Abstract
Excess fat accumulation and abnormal metabolism are involved in numerous diseases and thus the research on identification of compounds that can regulate energy homeostasis could significantly facilitate the current effort to prevent and/or treat metabolic disorders. Piceatannol, one of the natural stilbenes, was previously found to decrease lipid accumulation of 3T3-L1 adipocytes. However, its role in fat metabolism in vivo is not known. Thus, Caenorhabditis elegans as an animal model was used in the current study to determine the effect of piceatannol on fat accumulation and its underlying mechanisms. The results showed that 50 and 100 μM piceatannol significantly reduced fat accumulation of wild-type worms grown in normal and high-glucose conditions without altering the growth rate, worm length, pumping rate, or moving speed. The current study further indicated that piceatannol decreased the expression of sbp-1 (encodes an ortholog of mammalian sterol regulatory element-binding protein) and its target gene fasn-1 (encodes an ortholog of fatty acid synthase) as well as increased the expression of hosl-1 (encodes an ortholog of hormone-sensitive lipase) in glucose-treated worms. These data suggested that piceatannol reduced fat accumulation in C. elegans by suppression of genes involved in lipid synthesis and possibly through stimulation of lipolysis. Given that piceatannol exerts similar effects in both C. elegans and 3T3-L1 cells, our finding could provide a mechanistic insight into the role of piceatannol in lipid metabolism in mammals.
Keywords: : C. elegans, fat accumulation, lipid metabolism, piceatannol
Introduction
Currently, more than two-thirds of U.S. adults are overweight or obese.1 As it is known that excess fat accumulation and abnormal metabolism are involved in numerous diseases such as type 2 diabetes,2 the research on identification of compounds that can regulate energy homeostasis could significantly facilitate prevention and/or treatment of metabolic disorders. However, many compounds that are effective in in vitro assays fail to exhibit the desired response in vivo because of the sophistication of energy regulatory networks in organisms.3 One of the approaches to resolve this problem is to establish whole organism models to identify new fat regulatory compounds. Caenorhabditis elegans (C. elegans), a free-living nematode, has been extensively utilized as an animal model for the research involving aging, obesity, and other physiological processes because of its relatively short life span, quick turnover, easy maintenance, well-known genetic pathways, and large brood size, etc.4 Studies showed that the core mechanisms of regulatory pathways involving energy homeostasis are largely conserved from mammals to nematodes.5 C. elegans therefore offers great potential as an in vivo model to identify compounds that modulate fat storage.
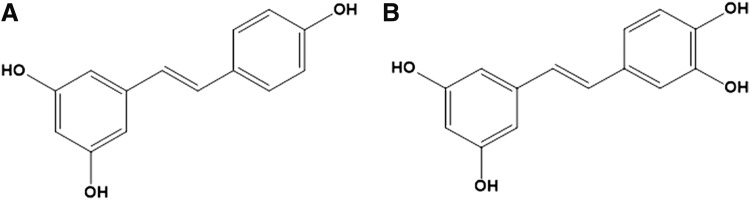
As the best known member of the stilbene family, resveratrol has gained widespread attention because of its diverse bioactivity, including antiobesity.6 Piceatannol is an analog and a metabolite of resveratrol, which possesses an additional hydroxyl group in position 3 of resveratrol (Fig. 1).7 Piceatannol can be found in many plant sources, including grapes, white tea, and passion fruits,8,9 and similar to resveratrol, many studies have reported the beneficial effects of piceatannol, such as estrogenic, anti-inflammatory, antioxidant, and anticancer activities.7 Moreover, it has been reported that the catechol group present in ring B of piceatannol formed by phenolic groups in positions 3′ and 4′ significantly increases its antioxidant activity,10 suggesting a possibility that piceatannol may have a greater beneficial effect than resveratrol. We have previously reported the life span-extending effect of piceatannol on C. elegans11 and piceatannol was previously found to decrease lipid accumulation of 3T3-L1 adipocytes12 and mice.13 This study on mice reported that the effects of piceatannol were slightly, although not significantly, more effective than that of resveratrol in mice.13 However, its role in fat metabolism of whole organisms has not yet been studied. Thus, the purpose of the current study was to determine the effects of piceatannol on fat accumulation of C. elegans in comparison with resveratrol as well as to determine the underlying mechanisms.
FIG. 1.
Structures of (A) resveratrol and (B) piceatannol.
Materials and Methods
Materials
All the strains of C. elegans were obtained from the Caenorhabditis Genetics Center, University of Minnesota, USA. Piceatannol (purity >98%) was from TCI America (Portland, OR) and resveratrol (purity ≥98%) was from MP Biomedicals (Santa Ana, CA). d-(+)-glucose (purity 99.5%) was from Sigma-Aldrich Co. (St. Louis, MO). Other chemicals were from Thermo Fisher Scientific, Inc., (Pittsburgh, PA) unless stated otherwise. Household bleach (The Clorox Company, Oakland, CA, USA) was used for bleaching the worms.
