Abstract
Intravenous immunoglobulin (IVIG) is used to treat or prevent severe viral infection, especially cytomegalovirus (CMV) infections. IVIG was characterized to understand its interaction with CMV-infected cells. IVIG retarded CMV spread and reduced virus yields depending on the neutralizing (NT) antibody titer. Immediate early protein synthesis was reduced by IVIG in 3 to 15 h, and IVIG specifically reduced the ratio of 66/68k protein synthesis among immediate early proteins in an NT antibody-dependent manner between 4 and 8 h after infection, indicating that antigenic modulation of CMV-infected cells by IVIG reduced viral protein synthesis and virus production. The half-life of antibody bound to CMV-infected cells was 3.8 h. NT antibody titers to varicella-zoster virus (VZV) and CMV in IVIG were dose dependently absorbed by cells infected with VZV and CMV, respectively, but the antibody titers to CMV and VZV, respectively, were not affected. NT antibody in 0.3 mL of IVIG (15 mg) was specifically absorbed by 108 CMV-infected cells and 107 VZV-infected cells, suggesting that the NT antibody in IVIG might be inactivated by one-tenth of a similar volume of CMV-infected or VZV-infected cells. Various antiviral activities of IVIG may contribute to control and alleviation of CMV infection.
Keywords: : cytomegalovirus, varicella-zoster virus, intravenous immunoglobulin, ADCC, antigenic modulation
Introduction
Cytomegalovirus (CMV) infection is one of the most devastating complications of birth (15,17,20,24,27,40,44), and it is also closely associated with rejection of transplanted organs (8,25,29). Maternal antibody prevents measles and varicella infection and alleviates CMV infection in neonates. Immunoglobulin (IgG) neutralizes viral infectivity with and without complement and mediates antibody-dependent cellular cytotoxicity (ADCC) toward infected cells (49). Thus, intravenous IgG (IVIG) is used to treat severe viral infections, especially CMV infections in congenitally CMV-infected (26) and immunosuppressed patients, such as transplant recipients (38,39,52). Although treatment with hyperimmune globulin did not significantly modify the course of primary CMV infection during pregnancy (19), CMV-specific hyperimmune globulin lowered the risk of maternal–fetal transmission and ameliorated the disease sequelae (31). Prophylactic administration of IVIG or valaganciclovir and IVIG benefits transplant recipients (6,12,23,30,32,48).
To characterize the role of anti-CMV antibody, we compared the neutralization (NT) of varicella-zoster virus (VZV) and CMV with and without complement and found that both NT activities were enhanced by the complement. The NT antibody titer of IVIG toward VZV and CMV was enhanced about three to six times by the complement (49). Antibody to VZV showed ADCC toward VZV-infected cells, but anti-CMV antibody failed to show significant ADCC toward CMV-infected cells. Thus, we showed the functional role of IVIG in the NT viral infectivity of VZV and CMV but contrasting results on ADCC between VZV and CMV infection in vitro. VZV-infected cells were efficiently eliminated by ADCC, whereas CMV-infected cells were not and may not be a target of ADCC. A rise in antibody against viral early antigens in the presence of anti-CMV antibody was observed in renal transplant recipients as assessed by immunofluorescent antibody (47) and immunoprecipitation (36), and this indicates the possible modification of CMV replication in the presence of anti-CMV antibody.
Antigenic modulation has first been reported in measles virus-infected cells treated with anti-measles virus antibody, and its treatment modified viral protein expression and localization (9–11). Anti-CMV antibody in IVIG showed the modification of viral protein synthesis, reduction of virus production in anti-CMV-treated infected cells and this may correspond to antigenic modulation in CMV infection.
In this study, we focused on the interaction of NT antibody of IVIG with spread of CMV infection, the modification of intracellular viral protein synthesis, and CMV-infected cell surface by observation of ADCC and reduction of NT antibody by adsorption with the respective infected cells. The half-life of antibody absorbed by CMV was determined. The results on the fate of IVIG may contribute to an understanding of the use and role of IVIG in the treatment of CMV infection.
Materials and Methods
Cells, viruses, and antiserum
Human embryonic lung (HEL) cells were propagated in Eagle's minimum essential medium (MEM) that was supplemented with 10% heat-inactivated fetal bovine serum (FBS) and maintained in the same medium with 2% FBS. Human lung cancer A549 cells were grown in MEM with 5% FBS (33,37,49).
Towne (21) and AD169 (4,35) stains of CMV were propagated in HEL cells, and cell-free virus was obtained by rapid freezing and thawing of infected cultures and stored at −70°C. AD169 strain was used for virus growth; viral protein synthesis in the presence of IVIG and Towne strain was used for ADCC, absorption of NT antibody, or a half-life of IgG bound to CMV-infected cells. A549 cells and HEL cells were used for the VZV NT test, and HEL cells were used for the CMV NT test. VZV was used for ADCC, absorption of NT antibody, in contrast to CMV.
The original Oka strain of VZV was propagated in HEL cells and stored as a cell-free virus in SPGC medium (phosphate-buffered saline [PBS] containing 5% sucrose, 0.1% sodium glutamate, and 10% FBS) (37,45) at −70°C.
Venoglobulin IH, an IVIG preparation for intravenous administration, was purchased from Japan Blood Product Organization Co.; its IgG concentration was 50 mg/mL.
Infectious center assay
HEL cells (in 25 cm2 plastic flasks) were infected with 0.001 plaque-forming units (pfu)/cell of AD169 strain for 1 h. After being washed three times with maintenance medium, the cells were incubated in maintenance medium in the presence or absence of IVIG (NT 1:64 to CMV) or various NT titers. The cells were trypsinized and suspended in 2 mL of the maintenance medium at the indicated times, and 0.2 mL aliquots of serially diluted cell suspensions were inoculated on the HEL monolayer (60 mm plastic dish) and overlaid with the nutrient agarose medium. After 2 weeks of incubation, the cells were fixed with 5% neutral formalin and stained with methylene blue. The numbers of infected cells forming plaques were counted under a binocular microscope.
Virus production in IVIG-treated cells
HEL cells in 25 cm2 flasks were infected with Towne strain at 2 pfu/cell and incubated in the absence or presence of IVIG at various NT titers for 72 h. The cultures were washed three times with maintenance medium, subjected to freezing and thawing three times, followed by centrifugation. The virus titers of their supernatants were determined in HEL cells by the plaque assay.
Immunoprecipitation of radiolabeled CMV proteins and SDS-PAGE
Isotopic labeling and immunoprecipitation, followed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), were based on the procedure previously used (34,36). HEL cell monolayers (100 mm plastic dish) were infected with mock or 2 pfu/cell of AD169 strain for 1 h. IVIG was dialyzed with MEM without methionine before use. The cells were incubated in the presence or absence of antibody with an NT titer of 1:64 or various other titers and labeled with 30 μCi/mL of 35S-methionine (>1,000 Ci/mmol; GE Healthcare, Tokyo, Japan) in the maintenance medium without methionine for 3 h. As the effect of IVIG on the ratio of 66 and 68k CMV proteins was obvious, the ratio was analyzed by using cells labeled 4 to 8 h after infection.
