Abstract
Background and Purpose
Adiponectin, the most abundant peptide secreted by adipocytes, is involved in the regulation of energy metabolism and vascular physiology. Here, we have investigated the effects of exogenous administration of adiponectin on metabolism, vascular reactivity and perivascular adipose tissue (PVAT) of mesenteric arteries in Wistar rats fed a high‐fat diet.
Experimental Approach
The effects of adiponectin on NO‐dependent and independent vasorelaxation were investigated in isolated mesenteric arteries from 12‐month‐old male Wistar rats (W12m) fed a high‐fat diet (HFD) for 4 months and compared with those from age‐matched rats given a control diet. Adiponectin ((96 μg·day−1) was administered by continuous infusion with a minipump, implanted subcutaneously, for 28 days.
Key Results
Chronic adiponectin treatment reduced body weight, total cholesterol, free fatty acids, fasting glucose and area under the curve of intraperitoneal glucose tolerance test, compared with HFD rats. It also normalized NO‐dependent vasorelaxation increasing endothelial NO synthase (eNOS) phosphorylation in mesenteric arteries of HFD rats. In PVAT from aged (W12m) and HFD rats there was increased expression of chemokines and pro‐inflammatory adipokines, the latter being important contributors to endothelial dysfunction. Infusion of adiponectin reduced these changes.
Conclusions and Implications
Adiponectin normalized endothelial cell function by a mechanism that involved increased eNOS phoshorylation and decreased PVAT inflammation. Detailed characterization of the adiponectin signalling pathway in the vasculature and perivascular fat is likely to provide novel approaches to the management of atherosclerosis and metabolic disease.
Linked Articles
This article is part of a themed section on Molecular Mechanisms Regulating Perivascular Adipose Tissue – Potential Pharmacological Targets? To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v174.20/issuetoc
Abbreviations
- DHE
dihydroethidium
- eNOS
endothelial NO synthase
- FFA
free fatty acids
- HFD
high‐fat diet
- IPGTT
intraperitoneal glucose tolerance test
- PVAT
perivascular adipose tissue
- SNP
sodium nitroprusside
Tables of Links
| TARGETS | |
|---|---|
| Other protein targets a | Enzymes b |
| Adipo 1 receptor | eNOS |
| Adipo 2 receptor |
| LIGANDS | |
|---|---|
| Adiponectin | Indomethacin |
| CCL2 | Leptin |
| CCL5 | NO |
These Tables list key protein targets and ligands in this article that are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Southan et al., 2016), and are permanently archived in the Concise Guide to PHARMACOLOGY 2015/16 (a,bAlexander et al., 2015a,b).
Introduction
The worldwide prevalence of obesity has increased significantly in recent years. Currently, obesity is thought to initiate a state of chronic inflammation which, if unresolved, potentially causes cardiovascular disease (Lumeng and Saltiel, 2011).
Perivascular adipose tissue (PVAT), because of its location surrounding blood vessels, plays a prominent role as a paracrine regulator of vascular function. This includes the secretion of metabolically active adipokines, chemokines and hormone‐like factors, such as leptin, adiponectin and resistin, free fatty acids (FFA) and vasoactive substances (Ouwens et al., 2010; Szasz and Webb, 2012). As with visceral adipose tissue, PVAT mass increases with obesity and exhibits macrophage infiltrates and paracrine dysfunction (Virdis et al., 2015). PVAT has been implicated in the pathogenesis of vascular dysfunction particularly in vessels commonly surrounded by adipose tissue such as mesenteric arteries. Interestingly, superoxide released by the vasculature and adiponectin released by ‘healthy’ PVAT appear to balance each other, suggesting a counter‐regulatory protective mechanism (Antonopoulos et al., 2015).
Ageing also leads to changes in adipose mass and function (Preis et al., 2010) with elevated production of some adipokines and decreased adiponectin levels (Fernandez‐Real et al., 2003). Adipokines have been shown to influence metabolism, coagulation, inflammation and vascular reactivity (Nakamura et al., 2014). Hypoadiponectinemia has been closely associated with obesity and metabolic syndrome (Weyer et al., 2001) and linked with reduced endothelium‐dependent dilatation and cardiovascular disease (Ouchi et al., 2003; Shimabukuro et al., 2003; Tan et al., 2004), predicting endothelial dysfunction.
Adiponectin performs many biological functions, including the regulation of fatty acid and glucose metabolism (Yamauchi et al., 2001; Berg et al., 2002). It also improves insulin resistance exhibiting insulin‐sensitizing properties, and a reduced production of adiponectin is closely coupled to insulin resistance (Kadowaki et al., 2006; Zhu et al., 2008). At the cellular level, adiponectin is known to have anti‐inflammatory and anti‐atherogenic effects, increasing bioavailability of endothelial NO (Zhu et al., 2008; Hui et al., 2012). The beneficial effects of adiponectin on the vasculature have been described both in clinical and animal model studies (Zhu et al., 2008). In vitro, adiponectin protects against vascular dysfunction through its actions in almost all the major types of cells in the vasculature (Zhu et al., 2008). Despite the described beneficial effects in vascular homeostasis, hyperadiponectinemia has been associated with increased cardiovascular mortality in patients with cardiac heart failure (Nakamura et al., 2006). Thus, further studies are required to understand if this peptide can be considered a therapeutic agent.
Adiponectin is an important vasoactive regulator, and its role in the modulation of PVAT properties in obesity remains unexplored. Based on these observations, we sought to investigate the effects of chronic adiponectin treatment on the PVAT phenotype in 12 month old Wistar rats fed a high‐fat diet (HFD). Additionally, we have studied its effects on endothelial function, oxidative stress and activation of endothelial NO synthase (eNOS) in the mesenteric arteries of HFD rats. We evaluated endothelial‐dependent and independent vascular relaxation, together with the inflammatory profile of the PVAT of mesenteric arteries and these variables were correlated with endothelial dysfunction.
Methods
Animals
All animal care and experimental procedures were in accordance with the Portuguese Law on Experimentation with Laboratory Animals, which is based on the principles of laboratory animal care, as adopted by Directive 2010/63/EU for animal experiments. Animal studies are reported in compliance with the ARRIVE guidelines (Kilkenny et al., 2010; McGrath and Lilley, 2015). Adult male Wistar rats were obtained from the local breeding colony in Faculty of Medicine of Coimbra (Portugal). Control animals were fed, ad libitum, with a standard commercial pellet chow (Diet AO3‐Panlab). Six‐month‐old rats (W6m) served as a young model of non‐obese rats. At 8 months old, rats were divided in two groups, the first comprising 12‐month‐old Wistar rats (W12m) was maintained with standard diet AO3 (5% of triglycerides and 45% of carbohydrates, SAFE, France), while the second one was maintained with the HFD (40% of triglycerides and 10% of carbohydrates, 231 HF, SAFE, France) for 4 months. This second group was subsequently divided into three subgroups: A group of rats was treated during the last 28 days with globular adiponectin (2.7 mg: 96.4 μg·day−1) through a subcutaneous mini pump (Alzet osmotic pump 2ML4) with continued release (HFDA) (n = 11). Another group (HFDV) was also subjected to mini pump implantation but infused with PBS only (in mM: NaCl 137, KCl 2.7, Na2HPO4 1.0, KH2PO4 1.8, pH 7.4; vehicle) (n = 11). A third subgroup was maintained with no treatment (HFD) (n = 11). A pilot study (data not shown) with two different concentrations of adiponectin (2.7 and 4.15 mg) showed that 96.4 μg·day−1 was better than 148.2 μg·day−1 as it had a better outcome in terms of metabolic and endothelial function. All animals were allowed free access to water; they were kept in rooms with 12 h periods of light and darkness. Animals were anaesthetized with ketamine/chlorpromazine [ketamine chloride (75 mg·kg−1, i.m., Parke‐Davis, Ann Arbor, MI, USA) and chlorpromazine chloride (2.65 mg·kg−1, i.m., Lab. Vitória, Portugal)]. Blood was collected at the end of treatment. The mesenteric arteries and PVAT were excised and used as described below.