C. elegans culture
M9 buffer, S-complete, and nematode growth medium (NGM) used for C. elegans culture were prepared as previously described.14–16 The C. elegans strain used in this study was N2, Bristol (wild type). Synchronized populations were obtained according to standard protocols.17 Treatments were started with synchronized L1 worms in 12-well plates at 20°C. Treatments were piceatannol (50 and 100 μM), resveratrol (50 and 100 μM), or 0.1% dimethyl sulfoxide (DMSO as a vehicle). For inclusion of glucose, additional glucose (0.5%, 0.75%, or 1%) was added during the treatment period. After 48 h of treatment, worms were collected for triglyceride and protein quantification. For Nile red staining, L1 worms were grown in liquid medium with 100 ng/mL Nile red (added as a 0.5 mg/mL stock solution in acetone at final concentration of acetone was 0.02%) and incubated for 48 h at 20°C.5,18
Growth rate, pumping rate, worm size, and locomotive activity
Growth rate was determined by counting the number of worms at each developmental stage after 48 h as previously described.5 For pumping rate, the number of pharyngeal contractions of 12 randomly selected nematodes was counted under the microscope for 1 min.19
Worm size and locomotion behavior were analyzed by WormLab tracking system (MBF Bio-science, Williston, VT), as described previously.11 Low-peptone NGM plates were covered with a thin layer of Escherichia coli OP50 spread at 5 min before tracking. Worms were transferred to the plates and allowed to acclimate for 30 min before the tracking started. A 2-min recording (7.99 frames/sec) was captured. WormLab software was then used to track and analyze the worm size (length and width) and moving behavior of 40–50 animals per treatment.
Triglycerides and protein assay
Worms were collected and washed three times with M9 buffer before sonication (Sonicator 505, Fisher Scientific) for 3 min. The homogenized samples were then used for triglyceride and protein determination. The samples were analyzed with commercial kits: Infinity™ Triglyceride Reagent from Thermo Fisher Scientific, Inc., (Middletown, VA) for triglyceride measurement and Bio-Rad DC protein assay kit from Bio-Rad Co., (Hercules, CA) for protein measurement, respectively, according to manufacturers' instructions. Absorbance was measured using the SpectraMax i3 Platform (Molecular Devices LLC, Sunnyvale, CA) at 510 and 630 nm, respectively, for triglyceride and protein determination. Triglyceride content was normalized to protein concentration.16,20
Nile red staining of C. elegans
Worms were collected and washed three times with M9 buffer before being anesthetized in 10 mM sodium azide and then placed on an agarose pad covered with a coverslip; 12–15 randomly selected worms were photographed for each treatment group under a confocal microscope (Eclipse 80i SOP; Nikon, Tokyo, Japan).5 The dye intensity was measured and quantified by ImageJ (Dr. Wayne Rasband, NIH, USA).
Real-time polymerase chain reaction
Total RNA was isolated with TRIzol. Reverse transcription to generate cDNA was performed using high-capacity cDNA reverse transcription kit (Thermo Fisher Scientific, Inc., Middletown, VA). Quantitative RT-PCR was carried out using the StepOnePlus™ Real-Time PCR system (Applied Biosystems, Foster City, CA) and results were analyzed using comparative Ct method. TaqMan gene expression assays used for sbp-1 (Ce02453000-m1), nhr-49 (Ce02412667-m1), hosl-1 (Ce02494529-m1), atgl-1 (Ce02406730-g1), pod-2 (Ce02427721_g1), fasn-1 (Ce02411648_g1), acs-2 (Ce02486193_g1), and ama-1 (Ce02462726-m1) (internal control) were purchased from Life Technologies Co. (Carlsbad, CA).
Statistical analysis
Data are presented as mean ± S.E. and analyzed with the Statistical Analysis System (SAS Institute, Cary, NC). Differences between groups were assessed with one-way analysis of variance, followed by Tukey's multiple range test. Significance of differences was defined at the P < .05 level.
