clinical importance of PVAT dysfunction in vascular health. Indeed, oxidative stress and increased production of pro‐inflammatory cytokines, as well as endothelial dysfunction, have been closely linked to the pathophysiology of obesity, hypertension, atherosclerosis and insulin resistance.
Moreover, PVAT can also reduce endothelium‐independent relaxation. In many studies, the presence of PVAT has no significant effect on relaxation to NO donors (Payne et al., 2008; Ma et al., 2010; Vallejo et al., 2011; Lee et al., 2014). However, Tune and co‐workers (Owen et al., 2013; Noblet et al., 2015) have shown that PVAT inhibits distinct subtypes of K+ channels in coronary smooth muscle of lean versus diet‐induced obese pigs. Another adipokine, nesfatin‐1, has also been shown to reduce smooth muscle cGMP production in mesenteric arteries and increase arterial blood pressure in rats (Yamawaki et al., 2012).
Evidence for relaxant factors from PVAT
In contrast to the aforementioned (pro)‐contractile actions, numerous PVAT‐derived mediators are vasorelaxants and therefore exert anti‐contractile effects; these have been the focus of a number of excellent reviews (e.g. Gollasch, 2012 and Withers et al., 2014). PVAT relaxants include adiponectin, omentin, leptin, Ang 1–7, NO, hydrogen peroxide and hydrogen sulphide (Dubrovska et al., 2004; Gao et al., 2007; Lee et al., 2009; Gil‐Ortega et al., 2010; Payne et al., 2010; Schleifenbaum et al., 2010). Again, isolated tension recording and bioassay experiments have been instrumental in establishing an anti‐contractile action of PVAT in arteries from rodents and humans. Of note, the presence of PVAT reduces contraction to some, but not all, vasoconstrictors (Soltis and Cassis, 1991; Lohn et al., 2002; Verlohren et al., 2004; Gao et al., 2005b; Malinowski et al., 2008; Greenstein et al., 2009). Under physiological conditions, adipocytes are thought to be the main cellular source of these factors, which are sometimes referred to as ADRFs (Soltis and Cassis, 1991; Lohn et al., 2002; Verlohren et al., 2004).
Diverse signalling mechanisms have been proposed, including endothelial NO release, cGMP generation, reactive oxygen species and opening of various K+ channel subtypes, but independent of COX products or sympathetic nerves (Gollasch, 2012 and Withers et al., 2014). Accumulating evidence suggests that the anti‐contractile effect of PVAT relies on smooth muscle K+ channels, specifically the activation of voltage‐gated K+ channels (KV7) and Ca2+‐activated K+ channels (BKCa) through endothelium‐independent and ‐dependent pathways respectively. Interestingly, in healthy rat coronary septal arteries, increases in PVAT also reduce Rho kinase‐dependent Ca2+ sensitivity in vascular smooth muscle (Aalbaek et al., 2015). This contrasts with the observation that PVAT from pig coronary artery enhances vasocontraction via Rho kinase (Owen et al., 2013).
As for a pathological role, a loss or reduced relaxant effect of PVAT is often reported in disease states. In spontaneously hypertensive rats, there is a loss of anti‐contractile effect in mesenteric arteries possibly due to a down‐regulation of KV7 channels in vascular smooth muscle or reduced PVAT‐induced production of Ang 1–7, one of the ADRF candidates (Galvez et al., 2006; Galvez‐Prieto et al., 2008b; Li et al., 2013). In experimental models of obesity and metabolic syndrome, increases in PVAT‐derived leptin (Payne et al., 2010; Ketonen et al., 2010), superoxide, hydrogen peroxide (Gao et al., 2005a; Ketonen et al., 2010; Rebolledo et al., 2010; Aghamohammadzadeh et al., 2016) or free fatty acid (Sun et al., 2013) also play a role in aorta, mesenteric or subcutaneous arteries. Although PVAT produces the vasorelaxants adiponectin and NO, obesity is associated with a reduction in PVAT‐derived adiponectin and reduced endothelial NO release and bioavailability, partly due to oxidative stress (cf. Figure 2). For example, in rodent mesenteric arteries, this can be prevented by superoxide dismutase and catalase, which remove superoxide and hydrogen peroxide respectively, or antioxidants (Marchesi et al., 2009; Aghamohammadzadeh et al., 2016). It has been shown in models of hypertension and obesity that, despite an up‐regulation of leptin, there is an impairment of leptin‐induced NO release from the endothelium (Beltowski et al., 2003; Rahmouni et al., 2005; Galvez‐Prieto et al., 2012). This implicates PVAT in vascular leptin resistance, which exacerbates the cardiovascular complications associated with obesity.
Other data suggest that, in obesity and diabetes, a down‐regulation of PVAT‐derived adiponectin might lead to an up‐regulation of superoxide and TNF‐α and reduced endothelial NO production and relaxation (cf. Table 2; Virdis et al., 2015; Hou et al., 2016; Nacci et al., 2016). It is often unclear why PVAT‐derived relaxants are down‐regulated in disease states, but hypoxia in PVAT might be a contributing factor (Withers et al., 2011). A local reduction of PVAT adiponectin (Virdis et al., 2015; Aghamohammadzadeh et al., 2016) without concomitant changes in its circulatory levels is also evident in human obesity (Dreier et al., 2016). Of particular relevance to insulin resistance and diabetes, adiponectin is known to activate AMP‐activated protein kinase (AMPK), a key intracellular energy sensor that improves insulin sensitivity, and modulate adipocyte metabolism and inflammation (see Almabrouk et al., 2014 for reivew). AMPK in endothelium and vascular smooth muscle also regulates vascular tone and remodelling (Ma et al., 2010; Meijer et al., 2013; Almabrouk et al., 2014) and probably contributes to the cardiovascular benefits of the anti‐diabetic drugs, glitazones, which are PPARγ agonists and AMPK activators. Interestingly, a recent study suggests that AMPK in PVAT is required for the secretion of adiponectin in mouse aorta (Almabrouk et al., 2016), providing a molecular mechanism for crosstalks with hypoxia and other PVAT‐derived vasoactive substances that activate or inhibit AMPK (Almabrouk et al., 2014; Virdis et al., 2015).
Anti‐contractile versus pro‐contractile effects of PVAT
The coexistence of pro‐contractile and anti‐contractile actions of PVAT may seem contradictory, but such dual effects have also been demonstrated in the same arteries and within the same studies (Soltis and Cassis, 1991; Lohn et al., 2002; Ketonen et al., 2010; Li et al., 2013; Aalbaek et al., 2015). Indeed, some of the PVAT‐derived factors such as leptin, TNF‐α, IL‐6 and hydrogen peroxide are known to have both contractile and relaxant properties (Brian and Faraci, 1998; Orshal and Khalil, 2004; Thakali et al., 2006; Virdis et al., 2015). The up‐regulation or down‐regulation of these factors can also compromise endothelial function (cf. Table 2). It is therefore not surprising that the balance between pro‐contractile and anti‐contractile function and how it is altered in disease conditions is under increasingly intense investigations.