Determination of metabolic and oxidative stress parameters
After a 15 h fast, animals were anaesthetized with ketamine/chlorpromazine and killed by decapitation. Blood was taken by heart puncture for determination of lipids and FFA levels. For glucose tolerance tests, rats were fasted overnight and were injected with glucose (i.p.; 1.75 g·kg−1) in PBS. Blood glucose was determined by sampling from the tail vein at 0, 30, 60 and 120 min after injection by a glucose‐oxidase method using a glucometer (Glucometer‐Elite‐Bayer, Portugal S.A.) and compatible reactive test strips. Lipids (total and HDL cholesterol and triglycerides) were quantified using commercially available kits. Plasma FFA levels were evaluated using enzymic assay kits (Roche Applied Science, Portugal) as previously (Sena et al., 2011). Serum concentrations of adiponectin and leptin were determined by ELISA using the Rat Adiponectin Immunoassay Kit and the Rat Leptin Immunoassay Kit (Invitrogen) respectively.
Isometric tension studies
Segments of second order superior mesenteric artery (approx. 200 μm external diameter) were trimmed free of fat and adhering connective tissue and mounted in a myograph according to the technique of Mulvany and Halpern (1977). Isometric tension was recorded and collected by a PowerLab data acquisition system (ADInstruments, UK) and recorded on a computer using the LabChart 7 data acquisition and analysis software (ADInstruments, UK). Segments were allowed to equilibrate for 30 min in modified Krebs–Henseleit solution (composition in mM: NaCl 119, KCl 4.7, CaCl2 1.6, MgSO4 1.2, NaHCO3 25, KH2PO4 1.2, glucose 11.0), maintained at 37°C and gassed with 5% CO2 in air. Each vessel was placed under a stretch equivalent to the in vivo arterial blood pressure in each individual rat according to the technique of Mulvany and Halpern (1977). Tissue contractility was assessed by exposure to KCl (125 mM) and then cumulative concentration–response curves were obtained to phenylephrine (0.1–30 μM). Tissues were allowed to re‐equilibrate for 30 min. The mesenteric rings were then used to study the response to one of the following agents: relaxation was assessed in response to either ACh (10−9 to 10−6 M), with or without indomethacin (10 μM) or sodium nitroprusside (SNP, 10−9 to 10−3 M). Relaxation responses were obtained following pre‐contraction with phenylephrine to approximately 50% of the maximum as determined from the phenylephrine concentration‐response curve. In a second series of experiments using mesenteric artery segments, the acute effects of NO synthase inhibition were assessed using L‐NAME. Responses to ACh were obtained as above and subsequently repeated in the presence of L‐NAME (300 μM). Relaxation responses to ACh and SNP were expressed as percentage of relaxation from a submaximal phenylephrine‐induced contraction, and concentration–response curves were obtained as previously described (Sena et al., 2008; 2011).
Detection of superoxide anions
Unfixed. frozen sections (6μm) of arteries were incubated with DHE (2 × 10−6 M) in PBS for 30 min at 37°C in a humidified chamber protected from light. DHE is oxidized on reaction with superoxide anions to ethidium bromide, which binds to DNA in the nucleus and fluoresces red (Sena et al., 2011). For ethidium bromide detection, images were obtained with a fluorescence microscope (Leica DMIRE200, Wetzlar, Germany). Fluorescence was detected with a 568 nm filter. Arteries were processed and imaged in parallel with identical settings. Microscope and camera settings were kept constant for all preparations. Fluorescence was quantified using ImageJ (1.40 g, NIH).
Assessment of mesenteric immunofluorescence
Sections (6 μm) of mesenteric arteries were washed with PBS and fixed in ice‐cold acetone, for 10 min. Sections were then permeabilized for 10 min in 1% Triton X‐100 in PBS, pH 7.4, and blocked with 10% goat serum, for 30 min. Primary antibodies were diluted in PBS containing 0.02% BSA (PBS/BSA). The primary antibodies were added, and the sections were incubated overnight at 4°C. After incubation, the sections were extensively washed with PBS/BSA solution. After, sections were incubated with the secondary antibodies, diluted in PBS/BSA for 1 h. The coverslips were washed before mounting with Glycergel Dako mounting medium (Dako, Carpinteria, CA, USA). Immunostained mesenteric sections were visualized with a Leica DMIRE200 fluorescence microscope. Immunostained mesenteric sections were counterstained with DAPI and examined, photographed and quantified as previously (Sena et al., 2011).
Western blot analysis
PVAT was washed with cold PBS and chilled in buffer containing in mM: Tris–HCl 50, NaCl 150, EDTA 1, EGTA 0.1, as well as NP‐40, 0.1%, SDS 0.1% and deoxycholate 0.5%. Phenylmethylsulfonyl fluoride (1 mM), aprotinin (10 μg·mL−1), leupeptin (10 μg·mL−1) and pepstatin (10 μg·mL−1) all from Sigma Chemicals (St. Louis, MO, USA) were added as protease inhibitors. Tissues were homogenized in a standard fashion, followed by centrifugation at 14000× g for 20 min at 4°C. The supernatants were collected, and total protein concentration was determined. Samples containing 20 μg of protein were loaded on to 12 or 15% SDS‐PAGE gel, run and electroblotted onto polyvinylidene difluoride membrane. Prestained molecular weight marker proteins were used as standards for the SDS‐PAGE. A Ponceau stain was performed to check the quality of the transfer and to ensure equal protein loading. Blots were blocked in 5% skimmed nonfat milk in PBS for 1 h, treated overnight with antibody against leptin, lipocalin‐2, CCL2 or CCL5 and then incubated with alkaline phosphatase secondary antibodies for 1 h. Immunoblots were developed with an ECF Western blotting detection system (Amersham Biosciences). Protein content was determined using a Pierce protein assay kit.
Data and statistical analysis
The data and statistical analysis comply with the recommendations on experimental design and analysis in pharmacology (Curtis et al., 2015). All data were analysed by standard computer programs (GraphPad Prism PC Software version 3.0, ANOVA) and are expressed as mean ± SE (n = 11 individual animals per group). Significant differences were evaluated using either the t‐test or ANOVA. P < 0.05 was considered significant. Dose–response curves were fitted by non‐linear regression with simplex algorithm. Relaxation responses are expressed as the percentage of phenylephrine‐induced pre‐contraction. Comparisons of dose–response curves were evaluated by two‐way ANOVA for repeated measures followed by Bonferroni post hoc test for individual comparisons.
Materials
Adiponectin was obtained from Hitag® Biotechnology (Cantanhede, Portugal) (Matafome et al., 2014). Phenylephrine, ACh and N‐nitro‐L‐arginine‐ methyl ester (L‐NAME) were obtained from SIGMA (St. Louis, MO, USA). Antibodies against eNOS, phosphorylated eNOS (p‐eNOS) and nitrotyrosine were obtained from Cell Signaling Technology (Danvers, MA, USA) and Upstate Biotechnology (Lake Placid, NY, USA) respectively. Antibodies against CCL2, β‐actin, CCL5, lipocalin‐2 and leptin were obtained from Chemicon International Inc. (Temecula, CA, USA) and Abcam plc (Cambridge, UK), whereas rat adipokine array kit, dihydroethidium (DHE), Rat Adiponectin and Leptin Immunoassay Kits were obtained from R&D Systems and Invitrogen (Barcelona, Spain) respectively. All other chemicals and reagents used in the study were of high grade and obtained from Merck Darmstad (Germany).
Results
Animal characteristics
Age‐matched Wistar rats used in our experiments exhibited similar body weight at the beginning of the study. Food consumption and water intake did not significantly change over the experimental period between the different groups studied (data not shown). At the end, body weight was significantly higher in HFD rats compared with age‐matched Wistar rats and adiponectin treatment significantly decreased this parameter (Table 1). Glycaemia, 2 h after glucose load and AUC in intraperitoneal glucose tolerance test (IPGTT) and FFA were elevated in HFD and HFDV rats when compared with age‐matched Wistar rats and with the young W6m rats (Figure 1A–D). Treatment with adiponectin for 28 days effectively reduced blood glucose (fasting and AUC in IPGTT) and circulating concentrations of FFA (Figure 1 and Table 1).