Results
Piceatannol decreased fat accumulation of C. elegans
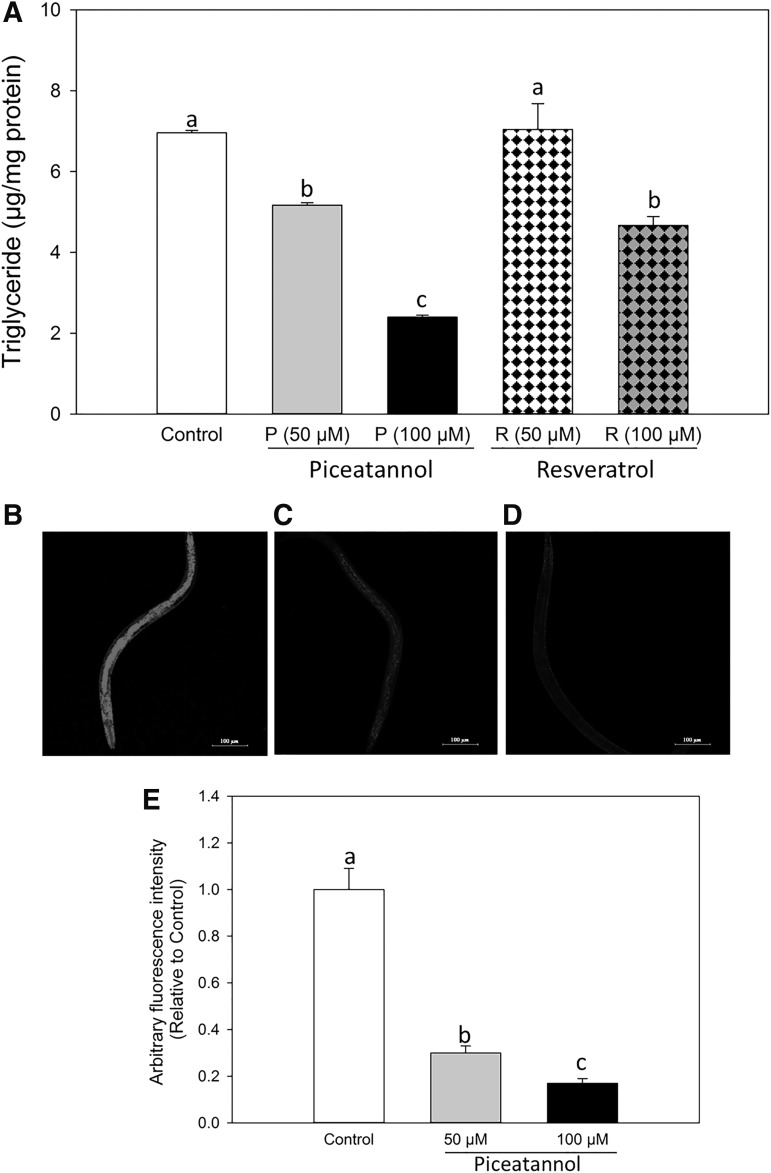
Since piceatannol is an analog and a metabolite of resveratrol, we measured the fat-reducing effect of both compounds at 50 and 100 μM. Figure 2A indicates that 100 μM resveratrol significantly decreased fat accumulation of C. elegans, 31% reduction compared with control, while 50 μM did not show any significant effect. In contrast, both 50 and 100 μM piceatannol treatments significantly reduced fat accumulation compared with the control group (25% and 63% reduction over control, respectively), indicating that piceatannol treatments were more effective than the same concentrations of resveratrol. The effect of piceatannol on fat accumulation was also confirmed by Nile red staining. As shown in Figure 2B–E, 50 and 100 μM piceatannol significantly decreased the fluorescence intensity of Nile red-stained intracellular fat by 70% and 83%, respectively, compared with control.
FIG. 2.
Piceatannol decreased fat accumulation of C. elegans. (A) Synchronized L1 worms were treated with 0.1% DMSO as control, piceatannol (50 or 100 μM), or resveratrol (50 or 100 μM) for 48 h. Triglyceride content was normalized by protein concentration. (B–E) Synchronized L1 worms were treated with control (B) or piceatannol at 50 (C) or 100 μM (D) and 100 ng/mL Nile Red for 48 h; 12–15 randomly selected worms were photographed for each treatment group under a confocal microscope. (E) The dye intensity was measured and quantified by ImageJ. Values represent different means±S.Es. (n = 3–15). a–cMeans with different letters are significantly different at P < 0.05. DMSO, dimethyl sulfoxide.
Piceatannol decreased fat accumulation of glucose-treated worms
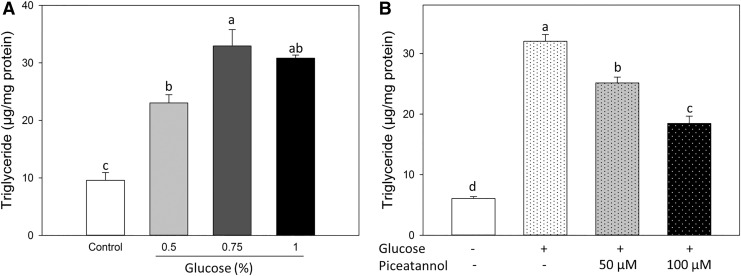
Glucose has been used to increase fat accumulation as an established obesity model in C. elegans.21 We next investigated the effect of piceatannol on glucose-stimulated fat accumulation in C. elegans. Our results showed that glucose increased fat accumulation of C. elegans (by 1.4 and 2.4-fold at 0.5% and 0.75%, respectively) and reached saturation at levels above 0.75% (Fig. 3A). Thus, we next tested the effect of piceatannol on worms cultured with 0.75% glucose. Piceatannol at both 50 and 100 μM reduced the fat accumulation of glucose-treated worms by 22% and 44% compared with control, respectively, but not to the level in control (Fig. 3B).
