Abstract
Background
Malawi has the highest age standardised rate of cervical cancer in the world. This study describes the presentation, management and short-term outcomes of patients with newly diagnosed cervical cancer at Queen Elizabeth Central Hospital (QECH), in Southern Malawi.
Methods
All patients with a new diagnosis of cervical cancer presenting to QECH between 1st January-1st July 2015 had demographic data, referral pathway, stage, histology and management prospectively recorded at presentation, and at two months after initial presentation.
Results
310 women presented with cervical cancer to QECH and 300 were included (mean age 44.9 years; HIV 47%), representing 8% of the estimated annual number of new presentations in Malawi. Mean age of patients with HIV was 6.9 years younger compared to those without HIV (p<0.05). 132 (44%) patients had stage 1 cervical cancer and 168 (56%) presented with more advanced disease (stage II-IV). There was a mean delay of 23.1 weeks between onset of symptoms and being seen by a clinician and a further 19 weeks before attending QECH. Most common management plans at initial consultation were: same day biopsy (n=112, 37.3%);, booking for curative surgery (n=76, 25.3%);, and referral to palliative care (n=93, 31%). At 2 months, 64 (57%) biopsies were reported, 31 (40.8%) operations were completed and 27 (29%) patients had attended the palliative clinic.
Conclusions
Patients presenting with cervical cancer to QECH were young, with a high prevalence of HIV, and late stage disease. The lack of pathological and surgical capacity and the absence of radiotherapy severely limited the possibility of curative treatment. Access to quality palliative care remains an important component of management in low resource settings. Improving awareness of cervical cancer in the community, and better recognition and management within the health service, are important in reducing the cancer burden for women in Malawi.
Introduction
Cervical cancer is the most common malignancy in women in Malawi, accounting for over 40% of female cancers; it is a major cause of mortality and morbidity.1–3 Accurate estimation of the numbers of women affected is complicated by the fact that many cancers may go unrecorded and that there is a lack of a national system of death certification. Two agencies, the Malawi Cancer Registry (MCR) and the International Agency for Research on Cancer's (IARC) GLOBOCAN programme, have made recent estimates which differ considerably.1,3 The MCR estimated the burden of new cases as 1236/year in 2009 and IARC 3684 cases/year (2012 data). The IARC GLOBOCAN figures show that, at 75.9/100,000, the country currently has the highest age-standardised incidence rate of cervical cancer in the world.3 Mortality, at 49.8/100,000, is also the highest recorded internationally and one Malawian study suggests 5-year survival is only 2.9%,2 lower than reports from Uganda and Zimbabwe, where 5-year survival is 17.7% and 26.5%, respectively.4,5 Survival rates achieved in high-resource settings are considerably higher; cancer.net reports a 68% 5-year survival for all women with cervical cancer in the USA.6
The reasons for poor cancer survival in Africa are widely reported.7–10 There are not effective screening programmes, community and healthcare worker cancer awareness is poor, plus there is shortage of trained professionals, oncology staff, treatment facilities and equipment. All these factors contribute to late presentation and diagnosis of advanced cervical cancer is common. A range of delays, often attributed to patient/community and healthcare determined delays have been reported, and the role of traditional healers is also cited.7
Cervical cancer is an AIDS defining condition, and as Malawi has one of the highest HIV prevalence rates in the world with 10.6% of the adult population (15–64 years) having HIV (12.8% women vs 8.2% in men)2 it remains an important risk factor. Other prominent risk factors include human papillomavirus (HPV) infection, smoking and early age of first sexual intercourse.
Malawians typically access conventional healthcare initially at local clinics and cervical screening using visual inspection with acetic acid is increasingly available through the National Cervical Cancer Control Programme.2 From clinics, patients can be referred to District Hospitals and to one of three Central Hospitals. One of Malawi's two oncology units is based at QECH. This is the tertiary referral centre for the Southern region where 45% of Malawi's population live. Simple extrapolation from GLOBOCAN estimates of 3684 incident cases of cervical cancer in Malawi each year, suggest that 1658 cases should therefore be presenting to QECH each year (829 in 6 months).3
At QECH, women with suspected cervical cancer predominantly present to, and are managed, by gynaecology or oncology departments. The Recent American Society of Clinical Oncology resource stratified guidelines would categorise Malawi's treatment capacity as ‘basic’.8 The main treatment options in Malawi are surgery, chemotherapy and referral to the palliative care service at Tiyanjane Clinic based within QECH.9,10 Radiotherapy, not yet available in Malawi, is an important treatment option in cervical cancer and potentially 60% to 70% of patients would benefit from it with possible cured.11 A small number of patients do access radiotherapy in other countries either self-funded or through a Malawi Government scheme.