Table 1.
Effects of HFD and of adiponectin on body weight and lipid levels of W12m and W6m rats
| – | W6m | W12m | HFD | HFDV | HFDA |
|---|---|---|---|---|---|
| Body weight (g) | 402.3 ± 10.0 | 519 ± 17.2 * | 623.3 ± 18.1 * , § | 584.8 ± 24.8 * | 521.7 ± 19.9* φ |
| Total cholesterol (mM) | 2.1 ± 0.08 | 2.8 ± 0.07 * | 3.13 ± 0.2 * | 2.84 ± 0.18 * | 2.45 ± 0.18φ |
| Non‐HDL cholesterol (mM) | 0.86 ± 0.04 | 1.25 ± 0.06 * | 1.42 ± 0.1 * | 1.28 ± 0.08 * | 1.08 ± 0.11 |
| Triglycerides (mM) | 0.85 ± 0.06 | 0.96 ± 0.08 | 1.06 ± 0.06 | 1.08 ± 0.1 | 0.95 ± 0.07 |
| FFA (mM) | 0.49 ± 0.03 | 0.48 ± 0.04 | 0.88 ± 0.06 * , § | 0.74 ± 0.04 * , § | 0.6 ± 0.06φ |
| Leptin (ng·mL−1) | 0.94 ± 0.2 | 1.97 ± 0.36 | 16.49 ± 3.1 * , § | 15.8 ± 3.04 * , § | 6.34 ± 0.88φ, Δ |
| Adiponectin (μg·mL−1) | 30.35 ± 2. 04 | 43.99 ± 6.0 | 46.15 ± 4.37 | 41.96 ± 4.99 | 45.1 ± 8.66 |
Data are expressed as mean ± SE (n = 11 animals in each group).
P < 0.05, significantly different from W6m rats;
P < 0.05, significantly different from W12m rats;
P < 0.05, significantly different from HFD rats;
P < 0.05, significantly different from HFDV rats.
Figure 1.
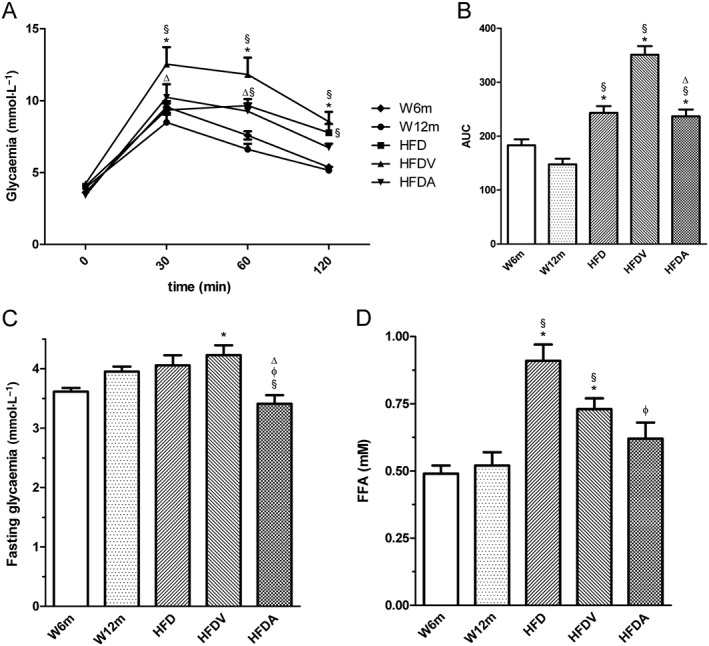
Effects of HFD and adiponectin on blood glucose levels during an IPGTT (A), AUC of IPGTT (B), fasting glycaemia (C) and plasma FFA (D) in W12m rats, compared with W6m rats. Blood glucose and FFA levels were determined at the end of treatment in all experimental groups [W6, W12m, HFD, HFDV or adiponectin (HFDA)]. The blood glucose was determined using a blood glucose monitoring system at 0, 30, 60 and 120 min after the glucose injection. Results are mean ± SE (n = 11 animals in each group). *P < 0.05, significantly different from W6m group; §P < 0.05, significantly different from W12m group; φP < 0.05, significantly different from HFD group; ΔP < 0.05, significantly different from HFDV group.
Total and non‐HDL cholesterol were significantly higher in W12m, HFD and HFDV rats when compared with W6m rats. Treatment with adiponectin reduced total cholesterol levels. Triglycerides did not significantly change between the different groups studied (Table 1).
NO‐dependent vascular relaxation in rat mesenteric arteries
W12m rats exhibited decreased endothelial‐dependent vasorelaxation in response to maximal ACh concentrations, compared with W6m rats (Figure 2A). HFD in 12‐month‐old rats exhibited impaired endothelium‐mediated relaxation of phenylephrine‐precontracted mesenteric arterial rings in response to ACh, compared with age‐matched W12m rats, with the maximal endothelium‐mediated relaxation to ACh decreasing by ~60% in HFD and HFDV groups (Figure 2A). The endothelium‐independent relaxations to SNP were also impaired in both HFD and HFDV groups, with maximal relaxations and vascular sensitivity being decreased (Figure 2B, Table 2). Preincubation of the arterial rings with the NOS inhibitor L‐NAME and the cyclooxygenase inhibitor indomethacin almost completely abolished relaxation to ACh in Wistar rats (data not shown). Treatment with exogenous adiponectin normalized endothelium‐dependent vascular relaxation during the HFD (Figure 2A) and ameliorated endothelium‐independent relaxation. Detailed data on maximal relaxations and EC50 values are summarized in Table 2. These results indicated that treatment with adiponectin for 28 days normalized this index of endothelial function in the HFD group.
Figure 2.
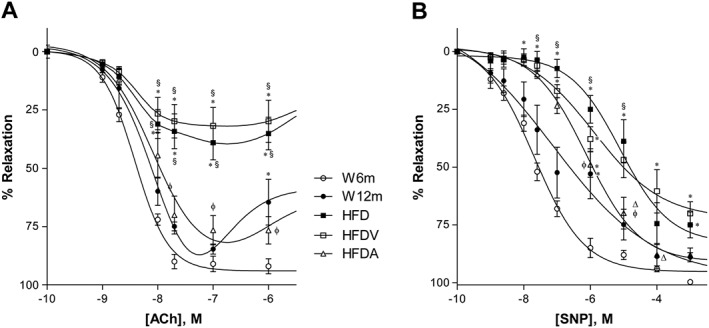
Effects of HFD and adiponectin treatment on relaxant responses to ACh (A) and sodium nitroprusside (B) in segments of mesenteric arteries from W12m rats, compared with W6m rats, after phenylephrine precontraction. Relaxation was measured using an isometric force displacement transducer in all groups including HFD treated with HFDV or adiponectin (HFDA). Data are expressed as mean ± SE (n = 11, 22 vascular ring preparations in 11 animals per group). *P < 0.05 significantly different from W6m group; §P < 0.05, significantly different from W12m group; φP < 0.05, significantly different from HFD group; ΔP < 0.05, significantly different from HFDV group.
Table 2.
Effects of HFD and of adiponectin on the maximal relaxation responses (%) and pEC50 for ACh or SNP. Data obtained in segments of mesenteric arteries from W6m and W12m rats.
| W6m | W12m | HFD | HFDV | HFDA | |
|---|---|---|---|---|---|
| ACh | – | ||||
| pEC50 | 8.4 ± 0.05 | 8.2 ± 0.35 | 8.4 ± 0.26 | 8.4 ± 0.34 | 8.1 ± 0.12 |
| Maximal relaxation (%) | 91 ± 3.3 | 85 ± 2.2 | 39 ± 7.3 * , § | 32 ± 7.9 * , § | 76 ± 6.2φ , Δ |
| SNP | |||||
| pEC50 | 7.7 ± 0.1 | 7.1 ± 0.59 | 5.1 ± 0.27 * , § | 5.99 ± 0.35 * | 6.1 ± 0.12 * |
| Maximal relaxation (%) | 99.7 ± 2.3 | 89.0 ± 2.0 | 75.0 ± 5.6 * | 70.0 ± 4.9 * , § | 88.0 ± 3.2Δ |
Data are expressed as mean ± SE (n = 11 animals in each group). pEC50 values are the negative logarithm (−logEC50) of the EC50 of the agonist shown.