Both relaxant and contractile actions of PVAT effects are detectable in healthy conditions, at least in thoracic aorta and mesenteric and coronary artery (Soltis and Cassis, 1991; Dubrovska et al., 2004; Verlohren et al. 2004; Payne et al., 2010). Many studies have proposed a predominantly anti‐contractile action in health, although it is possible that the net effect on vascular tone depends on the anatomical location and experimental conditions used. As discussed in the previous section, systemic arteries (including mesenteric artery and thoracic and abdominal aorta) and coronary arteries often show a reduced production or responsiveness to PVAT‐derived relaxants or other vasorelaxants in hypertension, obesity and diabetes. This, together with an underlying contraction induced by PVAT, which can also be enhanced in some forms of hypertension and obesity (cf. Table 1), would promote a net contractile action of PVAT (Figure 1). This may result in sustained vasoconstriction. Indeed, PVAT dysfunction is correlated with raised arterial blood pressure in obese rats (Aghamohammadzadeh et al., 2016). Genetic deletion of PPARγ in mouse perivascular adipocytes during development results in the absence of PVAT and hypotension, indicating a key role for PVAT in the regulation of blood pressure (Chang et al., 2012). However, we are yet to fully understand how PVAT function transitions from health to disease and how best to reverse the adverse effects of PVAT. In the case of obesity, weight loss through bariatric surgery or caloric restriction might reduce PVAT‐mediated inflammation and improve NO bioavailability, resulting in normalized blood pressure (Aghamohammadzadeh et al., 2013; Bussey et al., 2016).
Figure 1.
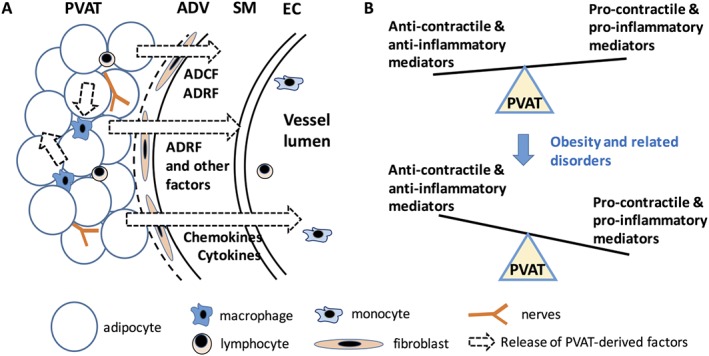
Regulation of vascular tone by PVAT in health and disease. (A) PVAT releases a diverse group of bioactive and diffusible substances, including leptin, adiponectin, Ang II, Ang 1–7, catecholamines, reactive oxygen species, NO, hydrogen sulphide, cytokines such as TNF‐α and IL‐6, and chemokines such as MCP‐1 and RANTES. These mediators modulate vascular tone through a paracrine action on the endothelium, vascular smooth muscle and immune cells. The chemokines and cytokines regulate the migration of immune cells into PVAT, and activated macrophages and lymphocytes within PVAT can also release additional cytokines. (B) In healthy conditions, PVAT tends to exert a net anti‐contractile effect. Pathophysiological stimuli for example in obesity, hypertension and diabetes alter the secretion pattern of PVAT, leading to increased pro‐contractile and decreased anti‐contractile actions. This imbalance is characteristic of PVAT dysfunction in disease states. Other changes in PVAT composition and function include adipocyte hypertrophy, infiltration of macrophages and lymphocytes, and inflammation within PVAT and vascular cells. ADV, adventitia; EC, endothelium; SM, smooth muscle.
Interactions between adipocytes and immune cells in PVAT
Aside from vascular reactivity, many of the PVAT‐derived mediators are also critical players in vascular inflammation. Evidence suggests that a pro‐inflammatory phenotype of PVAT is a common feature of hypertension, obesity, insulin resistance and atherosclerosis (Chatterjee et al., 2009; Almabrouk et al., 2014; Omar et al., 2014; Mikolajczyk et al., 2016). Adipocytes are the main component in PVAT, but immune cells such as macrophages and T‐lymphocytes also play an important role in regulating PVAT function and provide an alternative source of vasoactive mediators. As part of the pathological remodelling of adipose tissues, obese rodents and humans have a higher PVAT mass and adipocyte hypertrophy (Greenstein et al., 2009; Marchesi et al., 2009; Ma et al., 2010). The hypertrophied PVAT probably exceeds the diffusion limit of oxygen and suffers from hypoperfusion, leading to local hypoxia (Hosogai et al., 2007; Greenstein et al., 2009). The hypoxic state is linked to an increased expression of the chemokine monocyte chemoattractant protein‐1 (MCP‐1 also known as CCL2 ) in PVAT, which in turn promotes the recruitment and infiltration of macrophages, which act as a major source of TNF‐α (see Gustafson et al., 2007, for a review; Ketonen et al., 2010). From models of obesity, hypertension and metabolic syndrome, it has been demonstrated that PVAT also stimulates the recruitment of monocytes and lymphocytes in arteries by up‐regulating of the expression of the chemokines IL‐8 and RANTES and superoxide in vascular cells (Table 2) (Henrichot et al., 2005; Marchesi et al., 2009; Mikolajczyk et al., 2016). Figure 2 illustrates how the dysregulation of PVAT‐derived factors might occur in these disease states.
Figure 2.
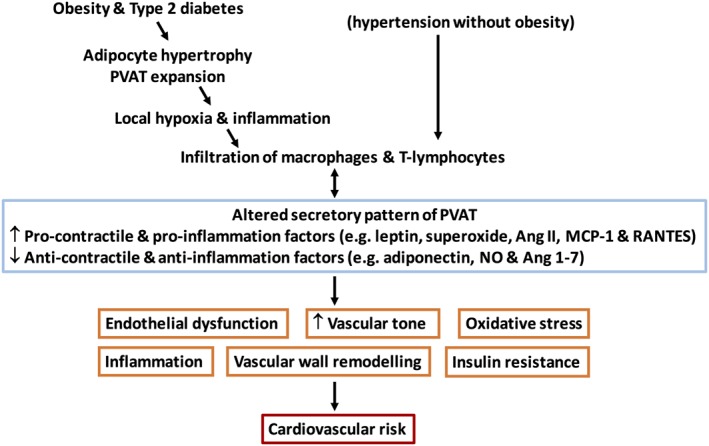
Proposed mechanisms of PVAT dysfunction in obesity, diabetes and hypertension. PVAT dysfunction is characterized by changes in its secretory pattern and increased occurrence of activated macrophages and lymphocytes in PVAT. In addition to adipocytes, activated immune cells within PVAT also release additional cytokines. In obesity and Type 2 diabetes, PVAT dysfunction is probably triggered by adipocyte hypertrophy and increases in PVAT mass. PVAT expansion is not a prerequisite for PVAT dysfunction, since the size of adipocytes and overall PVAT mass may be reduced in some forms of hypertension in the absence of obesity.