After three washes with PBS, the labeled cells were solubilized in 4 mL of RIPA buffer (20 mM Tris [pH 8.0], 150 mM NaC1, 1% sodium deoxycholate, 1% Triton X-100, 0.1% SDS, 1 mM phenylmethylsulfonyl fluoride) by sonication in an ice bath, and the cell lysates were clarified by centrifugation at 100,000 × g for 2 h at 4°C. One or 10 μL of serum was incubated with 400 μL of each antigen at 4°C overnight, and immune complexes were separated after addition of Protein A Sepharose (GE Healthcare, Tokyo, Japan) and analyzed by SDS-PAGE. Molecular weight markers used were from an Electrophoresis Calibration Kit (GE Healthcare, Tokyo, Japan).
Antibody-dependent cellular cytotoxicity
ADCC was examined as previously reported (49). Briefly, male Hartley guinea pigs (3 weeks old) were purchased from Sankyo Labs Service Co. Ltd., Tokyo, Japan. Guinea pig splenocytes were used as effector cells. Spleen cells were treated with hemolytic ACK buffer (150 mM ammonium chloride, 10 mM potassium hydrogen carbonate, 1 mM EDTA), washed twice with PRMI-1640 with 10% FBS, and resuspended in PRMI-1640 with 10% FBS at concentrations of 2 × 107 and 6 × 106 cells/mL. VZV-infected HEL cells, which showed 40–60% cytopathic effect, were removed from flasks with 0.1% EDTA-0.05% trypsin and resuspended in PRMI-1640 with 10% FBS at a concentration of 2 × 105 cells/mL. CMV (Towne strain)-infected cells were similarly suspended in PRMI-1640 with 10% FBS at a concentration of 2 × 105 cells/mL. We examined the effector-to-target (E:T) cell ratio of 100:1 and 30:1 in the ADCC assays to VZV- and CMV-infected cells with guinea pig spleen cells, and the E:T ratio of 100:1 showed better activity to VZV- and CMV-infected cells as previously reported (49). Aliquots of 0.1 mL of target cells (2 × 104 cells) were dispensed into round-bottomed tubes. IgG and/or 0.2 mL of effector cells was added to target cells to give the IgG concentration of no IgG, 1:25 and 1:100 dilutions and the E:T cell ratio of 100:1. The tubes were incubated for 4 h at 37°C in 5% CO2. At the end of incubation, 0.2 mL cell suspensions were subjected to infectious center (IFC) assays to examine the number of infected cells. ADCC was expressed as the mean ± standard deviation of six samples. All animal experimental procedures conformed to the National Institute of Health Guide for the Care and Use of Laboratory Animals and the Experimentation Guidelines of the University of Toyama.
Fate of IgG bound to CMV-infected cells
The fate of IgG bound to CMV-infected cells was examined by Western blot analysis. CMV-infected cells at 48 h after infection with 5 pfu/cell and uninfected cells in six-well plates were treated with 10 mg of IVIG for 2 h, washed three times, and incubated with MEM with 2% FBS. The cells were serially harvested 0 to 72 h after removal of IgG, and the amount of IgG bound to the cells was detected by Western blot analysis with HRP-conjugated rabbit anti-human IgG gamma-chains (Dako, Denmark). The amount of IgG detected by Western blot was determined with an HRP-conjugated anti-human IgG second antibody using chemiluminescence (Chemi-Lumi One L chemiluminescence detection system, Nakalai) quantitated using ImageQuant software (GE Healthcare Life Sciences) with a Fujifilm LAS-4000 imaging system.
Residual NT titer of IVIG after mixing with infected cells
Three hundred microliters of IVIG (15 mg of IgG) was mixed with 0 to 9.6 × 107 Towne strain-infected cells or 0 to 3.51 × 107 VZV-infected cells and incubated for 1 h at 37°C. Then, the mixtures were centrifuged at 3,000 rpm for 10 min at 4°C, and the supernatants were examined for their NT antibody titers to CMV and VZV.
NT tests were performed as previously described (33,34,43,49). Briefly, solutions of CMV (Towne strain) and VZV containing ∼200 pfu/80 μL were mixed with equal amounts of twofold serially diluted samples, and the mixtures were incubated at 37°C for 1 h. Eighty microliters of diluted globulin sample-virus mixtures were inoculated into HEL cells for CMV and A549 cells or HEL cells for VZV in six-well plastic dishes. Then, the mixtures were incubated at 37°C for 1 h to permit adsorption of non-neutralized virus. Nutrient methylcellulose medium was overlaid on the monolayers, and incubation was continued for 12 days for CMV and 7 to 8 days for VZV, until the plaques were large enough to count. The neutralizing activity was expressed as the reciprocal of the highest dilution of the concentration necessary to reduce the number of plaques by 50% (IC50).
Statistical analysis
The ADCC assessed by residual plaque formation was analyzed by one-way ANOVA followed by the Bonferroni/Dunn method, and the differences were considered significant if p values were less than 0.05.
Results
Spread of CMV in presence of IVIG
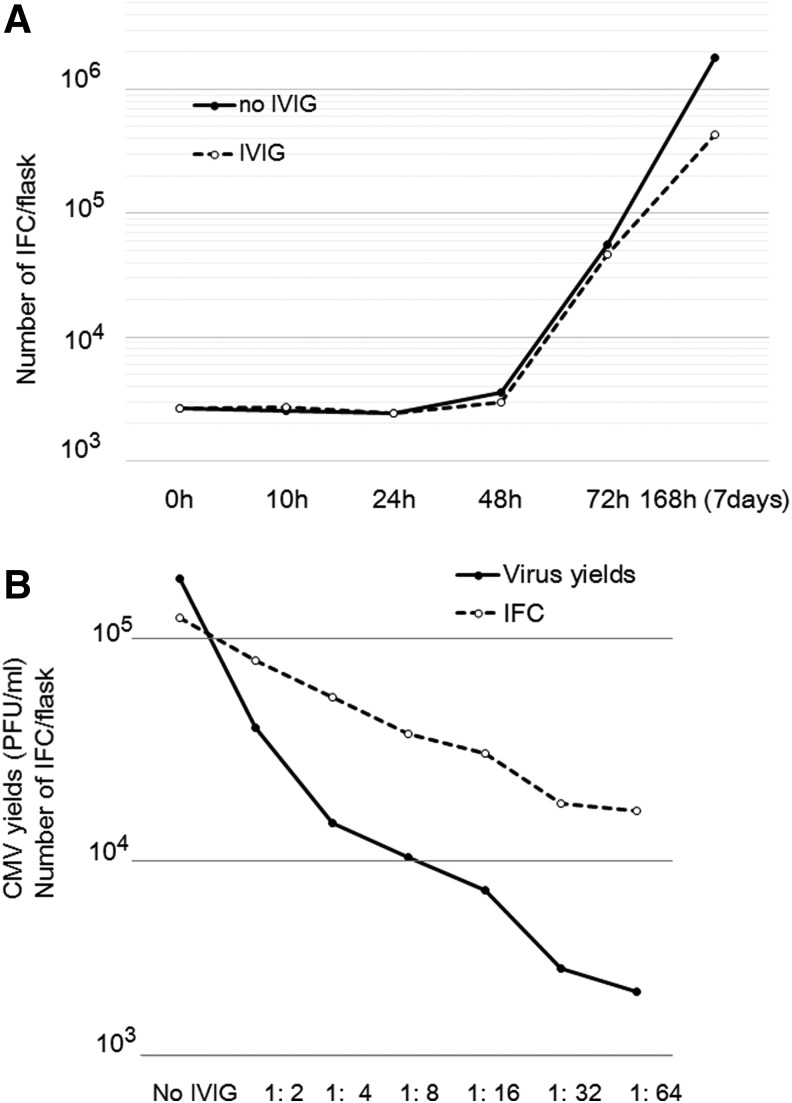
Figure 1A shows the spread of infection in the presence and absence of IVIG (NT 1:64) as assessed by the IFC assay. The number of infected cells began to increase at 48 h after virus infection in the presence or absence of antiserum. Because the number of infected cells was not affected by the NT antibody for a maximum of 48 h, the spread of CMV was retarded and the presence of IVIG suppressed the increase in the number of infected cells more than the absence of IVIG. The spread of CMV infection was suppressed in an NT antibody titer-dependent manner, as shown in Figure 1B. Suppression of intracellular virus production by IVIG was evaluated, and IVIG was shown to reduce the virus yields to about 1% in an NT antibody titer-dependent manner. Thus, IVIG suppressed the spread of infection and virus yields in an NT antibody titer-dependent manner.
