Reportedly, it was Hippocrates (ca. 460–370 BC) and Galenos (Galen of Pergamum; ca AD 129–199) who first stated that a poison, and not evil spirits, must be responsible for symptoms such as fever, vomiting and diarrhea [1]. Naturally, the nature of this ‘poison’ remained unclear. Richard Pfeiffer (1858–1945) discovered that a toxic substance was associated with the membrane of bacteria, and called it ‘endotoxin’ [2]. Endotoxin infusion mimics many of the inflammatory, metabolic, cardiovascular changes observed in sepsis patients and represents one of the main inducers of shock in sepsis. The discoverer of its receptor (the Toll-like receptor 4) [3], Nüsslein-Volhard was awarded the Nobel prize for this discovery in 1995, further propelling this field of research.
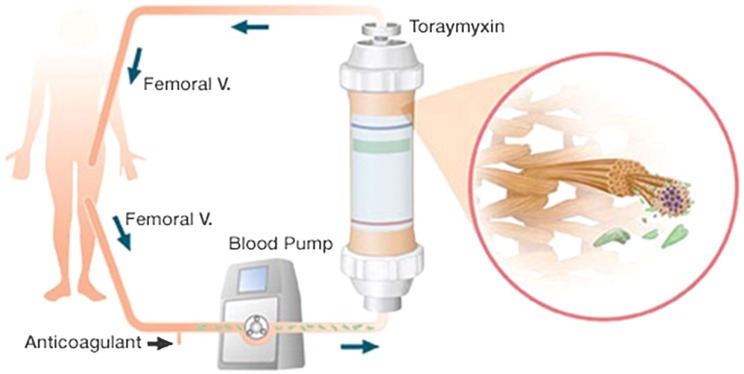
Clinical research has shown that endotoxin is indeed circulating in the blood of up to 50% of sepsis shock patients [4] and associated with impaired clinical outcome [5]. In view of the pivotal role of endotoxin in sepsis patients, the idea of ‘blood purification’ emerged. Polysterene fiber filters coated with polymyxin B are able to bind endotoxin (Fig. 1). A meta-analysis of trials primarily of Japanese origin indicated a survival benefit for patients treated with polymyxin B hemoperfusion [6]. Since then, two randomized trials from Europe and one from USA/Canada have been conducted.
Fig. 1.
Extracorporeal endotoxin binding by polymyxin B hemoperfusion
In 2009, the Euphas-trial (Early Use of Polymyxin B Hemoperfusion in Abdominal Septic shock), an open-label randomized study performed in Italy, was published [7]. Sixty-four patients with abdominal severe sepsis or septic shock were treated with PMX hemoperfusion (or standard of care) within 6 h following surgery. The primary end-point of this study was the change (from day 0 to 3) in hemodynamic stability. Secondary endpoints included change in SOFA-score and mortality. The authors reported an improvement in hemodynamic stability in the PMX hemoperfusion group, while this was not the case in the control group. In addition, survival time analysis showed a significant improvement in the treatment group. The trial was stopped prematurely based on these results. A discussion emerged [8], as it was highlighted that no significant differences between the 2 groups were present, and that polymyxine B hemoperfusion appears to prolong time to mortality, but did not significantly affect day 28 mortality. Early termination of the study was deemed unfortunate [8].
Six years later, the Abdomix-trial from France was published [9]. Again, abdominal septic shock patients were randomized to PMX-hemoperfusion treatment (n = 119) or to the control group (n = 113). The first hemoperfusion session started within 12 h following surgery and was repeated 24 h later. Adequacy of surgical procedure was blindly evaluated by an independent surgeon and classified as adequate, sufficient, or inadequate. The 28-day mortality was 28% in the PMX-group versus 20% in the control group (p = 0.1). Incomplete PMX sessions occurred in 11% of the patients due to circuit coagulation mainly during the 1st session. Importantly, also in the subgroup having received adequate surgery and two uneventful complete PMX sessions, no sign of a beneficial effect of treatment was observed. Finally, neither a more rapid improvement in SOFA-score in the treatment group nor a more rapid decrease in circulating cytokines was found in this study [10]. Potential reasons for these findings were discussed [11, 12].