At the same time as there is an up‐regulation of pro‐inflammatory mediators from PVAT (e.g. TNF‐α and IL‐6), there is also a down‐regulation of anti‐inflammatory mediators (e.g. adiponectin and IL‐10), from adipocytes and macrophages (Greenstein et al., 2009; Chatterjee et al., 2009; Lumeng et al., 2007). The resultant pro‐inflammatory phenotype has been linked to the loss of PVAT‐induced relaxation, and this deficit may be partially reversed by TNF‐α antagonists or IL‐6 antibodies (Greenstein et al., 2009; Ozen et al., 2015; Aghamohammadzadeh et al., 2016). Endothelium‐dependent relaxation is also likely to be compromised by PVAT‐derived TNF‐α and reactive oxygen species (Virdis et al., 2015). In macrophage‐deficient mice, the ability of hypoxia to inhibit PVAT‐mediated relaxation is greatly reduced, supporting a key role for these macrophages (Withers et al., 2011). In addition, there is an accumulation of leukocytes in PVAT, so much so that a deficit in P‐selectin glycoprotein ligand‐1, a ligand essential for leukocyte attachment and rolling at the endothelium, prevents the endothelial dysfunction and inflammation mediated by PVAT in obese mice (Wang et al., 2012).
In contrast to the conditions in obesity, the size of PVAT adipocytes and PVAT mass are often reduced in experimental models of hypertension, including spontaneously hypertensive and DOCA–salt hypertensive rats (Galvez et al., 2006; Ruan et al., 2010). Despite this, a recent study has demonstrated that Ang II‐induced hypertension increases PVAT‐mediated expression of RANTES, resulting in increased T‐lymphocyte infiltration and impaired endothelium‐dependent relaxation (Mikolajczyk et al., 2016; Figure 2). It should be noted that whilst adipokines such as chemerin, adiponectin and leptin are released from PVAT adipocytes, many of the PVAT‐derived chemokines, cytokines and reactive oxygen species might be produced by multiple cell types within PVAT, including adipocytes and immune cells (see also Szasz et al., 2013 and Pellegrinelli et al., 2016, for reviews). Furthermore, vascular cells are responsible for some of the pro‐ and anti‐inflammatory mediators produced by PVAT and also express receptors for these mediators. Therefore, the interplay among adipocytes, infiltrated immune cells and vascular cells needs to be explored further.
PVAT dysfunction in humans
The past 10 years has seen a growing interest in PVAT dysfunction. While the current data on human PVAT remain limited, they broadly agree with those obtained in animal models. For example, the anti‐contractile effects of PVAT are compromised in small arteries of patients with metabolic syndrome or obesity (Greenstein et al., 2009; Aghamohammadzadeh et al., 2013), and that human PVAT shows a distinctive pattern of expression of pro‐inflammatory mediators, including IL‐6, MCP‐1 and leptin, compared with subcutaneous adipose tissue (Rittig et al., 2012; Mauro et al., 2013). However, the functional significance of various ADCFs and ADRFs may differ. For example, adiponectin appears to play a more important role in PVAT relaxation in humans than in rodents (Fesus et al., 2007; Greenstein et al., 2009; Meijer et al., 2013). In patients undergoing coronary bypass surgery, initial experiments suggest that a saphenous vein graft with intact adventitia and PVAT, as opposed to a conventional PVAT‐free graft, reduces vasospasm and potentially improves its patency (Dashwood et al., 2009). Thus, it might be concluded that PVAT exerts a predominantly anti‐contractile effect in humans. In contrast, individuals with more PVAT in their brachial artery have a diminished hyperaemic blood flow (Rittig et al., 2008), suggesting a basal contractile effect of PVAT. Further characterization of PVAT‐derived factors from different vascular regions is needed. Thus far, mechanistic studies have been performed on the more accessible vessels from volunteers, namely, the internal thoracic artery (Gao et al., 2005a; Malinowski et al., 2008), small arteries in gluteal fat (Greenstein et al., 2009; Aghamohammadzadeh et al., 2013) and saphenous veins (Dashwood et al., 2009).
The Framingham Heart Study reported a correlation between periaortic fat mass and hypertension, and diabetes, irrespective of the body mass index, but a causal relationship is yet to be established (Lehman et al., 2010; Britton et al., 2012). In addition to reduced body weight, bariatric surgery in severely obese patients has been shown to restore PVAT‐induced relaxation, improve the inflammatory cytokine profile and NO bioavailability and reduce macrophage infiltration and systolic blood pressure (Aghamohammadzadeh et al., 2013). More recently, in diet‐induced obese rats, calorie restriction and sustained weight loss has similarly been found to reverse PVAT‐mediated vascular damage (Bussey et al., 2016). These data support the concept that PVAT dysfunction contributes to the pathogenesis of obesity and metabolic syndrome. An increase in the circulatory levels of chemerin has also been correlated with impaired endothelial function and increased arterial stiffness in hypertensive patients (Gu et al., 2015). The specific role played by PVAT relative to other fat depots merits further investigations, particularly in view of the differential responses to high‐fat diet in white and brown adipocytes (Fitzgibbons et al., 2011).
When assessing data from animal and human experimental studies, it is also important to consider the effect of ageing, an independent risk factor for cardiovascular diseases. Ageing exacerbates aortic PVAT dysfunction, with increases in oxidative stress and macrophage infiltration and a pro‐inflammatory secretion pattern of cytokines and chemokines (Bailey‐Downs et al., 2013; Mauro et al., 2013; Fleenor et al., 2014). This effect is at least partly mimicked by medium conditioned with PVAT from aged aorta and is accompanied by endothelial dysfunction in aorta, especially in diet‐induced obese mice (Bailey‐Downs et al., 2013). Thus, PVAT may also contribute to the endothelial dysfunction and vascular remodelling seen in ageing. Moreover, PVAT‐mediated relaxation is inhibited in ageing mice (Agabiti‐Rosei et al., 2017), hinting at an overall pro‐contractile action of PVAT during ageing. Further investigations are needed to clarify the function of PVAT in healthy versus pathological ageing. It is also worth noting that the age of rodents used in models of obesity varies (Lutz and Woods, 2012) and is generally younger than that of subjects involved in clinical studies.