P < 0.05, significantly different from W6m rats;
P < 0.05, significantly different from W12m rats;
P < 0.05, significantly different from HFD rats;
P < 0.05, significantly different from HFDV rats.
Oxidative stress in the vascular wall
We determined the potential impact of adiponectin on the generation of superoxide in the vasculature, as assessed by DHE. Interestingly, the aged rats (W12m) had a twofold increase in superoxide production in mesenteric arteries compared with the young W6m rats (Figure 3B, K). Superoxide levels were also about two‐fold higher in the mesenteric arteries of the HFD rats compared with the age‐matched W12m rats (Figure 3C, K). Adiponectin treatment reduced superoxide production in the HFD rats (Figure 3E, K). Similarly, L‐NAME (300 μM) reduced superoxide levels in this group of rats (data not shown). Accordingly, we sought to investigate whether the enhanced production of superoxide in mesenteric arteries of HFD rats was associated with peroxynitrite formation and the nitration of tyrosine residues. W12m rats also had increased immunoreactive nitrotyrosine levels in their mesenteric arteries, suggesting increased peroxynitrite‐mediated protein oxidation (Figure 3G, L). The high‐fat diet groups (HFD and HFDV) showed increased nitrotyrosine staining ( Figure 3H, I, L), which was reduced by adiponectin in the HFDA rats (Figure 3J, L).
Figure 3.
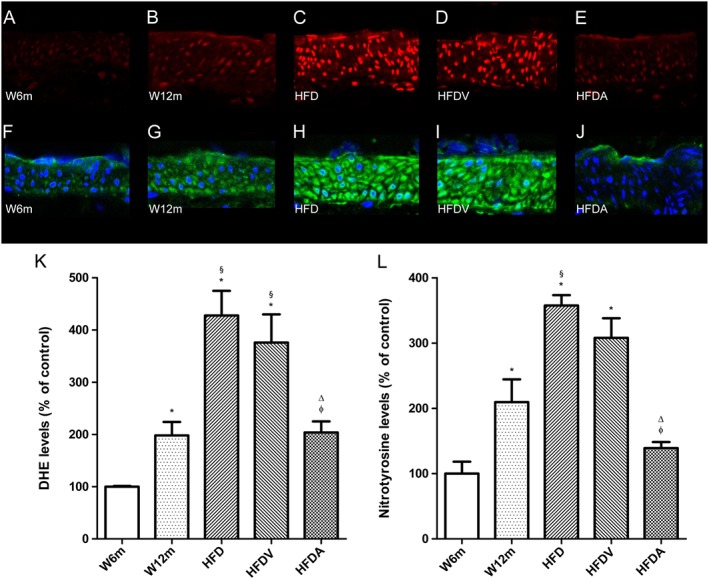
In situ detection of superoxide (A–E) and nitrotyrosine levels (F–J) in rat mesenteric arteries. Representative DHE‐stained mesenteric artery sections reflect production of superoxide (A–E) with the different treatments. The endothelium is facing up in all layers. At identical settings, fluorescence in sections from W12m rats (B) was increased compared those from W6m rats (A). Note the markedly increased fluorescence, reflecting superoxide levels in the endothelium, intima and media of HFD rats (HFD and HFDV; C and D respectively). Fluorescence decreased to basal levels (as in W6m rats) in the adiponectin (HFDA)‐treated group (E). Panel (K) shows quantification of the fluorescence ethidium signal in the different groups of arteries. Representative mesenteric sections showing nitrotyrosine staining (F–J), indicative of increased peroxynitrite formation, in W6m (F), W12m (G), HFD (H), HFDV (I) and HFD treated with adiponectin (HFDA; J) rats. Panel (L) contains quantification of the green fluorescence in the different groups of arteries. Data are mean ± SE (n = 11 animals per group). *P < 0.05, significantly different from W6m group; §P < 0.05, significantly different from W12m group; φ P < 0.05, significantly different from HFD group; ΔP < 0.05, HFDV group.
Phosphorylated and total eNOS in mesenteric arteries
The levels of phosphorylated eNOS at Ser1177 (p‐eNOS) is an indicator of eNOS activity. We measured p‐eNOS and total eNOS in the mesenteric arteries by immunofluorescence and found that p‐eNOS and the ratio of p‐eNOS/total eNOS were decreased in aged rats in all groups (W12m, HFD and HFDV), compared with young W6m rats (Figure 4B–D, F). Adiponectin significantly increased p‐eNOS and the ratio of p‐eNOS/total eNOS in endothelium of the HFD rats (Figure 4E, F).
Figure 4.
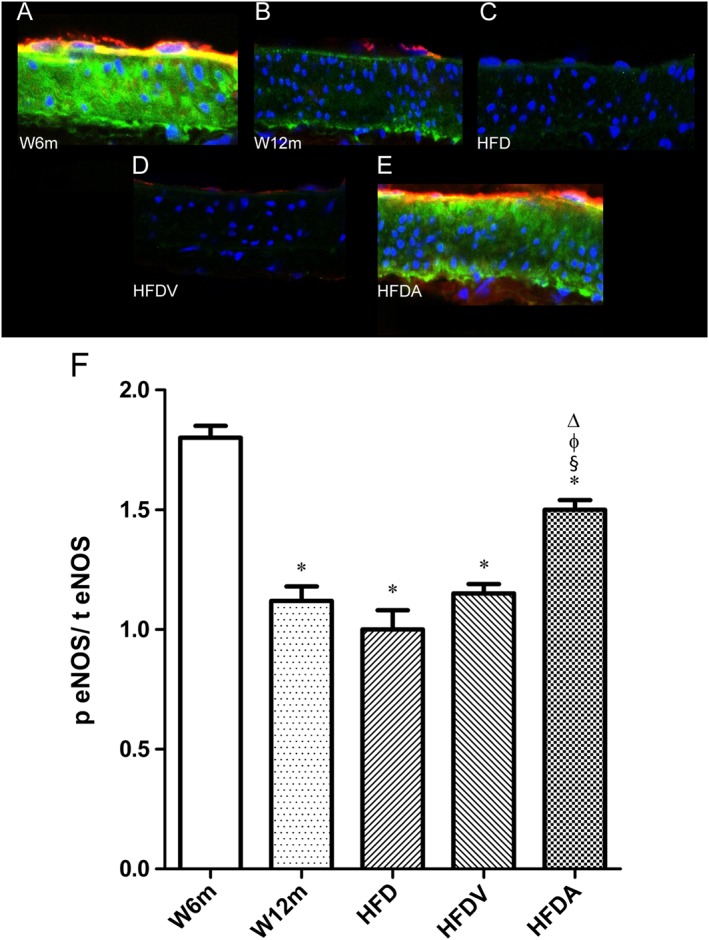
Effects of HFD and adiponectin treatment on mesenteric eNOS levels in W12m rats, compared with W6m rats. Representative mesenteric sections demonstrating decreased p‐eNOS staining in W12m, HFD and HFDV arteries (B–D). Panel presents mesenteric arteries from W6m (A), W12m (B), HFD (C), HFDV (D) and HFDA (E) groups of rats. Panel (F) contains quantification of the red (p eNOS) to green fluorescence (total eNOS;t eNOS) ratio in the different groups of arteries. Data are mean ± SE (n = 11 animals per group). *P < 0.05, significantly different from W6m group; § P < 0.05, significantly different from W12m group; φ P < 0.05, significantly different from HFD group; Δ P < 0.05, significantly different from HFDV group.
Inflammation biomarkers
Given the recent importance of PVAT in obesity vascular function, we next focused on local adipokines present in this tissue. PVAT leptin levels were increased in aged rats (W12m, HFD and HFDV) compared with W6m rats. HFD increased PVAT leptin levels, and adiponectin treatment decreased these levels (Figure 5B, C). Additionally, systemic leptin levels (Table 1) and the systemic leptin to adiponectin ratio (L/A) were increased in HFD fed rats (HFD and HFDV), and adiponectin also reduced these increases (Figure 5A, Table 1). Systemic levels of adiponectin did not significantly change between the different groups studied (Table 1).