FIG. 1.
Growth of CMV in the presence or absence of antiserum. (A) The final antibody titer used was 1:64 in the NT test, and the spread of CMV infection was assessed by an IFC assay. The solid line indicates the number of CMV infectious centers that grew in the absence of antiserum, and the dotted line indicates that of CMV infectious centers that grew in the presence of antiserum. (B) Effects of NT antibody titer on CMV replication at 72 h and spread of infection were assessed by a plaque assay (solid line) and IFC assay (dotted line), respectively. CMV, cytomegalovirus; IFC, infectious center.
Effect of IVIG on CMV protein synthesis
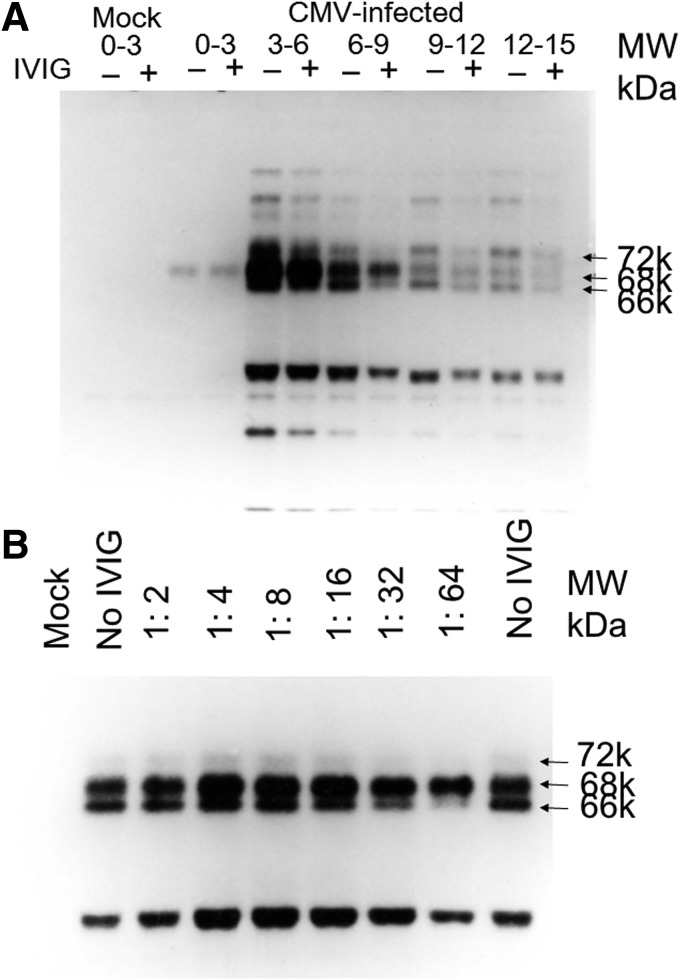
As shown in Figure 1B, intracellular virus yields were suppressed by anti-CMV antibody (IVIG) when the effect of antibody on CMV protein synthesis was analyzed in the presence and absence of anti-CMV antibody. The time course of viral protein synthesis is shown in Figure 2A. CMV stimulates cellular macromolecular synthesis of proteins (16,42). This was taken into consideration for the identification of CMV-specific proteins. The immediate early protein synthesis was reduced by anti-CMV antibody between 3 and 15 h, and the ratio of 66 over 68k was reduced in IVIG-treated cells (Fig. 2A). Anti-CMV antibody specifically reduced the 66k immediate early protein synthesis versus 68k protein in an NT-antibody-dependent manner between 4 and 8 h after infection (Fig. 2B).
FIG. 2.
Time course of CMV protein synthesis in the presence or absence of antiserum. Viral proteins were labeled with 35S methionine in the presence or absence of antiserum at the indicated time period (h) after infection and immunoprecipitated with IVIG. (A) IVIG (−) indicates that the cells were grown and labeled in the absence of antiserum, and IVIG (+) indicates that the cells were grown and labeled in the presence of IVIG at NT antibody titer of 1:64. (B) Effects of antibody titer of IVIG on the viral protein synthesis in CMV-infected cells 4–8 h after infection. The 66k protein was reduced by the increase in anti-CMV antibody titers, but 68k protein was not influenced.