Conclusion
In this review, we have focused on the effects of PVAT on vascular tone regulation. It is apparent that PVAT exerts both contractile and relaxant actions through the release of autocrine/paracrine factors from adipocytes and infiltrating inflammatory cells (Figure 1). PVAT is therefore an integral part of vascular function, including crosstalk with the endothelium, smooth muscle, immune cells and perivascular nerves. The balance between pro‐contractile and anti‐contractile effects maybe tissue‐specific, but its modulation by obesity and hypertension induces a shift towards a pro‐contractile, pro‐inflammatory and pro‐oxidative phenotype (Figure 2). This PVAT dysfunction may also occur in other obesity‐related disorders, including metabolic syndrome, diabetes and atherosclerosis. However, despite a much better understanding of the structure and function of PVAT, there are still many unanswered questions. The molecular mechanisms that regulate PVAT quantity and composition, and secretion of vasoactive factors in health and disease, which probably exist in a continuum, remain elusive. Although there is experimental evidence for PVAT dysfunction in the pathogenesis of hypercontractility in disease states, it is also possible that it has a protective and adaptive role in vascular homeostasis. Moreover, the function of PVAT relative to the systemic influence of visceral and subcutaneous fat remains to be clarified. Given the heterogeneity of PVAT function in different anatomical regions and species, more studies in human tissues are required.
Conflict of interest
The authors declare no conflicts of interest.
Ramirez, J. G. , O'Malley, E. J. , and Ho, W. S. V. (2017) Pro‐contractile effects of perivascular fat in health and disease. British Journal of Pharmacology, 174: 3482–3495. doi: 10.1111/bph.13767.
References
- Aalbaek F, Bonde L, Kim S, Boedtkjer E (2015). Perivascular tissue inhibits rho‐kinase‐dependent smooth muscle Ca2+ sensitivity and endothelium‐dependent H2S signalling in rat coronary arteries. J Physiol 593: 4747–4764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agabiti‐Rosei C, Favero G, de Ciuceis C, Rossini C, Porteri E, Rodella LF et al. (2017). Effect of long‐term treatment with melatonin on vascular markers of oxidative stress/inflammation and on the anticontractile activity of perivascular fat in aging mice. Hypertens Res 40: 41–50. [DOI] [PubMed] [Google Scholar]
- Aghamohammadzadeh R, Greenstein AS, Yadav R, Jeziorska M, Hama S, Soltani F et al. (2013). Effects of bariatric surgery on human small artery function: evidence for reduction in perivascular adipocyte inflammation, and the restoration of normal anticontractile activity despite persistent obesity. J Am Coll Cardiol 62: 128–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aghamohammadzadeh R, Unwin RD, Greenstein AS, Heagerty AM (2016). Effects of obesity on perivascular adipose tissue vasorelaxant function: nitric oxide, inflammation and elevated systemic blood pressure. J Vasc Res 52: 299–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahima RS, Flier JS (2000). Adipose tissue as an endocrine organ. Trends Endocrinol Metab 11: 327–332. [DOI] [PubMed] [Google Scholar]
- Alexander SP, Davenport AP, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015a). The Concise Guide to PHARMACOLOGY 2015/16: G protein‐coupled receptors. Br J Pharmacol 172: 5744–5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Catterall WA, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015b). The Concise Guide to PHARMACOLOGY 2015/16: Voltage‐gated ion channels. Br J Pharmacol 172: 5904–5941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Cidlowski JA, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015c). The Concise Guide to PHARMACOLOGY 2015/16: Nuclear hormone receptors. Br J Pharmacol 172: 5956–5978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Fabbro D, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015d). The Concise Guide to PHARMACOLOGY 2015/16: Enzymes. Br J Pharmacol 172: 6024–6109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almabrouk TA, Ewart MA, Salt IP, Kennedy S (2014). Perivascular fat, AMP‐activated protein kinase and vascular diseases. Br J Pharmacol 171: 595–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almabrouk TA, Ugusman AB, Katwan OJ, Ian P, Kennedy S (2016). Deletion of AMPKα1 attenuates the anticontractile effect of perivascular adipose tissue (PVAT) and reduces adiponectin release. Br J Pharmacol. https://doi.org/10.1111/bph.13633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aroor A, Demarco V, Jia G, Sun Z, Nistala R, Meininger G et al. (2013). The role of tissue renin–angiotensin–aldosterone system in the development of endothelial dysfunction and arterial stiffness. Front Endocrinol 4: 161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayala‐Lopez N, Martini M, Jackson W, Darios E, Burnett R, Seitz B et al. (2014). Perivascular adipose tissue contains functional catecholamines. Pharmacol Res Perspect 2: e00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey‐Downs LC, Tucsek Z, Toth P, Sosnowska D, Gautam T, Sonntag WE et al. (2013). Aging exacerbates obesity‐induced oxidative stress and inflammation in perivascular adipose tissue in mice: a paracrine mechanism contributing to vascular redox dysregulation and inflammation. J Gerontol 68: 780–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltowski J, Wójcicka G, Jamroz A (2003). Stimulatory effect of leptin on nitric oxide production is impaired in dietary‐induced obesity. Obes Res 11: 1571–1580. [DOI] [PubMed] [Google Scholar]
- Brian JE Jr, Faraci FM (1998). Tumor necrosis factor‐alpha‐induced dilatation of cerebral arterioles. Stroke 29: 509–515. [DOI] [PubMed] [Google Scholar]
- Britton KA, Pedley A, Massaro JM, Corsini EM, Murabito JM, Hoffmann U et al. (2012). Prevalence, distribution, and risk factor correlates of high thoracic periaortic fat in the Framingham heart study. J Am Heart Assoc 1: e004200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner H, Cockcroft JR, Deanfield J, Donald A, Ferrannini E, Halcox J et al. (2005). Endothelial function and dysfunction. Part II: association with cardiovascular risk factors and diseases. A statement by the working group on endothelins and endothelial factors of the European society of hypertension. J Hypertens 23: 233–246. [DOI] [PubMed] [Google Scholar]