Figure 5.
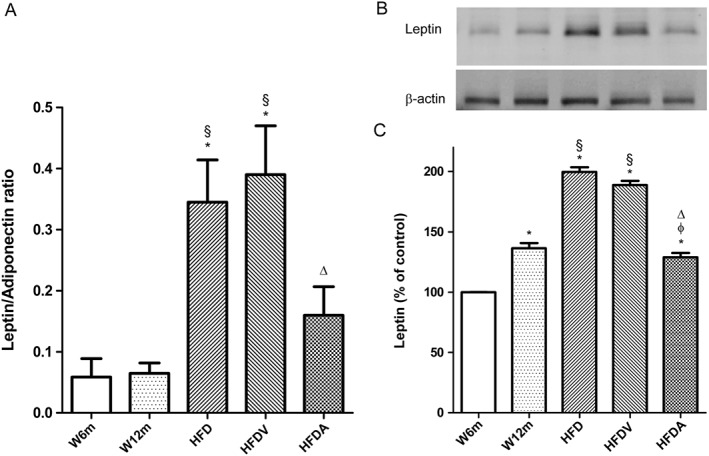
Effects of HFD and adiponectin treatment on systemic leptin/adiponectin ratio (A) and PVAT leptin levels (B, C) in W12m and W6m rats. PVAT lysates were analysed by SDS‐PAGE. Representative Western blot analyses of leptin expression in PVAT of the different groups of rats (B). Averaged densitometric data for the different groups expressed as a percentage of elevation over the W6m value, set to 100% after normalisation to actin by densitometry. Data are mean ± SE (n = 11 animals per group). * P < 0.05, significantly different from W6m group; § P < 0.05, significantly different from W12m group; φ P < 0.05, significantly different from HFD group; ΔP < 0.05, significantly different from HFDV group.
PVAT is the primary contributor to vascular inflammation due to both its proximity to the blood vessel wall and its pro‐inflammatory properties (Chatterjee et al., 2009). So we focused on evaluating other inflammatory mediators present in PVAT that surrounded mesenteric arteries of the different groups. Levels of the chemokines CCL2, one of the earliest molecular markers of vascular inflammation in atherogenesis (Piga et al., 2007), and of CCL5, a marker of atherosclerosis progression, were significantly increased in PVAT of aged rats (W12m, HFD and HFDV), compared with W6m rats (Figure 6A–D). HFD increased these chemokines, and adiponectin administration attenuated these inflammatory markers in the PVAT (Figure 6A–D).
Figure 6.
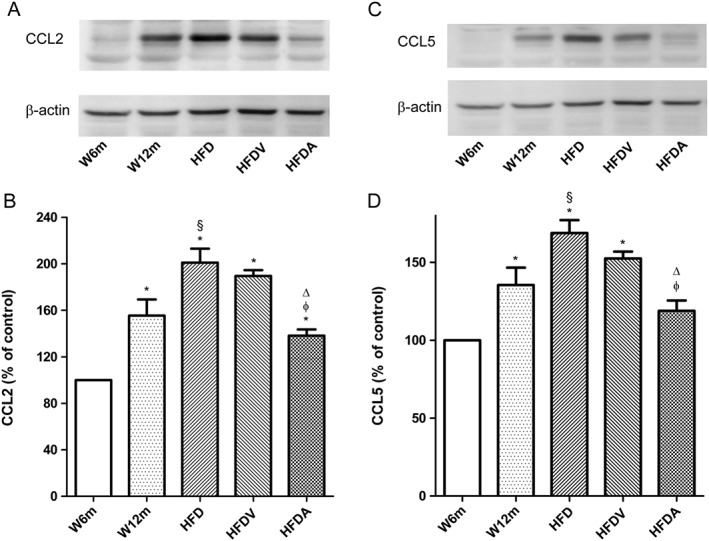
Effects of HFD and adiponectin treatment on chemokine (CCL2 and CCL5) levels in PVAT from W12m and W6m rats. (A, C) Representative Western blot analyses of CCL2 and CCL5 expressions in PVAT of the different groups of rats. (B, D) Averaged densitometric data for the different groups expressed as a percentage of elevation over the W6m value, set to 100% after normalisation to actin by densitometry. Data are mean ± SE (n = 11 animals per group). *P < 0.05, significantly different from W6m group; §P < 0.05, significantly different from W12m group; φ P < 0.05, significantly different from HFD group; ΔP < 0.05, significantly different from HFDV group.
We also evaluated PVAT levels of lipocalin‐2, another proinflammatory adipokine whose expression and secretion is induced in obesity and insulin resistance in mouse and humans (Wang, 2012). Levels of lipocalin‐2 in PVAT were increased in aged rats (W12m, HFD and HFDV), compared with those in W6m rats (Figure 7A, B). HFD increased lipocalin‐2 levels, and adiponectin administration attenuated this inflammatory marker in the PVAT (Figure 7A, B).
Figure 7.
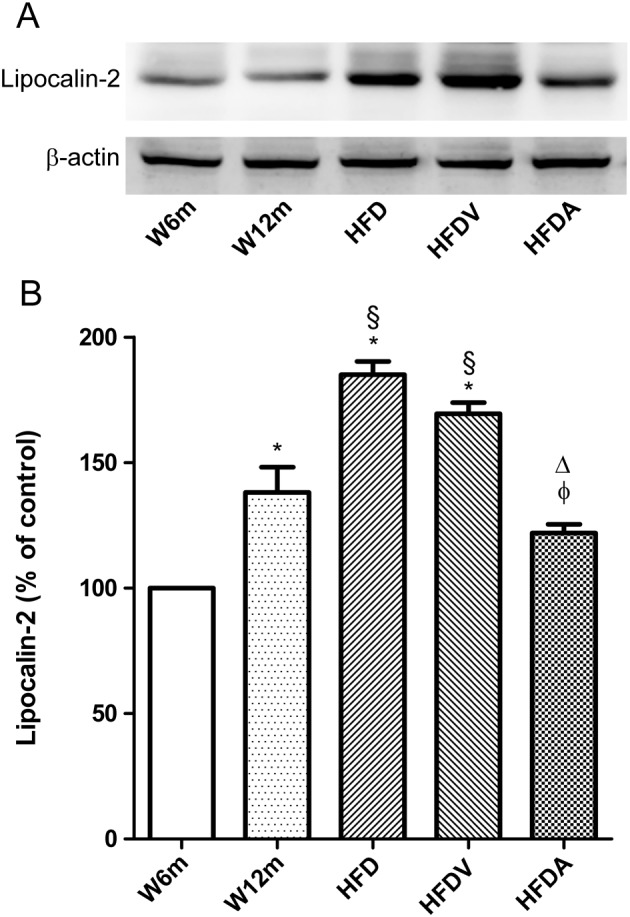
Effects of HFD and adiponectin treatment on lipocalin‐2 levels in PVAT from W12m and W6m rats. (A) Representative Western blot analyses of lipocalin‐2 expression in PVAT of mesenteric arteries of the different groups of rats. (B) Averaged densitometric data for groups expressed as a percentage of elevation over the W6m value, set to 100% after normalisation to actin by densitometry. Data are expressed as mean ± SE (n = 11 animals per group). *P < 0.05, significantly different from W6m group; §P < 0.05, significantly different from W12m group; φP < 0.05, significantly different from HFD group; ΔP < 0.05, significantly different from HFDV group.
Discussion
Adiponectin is one of the first described adipokines known to be dysregulated in obesity. We have demonstrated for the first time, that treatment with exogenous adiponectin improved the pro‐inflammatory PVAT phenotype present in obesity. Concomitantly, this effect was associated with an increased eNOS phosphorylation and a decreased oxidative stress, leading to normalized endothelial dysfunction in the mesenteric arteries.