FIG. 3.
Piceatannol reduced fat accumulation of glucose-treated worms. (A) Synchronized L1 worms were treated with d-glucose at (0%, 0.5%, 0.75%, or 1%) for 48 h. (B) Synchronized L1 worms were treated with 0.1% DMSO as blank, 0.75% d-glucose as positive control, or 0.75% d-glucose with piceatannol (50 or 100 μM) for 48 h. Triglyceride content was normalized with protein concentration. Values represent different means±S.Es. (n = 3). a–cMeans with different letters are significantly different at P < .05.
Effect of piceatannol on growth rate, worm size, pumping rate, and locomotive activity of glucose-treated worms
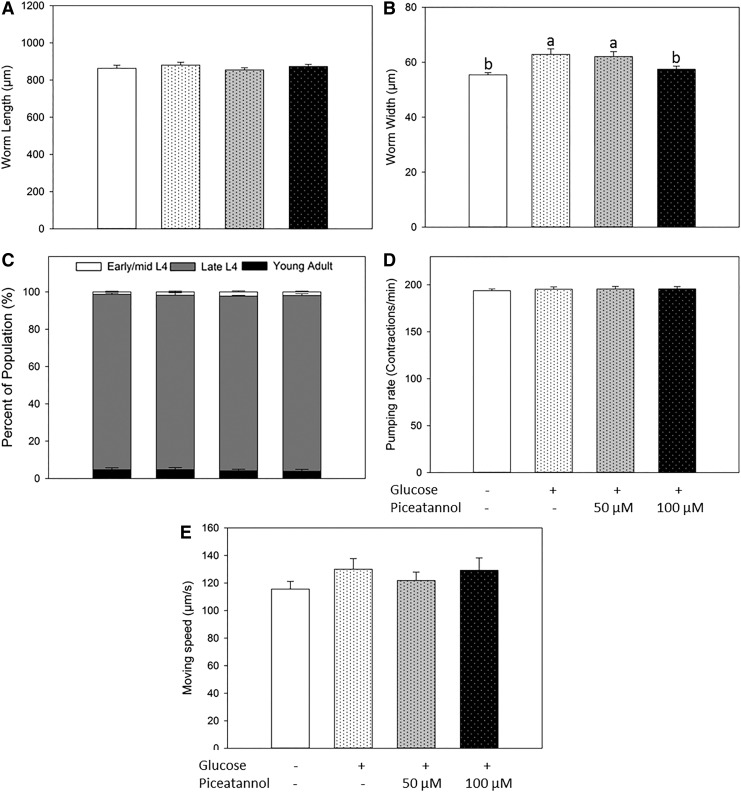
Our previous study showed that piceatannol did not alter any of the physiological states of wild-type nematodes.11 In the current study, we also determined whether it had any effects on glucose-treated worms, including worm size, growth rate, pumping rate, and locomotive activity. As shown in Figure 4A, piceatannol did not influence the worm length after 48 h of incubation compared with both nontreated worms and glucose-treated worms. Glucose (0.75%) slightly but significantly increased the width of C. elegans (Fig. 4B). After coincubation with piceatannol (100 μM), the worms showed reduced width compared with glucose-treated worms, although no effect was observed upon 50 μM treatment (Fig. 4B). Piceatannol did not influence the growth rate of glucose-treated worms as shown in Figure 4C. After 48 h of incubation, 93–95% of the worms reached late the L4 stage for all the four groups.
FIG. 4.
Effect of piceatannol on worm size, growth rate, pumping rate, and locomotive activity of glucose-treated worms. Synchronized L1 worms were treated with 0.1% DMSO as blank, 0.75% d-glucose as positive control, or 0.75% d-glucose with piceatannol (50 or 100 μM) for 48 h. (A) Worm length and (B) worm width were analyzed by WormLab software. (C) The number of worms at each developmental stage was counted. (D) The number of pharyngeal contractions of 12 randomly selected nematodes was counted under a microscope for 1 min. (E) WormLab software was used to track the average moving speed of each worm and 40–50 animals of each treatment were tracked and values calculated. Values represent different means±S.Es. (n = 40–50 for worm size and moving speed; n = 3 plates and ≈100 worms/plate for growth rate; n = 12 for pumping rate). a–bMeans with different letters are significantly different at P < .05.