ADCC of IVIG toward CMV-infected cells
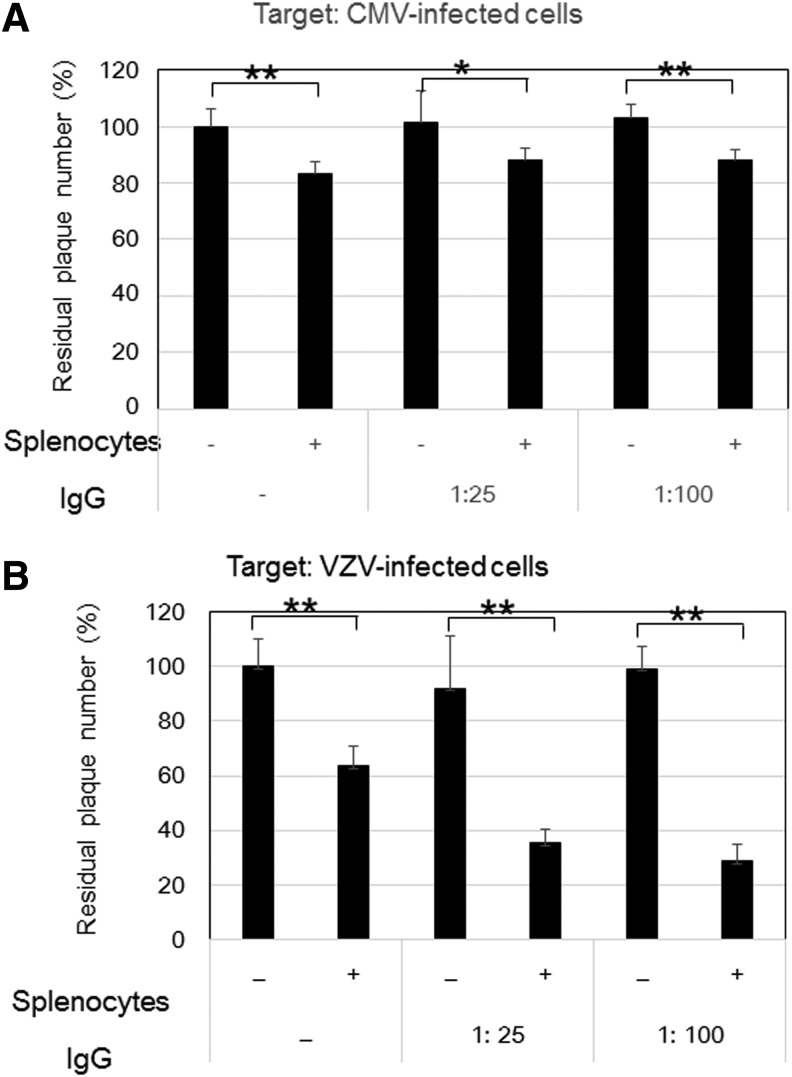
Figure 3 shows the comparative ADCC of anti-CMV antibody and anti-VZV antibody in IVIG against CMV- and VZV-infected cells. Our previous study showed that an optimal condition of ADCC was the E:T ratio of 100:1 and an IgG dilution of 1:25 and 1:100. IVIG alone had no effect on the plaque number. The residual number of VZV-infected cells treated with splenocytes was reduced to 60% of that of the control, indicating that the reduction was due to natural killer (NK) cell activity. The residual number of VZV-infected cells was further reduced to 35% by the addition of anti-VZV antibody in IVIG and splenocytes, indicating that 25% of infected cells were eliminated by the ADCC of anti-VZV antibody.
FIG. 3.
ADCC against CMV-infected cells (A) and VZV-infected cells (B). CMV-infected cells with 133 plaques (n = 6) and VZV-infected cells with 128 plaques (n = 6) were expressed as 100% as the residual infected cells, and the residual infected cells in the various treatments were expressed as the percentage + SD. ADCC assays were performed in the condition at the E:T ratio of 100:1 in both infected cells and no IVIG and its dilutions of 1:25 and 1:100 based on the results in the previous report (49). Common observations in CMV- and VZV infection were no effect of antibody alone and significant reduction of plaques by splenocytes, corresponding to NK cell activity. In contrast, splenocytes with IgG (ADCC) significantly reduced plaques in VZV-infected cells but not in CMV-infected cells, as previously reported (49). * and ** indicate a p-value less than 0.05 and 0.01, respectively. ADCC, antibody-dependent cellular cytotoxicity; IgG, immunoglobulin; VZV, varicella-zoster virus.
The effects of splenocytes and anti-CMV antibody in IVIG on CMV-infected cells were assessed by CMV plaque formation. Treatment with splenocytes reduced the residual number of CMV-infected cells to 80% of that of the control (Fig. 3A), and a further significant reduction of the residual number of infected cells was not observed with IVIG, in contrast to VZV-infected cells (Fig. 3B). Thus, in contrast to VZV-infected cells, ADCC of anti-CMV antibody in IVIG was not observed in CMV-infected cells, as previously reported (49).
Fate of IgG bound to CMV-infected cells
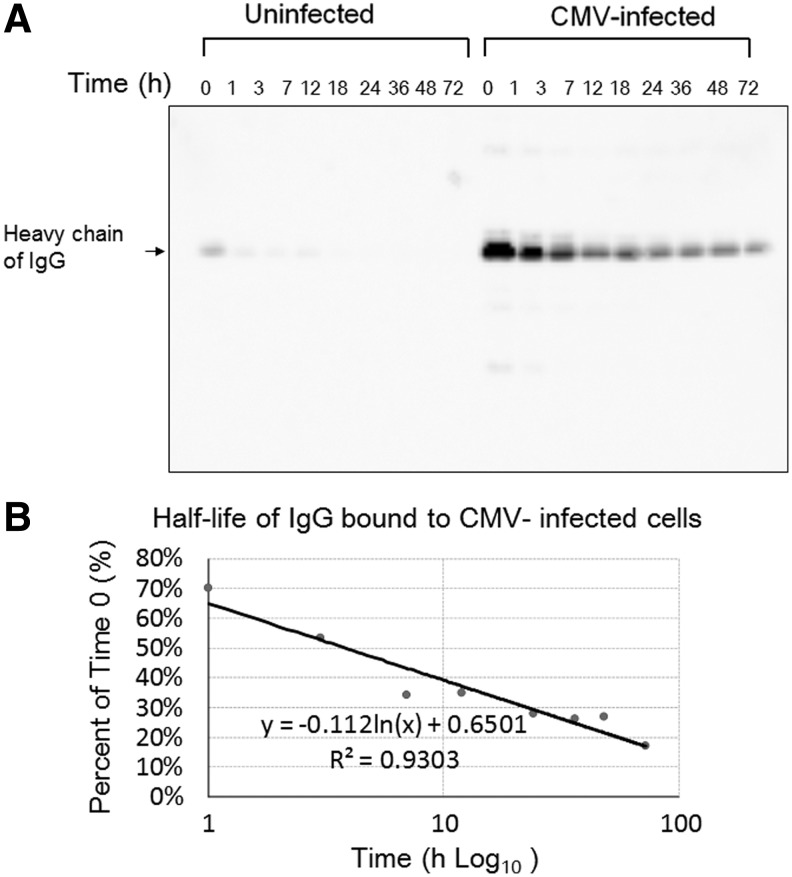
CMV-infected cells and uninfected cells were treated with 10 mg of IVIG (IgG), and the amount of IgG in the cellular fraction was visualized by Western blot analysis (Fig. 4). A small amount of IgG was bound to uninfected cells but disappeared quickly. In contrast, CMV-infected cells absorbed IgG, and IgG in the cellular fractions of CMV-infected cells was decreased in a time-dependent manner, as shown in Figure 4A. The half-life of IgG bound to CMV-infected cells as determined by the decay curve in the Western blot analysis was 3.8 h (Fig. 4B).
FIG. 4.