- Bulloch JM, Daly CJ (2014). Autonomic nerves and perivascular fat: interactive mechanisms. Pharmacol Ther 143: 61–73. [DOI] [PubMed] [Google Scholar]
- Bussey CE, Withers SB, Aldous RG, Edwards G, Heagerty AM (2016). Obesity‐related perivascular adipose tissue damage is reversed by sustained weight loss in the rat. Arterioscler Thromb Vasc Biol 36: 1377–1385. [DOI] [PubMed] [Google Scholar]
- Cassis LA, Police SB, Yiannikouris F, Thatcher SE (2008). Local adipose tissue renin–angiotensin system. Curr Hypertens Rep 10: 93–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassis L, Lynch K, Peach M (1988). Localization of angiotensinogen messenger RNA in rat aorta. Circ Res 62: 1259–1262. [DOI] [PubMed] [Google Scholar]
- Chang L, Villacorta L, Li R, Hamblin M, Xu W, Dou C et al. (2012). Loss of perivascular adipose tissue on peroxisome proliferator‐activated receptor‐γ deletion in smooth muscle cells impairs intravascular thermoregulation and enhances atherosclerosis. Circulation 126: 1067–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee TK, Stoll LL, Denning GM, Harrelson A, Blomkalns AL, Idelman G et al. (2009). Proinflammatory phenotype of perivascular adipocytes. Circ Res 104: 541–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darios E, Winner B, Charvat T, Krasinksi A, Punna S, Watts S (2016). The adipokine chemerin amplifies electrical field‐stimulated contraction in the isolated rat superior mesenteric artery. Am J Physiol Heart Circ Physiol 311: H498–H507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dashwood MR, Loesch A (2011). Does perivascular fat influence neural control of the saphenous vein? Implications in coronary artery bypass surgery (CABG). Curr Neurobiol 2: 71–74. [Google Scholar]
- Dashwood MR, Savage K, Tsui JC, Dooley A, Shaw SG, Fernandez Alfonso MS et al. (2009). Retaining perivascular tissue of human saphenous vein grafts protects against surgical and distension‐induced damage and preserves endothelial nitric oxide synthase and nitric oxide synthase activity. J Thorac Cardiovasc Surg 138: 334–340. [DOI] [PubMed] [Google Scholar]
- Dreier R, Asferg C, Berg JO, Andersen UB, Flyvbjerg A, Frystyk J et al. (2016). Similar adiponectin levels in obese normotensive and obese hypertensive men and no vasorelaxant effect of adiponectin on human arteries. Basic Clin Pharmacol Toxicol 118: 128–135. [DOI] [PubMed] [Google Scholar]
- Dubrovska G, Verlohren S, Luft FC, Gollasch M (2004). Mechanisms of ADRF release from rat aortic adventitial adipose tissue. Am J Physiol Heart Circ Physiol 286: H1107–H1113. [DOI] [PubMed] [Google Scholar]
- Engeli S, Gorzelniak K, Kreutz R, Runkel N, Distler A, Sharma AM (1999). Co‐expression of renin–angiotensin system genes in human adipose tissue. J Hypertens 17: 555–560. [DOI] [PubMed] [Google Scholar]
- Fernandez Alfonso MS, Gil‐Ortega M, Aranguez I, Souza D, Dreifaldt M, Somoza B et al. (2017). Role of PVAT on coronary atherosclerosis and vein graft patency: friend or foe? Br J Pharmacol. https://doi.org/10.1111/bph.13734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fesus G, Dubrovska G, Gorzelniak K, Kluge R, Huang Y, Luft FC et al. (2007). Adiponectin is a novel humoral vasodilator. Cardiovasc Res 75: 719–727. [DOI] [PubMed] [Google Scholar]
- Fitzgibbons TP, Kogan S, Aouadi M, Hendricks GM, Straubhaar J, Czech MP (2011). Similarity of mouse perivascular and brown adipose tissues and their resistance to diet‐induced inflammation. Am J Physiol Heart Circ Physiol 301: H1425–H1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleenor BS, Eng JS, Sindler AL, Pham BT, Kloor JD, Seals DR (2014). Superoxide signaling in perivascular adipose tissue promotes age‐related artery stiffness. Aging Cell 13: 576–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvez B, de Castro J, Herold D, Dubrovska G, Arribas S, Gonzalez MC et al. (2006). Perivascular adipose tissue and mesenteric vascular function in spontaneously hypertensive rats. Arterioscler Thromb Vasc Biol 26: 1297–1302. [DOI] [PubMed] [Google Scholar]
- Galvez‐Prieto B, Bolbrinker J, Stucchi P, de las Heras AI, Merino B, Arribas S et al. (2008a). Comparative expression analysis of the renin–angiotensin system components between white and brown perivascular adipose tissue. J Endocrinol 197: 55–64. [DOI] [PubMed] [Google Scholar]
- Galvez‐Prieto B, Dubrovska G, Cano MV, Delgado M, Aranguez I, Gonzalez MC et al. (2008b). A reduction in the amount and anti‐contractile effect of periadventitial mesenteric adipose tissue precedes hypertension development in spontaneously hypertensive rats. Hypertens Res 31: 1415–1423. [DOI] [PubMed] [Google Scholar]
- Galvez‐Prieto B, Somoza B, Gil‐Ortega M, Garcia‐Prieto CF, Gonzalez CM, Arribas S et al. (2012). Anticontractile effect of perivascular adipose tissue and leptin are reduced in hypertension. Front Pharmacol 3: 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao YJ (2007). Dual modulation of vascular function by perivascular adipose tissue and its potential correlation with adiposity/lipoatrophy‐related vascular dysfunction. Curr Pharm Des 13: 2185–2192. [DOI] [PubMed] [Google Scholar]
- Gao YJ, Holloway AC, Zeng ZH, Lim GE, Petrik JJ, Foster WG et al. (2005a). Prenatal exposure to nicotine causes postnatal obesity and altered perivascular adipose tissue function. Obes Res 13: 687–692. [DOI] [PubMed] [Google Scholar]
- Gao YJ, Lu C, Su LY, Sharma AM (2007). Modulation of vascular function by perivascular adipose tissue: the role of endothelium and hydrogen peroxide. Br J Pharmacol 151: 323–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao YJ, Takemori K, Su L, An W, Lu C, Sharma A et al. (2006). Perivascular adipose tissue promotes vasoconstriction: the role of superoxide anion. Cardiovasc Res 71: 363–373. [DOI] [PubMed] [Google Scholar]
- Gao YJ, Zeng ZH, Teoh K, Sharma AM, Abouzahr L, Cybulsky I et al. (2005b). Perivascular adipose tissue modulates vascular function in the human internal thoracic artery. J Thorac Cardiovasc Surg 130: 1130–1136. [DOI] [PubMed] [Google Scholar]