Obesity is associated with elevated levels of oxidative stress, inflammation and endothelial dysfunction both in humans and animal models (Iantorno et al., 2014). Feeding a HFD promoted this phenotype in normal Wistar rats. Furthermore, adiponectin treatment reduced oxidative stress as well as PVAT inflammation observed in this animal model, and these actions were associated with a recovery in endothelial function in mesenteric arteries.
Ageing is also related to endothelial dysfunction (Herrera et al., 2010). In agreement, we have observed that the W12m rats had impaired endothelium‐dependent vascular relaxation in response to higher ACh concentrations. compared with the younger W6m rats.
In W12m rats fed with HFD (the HFD group), blood glucose, triglyceride, cholesterol and FFA levels were increased, relative to those in the W6m rats. Adiponectin did not improve the triglyceride levels but it did decrease body weight, total cholesterol and FFA as well as fasting glucose and AUC of IPGTT which can partly explain the beneficial effects on vascular function. These results are consistent with other studies that have reported that adiponectin is an important metabolic regulator (Yamauchi et al., 2001; Berg et al., 2002).
Several reports have suggested the independent association between serum levels of adiponectin and endothelium‐dependent vasodilatation (Shimabukuro et al., 2003; Ouchi et al., 2003; Tan et al., 2004). Ex vivo incubation with adiponectin improved endothelial function in aortas of hyperlipidaemic rats by lowering oxidative stress (Li et al., 2007) and overexpression of adiponectin alleviated the atherosclerotic lesions in apolipoprotein E knockout mice (Yamauchi et al., 2003). Our present results agree with the earlier data showing that adiponectin ameliorates endothelial dysfunction (Xu and Vanhoutte 2012; Parker‐Buffen et al., 2013) by reducing the production of ROS, promoting the coupling and activity of eNOS and increasing NO bioavailability (Chen et al., 2003; Tan et al., 2004; Cheng et al., 2007; Li et al., 2007; Zhu et al., 2008; Margaritis et al., 2013). Others have also reported that adiponectin moderates endothelial function by inhibiting the pro‐inflammatory kinase JNK, suppressing endothelial cell activation and apoptosis and promoting endothelial repair (Xu and Vanhoutte, 2012; Meijer et al., 2013). These favourable effects of adiponectin are mediated through its pleiotropic actions on several types of cells in the vasculature, including mature endothelial cells, endothelial progenitor cells, monocytes and smooth muscle cells (Zhu et al., 2008).
Oxidative stress is now considered to play a key role in metabolic and vascular derangements with an imbalance arising from exaggerated production and reduced elimination of free radicals (Sena et al., 2014). In this study, we present evidence that adiponectin exerts an anti‐oxidative effect reducing superoxide anion and nitrotyrosine accumulation in mesenteric arteries. This anti‐oxidative effect of adiponectin is one important mechanism underlying the normalization in endothelial dysfunction observed. Production of ROS reduces the bioavailability of NO as a result of the uncoupling of eNOS. In addition, the increased level of superoxide anions leads to the formation of peroxynitrite, which further aggravates the impairment of eNOS activity and reduces NO production (Pacher et al., 2007; Sena et al., 2011; Sena et al., 2014). Adiponectin is known to inhibit both the basal and oxidized low density lipoprotein‐induced ROS generation, possibly through the inhibition of NADPH oxidase in bovine endothelial cells (Motoshima et al., 2004) and in human umbilical vein endothelial cells by increasing glutathione levels (Plant et al., 2008).
Here, we have shown a marked reduction in the levels of mesenteric p‐eNOS (Ser1177) during ageing and HFD. Adiponectin treatment increased p‐eNOS and normalized endothelium‐dependent relaxation in HFD rats. The benefits of adiponectin treatment could be due to its antioxidant properties that lead to increased eNOS activation and a consequently increased NO bioavailability. Adiponectin enhances eNOS activity by stimulating phosphorylation at Ser1177 through AMP‐activated protein kinase and stabilization of the complex of Hsp90 and eNOS (Chen et al., 2003; Xi et al., 2005). In a recent study in human vessels, local adiponectin promoted eNOS coupling, an effect mediated by PI3K/Akt‐mediated phosphorylation of eNOS and vascular tetrahydrobiopterin bioavailability (Margaritis et al., 2013).
The first evidence for a paracrine vasodilator effect of PVAT‐derived adiponectin was demonstrated by Greenstein et al. (2009)reported an increase in inflammatory cytokines in PVAT surrounding small arteries from obese subjects with metabolic syndrome, which was correlated with oxidative stress (Greenstein et al., 2009). Ageing is also known to exacerbate endothelial dysfunction and vascular inflammation in HFD mice (Bailey‐Downs et al., 2013). These studies suggested that, as we found, there is a synergy between age and obesity‐related alterations in PVAT that damage the vascular wall. HFD‐induced obesity promotes a marked proinflammatory shift in the profile of secreted cytokines and chemokines which is associated with oxidative stress in PVAT (Bailey‐Downs et al., 2013). PVAT of New Zealand obese mice also exhibits inflammation and an increase in oxidative stress, leading to endothelial dysfunction, the latter effect being due to decreased NO and enhanced superoxide generated by uncoupled eNOS (Marchesi et al., 2009).
Adiponectin has anti‐inflammatory properties confirmed by the ability to decrease systemic inflammatory biomarkers (Hui et al., 2012; Xu and Vanhoutte, 2012) as the ratio of L/A in this study. However, the effects of exogenous administration of adiponectin at the PVAT level are still undefined. In this study, we examined several characteristics of PVAT and observed that in aged and HFD rats, several adipokines, chemokines and hormones were changed in mesenteric PVAT. We have presented evidence that CCL2 and CCL5 were increased in PVAT of aged rats and were decreased after adiponectin treatment, indicating an inhibition of early inflammation in the PVAT of this animal model. Chemokines from PVAT, such as CCL2 and CCL5 induce the recruitment and infiltration of monocytes, lymphocytes and neutrophils into the blood vessel wall to stimulate local vascular inflammation (Libby et al., 2010). Augmented CCL5 levels are known to be linked with atherosclerosis progression due to several mechanisms, such as the CCL5‐related promotion of monocyte CCL2 production, macrophage accumulation and neointimal growth (Mason et al., 2015).
In agreement with previous studies, we observed increased PVAT leptin levels with age and HFD. Increased leptin levels in PVAT promote neointima formation independent of obesity and systemic hyperleptinemia (Schroeter et al., 2013). In Ossabaw swine with metabolic syndrome, increased leptin, derived from epicardial PVAT, exacerbated coronary endothelial dysfunction (Payne et al., 2010). Similarly, an up‐regulation of leptin in aortic PVAT was accompained by a reduced anti‐contractile effect of PVAT. In a mouse model of diet‐induced obesity, the increase in leptin levels correlated with a loss in PVAT‐derived NO and eNOS (Gil‐Ortega et al., 2009). Importantly, we have shown decreased levels of leptin in mesenteric PVAT after administration of adiponectin.
Lipocalin‐2 is another proinflammatory adipokine positively associated with adiposity and obesity‐related metabolic and vascular disorders (Wang, 2012). Lipocalin‐2 deficiency protects mice from developing ageing‐ and obesity‐induced insulin resistance (Law et al., 2011). It appears that lipocalin‐2 acts as a lipid carrier to promote endothelium‐dependent contractions and attenuate endothelium‐dependent relaxations, by inducing eNOS uncoupling and elevating cyclooxygenase expression in endothelial cells (Liu et al., 2012). Thus, lipocalin‐2 seems to be an important regulator of vascular function. Previous studies have shown a negative association between plasma adiponectin and other markers of inflammation and atherosclerosis such as lipocalin‐2 (Wang et al., 2007). In this study, we showed that lipocalin‐2 levels in mesenteric PVAT were increased with ageing and HFD, and adiponectin administration decreased this adipokine.
This is the first study to show increased levels of markers such as lipocalin‐2, leptin, CCL2 and CCL5 in the PVAT of mesenteric arteries of obese rats, and chronic adiponectin administration reduced these changes. The unique efficacy of adiponectin in the normalization of mesenteric endothelial dysfunction is presumably not an expression of one mechanism alone but of the diverse and manifold properties of this adipokine.