Within QECH concerted efforts are being made to implement multidisciplinary cancer care.12 As inpatient care becomes more standardised the potential for systematic data collection about patients, cancer type and stage is developing. However much about the patient pathway before and after hospital remains unclear. Understanding the clinical epidemiology of cervical cancer and patient management pathways is crucial to improving care. We therefore undertook an analysis of 6 months of cervical cancer presentations to QECH.
Methods
All patients presenting to gynaecology and oncology departments between 1st January and 1st July 2015 (outpatient or inpatient services) with a new clinical and/or histologically confirmed diagnosis of cervical cancer were included. New cases of suspected cervical cancer in other departments within the hospital are referred to gynaecology or oncology, therefore to ensure capture of all new cases in QECH patients were identified by daily search of inpatient admission books in Gynaecology and Oncology wards and identification in respective new patient clinics. Patients who did not provide informed consent or those with a previous diagnosis of cancer were excluded.
Patients were clinically assessed at first presentation by a research clinician (registrar or consultant) who, after gaining consent, completed a standard study proforma. Patient demographics, presenting symptoms, time and pathway to presentation, risk factors, stage of disease at presentation, initial investigation and treatment were recorded. Cervical cancer was staged with the revised International Federation of Gynaecology and Obstetrics (FIGO) staging system for cervical cancer.13
In all enrolled patients, follow up data was collected at one and two months after initial presentation to determine whether they had received their treatment/management documented at their initial consultation. We reviewed inpatient and outpatient medical records in addition to histopathology reports from the laboratory for biopsy results, theatre lists for confirmation of completed operations, attendance records at oncology and palliative care clinics, and attempted to phone all palliative care patients at 2 months for confirmation of outcome.
Data was anonymised at the point of capture, recorded on password protected computers in password protected files only accessible to the study team, and analysed using Microsoft Excel. We compared variables between patients with different stages of cervical cancer using standard parametric and non-parametric tests according to the distribution of variables, in addition to comparing patients with and without HIV. To facilitate analysis, we created two groups by combining patients with FIGO stage I-IIA disease (surgically resectable) and stage IIB-IV (non-resectable), referred to as early and advanced cervical cancer respectively.
Ethics approval for the study was granted by the College of Medicine Research Ethics Committee (reference P.01/15/1670).
Results
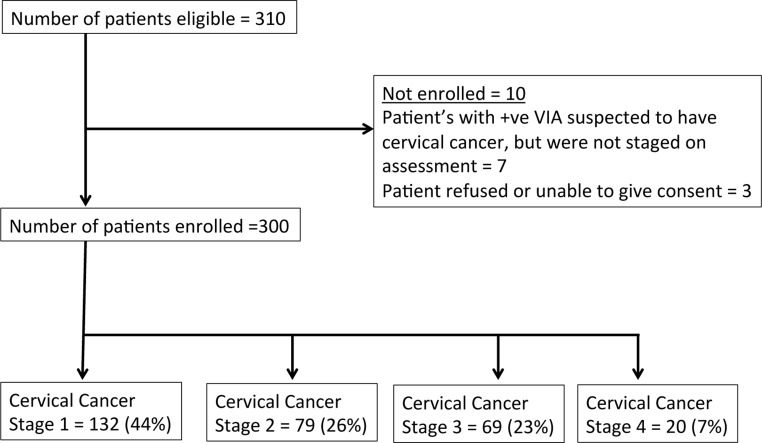
310 patients presented with a clinical diagnosis of cervical cancer between 1st January and 1st July 2015, and 300 of these were included in the analysis (Figure 1), representing 8% of the estimated total annual number (3684) of new presentations in Malawi.3 Two patients did not consent, one was unable to consent, and seven patients with a positive visual inspection by acetic acid (VIA) were excluded from the analysis as they were not staged. Considering 45% of the population live in the Southern Region, we estimate these 300 patients represent 36% of the expected cases in this region over this time period.