Pharyngeal pumping is used as an indicator of food intake for C. elegans.22 In addition, the automated worm tracking system was employed to determine moving behavior of worms as an indicator of energy expenditure in C. elegans.20 Our previous report demonstrated that piceatannol did not influence the pumping rate or moving behavior of C. elegans after short-term treatment (48 h).11 Thus, we determined the effects of piceatannol on pumping rate and movement in glucose-treated worms (Fig. 4D, E). Piceatannol, at both 50 and 100 μM, did not affect the pumping rate compared with glucose-treated worms (Fig. 4D), suggesting that there were no changes in food intake due to the treatments. As shown in Figure 4E, both 50 and 100 μM piceatannol did not show any effect on the moving speed of glucose-treated worms, suggesting no effects of piceatannol on energy expenditure.
Effect of piceatannol on the expression of genes involved in lipid metabolism
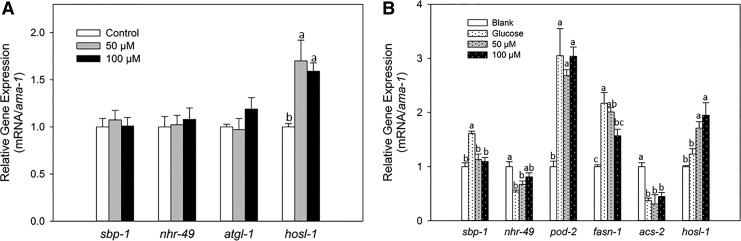
Real-time PCR was used to investigate the effect of piceatannol on the expression of genes involved in lipid metabolism of C. elegans. sbp-1 encodes a C. elegans ortholog of mammalian sterol regulatory element-binding protein (SREBP), which is a crucial regulator of fatty acid synthesis and lipid homeostasis of this organism.23–25 In C. elegans, the function of NHR-49 is close to mammalian peroxisome proliferator-activated receptors, which regulates β-oxidation and the expression of other genes that respond to dietary input.26 Our results showed that piceatannol did not affect the expression of these two genes (Fig. 5A) in wild-type worms. We also measured the mRNA levels of two lipases in C. elegans, adipose triacylglyceride lipase 1 (ATGL-1) and hormone-sensitive lipase (HOSL-1), which are responsible for more than 90% of triacylglyceride hydrolysis.27 Our results indicated that piceatannol did not affect atgl-1 expression, but significantly increased the expression of hosl-1 (Fig. 5A).
FIG. 5.
Effect of piceatannol on the expression of fat metabolism-related genes in C. elegans. (A) Synchronized L1 worms were treated with 0.1% DMSO as control or piceatannol (50 or 100 μM) for 48 h; (B) Synchronized L1 worms were treated with 0.1% DMSO as blank, 0.75% d-glucose as positive control, or 0.75% d-glucose with piceatannol (50 or 100 μM) for 48 h. Values represent different means±S.Es. (n = 3). a–cMeans with different letters are significantly different at P < .05.
On the other hand, glucose (0.75%) significantly increased the expression of sbp-1 (by 61%) and its targets, the lipid synthesis-related genes, pod-2 and fasn-1, by 3- and 2-fold over the control, respectively (Fig. 5B). C. elegans pod-2 and fasn-1 encode acetyl-CoA carboxylase and fatty acid synthase that are predicted to catalyze the first and second steps in de novo fatty acid biosynthesis, respectively.28 Glucose (0.75%) also repressed the expression of nhr-49 (by 47%) and its target gene acs-2 (by 63%) (Fig. 5B). acs-2 is predicted to encode a mitochondrial acyl-CoA synthetase, which activates fatty acids for transport into the mitochondrial matrix, where the fatty acids are then subject to β-oxidation.29 Moreover, an increased mRNA level of hosl-1 (by 23%) was observed upon glucose incubation (Fig. 5B).
Although piceatannol did not show any effect on sbp-1 mRNA level of wild-type worms (Fig. 5A), it significantly reduced the expression of sbp-1 by 30% and 32% at 50 and 100 μM compared with control, respectively, in glucose-treated worms (Fig. 5B). Piceatannol did not affect the mRNA level of pod-2 compared with glucose-treated control, but 100 μM treatment reduced the expression of fasn-1 by 28% (Fig. 5B). Piceatannol at 50 and 100 μM slightly increased the expression of nhr-49, but did not show any effect on its target gene acs-2 (Fig. 5B). As observed in nonglucose-treated worms, piceatannol at both 50 and 100 μM significantly increased the expression of hosl-1 (by 39% and 59%, respectively) over glucose-treated control (Fig. 5B).