In the recently finalized Euphrates trial [13] (Evaluating the Use of Polymyxin B Hemoperfusion in a Randomized controlled trial of Adults Treated for Endotoxemia and Septic shock), PMX hemoperfusion was applied in a blinded manner in patients with septic shock and confirmed endotoxemia, as measured by the endotoxin activity assay (EAA). The trial, conducted in the United States and Canada, was powered to enroll 360 patients to detect an effect on 28-day all-cause mortality. Similar to the previous trials, treatment consisted of two sessions of PMX hemoperfusion 24 h apart. Unique features of this trial included patient enrichment by use of EAA to confirm endotoxemia (EAA > 0.6) and use of a detailed “façade” hemoperfusion event as a blinding mechanism. Following the second interim analysis, the study was resized to 650 patients. However, after 446 evaluable patients were included, the trial was terminated. The study has not yet been published, but Spectral, the company of the EAA, has stated that it failed to meet its primary end-point. There was a non-significant 5% mortality reduction in the per protocol population, and it is mentioned that ‘other positive benefits were observed’ in treated septic shock patients compared to standard treatment [14]. Currently, no further details have been made public and it remains unclear to what extent timing of the intervention and the amount of endotoxin adsorped by the membrane may play a role.
New developments are emerging. Coupled plasma filtration adsorption is another extracorporeal blood purification therapy for sepsis which adsorbs both proinflammatory and anti-inflammatory mediators from filtered plasma. Effects on clinical outcome are awaited. The ‘Cytosorb’-filter, with polymer beads that have pores that can adsorb hydrophobic molecules in a size range of approximately 10–55 kD (sufficient to remove almost all known cytokines, including HMGB-1, but not endotoxin), has a huge absorption area of 40,000 m2, and several case series have been published. Unfortunately, in a small (n = 37 patients) blinded, randomized study in cardiac surgery patients, installment of the adsorber on the cardio-pulmonary bypass machine had no effect on surgery-induced cytokine release or hemodynamic stability measures [15]. The ‘oXiris’-filter is designed to adsorb endotoxin as well as cytokines, but no adequately powered human study is currently available.
While removal of endotoxin/cyokines is theoretically seen as a beneficial effect, other potentially detrimental effects may also occur. As both pro-and anti-inflammatory cytokines may decrease, the net effect is uncertain. In addition, other nutrients and therapeutic drugs (including antibiotics) may also be removed from the circulation, with potential negative impact on organ function and recovery.
The high incidence and morbidity, mortality, and associated costs of septic shock illustrate that the medical need for an adjuvant treatment is still unmet. Endotoxin, one of the most potent mediators of sepsis, is found in high levels in approximately half of patients with septic shock. Polymyxin B (PMX) hemoperfusion has been shown in numerous studies to successfully remove endotoxin and potentially improve outcomes. Although numerous case series and small studies suggest that these beneficial effects may also be present in sepsis patients, larger randomized controlled trials have not confirmed these findings. While blood purification in sepsis is a valid approach, the potential efficacy of LPS/cytokine elimination using these membranes currently cannot be estimated without positive clinical data from randomized trials.
Compliance with ethical standards
Conflicts of interest
P. Pickkers has received honoraria from Gambro-Baxter. D. Payen received unlimited grant from Toray Med Ltd (Japan) and Sté Meditor (France) to design and perform the ABDOMIX randomized Clinical Trial.
Contributor Information
Peter Pickkers, Email: peter.pickkers@radboudumc.nl.
Didier Payen, Email: dpayen1234@orange.fr.