Figure 1.
CONSORT diagram illustrating the number of patients with cervical cancer included in the study
Mean age of patients was 44.9 years (range 19–86 years) and this increased with stage of disease. 141 (47%) patients had HIV, 136 (45.3%) patients did not have HIV, and 23 (7.6%) did not know their HIV status (Table 1). Mean age of patients with HIV was 6.9 years younger than those without HIV (P < 0.01, 95% confidence interval 3.9 to 9.9). This age difference was consistent across all stages of cervical cancer. To facilitate analysis, we created two groups by coalescing stage — Early (FIGO Stage I-IIA) and Advanced (Stage IIB-IV). 184 (61.3%) patients had early disease (mean age 42.5), and 116 (38.6%) patients had advanced disease (mean age 48.7) at diagnosis (Table 2).
Table 1.
Stage and mean age of all women enrolled in the study, divided into those with HIV, without HIV, and in those in which HIV status was unknown
| Stage | All patients | Patients with HIV | Patients without HIV |
Patients with unknown HIV status |
||||
| Number | Mean age | Number | Mean age | Number | Mean age | Number | Mean age | |
| I | 132 | 42.1 | 63 | 39.4 | 59 | 44.4 | 10 | 45.0 |
| II | 79 | 45.7 | 41 | 41.5 | 34 | 49.6 | 4 | 55.5 |
| III | 69 | 47.8 | 29 | 42.2 | 34 | 51.5 | 6 | 54.3 |
| IV | 20 | 50.4 | 8 | 45.9 | 9 | 49.1 | 3 | 66.0 |
| All | 300 | 44.9 | 141 | 40.9 | 136 | 47.8 | 23 | 52.0 |
Table 2.
Number of patients presenting with early and advanced cancer classified by age group
| Age (years) |
Early cancer (stage I to IIA) |
Advanced cancer (stage IIB to IV) |
| < 30 | 24 | 2 |
| 30 to 39 | 62 | 29 |
| 40 to 49 | 51 | 35 |
| 50 to 59 | 26 | 23 |
| 60 to 69 | 9 | 20 |
| ≥ 70 | 12 | 7 |
| Total | 184 | 116 |
The patient pathway
Source of referral
Nine (3%) women presented asymptomatically through screening after having a VIA indicating a cancer. Of the 291 patients who presented with symptoms, 169 (58%) women had been referred from a clinic and 122 (42%) from other hospitals.
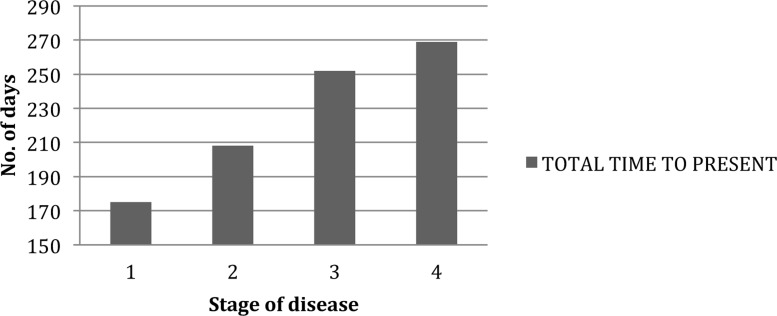
The mean delay between women recognizing symptoms and presenting to a clinician was 23.1 weeks with a further 19 weeks before presentation at QECH—meaning that the total delay between becoming aware of symptoms and QECH appointment was 42 weeks. Women with advanced disease at presentation experienced longer delays amounting to a month more between symptom recognition and QECH appointment (Table 3). When we compared time to presentation with stage of disease (I-IV), there was a trend of increased time to presentation with the more advanced disease at diagnosis (Figure 2).
Table 3.
