ABSTRACT
The recent escalation of occurrences of carbapenem-resistant Pseudomonas aeruginosa has been recognized globally and threatens to erode the widespread clinical utility of the carbapenem class of compounds for this prevalent health care-associated pathogen. Here, we compared the in vitro inhibitory activity of ceftazidime-avibactam and ceftolozane-tazobactam against 290 meropenem-nonsusceptible Pseudomonas aeruginosa nonduplicate clinical isolates from 34 U.S. hospitals using reference broth microdilution methods. Ceftazidime-avibactam and ceftolozane-tazobactam were active, with ceftolozane-tazobactam having significantly higher inhibitory activity than ceftazidime-avibactam. The heightened inhibitory activity of ceftolozane-tazobactam was sustained when the site of origin (respiratory, blood, or wound) and nonsusceptibility to other β-lactam antimicrobials was considered. An extensive genotypic search for enzymatically driven β-lactam resistance mechanisms revealed the exclusive presence of the VIM metallo-β-lactamase among only 4% of the subset of isolates nonsusceptible to ceftazidime-avibactam, ceftolozane-tazobactam, or both. These findings suggest an important role for both ceftazidime-avibactam and ceftolozane-tazobactam against carbapenem-nonsusceptible Pseudomonas aeruginosa. Further in vitro and in vivo studies are needed to better define the clinical utility of these novel therapies against the increasingly prevalent threat of multidrug-resistant Pseudomonas aeruginosa.
KEYWORDS: Pseudomonas aeruginosa, beta-lactams, ceftazidime-avibactam, ceftolozane-tazobactam, in vitro, potency
INTRODUCTION
Pseudomonas aeruginosa is responsible for a substantial proportion of nosocomial infections, including hospital-acquired bacterial pneumonia (HABP) and ventilator-associated bacterial pneumonia (VABP) (1, 2). Moreover, this organism is notoriously recognized for its predisposition to possess intrinsic and/or acquire a variety of resistance mechanisms, which frequently results in a phenotypic profile of multidrug resistance. Among β-lactam-resistant P. aeruginosa isolates, the carbapenem-resistant phenotype is especially worrisome due to its increasing prevalence and the frequency at which the carbapenems serve as the agents of choice for the treatment of severe P. aeruginosa infection (3, 4). Although carbapenem resistance in P. aeruginosa can be mediated by extrinsic mechanisms, such as acquired carbapenemases, including the Ambler class B metallo-β-lactamases and Klebsiella pneumoniae carbapenemase (KPC), it often originates from intrinsic mechanisms. These intrinsic mechanisms include overexpression of chromosomal cephalosporinases and hyperexpression of MexA-B and MexX-Y efflux pumps and OprD outer membrane protein mutations, which frequently occur simultaneously in P. aeruginosa (4, 5).
Ceftazidime-avibactam (CZA) and ceftolozane-tazobactam (C/T) are β-lactam/β-lactamase inhibitor antimicrobials that possess activity against P. aeruginosa and have been recently approved by the Food and Drug Administration (FDA). The current role of CZA and C/T as viable treatment options for P. aeruginosa infection has been reviewed in depth, but scarce data exist regarding their activity against carbapenem-resistant P. aeruginosa isolates (4, 5). Recognizing the apparent need for novel therapeutic agents against this alarming threat, the comparative in vitro inhibitory activities of both CZA and C/T against meropenem-nonsusceptible P. aeruginosa (MEM-NS-PSA) isolates were recently evaluated in two single-center studies (6, 7). The first investigated the susceptibility profile of CZA and C/T against 38 blood and respiratory tract isolates using a combination of broth microdilution and Etest methodologies (6). The authors found that while both CZA and C/T were active against 92% of the isolates, the MIC tended to be at the susceptibility breakpoint for CZA much more frequently than for C/T. In addition, a genotypic evaluation of these organisms revealed the presence of oprD mutations; however, definitive mechanisms other than porin mutations were not elucidated for either drug (6). The second study, using Etest methods, reported susceptibilities of 82% for CZA and 87% for C/T among 45 isolates obtained from blood, respiratory tract, urinary tract, or wound cultures (7).