We conclude that, in 12‐month old Wistar rats fed with a HFD, endothelial dysfunction is very pronounced in mesenteric arteries. Chronic administration of adiponectin normalized endothelial dysfunction enhancing eNOS phosphorylation and reducing oxidative stress at the mesenteric level in HFD rats. Additionally, the PVAT was modified with increased inflammatory and pro‐atherogenic biomarkers, and infusion of exogenous adiponectin normalized this PVAT phenotype. These findings support the concept of the central role of adiponectin as an important adipokine in the regulation of PVAT properties known to have vasocrine actions regulating vascular function. This has important implications for the treatment of vascular disease in patients with obesity and metabolic syndrome and suggests that globular adiponectin may have a therapeutic potential.
Author contributions
C.M.S, L.L. and R.M.S. designed the research study. C.M.S., A.P. and R.F. performed the research. L.L. studied the treatment of the animals with high‐fat diet. C.M.S. and A.P. analysed the data. R.M.S. contributed with essential reagents or tools and critically revised the manuscript. C.M.S. wrote the paper.
Conflict of interest
The authors declare no conflicts of interest.
Declaration of transparency and scientific rigour
This Declaration acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research recommended by funding agencies, publishers and other organisations engaged with supporting research.
Acknowledgements
The present work was supported by GAI, Faculty of Medicine, University of Coimbra, Banco Santander Totta and by PTDC/BIM‐MET/4447/2014; PEst FCT: UID/NEU/04539/2013; COMPETE: POCI‐01‐0145‐FEDER‐007440; PTDC/BIM‐MET/4447/2014; COMPETE: POCI‐01‐0145‐FEDER‐016784.
Sena, C. M. , Pereira, A. , Fernandes, R. , Letra, L. , and Seiça, R. M. (2017) Adiponectin improves endothelial function in mesenteric arteries of rats fed a high‐fat diet: role of perivascular adipose tissue. British Journal of Pharmacology, 174: 3514–3526. doi: 10.1111/bph.13756.
References
- Alexander SPH, Kelly E, Marrion N, Peters JA, Benson HE, Faccenda E et al. (2015a). The concise guide to pharmacology 2015/16: Overview. Br J Pharmacol 172: 5729–5743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Fabbro D, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015b). The concise guide to PHARMACOLOGY 2015/16: Enzymes. Br J Pharmacol 172: 6024–6109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonopoulos AS, Margaritis M, Coutinho P, Shirodaria C, Psarros C, Herdman L et al. (2015). Adiponectin as a link between type 2 diabetes mellitus and vascular NADPH‐oxidase activity in the human arterial wall: the regulatory role of perivascular adipose tissue. Diabetes 64: 2207–2219. [DOI] [PubMed] [Google Scholar]
- Bailey‐Downs LC, Tucsek Z, Toth P, Sosnowska D, Gautam T, Sonntag WE et al. (2013). Aging exacerbates obesity‐induced oxidative stress and inflammation in perivascular adipose tissue in mice: a paracrine mechanism contributing to vascular redox dysregulation and inflammation. J Gerontol A Biol Sci Med Sci 68: 780–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg AH, Combs TP, Scherer PR (2002). ACRP30/adiponectin: an adipokine regulating glucose and lipid metabolism. Trends Endocrinol Metab 13: 84–89. [DOI] [PubMed] [Google Scholar]
- Chatterjee TK, Stoll LL, Denning GM, Harrelson A, Blomkalns AL, Idelman G et al. (2009). Proinflammatory phenotype of perivascular adipocytes: influence of high‐fat feeding. Circ Res 104: 541–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Montagnani M, Funahashi T, Shimomura I, Quon MJ (2003). Adiponectin stimulates production of nitric oxide in vascular endothelial cells. J Biol Chem 278: 45021–45026. [DOI] [PubMed] [Google Scholar]
- Cheng KK, Lam KS, Wang Y, Huang Y, Carling D, Wu D et al. (2007). Adiponectin‐induced endothelial nitric oxide synthase activation and nitric oxide production are mediated by APPL1 in endothelial cells. Diabetes 56: 1387–1394. [DOI] [PubMed] [Google Scholar]
- Curtis MJ, Bond RA, Spina D, Ahluwalia A, Alexander SP, Giembycz MA et al. (2015). Experimental design and analysis and their reporting: new guidance for publication in BJP. Br J Pharmacol 172: 3461–3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez‐Real JM, Lopez‐Bermejo A, Casamitjana R, Ricart W (2003). Novel interactions of adiponectin with the endocrine system and inflammatory parameters. J Clin Endocrinol Metab 88: 2714–2718. [DOI] [PubMed] [Google Scholar]
- Gil‐Ortega M, Somoza B, Aranguez I, Ruiz‐Gayo M, Fernández‐Alfonso MS (2009). Changes in resistance artery function during the development of diet‐induced obesity. Hypertension 54: 105–106. [Google Scholar]
- Greenstein AS, Khavandi K, Withers SB, Sonoyama K, Clancy O, Jeziorska M et al. (2009). Local inflammation and hypoxia abolish the protective anticontractile properties of perivascular fat in obese patients. Circulation 119: 1661–1670. [DOI] [PubMed] [Google Scholar]
- Herrera MD, Mingorance C, Rodríguez‐Rodríguez R, Alvarez de Sotomayor M (2010). Endothelial dysfunction and aging: an update. Ageing Res Rev 9: 142–152. [DOI] [PubMed] [Google Scholar]
- Hui X, Lam KSL, Vanhoutte PM, Xu A (2012). Adiponectin and cardiovascular health: an update. Br J Pharmacol 165: 574–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iantorno M, Campia U, Di Daniele N, Nistico S, Forleo GB, Cardillo C et al. (2014). Obesity, inflammation and endothelial dysfunction. J Biol Regul Homeost Agents 28: 169–176. [PubMed] [Google Scholar]
- Kadowaki T, Yamauchi T, Kubota N, Hara K, Ueki K, Tobe K (2006). Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J Clin Invest 116: 1784–1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG (2010). Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol 160: 1577–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law IK, Xu A, Lam KS, Berger T, Mak TW, Vanhoutte PM et al. (2011). Lipocalin‐2 deficiency attenuates insulin resistance associated with aging and obesity. Diabetes 59: 872–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Wang WQ, Zhang H, Yang X, Fan Q, Christopher TA et al. (2007). Adiponectin improves endothelial function in hyperlipidemic rats by reducing oxidative/nitrative stress and differential regulation of eNOS/iNOS activity. Am J Physiol Endocrinol Metab 293: E1703–E1708. [DOI] [PubMed] [Google Scholar]
- Libby P, Okamoto Y, Rocha VZ, Folco E (2010). Inflammation in atherosclerosis: transition from theory to practice. Circ J 74: 213–220. [DOI] [PubMed] [Google Scholar]
- Liu JT, Song E, Xu A, Berger T, Mak TW, Tse HF et al. (2012). Lipocalin‐2 deficiency prevents endothelial dysfunction associated with dietary obesity: role of cytochrome P450 2C inhibition. Br J Pharmacol 165: 520–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumeng CN, Saltiel AR (2011). Inflammatory links between obesity and metabolic disease. J Clin Invest 121: 2111–2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchesi C, Ebrahimian T, Angulo O, Paradis P, Schiffrin EL (2009). Endothelial nitric oxide synthase uncoupling and perivascular adipose oxidative stress and inflammation contribute to vascular dysfunction in a rodent model of metabolic syndrome. Hypertension 54: 1384–1392. [DOI] [PubMed] [Google Scholar]
- Margaritis M, Antonopoulos AS, Digby J, Lee R, Reilly S, Coutinho P et al. (2013). Interactions between vascular wall and perivascular adipose tissue reveal novel roles for adiponectin in the regulation of endothelial nitric oxide synthase function in human vessels. Circulation 127: 2209–2221. [DOI] [PubMed] [Google Scholar]