Fate of IgG bound to CMV-infected cells. Western blot analysis of heavy chain of IgG in the cellular fraction of CMV-infected and -uninfected cells shows the specific binding of IgG to CMV-infected cells. (A) The amount of IgG bound to CMV-infected cells, as quantitated by Fujifilm LAS-4000 imaging system, decreased with time. (B) The half-life of IgG bound to CMV-infected cells was 3.8 h.
Absorption of IgG by VZV- and CMV-infected cells
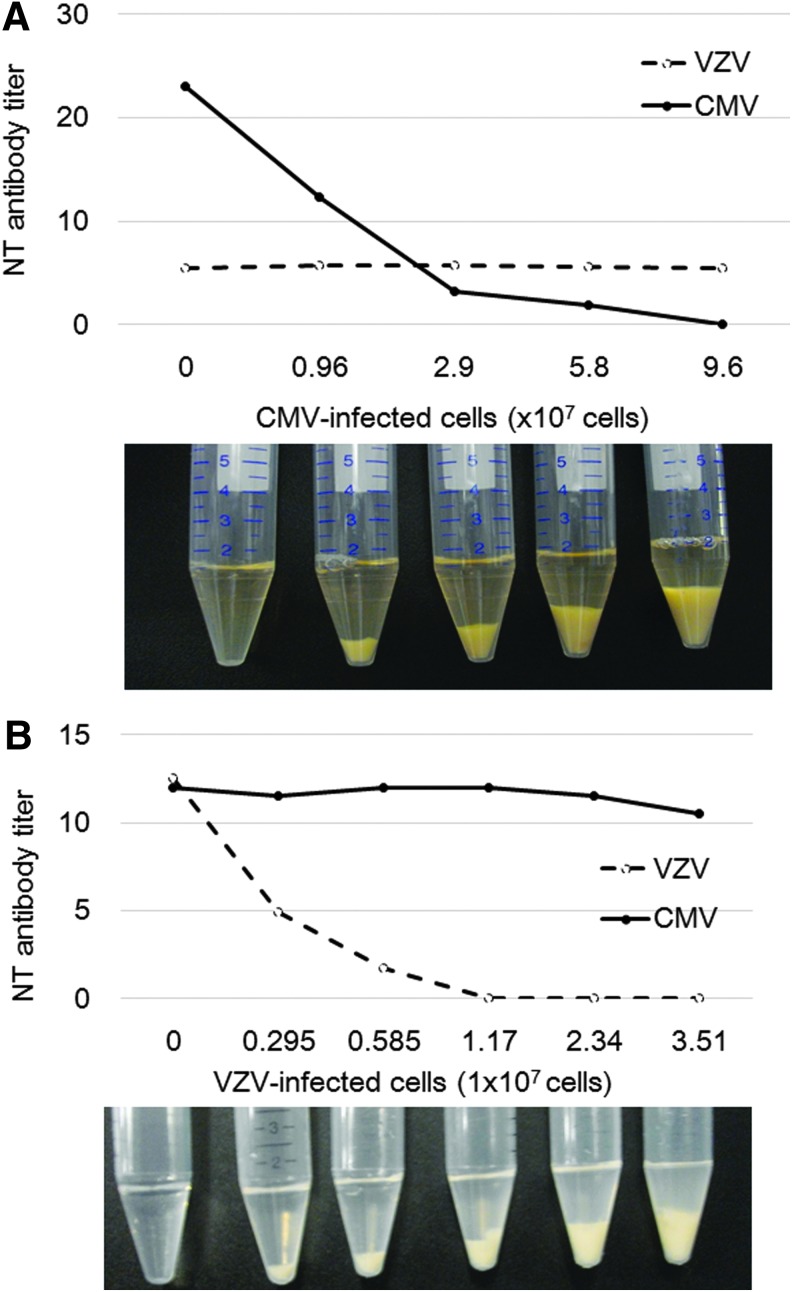
Figure 5 shows the profiles of adsorption of NT activity of IVIG by infected cells. Addition of CMV-infected cells dose dependently reduced the NT antibody titer to CMV but did not change the antibody titer to VZV (Fig. 5A). In contrast, the addition of VZV-infected cells dose dependently reduced the residual antibody titer to VZV but did not reduce the antibody titer to CMV (Fig. 5B). As NT antibody titer in IVIG was eliminated after treatment of the respective infected cells with no reduction in NT antibody titer of the other virus, absorbed antibody would be specific to the respective infected cells. These ratios of infected cells and the amount of IVIG would indicate the amount of IVIG needed to cover the corresponding amount of viral antigen on the infected cell surface. The anti-VZV antibody in 15 mg of IgG needed 1.17 × 107 VZV-infected cells to eliminate the NT antibody, and the anti-CMV antibody required 9.6 × 107 CMV-infected cells at 48 h after infection. About 10 times as many CMV-infected cells as VZV-infected cells were needed to completely abrogate the NT activity of 15 mg IgG, indicating that target NT antigens for CMV infectivity might be less expressed on the cell surface than those for VZV infectivity.
FIG. 5.
VZV- or CMV-infected cells and IgG (15 mg) from IVIG were mixed, and the residual NT antibody titer was determined. (A) Addition of CMV-infected cells gradually reduced the NT antibody titer to undetectable levels, but the NT antibody titer to VZV did not change. Photograph shows the amounts of CMV-infected cell pellets used for absorption of IVIG, and the supernatants were used for the NT tests. Thus, CMV-infected cells similarly reduced the NT antibody titer in IgG (IVIG 15 mg) without effects on VZV NT titer, and 9.6 × 107 infected cells absorbed the NT activity of IgG. (B) Addition of VZV-infected cells gradually reduced the NT antibody titer to undetectable levels, but anti-CMV NT antibody titer was not affected. Photograph shows the amounts of VZV-infected cell pellets used for absorption of IVIG, and the supernatants were used for the NT tests. The NT antibody titer of IgG (IVIG 1 mg) to VZV-infected cells was lost at 1.17 × 107 infected cells.
Discussion
One study showed that the antibody response to CMV proteins in bone marrow transplant recipients was similar to that of the natural infection of normal individuals (51). Antibody responses to CMV proteins were reported in congenital and perinatal infection (28) and in CMV mononucleosis (13). However, the appearance, duration, and titer of the antibody response to viral glycoproteins has been reported to be different in individuals when studied by using viral glycoproteins captured by monoclonal antibody (14). Antibody rises against viral early proteins were observed in active CMV infection (7) and in antibody responses to the immediate early protein of CMV in renal transplant recipients (36).
The effects of IVIG on the spread of CMV and protein synthesis in the early stage of infection were examined and showed that immediate early proteins were affected by the presence of antiserum. In this study designed to clarify the effects of IVIG on CMV-infected cells, antibody bound to CMV-infected cells was evaluated in vitro, and the half-life of bound IgG was determined.
IVIG treatment reduced the spread of CMV infection and intracellular virus production, and viral protein synthesis of the ratio of 66/68k was reduced, indicating that antibody bound to CMV-infected cells modified protein synthesis and suppressed the spread of infection and virus production.