The objectives of the current study were 2-fold. The first was to compare the in vitro inhibitory activity of CZA and C/T against MEM-NS-PSA isolates obtained from multiple U.S. hospitals. The second was to use genotypic profiling to evaluate the possible underlying enzymatic resistance mechanisms of isolates noted to be nonsusceptible to CZA and/or C/T.
RESULTS
Two-hundred ninety MEM-NS-PSA clinical isolates originating from respiratory tract (n = 195), blood (n = 35), and wound (n = 60) sources were available for evaluation. The phenotypic β-lactam susceptibility profile of this isolate population (n = 290) was as follows, as previously reported using similar MIC methodologies (8): cefepime (FEP), 42%; ceftazidime (CAZ), 46%; piperacillin-tazobactam (TZP), 36%; and aztreonam (ATM), 37%. In the current investigation, the CZA and C/T inhibitory activities for these MEM-NS-PSA were 81% and 91%, respectively. The main results of the in vitro inhibitory activity for CZA and C/T, stratified according to body site of origin, are presented in Table 1. The potency of C/T was higher than that of CZA, regardless of isolate origin (P = 0.0007, P = 0.0045, P = 0.0002, and P < 0.0001, for the overall population, respiratory tract, blood, and wound origins, respectively). For CZA, the MIC50 values were lower than or equal to the breakpoint of 8 mg/liter, whereas the MIC90 values for CZA were higher than the breakpoint for all sources. For C/T, the MIC50 values were lower than or equal to the breakpoint of 4 mg/liter, regardless of source. The MIC90 values for C/T were equal to the breakpoint when all the isolates were grouped and for isolates from the respiratory tract but were higher than the breakpoint when the isolate source was blood or wound.
TABLE 1.
MIC profiles of CZA and C/T stratified by body origin of MEM-NS-PSA isolatesa
| Isolate origin (n) | Antimicrobial | Range (mg/liter) | Mode | MIC50 | MIC90 | %S |
|---|---|---|---|---|---|---|
| All origins (290) | CZA | 0.25 to >64 | 8 | 4 | 16 | 81 |
| C/T | 0.25 to >64 | 1 | 1 | 4 | 91 | |
| Respiratory tract (195) | CZA | 0.25 to >64 | 8 | 4 | 16 | 84 |
| C/T | 0.25 to >64 | 1 | 1 | 4 | 92 | |
| Blood (35) | CZA | 1 to >64 | 4 | 8 | 32 | 77 |
| C/T | 0.25 to >64 | 0.5 | 1 | 8 | 89 | |
| Wound (60) | CZA | 0.5 to 64 | 4 | 4 | 32 | 72 |
| C/T | 0.5 to >64 | 1 | 1 | 8 | 88 |
Abbreviations: MEM-NS-PSA, meropenem-nonsusceptible P. aeruginosa; CZA, ceftazidime-avibactam; C/T, ceftolozane-tazobactam.
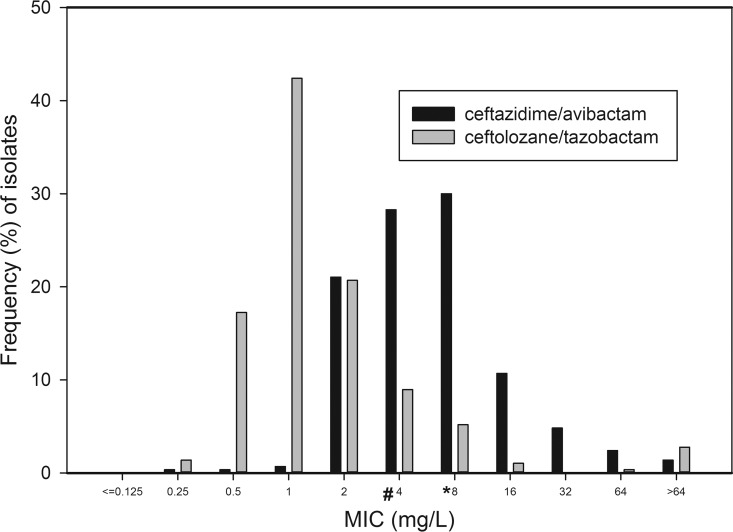
The overall rate of isolates with MIC at the susceptibility breakpoint (Fig. 1) was higher for CZA than for C/T (30% and 9%, respectively; P < 0.0001). The most prevalent MIC phenotype was susceptibility to both CZA and C/T, comprising 80.5%, 74.3%, and 71.6% of respiratory, blood, and wound isolates, respectively. Isolates NS to both CZA and C/T comprised 4.6%, 8.6%, and 11.6% of respiratory, blood, and wound isolates, respectively. Isolates susceptible to CZA but NS to C/T comprised 0.5%, 2.8%, and 0% of respiratory, blood, and wound isolates, respectively; a mirror phenotypic pattern of isolates susceptible to C/T but NS to CZA was evident in 11.3%, 14.3%, and 16.7% of respiratory, blood, and wound isolates, respectively.