- Gentile MT, Vecchione C, Marino G, Aretini A, Di Pardo A, Antenucci G et al. (2008). Resistin impairs insulin‐evoked vasodilation. Diabetes 57: 577–583. [DOI] [PubMed] [Google Scholar]
- Gil‐Ortega M, Somoza B, Huang Y, Gollasch M, Fernández‐Alfonso MS (2015). Regional differences in perivascular adipose tissue impacting vascular homeostasis. Trends Endocrinol Metab 26: 367–375. [DOI] [PubMed] [Google Scholar]
- Gil‐Ortega M, Stucchi P, Guzman‐Ruiz R, Cano V, Arribas S, Gonzalez MC et al. (2010). Adaptative nitric oxide overproduction in perivascular adipose tissue during early diet‐induced obesity. Endocrinology 151: 3299–3306. [DOI] [PubMed] [Google Scholar]
- Gollasch M (2012). Vasodilator signals from perivascular adipose tissue. Br J Pharmacol 165: 633–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graus‐Nunes F, Rachid TL, de Oliveira Santos F, Barbosa‐da‐Silva S, Souza‐Mello V (2017). AT1 receptor antagonist induces thermogenic beige adipocytes in the inguinal white adipose tissue of obese mice. Endocrine 55: 786–798. [DOI] [PubMed] [Google Scholar]
- Greenberg S, Xie J, Wang Y, Cai B, Kolls J, Nelson S et al. (1985). Tumor necrosis factor‐alpha inhibits endothelium‐dependent relaxation. J Appl Physiol 74: 2394–2403. [DOI] [PubMed] [Google Scholar]
- Greenstein AS, Khavandi K, Withers SB, Sonoyama K, Clancy O, Jeziorska M et al. (2009). Local inflammation and hypoxia abolish the protective anticontractile properties of perivascular fat in obese patients. Circulation 119: 1661–1670. [DOI] [PubMed] [Google Scholar]
- Gu P, Cheng M, Hui X, Lu B, Jiang W, Shi Z (2015). Elevating circulation chemerin level is associated with endothelial dysfunction and early atherosclerotic changes in essential hypertensive patients. J Hypertens 33: 1624–1632. [DOI] [PubMed] [Google Scholar]
- Gustafson B, Hammarstedt A, Andersson CX, Smith U (2007). Inflamed adipose tissue: a culprit underlying the metabolic syndrome and atherosclerosis. Arterioscler Thromb Vasc Biol 27: 2276–2283. [DOI] [PubMed] [Google Scholar]
- Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S et al. (2007). Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med 204: 2449–2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henrichot E, Juge‐Aubry CE, Pernin A, Pache J, Velebit V, Dayer J et al. (2005). Production of chemokines by perivascular adipose tissue. Arterioscler Thromb Vasc Biol 25: 2594–2599. [DOI] [PubMed] [Google Scholar]
- Hosogai N, Fukuhara A, Oshima K, Miyata Y, Tanaka S, Segawa K et al. (2007). Adipose tissue hypoxia in obesity and its impact on adipocytokine dysregulation. Diabetes 56: 901–911. [DOI] [PubMed] [Google Scholar]
- Hou N, Liu Y, Han F, Wang D, Sun X (2016). Irisin improves perivascular adipose tissue dysfunction via regulation of the heme oxygenase‐1/adiponectin axis in diet‐induced obese mice. J Mol Cell Cardiol 99: 188–196. [DOI] [PubMed] [Google Scholar]
- Ishida K, Matsumoto T, Taguchi K, Kamata K, Kobayashi T (2012). Protein kinase C delta contributes to increase in EP3 agonist‐induced contraction in mesenteric arteries from Type 2 diabetic Goto‐Kakizaki rats. Pflugers Arch 463: 593–602. [DOI] [PubMed] [Google Scholar]
- Karlsson C, Lindell K, Ottosson M, Sjöström L, Carlsson B, Carlsson L (1998). Human adipose tissue expresses angiotensinogen and enzymes required for its conversion to angiotensin II. J Clin Endocrinol Metab 83: 3925–3929. [DOI] [PubMed] [Google Scholar]
- Ketonen J, Shi J, Martonen E, Mervaala E (2010). Periadventitial adipose tissue promotes endothelial dysfunction via oxidative stress in diet‐induced obese C57Bl/6 mice. Circ J 74: 1479–1487. [DOI] [PubMed] [Google Scholar]
- Kougias P, Chai H, Lin PH, Lumsden AB, Yao Q, Chen C (2005). Adipocyte‐derived cytokine resistin causes endothelial dysfunction of porcine coronary arteries. J Vasc Surg 41: 691–698. [DOI] [PubMed] [Google Scholar]
- Kunimoto H, Kazama K, Takai M, Oda M, Okada M, Yamawaki H (2015). Chemerin promotes the proliferation and migration of vascular smooth muscle and increases mouse blood pressure. Am J Physiol Heart Circ Physiol 309: H1017–H1028. [DOI] [PubMed] [Google Scholar]
- Lee MH, Chen SJ, Tsao CM, Wu CC (2014). Perivascular adipose tissue inhibits endothelial function of rat aortas via caveolin‐1. PLoS One 9: e99947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RMKW, Lu C, Su L, Gao YJ (2009). Endothelium‐dependent relaxation factor released by perivascular adipose tissue. J Hypertens 27: 782–790. [DOI] [PubMed] [Google Scholar]
- Lehman SJ, Massaro JM, Schlett CL, O'Donnell CJ, Hoffmann U, Fox CS (2010). Peri‐aortic fat, cardiovascular disease risk factors, and aortic calcification: the Framingham heart study. Atherosclerosis 210: 656–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Andersen I, Aleke J, Golubinskaya V, Gustafsson H, Nilsson H (2013). Reduced anti‐contractile effect of perivascular adipose tissue on mesenteric small arteries from spontaneously hypertensive rats: role of Kv7 channels. Eur J Pharmacol 698: 310–315. [DOI] [PubMed] [Google Scholar]
- Lobato NS, Neves KB, Filgueira FP, Fortes ZB, Carvalho MHC, Webb RC et al. (2012). The adipokine chemerin augments vascular reactivity to contractile stimuli via activation of the MEK‐ERK1/2 pathway. Life Sci 91: 600–606. [DOI] [PubMed] [Google Scholar]
- Lohn M, Dubrovska G, Lauterbach B, Luft FC, Gollasch M, Sharma AM (2002). Periadventitial fat releases a vascular relaxing factor. FASEB J 16: 1057–1063. [DOI] [PubMed] [Google Scholar]
- Lu C, Su L, Lee R, Gao YJ (2010). Mechanisms for perivascular adipose tissue‐mediated potentiation of vascular contraction to perivascular neuronal stimulation: the role of adipocyte‐derived angiotensin II. Eur J Pharmacol 634: 107–112. [DOI] [PubMed] [Google Scholar]