Antibody to viral protein bound to infected cells modifies intracellular viral protein synthesis, and this modification is designated “antigenic modulation” (9,10,18,33). Anti-measles virus antibody eliminates viral antigens in the infected cells, and after removal of the antibody, viral antigens reappear in the cells. Anti-VZV antibody treatment of VZV-infected cells was limited compared with neutralizing anti-glycoprotein H (gH) monoclonal antibody (43), anti-gH neutralizing monoclonal antibody to VZV eliminated antigen expression, and the treated infected cells became noninfectious (33). However, anti-VZV polyclonal antibody in IVIG failed to render VZV-infected cells noninfectious, possibly due to the low NT activity (33). Antigenic modulation is known to function as therapeutic tools, such as Rituximab (anti-CD20 antibody), to a variety of malignant and autoimmune disorders (2,3,46). Antigenic modulation by antibodies has been reported in a variety of fields, but its exact mechanism in the modulation of protein synthesis has not been clarified. This antigenic modulation by anti-CMV antibody might have modified intracellular virus production by the presence of antibody in the culture medium, as shown in Figures 1B and 2.
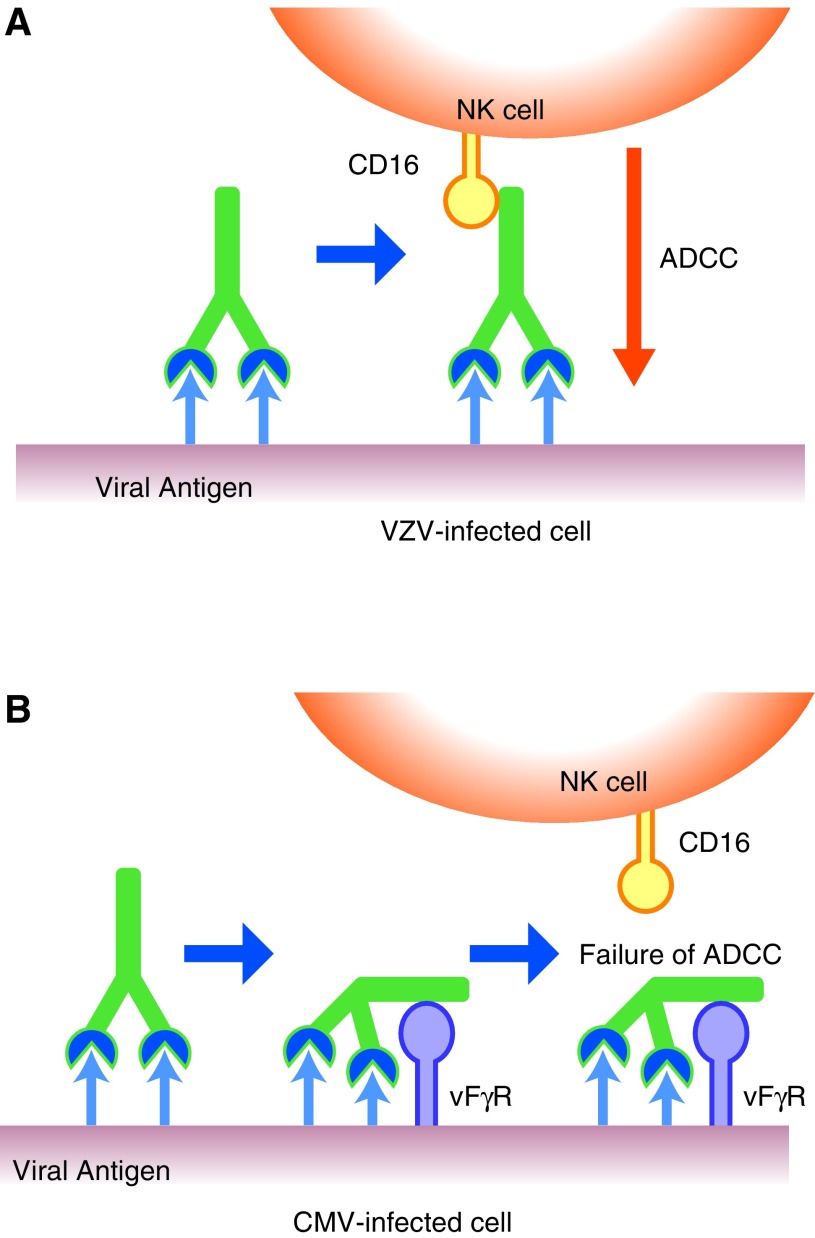
NK cell activity against CMV-infected cells was observed, but significant ADCC activity was not detected. These results are consistent with our previous study (50). Both NK cells and ADCC toward VZV-infected cells were observed, and 60% of infected cells were eliminated by ADCC, indicating the importance of interaction of IgG with the infected cell surface. Thus, CMV-infected cells were resistant to ADCC and CMV-infected cells expressed the target antigen for IgG antibody as well as viral Fc receptors (1,5,22,41). The Fab portion of IgG binds target antigens, and the Fc portion binds viral Fc receptors, resulting in a little outward-directed Fc portion exhibiting an ADCC effector function, as illustrated in Figure 6.
FIG. 6.
Schematic presentation of the difference between ADCC against VZV- and CMV-infected cells. (A) ADCC was exhibited against VZV-infected cells through the bound IgG on the cell surface and with NK cells as the effector. (B) CMV-infected cells express vFcγR on the cell surface. Anti-CMV antibodies recognize viral glycoproteins expressed on the CMV-infected cells, but the vFcγR binds to the Fc portion of the antibody (IgG). Thus, the Fc portion of IgG bound to viral proteins was captured by the vFcγR, and the Fc portion lost the binding to Fc receptors of NK cells. Consequently, CMV-infected cells interfere with the interaction of the Fc portion of IgG with Fc receptors of NK cells, leading to ADCC by the vFcγR. vFcγR, viral Fcγ receptor.
The activity of IgG bound to CMV-infected cells decreased with time, and the half-life of bound IgG was 3.8 h. The amount of IgG bound to infected cells also decreased with time, and the infected cells recovered binding activity with addition of fresh IgG at 24 h after removal of IgG. Thus, CMV-infected cells continuously absorbed NT antibody and recovered IgG binding activity after 24 h (data not shown).
Fifteen milligrams of IgG was bound to 108 CMV-infected cells and 107 VZV-infected cells at 48 h after infection. The fact that VZV-infected cells needed a smaller volume than CMV-infected cells may indicate that less antigen is expressed in CMV-infected cells than VZV-infected cells. This, in turn, indicated that estimation of the CMV- or VZV-infected cell number might be possible by the administration of IVIG. Antibody might be specifically absorbed by the infected cells, and the resultant NT antibody titer might be used to estimate the number of infected cells or the magnitude of infection in the body.