- Mason RP, Corbalan JJ, Jacob RF, Dawoud H, Malinski T (2015). Atorvastatin enhanced nitric oxide release and reduced blood pressure, nitroxidative stress and RANTES levels in hypertensive rats with diabetes. J Physiol Pharmacol 66: 65–72. [PubMed] [Google Scholar]
- Matafome P, Rodrigues T, Pereira A, Letra L, Azevedo H, Paixão A et al. (2014). Long‐term globular adiponectin administration improves adipose tissue dysmetabolism in high‐fat diet‐fed Wistar rats. Arch Physiol Biochem 120: 147–157. [DOI] [PubMed] [Google Scholar]
- McGrath JC, Lilley E (2015). Implementing guidelines on reporting research using animals (ARRIVE etc.): new requirements for publication in BJP. Br J Pharmacol 172: 3189–3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer RI, Bakker W, Alta C‐L AF, Sipkema P, Yudkin JS, Viollet B et al (2013). Perivascular adipose tissue control of insulin‐induced vasoreactivity in muscle is impaired in db/db mice. Diabetes 62: 590–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motoshima H, Wu X, Mahadev K, Goldstein BJ (2004). Adiponectin suppresses proliferation and superoxide generation and enhances eNOS activity in endothelial cells treated with oxidized LDL. Biochem Biophys Res Commun 315: 264–271. [DOI] [PubMed] [Google Scholar]
- Mulvany MJ, Halpern W (1977). Contractile properties of small arterial resistance vessels in spontaneously hypertensive and normotensive rats. Circulation 41: 19–26. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Funayama H, Kubo N, Yasu T, Kawakami M, Saito M et al. (2006). Association of hyperadiponectinemia with severity of ventricular dysfunction in congestive heart failure. Circ J 70: 1557–1562. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Fuster JJ, Walsh K (2014). Adipokines: a link between obesity and cardiovascular disease. J Cardiol 63: 250–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouchi N, Ohishi M, Kihara S, Funahashi T, Nakamura T, Nagaretani H et al. (2003). Association of hypoadiponectinemia with impaired vasoreactivity. Hypertension 42: 231–234. [DOI] [PubMed] [Google Scholar]
- Ouwens DM, Sell H, Greulich S, Eckel J (2010). The role of epicardial and perivascular adipose tissue in the pathophysiology of cardiovascular disease. J Cell Mol Med 14: 2223–2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacher P, Beckman JS, Liaudet L (2007). Nitric oxide and peroxynitrite in health and disease. Physiol Rev 87: 315–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker‐Buffen JL, Nakamura K, Silver M, Kikuchi R, Tigges U, Yoshida S et al. (2013). T‐cadherin is essential for adiponectin mediated revascularization. J Biol Chem 288: 24886–24897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne GA, Borbouse L, Kumar S, Neeb Z, Alloosh M, Sturek M et al. (2010). Epicardial perivascular adipose‐derived leptin exacerbates coronary endothelial dysfunction in metabolic syndrome via a protein kinase C‐beta pathway. Arterioscler Thromb Vasc Biol 30: 1711–1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piga R, Naito Y, Kokura S, Handa O, Yoshikawa T (2007). Short‐term high glucose exposure induces monocyte‐endothelial cells adhesion and transmigration by increasing VCAM‐1 and MCP‐1 expression in human aortic endothelial cells. Atherosclerosis 193: 328–334. [DOI] [PubMed] [Google Scholar]
- Plant S, Shand B, Elder P, Scott R (2008). Adiponectin attenuates endothelial dysfunction induced by oxidised low‐density lipoproteins. Diab Vasc Dis Res 5: 102–108. [DOI] [PubMed] [Google Scholar]
- Preis SR, Massaro JM, Robins SJ, Hoffmann U, Vasan RS, Irlbeck T et al. (2010). Abdominal subcutaneous and visceral adipose tissue and insulin resistance in the Framingham heart study. Obesity 18: 2191–2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeter MR, Eschholz N, Herzberg S, Jerchel I, Leifheit‐Nestler M, Czepluch FS et al. (2013). Leptin‐dependent and leptin‐independent paracrine effects of perivascular adipose tissue on neointima formation. Arterioscler Thromb Vasc Biol 33: 980–987. [DOI] [PubMed] [Google Scholar]
- Sena CM, Nunes E, Louro T, Proença T, Fernandes R, Boarder MR et al. (2008). Effects of α‐lipoic acid on endothelial function in aged diabetic and high‐fat fed rats. Br J Pharmacol 153: 894–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sena CM, Matafome P, Louro T, Nunes E, Fernandes R, Seiça RM (2011). Metformin restores endothelial function in aorta of diabetic rats. Br J Pharmacol 163: 424–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sena CM, Pereira AM, Seiça R (2014). Endothelial dysfunction – a major mediator of diabetic vascular disease. Biochim Biophys Acta 1832: 2216–2231. [DOI] [PubMed] [Google Scholar]
- Shimabukuro M, Higa N, Asahi T, Oshiro Y, Takasu N, Tagawa T et al. (2003). Hypoadiponectinemia is closely linked to endothelial dysfunction in man. J Clin Endocrinol Metab 88: 3236–3240. [DOI] [PubMed] [Google Scholar]
- Southan C, Sharman JL, Benson HE, Faccenda E, Pawson AJ, Alexander SP et al. (2016). The IUPHAR/BPS guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucleic Acids Res 44: D1054–D1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szasz T, Webb RC (2012). Perivascular adipose tissue: more than just structural support. Clin Sci (Lond) 122: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan KCB, Xu A, Chow WS, Lam MCW, Ai VHG, Tam SCF et al. (2004). Hypoadiponectinemia is associated with impaired endothelium dependent vasodilation. J Clin Endocrinol Metab 89: 765–769. [DOI] [PubMed] [Google Scholar]
- Virdis A, Duranti E, Rossi C, Dell'Agnello U, Santini E, Anselmino M et al. (2015). Tumour necrosis factor‐alpha participates on the endothelin‐1/nitric oxide imbalance in small arteries from obese patients: role of perivascular adipose tissue. Eur Heart J 36: 784–794. [DOI] [PubMed] [Google Scholar]
- Wang Y (2012). Small lipid‐binding proteins in regulating endothelial and vascular functions: focusing on adipocyte fatty acid binding protein and lipocalin‐2. Br J Pharmacol 165: 603–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Lam KS, Kraegen EW, Sweeney G, Zhang J, Tso AW et al. (2007). Lipocalin‐2 is an inflammatory marker closely associated with obesity, insulin resistance, and hyperglycemia in humans. Clin Chem 53: 34–41. [DOI] [PubMed] [Google Scholar]
- Weyer C, Funahashi T, Tanaka S, Hotta K, Matsuzawa Y, Pratley RE et al. (2001). Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. J Clin Endocrinol Metab 86: 1930–1935. [DOI] [PubMed] [Google Scholar]
- Xi W, Satoh H, Kase H, Suzuki K, Hattori Y (2005). Stimulated HSP90 binding to eNOS and activation of the PI3‐Akt pathway contribute to globular adiponectin‐induced NO production: vasorelaxation in response to globular adiponectin. Biochem Biophys Res Commun 332: 200–205. [DOI] [PubMed] [Google Scholar]
- Xu A, Vanhoutte PM (2012). Adiponectin and adipocyte fatty acid binding protein in the pathogenesis of cardiovascular disease. Am J Physiol Heart Circ Physiol 302: H1231–H1240. [DOI] [PubMed] [Google Scholar]
- Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K et al. (2001). The fat‐derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med 7: 941–946. [DOI] [PubMed] [Google Scholar]
- Yamauchi T, Kamon J, Waki H, Imai Y, Shimozawa N, Hioki K et al. (2003). Globular adiponectin protected ob/ob mice from diabetes and ApoE‐deficient mice from atherosclerosis. J Biol Chem 278: 2461–2468. [DOI] [PubMed] [Google Scholar]
- Zhu W, Cheng KK, Vanhoutte PM, Lam KS, Xu A (2008). Vascular effects of adiponectin: molecular mechanisms and potential therapeutic intervention. Clin Sci (Lond) 114: 361–374. [DOI] [PubMed] [Google Scholar]