- Lu H, Rateri DL, Feldman DL, Jr RJ, Fukamizu A, Ishida J et al. (2008). Renin inhibition reduces hypercholesterolemia‐induced atherosclerosis in mice. J Clin Invest 118: 984–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumeng CN, Bodzin JL, Saltiel AR (2007). Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest 117: 175–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz TA, Woods SC (2012). Overview of animal models of obesity. Curr Protoc Pharmacol. https://doi.org/10.1002/0471141755.ph0561s58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Ma S, He H, Yang D, Chen X, Luo Z et al. (2010). Perivascular fat‐mediated vascular dysfunction and remodeling through the AMPK/mTOR pathway in high‐fat diet‐induced obese rats. Hypertens Res 33: 446–453. [DOI] [PubMed] [Google Scholar]
- Malinowski M, Deja MA, Gołba KS, Roleder T, Biernat J, Wos S (2008). Perivascular tissue of internal thoracic artery releases potent nitric oxide and prostacyclin‐independent anticontractile factor. Eur J Cardiol Thorac Surg 33: 225–231. [DOI] [PubMed] [Google Scholar]
- Marchesi C, Ebrahimian T, Angulo O, Paradis P, Schiffrin EL (2009). Endothelial nitric oxide synthase uncoupling and perivascular adipose oxidative stress and inflammation contribute to vascular dysfunction in a rodent model of metabolic syndrome. Hypertension 54: 1384–1392. [DOI] [PubMed] [Google Scholar]
- Mauro CR, Ilonzo G, Nguyen BT, Yu P, Tao M, Gao I et al. (2013). Attenuated adiposopathy in perivascular adipose tissue compared with subcutaneous human adipose tissue. Am J Surg 206: 241–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer RI, Bakker W, Alta CL, Sipkema P, Yudkin JS, Viollet B et al. (2013). Perivascular adipose tissue control of insulin‐induced vasoreactivity in muscle is impaired in db/db mice. Diabetes 62: 590–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendizabal Y, Llorens S, Nava E (2013). Vasoactive effects of prostaglandins from the perivascular fat of mesenteric resistance arteries in WKY and SHROB rats. Life Sci 93: 1023–1032. [DOI] [PubMed] [Google Scholar]
- Meyer MR, Fredette NC, Barton M, Prossnitz ER (2013). Regulation of vascular smooth muscle tone by adipose‐derived contracting factor. PLoS One 8: e79245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao CY, Li ZY (2012). The role of perivascular adipose tissue in vascular smooth muscle cell growth. Br J Pharmacol 165: 643–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikolajczyk TP, Nosalski R, Szczepaniak P, Budzyn K, Osmenda G, Skiba D et al. (2016). Role of chemokine RANTES in the regulation of perivascular inflammation, T‐cell accumulation, and vascular dysfunction in hypertension. FASEB J 30: 1987–1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nacci C, Leo V, De Benedictis L, Potenza MA, Sgarra L, De Salvia MA et al. (2016). Infliximab therapy restores adiponectin expression in perivascular adipose tissue and improves endothelial nitric oxide‐mediated vasodilation in mice with type 1 diabetes. Vascul Pharmacol. https://doi.org/10.1016/j.vph.2016.08.007. [DOI] [PubMed] [Google Scholar]
- Nguyen KD, Qiu Y, Cui X, Goh YP, Mwangi J, David T et al. (2011). Alternatively activated macrophages produce catecholamines to sustain adaptive thermogenesis. Nature 480: 104–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noblet JN, Goodwill AG, Sassoon DJ, Kiel AM, Tune JD (2016). Leptin augments coronary vasoconstriction and smooth muscle proliferation via a Rho‐kinase‐dependent pathway. Basic Res Cardiol 111: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noblet JN, Owen MK, Goodwill AG, Sassoon DJ, Tune JD (2015). Lean and obese coronary perivascular adipose tissue impairs vasodilation via differential inhibition of vascular smooth muscle K+ channels. Arterioscler Thromb Vasc Biol 35: 1393–1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omar A, Chatterjee TK, Tang Y, Hui DY, Weintraub NL (2014). Proinflammatory phenotype of perivascular adipocytes. Arterioscler Thromb Vasc Biol 34: 1631–1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orshal JM, Khalil RA (2004). Interleukin‐6 impairs endothelium‐dependent NO–cGMP‐mediated relaxation and enhances contraction in systemic vessels of pregnant rats. Am J Physiol Regul Integr Comp Physiol 286: R1013–R1023. [DOI] [PubMed] [Google Scholar]
- Owen M, Witzmann F, McKenney M, Lai X, Berwick Z, Moberly S et al. (2013). Perivascular adipose tissue potentiates contraction of coronary vascular smooth muscle: influence of obesity. Circulation 128: 9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozen G, Daci A, Norel X, Topal G (2015). Human perivascular adipose tissue dysfunction as a cause of vascular disease: focus on vascular tone and wall remodeling. Eur J Pharmacol 766: 16–24. [DOI] [PubMed] [Google Scholar]
- Padilla J, Jenkins NT, Vieira‐Potter VJ, Laughlin MH (2013). Divergent phenotype of rat thoracic and abdominal perivascular adipose tissues. Am J Physiol Regul Integr Comp Physiol 304: R543–R552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne GA, Bohlen HG, Dincer UD, Borbouse L, Tune JD (2009). Periadventitial adipose tissue impairs coronary endothelial function via PKC‐beta‐dependent phosphorylation of nitric oxide synthase. Am J Physiol Heart Circ Physiol 297: H460–H465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne GA, Borbouse L, Bratz IN, Roell WC, Bohlen HG, Dick GM et al. (2008). Endogenous adipose‐derived factors diminish coronary endothelial function via inhibition of nitric oxide synthase. Microcirculation 15: 417–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne GA, Borbouse L, Kumar S, Neeb Z, Alloosh M, Sturek M et al. (2010). Epicardial perivascular adipose‐derived leptin exacerbates coronary endothelial dysfunction in metabolic syndrome via a protein kinase C‐beta pathway. Arterioscler Thromb Vasc Biol 30: 1711–1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrinelli V, Carobbio S, Vidal‐Puig A (2016). Adipose tissue plasticity: how fat depots respond differently to pathophysiological cues. Diabetologia 59: 1075–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Police SB, Thatcher SE, Charnigo R, Daugherty A, Cassis LA (2009). Obesity promotes inflammation in periaortic adipose tissue and angiotensin II‐induced abdominal aortic aneurysm formation. Arterioscler Thromb Vasc Biol 29: 1458–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahmouni K, Morgan DA, Morgan GM, Mark AL, Haynes WG (2005). Role of selective leptin resistance in diet‐induced obesity hypertension. Diabetes 54: 2012–2018. [DOI] [PubMed] [Google Scholar]