FIG 1.
MIC distribution (%) for ceftazidime-avibactam and ceftolozane-tazobactam of 290 meropenem-nonsusceptible blood, respiratory, and wound P. aeruginosa isolates. The susceptibility breakpoints for ceftazidime-avibactam and ceftolozane-tazobactam are 8 mg/liter (*) and 4 mg/liter (#), respectively.
While we did not directly compare the in vitro inhibitory activities of CZA and C/T to those of FEP, CAZ, TZP, and ATM, given that the values for the last set of drugs were fully obtained in another study (8), we did compare the in vitro inhibitory activities of CZA and C/T, stratified by NS to the other β-lactam agents (Table 2). The CZA inhibitory activity was significantly lower than that of C/T, whenever NS to ≥1 specific β-lactam agent was present (including the specific case of all 4 β-lactam agents). A phenotype of susceptibility to all 4 β-lactam agents was manifested by 18.3% (53/290) of all isolates, with identical inhibitory activity rates of 98% (52/53) for both CZA and C/T. Another perspective of the overall isolates' data reveals that among CZA-NS isolates (56/290) and C/T-NS isolates (27/290), the rate of those susceptible to ≥1 β-lactam agent was similar, 17.8% (10/56) and 18.5% (5/27), respectively (P = 1.00). There was a positive correlation between CZA and C/T MICs (r = 0.595), between CZA MICs and the MICs of FEP and ATM (r, 0.532 and 0.683, respectively), and between C/T MICs and the MICs of FEP, CAZ, ATM, and TZP (r ranges, 0.516 to 0.708). A positive correlation was also found between CZA MICs and the MICs of CAZ and TZP (r ranges, 0.451 to 0.49). The MICs of FEP, CAZ, ATM, and TZP had a positive correlation one with the other (r ranges, 0.604 to 0.794). Positive correlation was additionally found between the MEM MIC and the MICs of CZA, C/T, FEP, CAZ, ATM, and TZP (r ranges, 0.29 to 0.443).
TABLE 2.
Comparison of susceptibility rates for ceftazidime-avibactam (CZA) and ceftolozane-tazobactam (C/T) among meropenem-nonsusceptible P. aeruginosa isolates stratified by the presence of nonsusceptibility to other β-lactam agents
| β-Lactam agent(s)a to which isolates were NS (no. of isolates/total, %) | S to CZA (no. of isolates, %) | S to C/T (no. of isolates, %) | P valueb |
|---|---|---|---|
| FEP (168/290, 58) | 114, 68 | 142, 85 | 0.0003 |
| CAZ (157/290, 54) | 105, 67 | 132, 84 | 0.0006 |
| TZP (185/290, 64) | 133, 72 | 159, 86 | 0.0013 |
| ATM (183/290, 63) | 132, 72 | 159, 87 | 0.0007 |
| FEP and CAZ (133/290, 46) | 82, 62 | 108, 81 | 0.0006 |
| FEP and TZP (147/290, 51) | 97, 66 | 122, 83 | 0.0012 |
| FEP and ATM (131/290, 45) | 82, 63 | 108, 82 | 0.0005 |
| CAZ and TZP (145/290, 50) | 95, 66 | 121, 83 | 0.0007 |
| CAZ and ATM (121/290, 42) | 73, 60 | 99, 82 | 0.0004 |
| TZP and ATM (148/290, 51) | 99, 67 | 125, 85 | 0.0006 |
| FEP, CAZ, and TZP (127/290, 44) | 78/127, 61 | 103/127, 81 | 0.0008 |
| FEP, CAZ, and ATM (106/290, 37) | 59/106, 56 | 84/106, 79 | 0.0004 |
| FEP, TZP, and ATM (121/290, 42) | 73/121, 60 | 98/121, 81 | 0.0006 |
| CAZ, TZP, and ATM (118/290, 41) | 70/118, 59 | 96/118, 81 | 0.0003 |
| All 4 β-lactam agents (103/290, 36) | 56/103, 54 | 81/103, 79 | 0.0004 |
Abbreviations: S, susceptible; NS, nonsusceptible; ATM, aztreonam; FEP, cefepime; CZA, ceftazidime-avibactam; C/T, ceftolozane-tazobactam; CAZ, ceftazidime; TZP, piperacillin-tazobactam.