IVIG reduced the spread of infection and virus production by antigenic modulation of CMV-infected cells. Interaction of IgG with VZV-infected cells reduced the number of infected cells by ADCC, but significant ADCC activity toward CMV-infected cells was not observed. Circulating antibody may react with virus particles, circulating soluble antigen, and lysed cell fragments in addition to the surface of infected cells in vivo. Ten times more IgG bound to VZV-infected cells than to CMV-infected cells, indicating that CMV-infected cells expressed less viral antigen on the infected cell surface than VZV-infected cells. The half-life of IgG bound to CMV-infected cells was 3.8 h. Less virus-specific antibody binding or less viral antigen presentation on the cell surface and the escape from ADCC showed the immune evasive nature of CMV-infected cells than VZV-infected cells, although IVIG inhibited spread of CMV infection and a reduction in CMV production. Low level of virus-specific antigen expression on the CMV-infected cells and absorption of NT antibody might contribute to the immune evasion of CMV infection.
Thus, we characterized the antiviral function of IVIG and contrasting functions of IgG against CMV-infected cells and VZV-infected cells. Anti-CMV IgG antibody showed anti-CMV activity to virus production and possibly spread due to antigenic modulation in this study. These activities of anti-CMV antibody against CMV-infected cells may alleviate CMV disease in patients with CMV infection by limiting the spread of infection and reducing virus production, in addition to neutralization of virus infectivity by neutralizing antibody in patient serum.
Acknowledgments
This work was partly supported by grants from JSPS KAKENHI Grant No. 25293108 from the Ministry of Education, Culture, Sports, Science and Technology of Japan and Japan Blood Products Organization. The authors thank Ms. Katherine Ono for editing this article.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Atalay R, Zimmermann A, Wagner M, et al. Identification and expression of human cytomegalovirus transcription units coding for two distinct Fcgamma receptor homologs. J Virol 2002;76:8596–8608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beers SA, French RR, Chan HT, et al. Antigenic modulation limits the efficacy of anti-CD20 antibodies: implications for antibody selection. Blood 2010;115:5191–5201 [DOI] [PubMed] [Google Scholar]
- 3.Beum PV, Mack DA, Pawluczkowycz AW, Lindorfer MA, and Taylor RP. Binding of rituximab, trastuzumab, cetuximab, or mAb T101 to cancer cells promotes trogocytosis mediated by THP-1 cells and monocytes. J Immunol 2008;181:8120–8132 [DOI] [PubMed] [Google Scholar]
- 4.Cockley KD, Shiraki K, and Rapp F. A human cytomegalovirus function inhibits replication of herpes simplex virus. J Virol 1988;62:188–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Corrales-Aguilar E, Trilling M, Hunold K, et al. Human cytomegalovirus Fcgamma binding proteins gp34 and gp68 antagonize Fcgamma receptors I, II and III. PLoS Pathog 2014;10:e1004131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cowan J, Cameron DW, Knoll G, and Tay J. Protocol for updating a systematic review of randomised controlled trials on the prophylactic use of intravenous immunoglobulin for patients undergoing haematopoietic stem cell transplantation. BMJ Open 2015;5:e008316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cremer NE, Cossen CK, Shell GR, and Pereira L. Antibody response to cytomegalovirus polypeptides captured by monoclonal antibodies on the solid phase in enzyme immunoassays. J Clin Microbiol 1985;21:517–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fishman JA. Overview: cytomegalovirus and the herpesviruses in transplantation. Am J Transplant 2013;13 Suppl 3:1–8; quiz 8. [DOI] [PubMed] [Google Scholar]
- 9.Fujinami RS, Norrby E, and Oldstone MB. Antigenic modulation induced by monoclonal antibodies: antibodies to measles virus hemagglutinin alters expression of other viral polypeptides in infected cells. J Immunol 1984;132:2618–2621 [PubMed] [Google Scholar]
- 10.Fujinami RS. and Oldstone MB. Alterations in expression of measles virus polypeptides by antibody: molecular events in antibody-induced antigenic modulation. J Immunol 1980;125:78–85 [PubMed] [Google Scholar]
- 11.Fujinami RS. and Oldstone MB: Antiviral antibody reacting on the plasma membrane alters measles virus expression inside the cell. Nature 1979;279:529–530 [DOI] [PubMed] [Google Scholar]
- 12.Goldstein G, Rutenberg TF, Mendelovich SL, et al. The role of immunoglobulin prophylaxis for prevention of cytomegalovirus infection in pediatric hematopoietic stem cell transplantation recipients. Pediatr Blood Cancer 2017;DOI: 10.1002/pbc.26420 [DOI] [PubMed] [Google Scholar]
- 13.Hayes K. Prenatal viral infection with particular reference to cytomegaloviruses. Aust Paediatr J 1974;10:56–63 [DOI] [PubMed] [Google Scholar]
- 14.Hayes K, Alford C, and Britt W. Antibody response to virus-encoded proteins after cytomegalovirus mononucleosis. J Infect Dis 1987;156:615–621 [DOI] [PubMed] [Google Scholar]
- 15.Hyde TB, Schmid DS, and Cannon MJ. Cytomegalovirus seroconversion rates and risk factors: implications for congenital CMV. Rev Med Virol 2010;20:311–326 [DOI] [PubMed] [Google Scholar]
- 16.Jeor SC, Albrecht TB, Funk FD, and Rapp F. Stimulation of cellular DNA synthesis by human cytomegalovirus. J Virol 1974;13:353–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson JM. and Anderson BL. Cytomegalovirus: should we screen pregnant women for primary infection? Am J Perinatol 2013;30:121–124 [DOI] [PubMed] [Google Scholar]
- 18.Joseph BS. and Oldstone MB. Immunologic injury in measles virus infection. II. Suppression of immune injury through antigenic modulation. J Exp Med 1975;142:864–876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Juckstock J, Rothenburger M, Friese K, and Traunmuller F. Passive immunization against congenital cytomegalovirus infection: current state of knowledge. Pharmacology 2015;95:209–217 [DOI] [PubMed] [Google Scholar]
- 20.Koyano S, Inoue N, Oka A, Moriuchi H, et al. Screening for congenital cytomegalovirus infection using newborn urine samples collected on filter paper: feasibility and outcomes from a multicentre study. BMJ 2011. DOI: 10.1136/bmjopen-2011-000118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuramoto T, Daikoku T, Yoshida Y, et al. Novel anticytomegalovirus activity of immunosuppressant mizoribine and its synergism with ganciclovir. J Pharmacol Exp Ther 2010;333:816–821 [DOI] [PubMed] [Google Scholar]