Mean time period (weeks) between symptom onset, first clinical assessment, and clinic appointment in patients with early and advanced cervical cancer at Queen Elizabeth Central Hospital
| Cancer stage | Number of women | Mean time between symptoms to first healthcare visit (weeks) |
Mean time between first healthcare visit to QECH appointment (weeks) |
Mean total time between symptoms to QECH appointment (weeks) |
| Early (stage I to IIA) |
184 (61.3%) |
22.4 | 17.4 | 39.9 |
| Advanced (stage IIB to IV) |
116 (38.7%) |
23.9 | 21.4 | 45.3 |
| All cases | 300 | 23.1 | 18.7 | 42.0 |
Figure 2.
Time to present to hospital from symptom onset according to stage of disease
We also compared the healthcare delay between first healthcare visit at a community clinic (169 women) or a hospital setting (122 women) to diagnosis at QECH. The delay was 16.3 and 21.7 weeks, respectively. There was a trend towards quicker referrals from a clinic. Despite these trends, there were no statistical differences in delays attributable to early/advanced stage of disease or to source of referral.
Initial symptoms
The commonest initial symptom reported was vaginal bleeding (often post coital) in 105 (35.0%) women, and vaginal discharge in 62 (20.5%) with dysuria (n = 7, 2.3%), abdominal pain (n = 3, 1.0%), vomiting and weight loss (n = 3, 1.0%) also featuring.
Risk factors
Median age of first sexual intercourse was 17 (IQR 15 to 19) and 124 women (41.3%) had their first birth under 20 years of age. 87 (29%) said they had more than three sexual partners and 45 (15%) said they had previously had a sexually transmitted disease. 156 (52%) patients had five or more children. Six (2%) patients had a family history of cervical cancer, and 26 (8.7%) were smokers (Figure 3).
128 (42.6%) said they were aware of cervical screening, however only 7 (2.3%) of these patients knew the correct age that screening is offered in Malawi.9 (3%) said that they had heard of the HPV vaccine; none of 300 patients were vaccinated. 100 (33.3%) patients had never had a speculum or VIA examination before presentation to specialist services at QECH.
Management
286 (95%) patients were reviewed by gynaecology and 14 (5%) by Oncology; we recorded the management plan at this first clinic appointment. The commonest plan, in 112 cases (37.3%), was to have a same day biopsy and return for review when results were available. In the remainder, direct referrals were made at that first appointment to: palliative care (n = 93, 31%), surgery (n = 76, 25.3%), oncology for chemotherapy (n = 8, 2.6%), and radiotherapy (n = 1, 0.3%). Ten (3.3%) patients were asked to attend QECH at a later date with no management plan recorded due to overbooked clinics.
Short-term outcomes
Surgery
Of the 76 operations that were planned, 31 (40.8%) were carried out, 8 (10.5%) within 1 month of appointment, 10 (13.2%) within two months of appointment, and 13 (17%) after two months. The remainder (n = 45; 59.2%) were not carried out due factors including progression of disease and cancellation of surgery by clinician or surgical coordinator. Two patients were unable to attend due to no money for transport to the hospital. Three surgeries were delayed due to water shortages. Mean time from initial appointment to surgery was 31.8 days (range 2 to 109 days). Of the 31 patients with surgical findings documented, 30 were early surgically resectable cancers and one was advanced (Stage IIB).
Pathology
229 women (76%) had a biopsy taken and 126 (55%) reports were available. 31(25%) were reported within one month, 72 (57%) by 2 months, the remaining 53 (42%) after this. Of the 71 patients who were not biopsied, 54 (76.1%) had advanced disease (stage II to IV).
91(39.7%) biopsies confirmed cervical cancer (87 Squamous cell carcinoma, 3 adenocarcinoma, 1 Kaposi's sarcoma) and 9 (3.9%) demonstrated cervical intraepithelial neoplasia. The remaining reports showed cervicitis (n = 14), dysplasia (n = 5), wart (n = 1) and a nabothian cyst (n = 1). The remaining 5 were suboptimal samples. 54 (59%) of the 91 confirmed cancer cases were available 2 months or more after the date of biopsy.
Palliative care
Of 93 patients referred to palliative care, 27 (29%) were seen locally in the Tiyanjane palliative care clinic at QECH. In addition we attempted to phone all palliative care patients at 2 months: 42 patients had no mobile phone; there was no answer in 23; 10 had been to palliative care and were alive; 8 were alive and had not been to palliative care; 2 were awaiting chemotherapy; 5 were on chemotherapy; and 3 had died.