Comparisons were conducted using Fisher's exact test.
The Acuitas Resistome molecular test detected the presence of genes encoding OXA-50 in 85.1% (40/47), OXA-10 in 4.2% (2/47), SHV-G156 in 2.1% (1/47), and SHV-G2385/E240K in 2.1% (1/47) of selected isolates. Of the 12 isolates that were NS to both CZA and C/T, 2 were found to be positive for VIM metallo-β-lactamase and had MICs of ≥64 mg/liter for CZA and C/T.
DISCUSSION
The current large-scale comparative MIC study clearly shows that the in vitro inhibitory activity of C/T is higher than that of CZA. These observations are sustained, according to our results, when considering variability in the isolate source and NS to other specific β-lactam agents with antipseudomonal activity. Although our results are in general agreement with the data derived from the single-center studies by Buehrle et al. and Gonzales et al., differences do exist (6, 7). In the current study and that conducted by Gonzales and colleagues, similar CZA susceptibilities for the MEM-NS-PSA were observed (81% and 82%, respectively); however, this inhibitory activity was lower than the 91% quoted by Buehrle and colleagues (6, 7). In addition, while the enhanced inhibitory activity of C/T versus that of CZA is directly reflected in our results, a more subtle observation (observed in the current study too) of a higher proportion of isolates at the susceptibility breakpoint for CZA than for C/T is provided as the sole support for C/T heightened inhibitory activity by Buehrle and colleagues (6). Similar to what was done in the study of Gonzales and colleagues (7), the current study also included isolates originating from wound samples in an attempt to incorporate infection sources in which P. aeruginosa may play an important role and resistance to other commonly utilized agents may be prominent.
Moreover, our results appear to agree with the recently published U.S. data for CZA and C/T using broth microdilution methods, as the reported susceptibility rates for MEM-NS-PSA were 86.2% and 92.8%, respectively. Since the aforementioned extensive reports do not provide a direct comparison of the inhibitory activities of CZA and C/T (9, 10), the current study provides an intriguing opportunity to compare the MIC distributions of CZA and C/T within the same isolate population. While the MIC50 values for both drugs were lower than or at their respective breakpoint for all isolates, regardless of origin, when considering the MIC90 values, C/T maintained a more favorable inhibitory activity profile, albeit influenced by source. To elaborate, when assessing the origin of the isolate (especially for CZA), a clear elevation of the MIC90 values for both CZA and C/T was observed. Notably, in the case of blood and wound isolates, the MIC90 value for C/T was only 1-fold dilution higher than the breakpoint, while a ≥2-fold dilution was observed with the corresponding CZA breakpoint. These results provide additional support to the higher in vitro inhibitory activity of C/T than of CZA and further emphasize the importance of incorporating possible sources of variability (e.g., isolate origin) into the analysis of in vitro inhibitory activity studies.