Discussion
In the present study, piceatannol (50 and 100 μM) significantly reduced fat accumulation in both nonglucose-treated worms and glucose-treated worms. Piceatannol at 100 μM reduced the width of glucose-treated nematodes, likely due to reduced fat accumulation, without altering other physiological properties (worm length, growth rate, pumping rate, and locomotive activity). Real-time PCR assay revealed that piceatannol reduced the expression of fatty acid synthesis-related genes, sbp-1 and fasn-1, as well as increased the expression of hosl-1 in glucose-treated worms.
Clinical studies on the bioavailability of resveratrol in humans reported that oral administration of resveratrol can achieve 0.1–6 μM blood concentrations for several hours.30 However, little information is known on the physiologically relevant concentrations of piceatannol in humans. In the current study, 50 and 100 μM concentrations of piceatannol were used based on a previous study, where 25 and 50 μM piceatannol decreased the lipid accumulation of 3T3-L1 adipose without any cytotoxicity.12 We found that only 50 and 100 μM, but not 25 μM (data not shown), significantly decreased the fat accumulation in C. elegans. This inconsistency may be attributed to the difference of study models and method to quantify fat content that we used and/or chemical stability of piceatannol in treatment conditions.
Glucose has been used to establish the C. elegans obesity model in many studies with the concentration ranging from 0.18% to 2%.21,31–33 In the current study, glucose dose-dependently elevated the fat accumulation of C. elegans and reached its maximum effect at 0.75%. Thus, we used this concentration for further research to investigate the antiobesity property of piceatannol. In mammals, triglyceride levels increase through upregulation of SREBP under the influence of nutritional status, such as glucose treatment.34,35 The same effect of glucose on the sbp-1 expression in C. elegans was also observed in our study and previous report.31,32 Together with the elevated expression of pod-2 and fasn-1, these data demonstrated increased fat synthesis in glucose-treated worms. In contrast, as the same effect on PPARα in mammals,36,37 glucose also reduced the mRNA levels of nhr-49 and its target acs-2 in C. elegans, suggesting lower fatty acid β-oxidation in these nematodes. Collectively, increased fat synthesis and reduced β-oxidation upon glucose treatment eventually lead to the nematodes with high fat accumulation.
Piceatannol significantly reduced the mRNA level of sbp-1 in glucose-treated worms. SBP-1 positively regulates fatty acid synthesis and lipid homeostasis of this organism.31 In addition, the expression of its target gene fasn-1 was also decreased upon 100 μM treatment. POD-2 and FASN-1 are predicted to catalyze the first and second step in de novo fatty acid biosynthesis, respectively.28 Since piceatannol only affected the expression of fasn-1, but not pod-2, it may act mainly on the second step in de novo fatty acid biosynthesis, namely the process from malonyl-CoA to saturated fatty acyl-CoAs. However, even though piceatannol had a slight effect on nhr-49, piceatannol did not influence its target acs-2, indicating that this may not be a significant contribution to overall fat content in this model.
HOSL is a multifunctional enzyme capable of hydrolyzing a variety of acylesters, including triacylglycerol, diacylglycerol, and monoacylglycerol.38 HOSL-1 is predicted to function in the regulation of energy homeostasis and fat metabolism by catalyzing the first rate-limiting step of triacylglyceride hydrolysis.39 The activity of HOSL-1 is regulated both post-translationally by phosphorylation and by pretranslational mechanisms.40 A study found that the amount of HOSL protein and its mRNA levels in subcutaneous adipocytes show a strong correlation with its lipolytic activity.41 Based on our results, piceatannol may increase the activity of HOSL-1 at the transcriptional level; however, its influence on the post-translational level of HOSL-1 needs to be further investigated.
In summary, the current study shows that piceatannol (50 and 100 μM) significantly reduced fat accumulation in C. elegans through the reduction of fat synthesis and potential elevation of lipolysis. Given that piceatannol exerts similar effects in both C. elegans and 3T3-L1 adipocytes, C. elegans can be a useful in vivo model to investigate any bioactives that may target lipid metabolism.
