The Society of Urologic Oncology Clinical Trials Consortium (SUO-CTC) is a clinical research investigator network of more than 300 members from more than 180 clinical sites in the United States and Canada. Through industry collaboration, SUO-CTC provides access to active, influential investigators in urologic oncology inclusive of both academic and community practices. It establishes and promotes synergistic partnerships between physician leaders and their respective practices and research entities (pharmacology and biotechnology industries) dedicated to evaluating breakthrough biomarkers and therapies through thoughtful and achievable clinical trials. This alliance of leading academic and community-based uro-oncologists is committed to furthering urology research for patients with urologic cancers.
Founding
Leaders within the SUO formed the SUO-CTC in 2008 as a nonprofit organization. SUO leaders formed SUO-CTC to foster higher levels of participation between the urologic oncology community and cutting-edge clinical trials because they realized that identifying targets and managing clinical trials was becoming increasingly complex. Additionally, SUO leaders determined that a multidisciplinary approach of various medical specialties and entities collaborating with industry sponsors would serve the evolution of clinical science and promote healthcare improvement. SUOCTC works assiduously to facilitate these goals.
SUO-CTC launched its first call for trial sites to more than 200 members in January 2010. Since then, the SUO’s network sites have participated in 18 trials in bladder cancer, prostate cancer, and renal cell carcinoma. The research generated 13 abstracts to date, with additional publications in active development and review.
SUO’s mission is outlined in three ways:
Offer and provide for the best possible approach to disease education and management for better patient outcomes
Continually provide professionals with opportunities to further their education
Inform professionals of existing research through collaborative educational processes, which benefits patients through the development of better therapies and clinical understanding.
Research
The SUO-CTC offers clinical research services to include protocol review and consultation on concept development, site selection, site communications, meetings with investigators, and consultancy agreements.
SUO-CTC conducts clinical trials with the pharmaceutical industry on the safety and efficacy of therapies, diagnostics, biomarkers, and devices for urologic cancers. Its vast network can identify key patient populations to deliver rapid and timely patient accrual. Additionally, the SUO-CTC involvement allows increased trial, product, and company visibility among SUO’s growing membership. The broad member and site demographics, which include academic, community, and large urology group practices (LUGPA), is an important strategy to the conduction of clinical research and the successful patient recruitment to clinical trials. To date, the SUO-CTC has contributed nearly 1000 patients to clinical trials (Figure 1).
Figure 1.
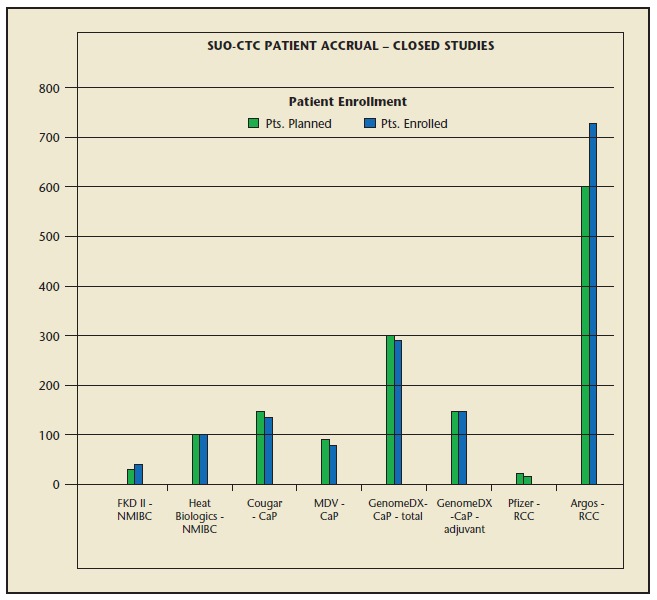
Society of Urologic Oncology Clinical Trials Consortium (SUO-CTC) patient accrual. CaP, prostate cancer; NMIBC, non–muscle-invasive bladder cancer; Pts, patients; RCC, randomized clinical trial. Argos Therapeutics, Charlotte, NC; Cougar Biotechnology, Los Angeles, CA; FDK Therapies Oy, Kuopio, Finland; GenomeDX Biosciences, Vancouver, Canada; Heat Biologics, Durham, NC; Medivation, San Francisco, CA; Pfizer, New York, NY.
Currently, SUO-CTC has five active trials open to patient accrual or in the process of final site selection. Open to patient accrual:
A Study to Evaluate INSTILIDRIN® in Patients with High Grade, Bacillus Calmette- Guerin (BCG) Unresponsive NMIBC (NCT02773849)
A Phase 3 Randomized, Double-blind, Multicenter Study of Adjuvant Nivolumab Versus Placebo in Subjects With High Risk Invasive Urothelial Carcinoma (NCT02632409)
A Study of Apalutamide (JNJ-56021927, ARN-509) Plus Androgen Deprivation Therapy (ADT) Versus ADT in Participants With mHSPC (TITAN; NCT02489318)
A Phase III, Open-label, Multicenter, Randomized Study of Atezolizumab (Anti-PD-L1 Antibody) Versus Observation as Adjuvant Therapy in Patients With High Risk Muscle-Invasive Urothelial Carcinoma After Surgical Resection (IMvigor010; NCT02450331)
A Phase III, Multicenter, Randomized, Placebo-controlled, Double Blind Study of ATEZOLIZUMAB (Anti- PD-L1 Antibody) as Adjuvant Therapy in Patients With PD-L1 Selected Renal Cell Carcinoma at Intermediate to High Risk of Developing Metastasis following Nephrectomy (IMmotion010; NCT03024996)
Finalizing site selection:
Prospective Validation of Prostate Biomarkers for Repeat Biopsy (PRIORITY; NCT03082274)
Membership
SUO-CTC has more than 300 academic, community, LUGPA, and military members who operate at leading sites in the United States and Canada. SUO’s physician leaders are dedicated to increasing urologic oncology community involvement in pivotal clinical trials. They are accountable, committed, and invested in contributing to the scientific body of knowledge, the success of the process, and the completion of SUO-CTC trials (Figure 2).
Figure 2.
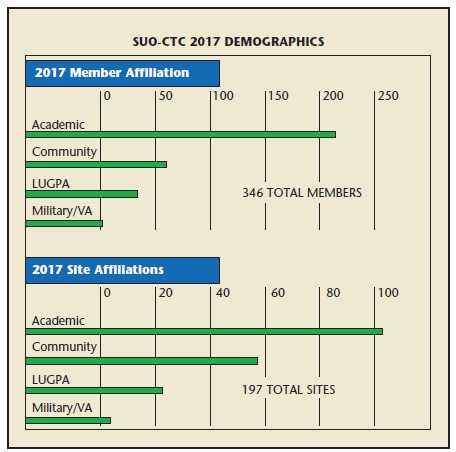
Society of Urologic Oncology Clinical Trials Consortium (SUO-CTC) 2017 demographics. VA, Veteran’s Administration.
We are very proud of our efforts to further our knowledge in urologic oncology and of our members’ efforts to involve themselves in research while learning and networking with other physicians and industry representatives. The organization remains committed to improving patient care and patient outcomes through our commitment to clinical research.
For more information, please contact SUO-CTC by telephone at 847-264-5901 or visit http://suoctc. org.