The significantly higher rate of isolates at the breakpoint dilution for CZA than for C/T has two important implications. First, as MIC results provided by automated antimicrobial susceptibility testing systems used in most clinical laboratories are known to have various degrees of disagreement with reference broth microdilution method (11), it is plausible for a higher rate of MEM-NS-PSA isolates to be misidentified as both susceptible and NS to CZA than in the case of testing C/T. Second, it is clear that the higher the MIC, the more challenging it becomes to attain the desired pharmacodynamic target exposure. Although a population pharmacokinetic/pharmacodynamic analysis of patients with normal renal function supports a 98.3% probability of target attainment (PTA) in the plasma against P. aeruginosa at MICs of ≤8 mg/liter with a regimen of 2.5 g of CZA every 8 h (q8h) in a 2-h infusion, the PTA drops sharply at the MIC of 16 mg/liter, to only 50.8% (12). Given that scarce direct clinical data are available to ascertain the simulation-derived high PTA at the breakpoint, a difference of that magnitude in the PTA across only a 1-fold dilution raises concerns from a clinical point of view, encouraging to adopt a cautious attitude when making treatment decisions regarding isolates with CZA MICs that are at the breakpoint of 8 mg/liter. Similar considerations can be exemplified in the case of C/T (breakpoint, 4 mg/liter), for which the PTA in the plasma against Enterobacteriaceae and P. aeruginosa with a MIC of ≤4 mg/liter in simulated patients with normal renal function following the approved dosing regimen of 1.5 g over 1 h q8h and using a conservative efficacy criterion of free time above the MIC of ≥50%, was 95%, declining to 80% at the MIC of 8 mg/liter. Conversely, simulation of a 3-g C/T infusion regimen, applying the same efficacy criterion, resulted in high PTAs for both plasma and the pulmonary epithelial lining fluid against Enterobacteriaceae and P. aeruginosa isolates with a MIC of 8 mg/liter, of 95% and 87.7%, respectively; consequently, the use of a regimen of 3 g C/T over 1 h q8h for nosocomial pneumonia is currently being tested in a clinical trial (13, 14).
While the phenotypic profile of P. aeruginosa is easily determined for both CZA and C/T, the exact resistance mechanism(s) responsible for the NS of any given isolate remains elusive. Similar to what was previously reported by Buehrle et al. (6), we also found a positive correlation between the MICs of these novel therapies and the other four antipseudomonal β-lactam agents tested (albeit in another study [8], using the exact same methodology). Congruent with other studies (6, 15) are the lower inhibitory activity rates for both CZA and C/T when stratification based on NS to other β-lactams is applied. Our results also indicate similar inhibitory activity for both CZA and C/T in the presence of susceptibility to all four β-lactam agents as well as a similar rate of susceptibility to ≥1 β-lactam agent for isolates that are CZA-NS or C/T-NS. While these findings suggest that some degree of cross-resistance for all the antimicrobials investigated in the current study exists, they do not provide definitive evidence regarding the actual resistance mechanisms. As such, we undertook the genotypic profiling studies in an attempt to more accurately define the enzymatic drivers of resistance. Unfortunately, the results of this analysis argue against the predominance of enzyme-mediated mechanisms explaining the observed NS to CZA and/or C/T, as only 2 (4%) of the 47 isolates displayed evidence for such a mechanism (i.e., a metallo-β-lactamase). These findings agree with other studies of P. aeruginosa conducted in the United States (6, 16, 17). In a comprehensive study by Winkler and colleagues (18), general, nonspecifically identified membrane permeability and drug efflux factors were suggested as the underlying resistance mechanisms of P. aeruginosa for CZA. In another study (19), high-level resistance to CZA was selected at very low frequency in 3 P. aeruginosa strains carrying a derepressed AmpC allele, and structural modifications found in AmpC caused it to be less effectively inhibited by avibactam. For the development of P. aeruginosa resistance to C/T, it has been hypothesized that multiple mutations leading to overexpression and structural modifications of AmpC are needed, based on investigating a hypermutator P. aeruginosa strain (20). Further support for the last hypothesis is given in the study by Castanheira and colleagues (21), in which the median C/T MIC was significantly higher among P. aeruginosa isolates demonstrating chromosomal AmpC overexpression than among isolates that had AmpC expression levels similar to those of the baseline strain.