Acknowledgments
This material is based upon work supported, in part, by the National Institute of Food and Agriculture, U.S. Department of Agriculture, the Massachusetts Agricultural Experiment Station, and the Department of Food Science, the University of Massachusetts Amherst, under project numbers MAS00450 and MAS00492. All the strains were provided by the CGC, which is funded by NIH Office of Research Infrastructure Programs (P40 OD010440). The China Scholarship Council supported Ms. Peiyi Shen. The authors thank Ms. Jayne M. Storkson for help with preparing this article.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Ogden CL, Carroll MD, Kit BK, Flegal KM: Prevalence of childhood and adult obesity in the United States, 2011–2012. Jama 2014;311:806–814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kopelman PG: Obesity as a medical problem. Nature 2000;404:635–643 [DOI] [PubMed] [Google Scholar]
- 3.Knight ZA, Shokat KM: Chemical genetics: Where genetics and pharmacology meet. Cell 2007;128:425–430 [DOI] [PubMed] [Google Scholar]
- 4.Shen P, Yue Y, Park Y: A Living Model for Obesity and Aging Research: Caenorhabditis elegans. Crit Rev Food Sci Nutr 2017; [Epub ahead of print]; DOI: 10.1080/10408398.2016.1220914 [DOI] [PubMed] [Google Scholar]
- 5.Lemieux GA, Liu J, Mayer N, Bainton RJ, Ashrafi K, Werb Z: A whole-organism screen identifies new regulators of fat storage. Nat Chem Bioil 2011;7:206–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang CC, Lin KY, Peng KY, Day YJ, Hung LM: Resveratrol exerts anti-obesity effects in high-fat diet obese mice and displays differential dosage effects on cytotoxicity, differentiation, and lipolysis in 3T3-L1 cells. Endocr J 2016;63:169–178 [DOI] [PubMed] [Google Scholar]
- 7.Piotrowska H, Kucinska M, Murias M: Biological activity of piceatannol: Leaving the shadow of resveratrol. Mutat Res 2012;750:60–82 [DOI] [PubMed] [Google Scholar]
- 8.Wesolowska O, Kuzdzal M, Strancar J, Michalak K: Interaction of the chemopreventive agent resveratrol and its metabolite, piceatannol, with model membranes. Biochim Biophys Acta 2009;1788:1851–1860 [DOI] [PubMed] [Google Scholar]
- 9.Matsui Y, Sugiyama K, Kamei M, et al. : Extract of passion fruit (Passiflora edulis) seed containing high amounts of piceatannol inhibits melanogenesis and promotes collagen synthesis. J Agric Food Chem 2010;58:11112–11118 [DOI] [PubMed] [Google Scholar]
- 10.Ruweler M, Gulden M, Maser E, Murias M, Seibert H: Cytotoxic, cytoprotective and antioxidant activities of resveratrol and analogues in C6 astroglioma cells in vitro. Chem Biol Interact 2009;182:128–135 [DOI] [PubMed] [Google Scholar]
- 11.Shen P, Yue Y, Sun Q, Kasireddy N, Kim KH, Park Y: Piceatannol extends the lifespan of Caenorhabditis elegans via DAF-16. Biofactors 2017; [Epub ahead of print]; DOI: 10.1002/biof.1346 [DOI] [PubMed] [Google Scholar]
- 12.Kwon JY, Seo SG, Heo YS, et al. : Piceatannol, natural polyphenolic stilbene, inhibits adipogenesis via modulation of mitotic clonal expansion and insulin receptor-dependent insulin signaling in early phase of differentiation. J Biol Chem 2012;287:11566–11578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tung YC, Lin YH, Chen HJ, et al. : Piceatannol exerts anti-obesity effects in C57BL/6 mice through modulating adipogenic proteins and gut microbiota. Molecules 2016;21:E1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Solis GM, Petrascheck M: Measuring Caenorhabditis elegans life span in 96 well microtiter plates. J Vis Exp 2011;18:pii: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stiernagle T: Maintenance of C. elegans. WormBook 2006;11:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Colmenares D, Sun Q, Shen P, Yue Y, McClements DJ, Park Y: Delivery of dietary triglycerides to Caenorhabditis elegans using lipid nanoparticles: Nanoemulsion-based delivery systems. Food Chem 2016;202:451–457 [DOI] [PubMed] [Google Scholar]
- 17.Kenyon C, Chang J, Gensch E, Rudner A, Tabtland R: A C. elegans mutant that lives twice as long as wile type. Nature 1993;366:461–464 [DOI] [PubMed] [Google Scholar]
- 18.Yen K, Le TT, Bansal A, Narasimhan SD, Cheng JX, Tissenbaum HA: A comparative study of fat storage quantitation in nematode Caenorhabditis elegans using label and label-free methods. PloS One 2010;16:e12810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen Y, Onken B, Chen H, et al. : Mechanism of longevity extension of Caenorhabditis elegans induced by pentagalloyl glucose isolated from eucalyptus leaves. J Agric Food Chem 2014;62:3422–3431 [DOI] [PubMed] [Google Scholar]