Oncology
Chemotherapy was initiated within a month for 6 of the 8 patients where this was planned (although one subsequently died between 1 and 2 months). One patient had private radiotherapy abroad and started this between 1 and 2 months. 2 were on no treatment, alive and well.
Discussion
Case numbers
This is a large case series of accurately clinically staged cervical cancer reported from an African country. We show that 300 cases present to QECH in 6 months, a caseload consistent with Malawi Cancer Registry figures.1 The Malawi Cancer registry largely relies on returns from QECH for their data collection so it is reassuring that our findings are of a similar scale. However they are considerably below GLOBOCAN estimates of 1658 new cases in Southern region, which implies that many women with cervical cancer, if they receive formal healthcare, are treated in local clinics or hospitals and never reach QECH.3 It may be that not all patients are referred from community clinics, patients may not have the means to get there, or they may choose to have alternative therapies and see no need in attending the hospital.
Patient characteristics
As in many Low and Middle Income Countries, the spectrum of presentation is skewed towards more advanced disease. This is complex to treat and an annual caseload of 600 cases is large for any hospital. It is a certainly a logistical challenge for QECH with its small clinical workforce and limited resources.8 The average age of our patients was 42 years, which is younger in comparison to 46.5 years in Zimbabwe, and 49 years in the US.14 The reasons for this are complex and unknown but highlights the need for public education on cervical cancer screening to start in younger age groups. The impact of HIV is obvious with 47% having HIV and as a group an average of 7 years younger than women without HIV. These points plus the fact that other characteristics regarding parity and sexually transmitted disease were consistent with published data gives us reassurance that we have a nationally representative sample of cervical cancer patients.15,16 HIV positive women in our sample were almost all on antiretroviral medication. This reflects the success of Malawi's AIDS treatment programme within which VIA is promoted and whose staff could be helpfully given training about detecting cancer early.16,17
Awareness of cervical cancer and screening
Improving public awareness that cervical cancer is one of the largest health problems for women in Malawi is crucial. Knowledge about the two most important preventive measures available, cervical screening and the HPV vaccine, was low in our sample of women with cervical cancer, absolutely the target group for preventive messages. The Malawi SRHR policy for cervical cancer prevention and control mentions neither the vaccine nor the age at which screening should start.18 This information needs to be clearly communicated to the public. Clear referral pathways from community hospitals/clinics with time targets should be established in order to improve coordination between primary, secondary and tertiary services. Information for clinicians and women attending ARV clinic and a referral pathway to screening would improve awareness and screening rates of asymptomatic women.
Delays in presentation
In the UK, NHS England has national guidelines for patients with a suspected cancer, stating that they must be seen in a specialist clinic within 2 weeks of symptom onset, and for definitive treatment to be initiated within 62 days.19 The overall delay from symptoms onset to first QECH clinic appointment of 42 weeks in our study is long, and is likely to have contributed to the presentation of advanced stage disease. The common causes of delays seen elsewhere in presenting to healthcare with symptoms suggestive of cancer were identified.20 We were unable, however to detect any sub group of women with particular types of delay by age or source of referral. Certainly we need to investigate the causes of delayed cancer presentation in Malawi, and discover why these may be different if at all to other types of disease. Reviewing the existing Malawian evidence about uptake and adherence to cervical screening and other fields such as tuberculosis and AIDS and thinking how this could transfer to cancer care would be useful.18 The healthcare component in the delay (between contact with the referring hospital or clinic and being seen at QECH) was still almost 19 weeks, nearly half of the total. We have accumulated examples of substandard practice such as lack of clinical assessment using a speculum at local centers and prolonged antibiotic use before referral. Given that a relatively small number of hospitals and clinics made the referrals, some focused training should be possible. Clinicians uniformly mention the trust women place in traditional healers and herbalist and the large proportion of women who received care from them prior to coming to the health service likely resulted in delay. Consequently the traditional healer cadre could be offered some basic training to alert them to suspicious symptoms and refer these women to the health service. With enhanced training in signs and symptoms of cancer, clinicians would quickly become more skilled at cancer detection and outcomes could improve sharply.21 Clinicians and support staff should also be supported to develop referral systems and methods of feedback so that referring clinicians learn from their decisions and standards improve.