Although the genotypic profiling as conducted here did not provide a substantial enhancement of our understanding regarding the observed NS of CZA and/or C/T, a further look at the results of CZA inhibitory activity when stratified based upon CAZ-NS isolates is of special interest in that context, as CZA had inhibitory activity against 67% of these isolates. Since CZA showed a beneficial susceptibility profile over CAZ in the absence of demonstrated carbapenemase- or extended-spectrum β-lactamase (ESBL)-mediated resistance mechanisms, it is very reasonable to deduce that inhibition of a chromosomally encoded AmpC that was undetected by the Resistome test (even though this test has an extensive ability to detect plasmid-borne Amp-C genes) is responsible for this observation (22). To the best of our understanding, the existence of a chromosomal AmpC genotypic resistance was not rejected in the study by Buehrle and colleagues, given their detailed methods; testing was restricted for the presence of the plasmid-borne AmpC genes encoding cephamycins (CMY), moxalactam (MOX), cefoxitin (FOX), AmpC type (ACT), and 2,8-dihydroxyadenine (DHA) (6). Moreover, our observation that C/T had inhibitory activity against 84% of the CAZ-NS isolates is also supportive for the presumed presence of an AmpC resistance mechanism (known to be responsible for CAZ NS), since C/T has been previously shown to exhibit excellent stability against AmpC (23, 24).
The current study is the largest to compare head to head the inhibitory activities of CZA and C/T among highly resistant P. aeruginosa isolates using the reference standard broth microdilution methods. Clearly, one must be prudent in making clinical inferences from in vitro data, but the current study suggests that in the United States, both ceftazidime-avibactam and ceftolozane-tazobactam may serve as appropriate treatment options when encountering an infection caused by a carbapenem-nonsusceptible P. aeruginosa strain. Ceftolozane-tazobactam appears to have higher inhibitory activity than ceftazidime-avibactam. Further studies should include relevant pharmacokinetic/pharmacodynamics and clinical data to help translate these findings into the real-world clinical arena.
MATERIALS AND METHODS
Nonduplicate MEM-NS-PSA isolates from the blood, respiratory tract, and wounds were identified from an adult inpatient sampling of 34 U.S. hospitals in 2013 and 2014 (8). Clinical and Laboratory Standards Institute (CLSI)-defined broth microdilution methods were employed to determine the MICs in triplicates for CZA and C/T (25). MIC trays were prepared in-house using the Biomek 3000 instrument (Beckman Instruments, Inc., Fullerton, CA) and stored at −80°C until use. Cation-adjusted Mueller-Hinton 11 broth was purchased from Becton, Dickinson Co. (Sparks, MD) and was used to dilute the MIC trays. Merck Pharmaceuticals provided C/T, avibactam was purchased from Tecoland Corporation (Irvine, CA), and all other antibiotics were purchased from Sigma (St. Louis, MO). Isolates were stored in skim milk at −80°C until used. They were then transferred twice onto Trypticase soy agar plates containing 5% blood from Becton, Dickinson Co. (Sparks, MD) for MIC determination. For each isolate, triplicates for CZA MICs were all performed on the same day. For C/T, duplicate MICs for each isolate were conducted on the same day as the CZA triplicates, with the remaining replicate of C/T MIC available from a previous run of MICs. All MIC testing was conducted using the exact same methods as those outlined above and included triplicate MICs for FEP, CAZ, TZP, and ATM (8). Results of MIC studies for each isolate are reported using the mode of triplicate tests conducted for each agent. Klebsiella pneumoniae ATCC 700603 was used for quality control (QC) testing of CZA and C/T MICs as defined by CLSI (25). The FDA breakpoint of 8 mg/liter and the CLSI breakpoint of 4 mg/liter were used to define susceptibility for CZA and C/T, respectively (25, 26). CLSI-defined susceptibility breakpoints of 8 mg/liter, 8 mg/liter, 16 mg/liter, and 8 mg/liter were used for FEP, CAZ, TZP, and ATM, respectively (25). The term “susceptible,” used throughout this paper, denotes that the isolate has a MIC value that is less than or equal to the breakpoint.
A comprehensive genotypic screen for β-lactam enzymatically driven resistance mechanisms was conducted using the Acuitas Resistome test (OpGen Inc., Gaithersburg, MD) on blood and respiratory sample-derived isolates. The aforementioned molecular test detects the presence of 49 major antimicrobial resistance genes, inclusive of those encoding carbapenemases (25 genes, 208 subtypes), extended-spectrum β-lactamases (13 genes, 557 subtypes), and plasmid-mediated AmpC (11 genes, 147 subtypes). Of all blood and respiratory isolates, 47 exhibited the following phenotypes: C/T susceptible (C/T-S) and CZA-NS (n = 27), C/T-NS and CZA-S (n = 8), and C/T-NS and CZA-NS (n = 12); they were subjected to this genotypic screening process.