- 20.Sun Q, Yue Y, Shen P, Yang JJ, Park Y: Cranberry product decreases fat accumulation in Caenorhabditis elegans. J Med Food 2016;19:427–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matsuyama S, Moriuchi M, Suico MA, et al. : Mild electrical stimulation increases stress resistance and suppresses fat accumulation via activation of LKB1-AMPK signaling pathway in C. elegans. PloS One 2014;9:e114690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raizen D, Song BM, Trojanowski N, You YJ: Methods for measuring pharyngeal behaviors. WormBook 2012;18:1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ashrafi K, Chang FY, Watts JL, Fraser AG, Kamath RS, Ahringer J, Ruvkun G: Genome-wide RNAi analysis of Caenorhabditis elegans fat regulatory genes. Nature 2003;421:268–272 [DOI] [PubMed] [Google Scholar]
- 24.McKay RM, McKay JP, Avery L, Graff JM: C elegans: A model for exploring the genetics of fat storage. Dev Cell 2003;4:131–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang F, Vought BW, Satterlee JS, et al. : An ARC/mediator subunit required for SREBP control of cholesterol and lipid homeostasis. Nature 2006;442:700–704 [DOI] [PubMed] [Google Scholar]
- 26.Van Gilst MR, Hadjivassiliou H, Yamamoto KR: A Caenorhabditis elegans nutrient response system partially dependent on nuclear receptor NHR-49. Proc Natl Acad Sci U S A. 2005;102:13496–13501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schweiger M, Schreiber R, Haemmerle G, et al. : Adipose triglyceride lipase and hormone-sensitive lipase are the major enzymes in adipose tissue triacylglycerol catabolism. J Biol Chem 2006;281:40236–40241 [DOI] [PubMed] [Google Scholar]
- 28.Ding Y, Zou X, Jiang X, et al. : Pu-erh tea down-regulates sterol regulatory element-binding protein and stearyol-CoA desaturase to reduce fat storage in Caenorhaditis elegans. PloS One 2015;10:e0113815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van Gilst MR, Hadjivassiliou H, Jolly A, Yamamoto KR: Nuclear hormone receptor NHR-49 controls fat consumption and fatty acid composition in C. elegans. PLoS Biol 2005;3:e53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Farrand L, Byun S, Kim JY, et al. : Piceatannol enhances cisplatin sensitivity in ovarian cancer via modulation of p53, X-linked inhibitor of apoptosis protein (XIAP), and mitochondrial fission. J Biol Chem 2013;288:23740–23750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nomura T, Horikawa M, Shimamura S, Hashimoto T, Sakamoto K: Fat accumulation in Caenorhabditis elegans is mediated by SREBP homolog SBP-1. Genes Nutr 2010;5:17–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee D, Jeong DE, Son HG, et al. : SREBP and MDT-15 protect C. elegans from glucose-induced accelerated aging by preventing accumulation of saturated fat. Genes Dev 2015;29:2490–2503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peng H, Wei Z, Luo H, et al. : Inhibition of fat accumulation by Hesperidin in Caenorhabditis elegans. J Agric Food Chem. 2016;64:5207–5214 [DOI] [PubMed] [Google Scholar]
- 34.Commerford SR, Peng L, Dubé JJ, O′Doherty RM: In vivo regulation of SREBP-1c in skeletal muscle: Effects of nutritional status, glucose, insulin, and leptin. Am J Physiol Regul Integr Comp Physiol 2004;287:R218–R227 [DOI] [PubMed] [Google Scholar]
- 35.Foretz M, Pacot C, Dugail I, et al. : ADD1/SREBP-1c is required in the activation of hepatic lipogenic gene expression by glucose. Mol Cell Biol 1999;19:3760–3768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roduit R, Morin J, Masse F, et al. : Glucose down-regulates the expression of the peroxisome proliferator-activated receptor-alpha gene in the pancreatic beta-cell. J Biol Chem 2000;275:35799–35806 [DOI] [PubMed] [Google Scholar]
- 37.Ravnskjaer K, Boergesen M, Dalgaard LT, Mandrup S: Glucose-induced repression of PPARalpha gene expression in pancreatic beta-cells involves PP2A activation and AMPK inactivation. J Mol Endocrinol 2006;36:289–299 [DOI] [PubMed] [Google Scholar]
- 38.Lass A, Zimmermann R, Oberer M, Zechner R: Lipolysis—a highly regulated multi-enzyme complex mediates the catabolism of cellular fat stores. Prog Lipid Res 2011;50:14–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ahmadian M, Duncan RE, Jaworski K, Sarkadi-Nagy E, Sul HS: Triacylglycerol metabolism in adipose tissue. Future lipidol 2007;2:229–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kraemer FB: Hormone-sensitive lipase: Control of intracellular tri-(di-)acylglycerol and cholesteryl ester hydrolysis. The Journal of Lipid Research 2002;43:1585–1594 [DOI] [PubMed] [Google Scholar]
- 41.Large V, Arner P, Reynisdottir S, et al. : Hormone-sensitive lipase expression and activity in relation to lipolysis in human fat cells. J Lipid Res 1998;39:1688–1695 [PubMed] [Google Scholar]