Diagnosis
In resource-poor countries such as Malawi, specialists in diagnostic specialties such as pathology and radiology are particularly rare. Pathology services at QECH face significant challenges and under half of specimens taken had reports available two months after biopsy. While rapid initiation of treatment informed by pathology was often not possible, those patients who had surgery were treated an average of just over one month from first being seen — a speed that rivals the developed world. The women who had operations were overwhelmingly early stage cancers where cure is feasible highlighting that the correct clinical prioritization process took place. A high proportion of women with advanced disease were referred directly to palliative services.
Treatment
However, about half of operations did not take place as intended. While this loss to follow-up requires further investigation, it is highly unlikely that the surgical capacity to tackle this oncology workload will exist in the foreseeable future in Malawi. Radiotherapy offers the possibility of cure for many women who currently cannot access potentially curative therapy. As data from the International Atomic Energy Authority show that radiotherapy would benefit some 60% of these women, this emphasises the importance of realising the National Non Communicable Disease strategy and bringing radiotherapy to Malawi.11,22,23 A specialist cervical cancer nurse could be appointed to coordinate this group of patients. A key responsibility would be coordinating a multidisciplinary team meeting between gynaecology, oncology and palliative care teams.
In a review of cervical cancer research in Africa, Finnocchario-Kessler et al highlighted the importance of severely affected countries such as Malawi increasing the amount of local research into cervical cancer treatment. This work demonstrates the large numbers of women are coming forward for treatment. It illustrates the challenges they pose for treatment services and adds to recent large reports from Malawi on cervical screening and palliative care in cervical cancer patients.10,24 This is important in helping to complete the picture of the clinical epidemiology of the condition in the country.
Limitations
Whilst QECH provides the oncological service for Southern Malawi, we are aware our population may not represent all the cases of cervical cancer in the region due, in part, to the logistical and financial challenges of travelling large distances for tertiary care. Collecting data prospectively improves accuracy of data capture as we do not have to rely on finding missing patient notes. The complete ascertainment of cases gives a comprehensive picture of the workload and their large number allows statistical analysis and confident conclusions. Our ambitions to collect follow up data were compromised by this well-recognised difficulty in resource poor settings. Tiyanjane Clinic in a 2015 cervical cancer palliative care case series experienced 33% loss to follow-up.10 Our efforts to contact patients by telephone to ascertain their status were largely unsuccessful and we are working with the MCR to develop more robust follow-up systems to collect outcome data.
Conclusions
Our results indicate that patients presenting with cervical cancer to QECH are young, with a high prevalence of HIV, and late stage disease. The cohort was largely unaware of preventive initiatives such as HPV vaccination and cervical screening, and awareness programmes such as the ‘Cancer below the belt’ campaign should be promoted. VIA is widely available in Malawi and should, where possible, be routinely performed along with a speculum exam during every gynaecological or pelvic examination, particularly in patients with HIV whom are at a much increased risk of cervical cancer. The lack of pathological and surgical capacity and the absence of radiotherapy severely limits the possibility of curative treatment. While the Government's plans to increase the number of staff working in cancer care are important, a significant advance for cervical cancer patients will come with the widespread use of HPV vaccination and the arrival of radiotherapy in the country.
Acknowledgements
Edinburgh Malawi Cancer Partnership and College of Medicine QECH.
Competing interests
All authors declare that they have no competing interests related to this work.
References
- 1.Msyamboza K, et al. Burden of cancer in Malawi; common types, incidence and trends: National population-based cancer registry. BMC Research Notes. 2012;5(1):149. doi: 10.1186/1756-0500-5-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Msyamboza KP, et al. Cervical cancer screening uptake and challenges in Malawi from 2011 to 2015: retrospective cohort study. BMC Public Health. 2016;16:806. doi: 10.1186/s12889-016-3530-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferlay J SI, Ervik M, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11. Lyon, France: International Agency for Research on Cancer; 2013. [Google Scholar]
- 4.Gondos A, et al. Cancer survival in Kampala, Uganda. Br J Cancer. 2005;92(9):1808–1812. doi: 10.1038/sj.bjc.6602540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gondos A, et al. Cancer survival in a southern African urban population. Int J Cancer. 2004;112(5):860–864. doi: 10.1002/ijc.20471. [DOI] [PubMed] [Google Scholar]
- 6. http://www.cancer.net/cancer-types/cervical-cancer/statistics.