Comparisons of categorical values and correlations were conducted using the Fisher exact test and the Spearman rank correlation coefficient, respectively (SigmaPlot Version 13; Systat Inc., San Jose, CA). A 2-tailed P value of <0.05 was considered statistically significant, and the interpretation of the correlation coefficient, r, was used to assess agreement.
ACKNOWLEDGMENTS
We thank Jennifer Tabor-Rennie, Debora Santini, Sara Giovagnoli, Kimelyn Greenwood, and Elizabeth Cyr for their collective efforts with MIC determinations.
Funding for the original surveillance program as well as the genotypic profiling in this study was supported by Merck & Co., Inc.; the phenotypic profiling of CZA and C/T and the subsequent analysis were conducted with funds from the Center for Anti-Infective Research and Development.
Competing interests: David Nicolau has acted as a consultant and speaker bureau member and has received research funding from Merck & Co., Inc. Mordechai Grupper and Christina Sutherland have no conflicts of interest to declare.
REFERENCES
- 1.Centers for Disease Control and Prevention. 2016. Antibiotic/antimicrobial resistance. http://www.cdc.gov/drugresistance/biggest_threats.html Accessed 15 September 2016.
- 2.Barbier F, Andremont A, Wolff M, Bouadma L. 2013. Hospital-acquired pneumonia and ventilator-associated pneumonia: recent advances in epidemiology and management. Curr Opin Pulm Med 19:216–228. doi: 10.1097/MCP.0b013e32835f27be. [DOI] [PubMed] [Google Scholar]
- 3.Zilberberg MD, Shorr AF. 2013. Prevalence of multidrug-resistant Pseudomonas aeruginosa and carbapenem-resistant Enterobacteriaceae among specimens from hospitalized patients with pneumonia and bloodstream infections in the United States from 2000 to 2009. J Hosp Med 8:559–563. doi: 10.1002/jhm.2080. [DOI] [PubMed] [Google Scholar]
- 4.van Duin D, Bonomo RA. 2016. Ceftazidime/avibactam and ceftolozane/tazobactam: second-generation β-lactam/β-lactamase inhibitor combinations. Clin Infect Dis 15:234–241. doi: 10.1093/cid/ciw243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liscio JL, Mahoney MV, Hirsch EB. 2015. Ceftolozane/tazobactam and ceftazidime/avibactam: two novel β-lactam/β-lactamase inhibitor combination agents for the treatment of resistant Gram-negative bacterial infections. Int J Antimicrob Agents 46:266–271. doi: 10.1016/j.ijantimicag.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 6.Buehrle DJ, Shields RK, Chen L, Hao B, Press EG, Alkrouk A, Potoski BA, Kreiswirth BN, Clancy CJ, Nguyen MH. 2016. Evaluation of the in vitro activity of ceftazidime-avibactam and ceftolozane-tazobactam against meropenem-resistant Pseudomonas aeruginosa isolates. Antimicrob Agents Chemother 22:3227–3231. doi: 10.1128/AAC.02969-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gonzalez MD, McMullen AR, Wallace MA, Crotty MP, Ritchie DJ, Burnham CA. 2017. Susceptibility of ceftolozane-tazobactam and ceftazidime-avibactam against a collection of β-lactam-resistant gram-negative bacteria. Ann Lab Med 37:174–176. doi: 10.3343/alm.2017.37.2.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sutherland CA, Verastegui JE, Nicolau DP. 2016. In vitro potency of amikacin and comparators against E. coli, K. pneumoniae and P. aeruginosa respiratory and blood isolates. Ann Clin Microbiol Antimicrob 15:39. doi: 10.1186/s12941-016-0155-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sader HS, Huband MD, Castanheira M, Flamm RK. 2017. Pseudomonas aeruginosa antimicrobial susceptibility results from four years (2012 to 2015) of the International Network for Optimal Resistance Monitoring program in the United States. Antimicrob Agents Chemother 61:e02252-16. doi: 10.1128/AAC.02252-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farrell DJ, Flamm RK, Sader HS, Jones RN. 2013. Antimicrobial activity of ceftolozane-tazobactam tested against Enterobacteriaceae and Pseudomonas aeruginosa with various resistance patterns isolated in U.S. hospitals (2011-2012). Antimicrob Agents Chemother 57:6305–6310. doi: 10.1128/AAC.01802-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bulik CC, Fauntleroy KA, Jenkins SG, Abuali M, LaBombardi VJ, Nicolau DP, Kuti JL. 2010. Comparison of meropenem MICs and susceptibilities for carbapenemase-producing Klebsiella pneumoniae isolates by various testing methods. J Clin Microbiol 48:2402–2406. doi: 10.1128/JCM.00267-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.US Food and Drug Administration Anti-Infective Drugs Advisory Committee. 2014. Cerexa Ia subsidiary of Actavis plc, ceftazidime-avibactam for injection. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2015/206494Orig1s000CllinPharmR.pdf Accessed 27 July 2017.