- 22.Lilley BN, Ploegh HL, and Tirabassi RS. Human cytomegalovirus open reading frame TRL11/IRL11 encodes an immunoglobulin G Fc-binding protein. J Virol 2001;75:11218–11221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lopez Garcia-Gallo C, Garcia Fadul C, Laporta R, Portero F, Millan I, and Ussetti P. Cytomegalovirus immunoglobulin for prophylaxis and treatment of cytomegalovirus infection in the (Val)Ganciclovir era: a single-center experience. Ann Transplant 2015;20:661–666 [DOI] [PubMed] [Google Scholar]
- 24.Morton CC. and Nance WE. Newborn hearing screening—a silent revolution. N Engl J Med 2006;354:2151–2164 [DOI] [PubMed] [Google Scholar]
- 25.Mumtaz K, Faisal N, Husain S, Morillo A, Renner EL, and Shah PS. Universal prophylaxis or preemptive strategy for cytomegalovirus disease after liver transplantation: a systematic review and meta-analysis. Am J Transplant 2015;15:472–481 [DOI] [PubMed] [Google Scholar]
- 26.Negishi H, Yamada H, Hirayama E. Intraperitoneal administration of cytomegalovirus hyperimmunoglobulin to the cytomegalovirus-infected fetus. J Perinatol 1998;18:466–469 [PubMed] [Google Scholar]
- 27.Ogawa H, Suzutani T, Baba Y, et al. Etiology of severe sensorineural hearing loss in children: independent impact of congenital cytomegalovirus infection and GJB2 mutations. J Infect Dis 2007;195:782–788 [DOI] [PubMed] [Google Scholar]
- 28.Pereira L, Stagno S, Hoffman M, and Volanakis JE. Cytomegalovirus-infected cell polypeptides immune-precipitated by sera from children with congenital and perinatal infections. Infect Immun 1983;39:100–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rawal BB, Shadrou S, Abubacker F, and Ghahramani N. A systematic review and meta-analysis of prophylactic versus pre-emptive strategies for preventing cytomegalovirus infection in renal transplant recipients. Int J Organ Transplant Med 2012;3:10–17 [PMC free article] [PubMed] [Google Scholar]
- 30.Rea F, Potena L, Yonan N, Wagner F, and Calabrese F. Cytomegalovirus hyper immunoglobulin for CMV prophylaxis in thoracic transplantation. Transplantation 2016;100 Suppl 3:S19–S26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Revello MG, Lazzarotto T, Guerra B, et al. A randomized trial of hyperimmune globulin to prevent congenital cytomegalovirus. N Engl J Med 2014;370:1316–1326 [DOI] [PubMed] [Google Scholar]
- 32.Sarmiento E, Diez P, Arraya M, et al. Early intravenous immunoglobulin replacement in hypogammaglobulinemic heart transplant recipients: results of a clinical trial. Transpl Infect Dis 2016;18:832–843 [DOI] [PubMed] [Google Scholar]
- 33.Shiraki K, Daikoku T, Takemoto M, et al. Neutralizing anti-gH antibody of Varicella-zoster virus modulates distribution of gH and induces gene regulation, mimicking latency. J Virol 2011;85:8172–8180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shiraki K, Hayakawa Y, Mori H, et al. Development of immunogenic recombinant Oka varicella vaccine expressing hepatitis B virus surface antigen. J Gen Virol 1991;72 (Pt 6):1393–1399 [DOI] [PubMed] [Google Scholar]
- 35.Shiraki K, Ishibashi M, Okuno T, et al. Effects of cyclosporine, azathioprine, mizoribine, and prednisolone on replication of human cytomegalovirus. Transplant Proc 1990;22:1682–1685 [PubMed] [Google Scholar]
- 36.Shiraki K, Ishibashi M, Okuno T, et al. Antibody response to the immediate early protein of cytomegalovirus in renal transplant recipients. J Med Virol 1991;34:280–283 [DOI] [PubMed] [Google Scholar]
- 37.Shiraki K, Yoshida Y, Asano Y, Yamanishi K, and Takahashi M. Pathogenetic tropism of varicella-zoster virus to primary human hepatocytes and attenuating tropism of Oka varicella vaccine strain to neonatal dermal fibroblasts. J Infect Dis 2003;188:1875–1877 [DOI] [PubMed] [Google Scholar]
- 38.Snydman DR. Cytomegalovirus immunoglobulins in the prevention and treatment of cytomegalovirus disease. Rev Infect Dis 1990;12 Suppl 7:S839–S848 [DOI] [PubMed] [Google Scholar]
- 39.Snydman DR. Historical overview of the use of cytomegalovirus hyperimmune globulin in organ transplantation. Transpl Infect Dis 2001;3 Suppl 2:6–13 [DOI] [PubMed] [Google Scholar]
- 40.Soper DE. Congenital cytomegalovirus infection: an obstetrician's point of view. Clin Infect Dis 2013;57 Suppl 4:S171–S173 [DOI] [PubMed] [Google Scholar]
- 41.Sprague ER, Reinhard H, Cheung EJ, et al. The human cytomegalovirus Fc receptor gp68 binds the Fc CH2-CH3 interface of immunoglobulin G. J Virol 2008;82:3490–3499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stinski MF. Synthesis of proteins and glycoproteins in cells infected with human cytomegalovirus. J Virol 1977;23:751–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Suzuki K, Akahori Y, Asano Y, Kurosawa Y, and Shiraki K. Isolation of therapeutic human monoclonal antibodies for varicella-zoster virus and the effect of light chains on the neutralizing activity. J Med Virol 2007;79:852–862 [DOI] [PubMed] [Google Scholar]
- 44.Swanson EC, and Schleiss MR. Congenital cytomegalovirus infection: new prospects for prevention and therapy. Pediatr Clin North Am 2013;60:335–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Takahashi M, Okuno Y, Otsuka T, Osame J, and Takamizawa A. Development of a live attenuated varicella vaccine. Biken J 1975;18:25–33 [PubMed] [Google Scholar]
- 46.Taylor RP, and Lindorfer MA. Antigenic modulation and rituximab resistance. Semin Hematol 2010;47:124–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.The TH, Andersen HK, Spencer ES, and Klein G. Antibodies against cytomegalovirus-induced early antigens (CMV-EA) in immunosuppressed renal-allograft recipients. Clin Exp Immunol 1977;28:502–505 [PMC free article] [PubMed] [Google Scholar]
- 48.van Gent R, Metselaar HJ, and Kwekkeboom J. Immunomodulation by hyperimmunoglobulins after solid organ transplantation: beyond prevention of viral infection. Transplant Rev 2017. DOI:http://dx.doi.org/10.1016/j.trre.2017.01.001 [DOI] [PubMed]
- 49.Yajima M, Shiraki A, Daikoku T, Oyama Y, Yoshida Y, and Shiraki K. Functional differences between antiviral activities of sulfonated and intact intravenous immunoglobulin preparations toward varicella-zoster virus and cytomegalovirus. J Infect Chemother 2015;21:427–433 [DOI] [PubMed] [Google Scholar]
- 50.Yamamura J, Kageyama S, Uwano T, Kurokawa M, Imakita M, and Shiraki K. Long-term gene expression in the anterior horn motor neurons after intramuscular inoculation of a live herpes simplex virus vector. Gene Ther 2000;7:934–941 [DOI] [PubMed] [Google Scholar]
- 51.Zaia JA. The biology of human cytomegalovirus infection after bone marrow transplantation. Int J Cell Cloning 1986;4 Suppl 1:135–154 [DOI] [PubMed] [Google Scholar]
- 52.Zaia JA. Prevention and treatment of cytomegalovirus pneumonia in transplant recipients. Clin Infect Dis 1993;17 Suppl 2:S392–S399 [DOI] [PubMed] [Google Scholar]