- 7.Zuma T, et al. The role of traditional health practitioners in Rural KwaZulu-Natal, South Africa: generic or mode specific? BMC Complementary and Alternative Medicine. 2016;16(1):304. doi: 10.1186/s12906-016-1293-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chuang LT, et al. Management and Care of Women With Invasive Cervical Cancer: ASCO Resource-Stratified Clinical Practice Guideline. J Clin Oncol. 2016;34(27):3354–3355. doi: 10.1200/JCO.2016.68.3789. [DOI] [PubMed] [Google Scholar]
- 9.Tapsfield JB, Bates M Jane. Hospital based palliative care in sub-Saharan Africa; a six month review from Malawi. BMC Palliative Care. 2011;10(1):12. doi: 10.1186/1472-684X-10-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bates MJ, Mijoya A. A review of patients with advanced cervical cancer presenting to palliative care services at Queen Elizabeth Central Hospital in Blantyre, Malawi. Malawi Medical Journal. 2015;27(3):93–95. doi: 10.4314/mmj.v27i3.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.AGENCY, I.A.E., author Planning National Radiotherapy Services: A Practical Tool. IAEA Human Health Series. Vienna: INTERNATIONAL ATOMIC ENERGY AGENCY; 2011. [Google Scholar]
- 12.Brown E, et al. The Edinburgh Malawi Cancer Partnership: helping to establish multidisciplinary cancer care in Blantyre, Malawi. J R Coll Physicians Edinb. 2016;46(1):14–17. doi: 10.4997/JRCPE.2016.104. [DOI] [PubMed] [Google Scholar]
- 13.Prat J, F.C.o.G. Oncology FIGO's staging classification for cancer of the ovary, fallopian tube, and peritoneum: abridged republication. Journal of Gynecologic Oncology. 2015;26(2):87–89. doi: 10.3802/jgo.2015.26.2.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. https://qap.sdsu.edu/screening/cervicalcancer/facts.html.
- 15.Malawi, U.N.P.F., author Brief Report Malawi Country Office Summary 2015. Lilongwe: 2016. [Google Scholar]
- 16.Malawi, G.o., author Malawi AIDS Response Progress Report 2015. Lilongwe: 2015. [Google Scholar]
- 17.Government, M., author; M.o. Health, editor. Malawi Population-Based HIV Impact Assessment - Preliminary Findings. Lilongwe, Malawi: 2016. [Google Scholar]
- 18.Maseko FC, Chirwa ML, Muula AS. Health systems challenges in cervical cancer prevention program in Malawi. Global Health Action. 2015;8 doi: 10.3402/gha.v8.26282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. https://www.england.nhs.uk/wp-content/uploads/2015/03/delivering-cancer-wait-times.pdf.
- 20.Sylla BS, Wild CP. A million Africans a year dying from cancer by 2030: What can cancer research and control offer to the continent? International journal of cancer Journal international du cancer. 2012;130(2):245–250. doi: 10.1002/ijc.26333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Masamba LPL, et al. Tuberculosis Diagnosis Delaying Treatment of Cancer: Experience From a New Oncology Unit in Blantyre, Malawi. Journal of Global Oncology. 2016 doi: 10.1200/JGO.2015.000299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Masamba L. The state of oncology in Malawi in 2015. Malawi Medical Journal. 2015;27(3):77–78. [PMC free article] [PubMed] [Google Scholar]
- 23.Government of Malawi, M.o.H., author National Action Plan for prevention and management of non-communicable diseases in Malawi (2012–2016) Lilongwe: 2013. [Google Scholar]
- 24.Finocchario-Kessler S, et al. Cervical cancer prevention and treatment research in Africa: a systematic review from a public health perspective. BMC Women's Health. 2016;16(1):29. doi: 10.1186/s12905-016-0306-6. [DOI] [PMC free article] [PubMed] [Google Scholar]