References
- 1.Beutler B, Rietschel ET. Innate immune sensing and its roots: the story of endotoxin. Nat Rev Immunol. 2003;3:169–176. doi: 10.1038/nri1004. [DOI] [PubMed] [Google Scholar]
- 2.Pfeiffer R. Untersuchungen über das Choleragift. Z Hyg Infektionskr. 1892;11:393–412. doi: 10.1007/BF02284303. [DOI] [Google Scholar]
- 3.Anderson KV, Bokla L, Nusslein-Volhard C. Establishment of dorsal-ventral polarity in the Drosophila embryo: the induction of polarity by the Toll gene product. Cell. 1985;42:791–798. doi: 10.1016/0092-8674(85)90275-2. [DOI] [PubMed] [Google Scholar]
- 4.Marshall JC, Walker PM, Foster DM, Harris D, Ribeiro M, Paice J, Romaschin AD, Derzko AN. Measurement of endotoxin activity in critically ill patients using whole blood neutrophil dependent chemiluminescence. Critical Care (Lond) 2002;6:342–348. doi: 10.1186/cc1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Opal SM, Scannon PJ, Vincent JL, White M, Carroll SF, Palardy JE, Parejo NA, Pribble JP, Lemke JH. Relationship between plasma levels of lipopolysaccharide (LPS) and LPS-binding protein in patients with severe sepsis and septic shock. J Infect Dis. 1999;180:1584–1589. doi: 10.1086/315093. [DOI] [PubMed] [Google Scholar]
- 6.Zhou F, Peng Z, Murugan R, Kellum JA. Blood purification and mortality in sepsis: a meta-analysis of randomized trials. Crit Care Med. 2013;41:2209–2220. doi: 10.1097/CCM.0b013e31828cf412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cruz DN, Antonelli M, Fumagalli R, Foltran F, Brienza N, Donati A, Malcangi V, Petrini F, Volta G, Bobbio Pallavicini FM, Rottoli F, Giunta F, Ronco C. Early use of polymyxin B hemoperfusion in abdominal septic shock: the EUPHAS randomized controlled trial. JAMA. 2009;301:2445–2452. doi: 10.1001/jama.2009.856. [DOI] [PubMed] [Google Scholar]
- 8.Vincent JL. Polymyxin B hemoperfusion and mortality in abdominal septic shock. JAMA. 2009;302:1968. doi: 10.1001/jama.2009.1606. [DOI] [PubMed] [Google Scholar]
- 9.Payen DM, Guilhot J, Launey Y, Lukaszewicz AC, Kaaki M, Veber B, Pottecher J, Joannes-Boyau O, Martin-Lefevre L, Jabaudon M, Mimoz O, Coudroy R, Ferrandiere M, Kipnis E, Vela C, Chevallier S, Mallat J, Robert R, Group A Early use of polymyxin B hemoperfusion in patients with septic shock due to peritonitis: a multicenter randomized control trial. Intensive Care Med. 2015;41:975–984. doi: 10.1007/s00134-015-3751-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coudroy R, Payen D, Launey Y, Lukaszewicz AC, Kaaki M, Veber B, Collange O, Dewitte A, Martin-Lefevre L, Jabaudon M, Kerforne T, Ferrandiere M, Kipnis E, Vela C, Chevalier S, Mallat J, Charreau S, Lecron JC, Robert R, group A Modulation by polymyxin-b hemoperfusion of inflammatory response related to severe peritonitis. Shock. 2017;47:93–99. doi: 10.1097/SHK.0000000000000725. [DOI] [PubMed] [Google Scholar]
- 11.Antonelli M, Cutuli SL, Ronco C. Polymyxin B hemoperfusion in septic shock: just look at the evidence! Intensive Care Med. 2015;41:1731–1732. doi: 10.1007/s00134-015-3931-x. [DOI] [PubMed] [Google Scholar]
- 12.Darmon M, Bagshaw SM, Forni LG. Balancing the “humors” in severe sepsis: still a role for extracorporeal therapies? Intensive Care Med. 2015;41:1132–1134. doi: 10.1007/s00134-015-3801-6. [DOI] [PubMed] [Google Scholar]
- 13.Klein DJ, Foster D, Schorr CA, Kazempour K, Walker PM, Dellinger RP. The EUPHRATES trial (Evaluating the Use of Polymyxin B Hemoperfusion in a Randomized controlled trial of Adults Treated for Endotoxemia and Septic shock): study protocol for a randomized controlled trial. Trials. 2014;15:218. doi: 10.1186/1745-6215-15-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mass Device (2016) Spectral Medical’s Toraymyxin fails pivotal trial. http://ift.tt/2dyhRgo
- 15.Bernardi MH, Rinoesl H, Dragosits K, Ristl R, Hoffelner F, Opfermann P, Lamm C, Preissing F, Wiedemann D, Hiesmayr MJ, Spittler A. Effect of hemoadsorption during cardiopulmonary bypass surgery - a blinded, randomized, controlled pilot study using a novel adsorbent. Critical Care (Lond) 2016;20:96. doi: 10.1186/s13054-016-1270-0. [DOI] [PMC free article] [PubMed] [Google Scholar]