- Rebolledo A, Rebolledo OR, Marra CA, García ME, Roldan Palomo AR, Rimorini L et al. (2010). Early alterations in vascular contractility associated to changes in fatty acid composition and oxidative stress markers in perivascular adipose tissue. Cardiovasc Diabetol 9: 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedel J, Badewien‐Rentzsch B, Kohn B, Hoeke L, Einspanier R (2016). Characterization of key genes of the renin‐angiotensin system in mature feline adipocytes and during in vitro adipogenesis. J Anim Physiol Anim Nutr 100: 1139–1148. [DOI] [PubMed] [Google Scholar]
- Rittig K, Dolderer JH, Balletshofer B, Machann J, Schick F, Meile T et al. (2012). The secretion pattern of perivascular fat cells is different from that of subcutaneous and visceral fat cells. Diabetologia 55: 1514–1525. [DOI] [PubMed] [Google Scholar]
- Rittig K, Staib K, Machann J, Böttcher M, Peter A, Schick F et al. (2008). Perivascular fatty tissue at the brachial artery is linked to insulin resistance but not to local endothelial dysfunction. Diabetologia 51: 2093–2099. [DOI] [PubMed] [Google Scholar]
- Romacho T, Sánchez‐Ferrer CF, Peiró C (2013). Visfatin/Nampt: an adipokine with cardiovascular impact. Mediators Inflamm 2013: 946427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan CC, Zhu DL, Chen QZ, Chen J, Guo SJ, Li XD et al. (2010). Perivascular adipose tissue‐derived complement 3 is required for adventitial fibroblast functions and adventitial remodeling in deoxycorticosterone acetate–salt hypertensive rats. Arterioscler Thromb Vasc Biol 30: 2568–2574. [DOI] [PubMed] [Google Scholar]
- Schleifenbaum J, Köhn C, Voblova N, Dubrovska G, Zavarirskaya O, Gloe T et al. (2010). Systemic peripheral artery relaxation by KCNQ channel openers and hydrogen sulfide. J Hypertens 28: 1875–1882. [DOI] [PubMed] [Google Scholar]
- Soltis EE, Cassis LA (1991). Influence of perivascular adipose tissue on rat aortic smooth muscle responsiveness. Clin Exp Hypertens 13: 277–296. [DOI] [PubMed] [Google Scholar]
- Southan C, Sharman JL, Benson HE, Faccenda E, Pawson AJ, Alexander SP et al. (2016). The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucleic Acids Res 44: D1054–D1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiroglou SG, Kostopoulos CG, Varakis JN, Papadaki HH (2010). Adipokines in periaortic and epicardial adipose tissue: differential expression and relation to atherosclerosis. J Atheroscler Thromb 17: 115–130. [DOI] [PubMed] [Google Scholar]
- Sun X, Hou N, Han F, Guo Y, Hui Z, Du G et al. (2013). Effect of high free fatty acids on the anti‐contractile response of perivascular adipose tissue in rat aorta. J Mol Cell Cardiol 63: 169–174. [DOI] [PubMed] [Google Scholar]
- Szasz T, Bomfim GF, Webb RC (2013). The influence of perivascular adipose tissue on vascular homeostasis. Vasc Health Risk Manag 9: 105–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakali K, Davenport L, Fink GD, Watts SW (2006). Pleiotropic effects of hydrogen peroxide in arteries and veins from normotensive and hypertensive rats. Hypertension 47: 482–487. [DOI] [PubMed] [Google Scholar]
- Thalmann S, Meier C (2007). Local adipose tissue depots as cardiovascular risk factors. Cardiovasc Res 75: 690–701. [DOI] [PubMed] [Google Scholar]
- Traupe T, Lang M, Goettsch W, Münter K, Morawietz H, Vetter W et al. (2002). Obesity increases prostanoid‐mediated vasoconstriction and vascular thromboxane receptor gene expression. J Hypertens 20: 2239–2245. [DOI] [PubMed] [Google Scholar]
- Vallejo S, Romacho T, Angulo J, Villalobos LA, Cercas E, Leivas A et al. (2011). Visfatin impairs endothelium‐dependent relaxation in rat and human mesenteric microvessels through nicotinamide phosphoribosyltransferase activity. PLoS One 6: e27299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargovic P, Ukropec J, Laukova M, Cleary S, Manz B, Pacak K et al. (2011). Adipocytes as a new source of catecholamine production. FEBS Lett 585: 2279–2284. [DOI] [PubMed] [Google Scholar]
- Verlohren S, Dubrovska G, Tsang S, Essin K, Luft FC, Huang Y et al. (2004). Visceral periadventitial adipose tissue regulates arterial tone of mesenteric arteries. Hypertension 44: 271–276. [DOI] [PubMed] [Google Scholar]
- Villacorta L, Chang L (2015). The role of perivascular adipose tissue in vasoconstriction, arterial stiffness, and aneurysm. Horm Mol Biol Clin Invest 21: 137–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virdis A, Duranti E, Rossi C, Dell'Agnello U, Santini E, Anselmino M et al. (2015). Tumour necrosis factor‐alpha participates on the endothelin‐1/nitric oxide imbalance in small arteries from obese patients: role of perivascular adipose tissue. Eur Heart J 36: 784–794. [DOI] [PubMed] [Google Scholar]
- Wang H, Luo W, Guo C, Wolffe SL, Bodary PF, Eitzman DT (2012). Obesity‐induced endothelial dysfunction is prevented by deficiency of P‐selectin glycoprotein ligand‐1. Diabetes 61: 3219–3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts S, Dorrance A, Penfold M, Rourke J, Sinal C, Seitz B et al. (2013). Chemerin connects fat to arterial contraction. Arterioscler Thromb Vasc Biol 33: 1320–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Withers SB, Agabiti‐Rosei C, Livingstone DM, Little MC, Aslam R, Malik RA et al. (2011). Macrophage activation is responsible for loss of anticontractile function in inflamed perivascular fat. Arterioscler Thromb Vasc Biol 31: 908–913. [DOI] [PubMed] [Google Scholar]
- Withers SB, Simpson L, Fattah S, Werner ME, Heagerty AM (2014). cGMP‐dependent protein kinase (PKG) mediates the anticontractile capacity of perivascular adipose tissue. Cardiovasc Res 101: 130–137. [DOI] [PubMed] [Google Scholar]
- Yamawaki H, Takahashi M, Mukohda M, Morita T, Okada M, Hara Y (2012). A novel adipocytokine, nesfatin‐1 modulates peripheral arterial contractility and blood pressure in rats. Biochem Biophys Res Commun 418: 676–681. [DOI] [PubMed] [Google Scholar]
- Yudkin JS, Eringa E, Stehouwer CD (2005). “Vasocrine” signalling from perivascular fat: a mechanism linking insulin resistance to vascular disease. Lancet 365: 1817–1820. [DOI] [PubMed] [Google Scholar]