- 13.Xiao AJ, Miller BW, Huntington JA, Nicolau DP. 2016. Ceftolozane/tazobactam pharmacokinetic/pharmacodynamic-derived dose justification for phase 3 studies in patients with nosocomial pneumonia. J Clin Pharmacol 56:56–66. doi: 10.1002/jcph.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cubist Pharmaceuticals LLC. 2000. A prospective, randomized, double-blind, multicenter, phase 3 study to assess the safety and efficacy of intravenous ceftolozane/tazobactam compared with meropenem in adult patients with ventilated nosocomial pneumonia. ClinicalTrials.gov NLM identifier NCT02070757. https://clinicaltrials.gov/ct2/show/NCT02070757.
- 15.Tato M, García-Castillo M, Bofarull AM, Cantón R, CENIT Study Group. 2015. In vitro activity of ceftolozane/tazobactam against clinical isolates of Pseudomonas aeruginosa and Enterobacteriaceae recovered in Spanish medical centres: results of the CENIT study. Int J Antimicrob Agents 46:502–510. doi: 10.1016/j.ijantimicag.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 16.Fehlberg LC, Xavier DE, Peraro PP, Marra AR, Edmond MB, Gales AC. 2012. Beta-lactam resistance mechanisms in Pseudomonas aeruginosa strains causing bloodstream infections: comparative results between Brazilian and American isolates. Microb Drug Resist 18:402–407. doi: 10.1089/mdr.2011.0174. [DOI] [PubMed] [Google Scholar]
- 17.Mesaros N, Nordmann P, Plésiat P, Roussel-Delvallez M, Van Eldere J, Glupczynski Y, Van Laethem Y, Jacobs F, Lebecque P, Malfroot A, Tulkens PM, Van Bambeke F. 2007. Pseudomonas aeruginosa: resistance and therapeutic options at the turn of the new millennium. Clin Microbiol Infect 13:560–578. doi: 10.1111/j.1469-0691.2007.01681.x. [DOI] [PubMed] [Google Scholar]
- 18.Winkler ML, Papp-Wallace KM, Hujer AM, Domitrovic TN, Hujer KM, Hurless KN, Tuohy M, Hall G, Bonomo RA. 2015. Unexpected challenges in treating multidrug-resistant Gram-negative bacteria: resistance to ceftazidime-avibactam in archived isolates of Pseudomonas aeruginosa. Antimicrob Agents Chemother 59:1020–1029. doi: 10.1128/AAC.04238-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lahiri SD, Walkup GK, Whiteaker JD, Palmer T, McCormack K, Tanudra MA, Nash TJ, Thresher J, Johnstone MR, Hajec L, Livchak S, McLaughlin RE, Alm RA. 2015. Selection and molecular characterization of ceftazidime/avibactam-resistant mutants in Pseudomonas aeruginosa strains containing derepressed AmpC. J Antimicrob Chemother 70:1650–1608. doi: 10.1093/jac/dkv131. [DOI] [PubMed] [Google Scholar]
- 20.Cabot G, Bruchmann S, Mulet X, Zamorano L, Moyà B, Juan C, Haussler S, Oliver A. 2014. Pseudomonas aeruginosa ceftolozane-tazobactam resistance development requires multiple mutations leading to overexpression and structural modification of AmpC. Antimicrob Agents Chemother 58:3091–3099. doi: 10.1128/AAC.02462-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Castanheira M, Mills JC, Farrell DJ, Jones RN. 2014. Mutation-driven β-lactam resistance mechanisms among contemporary ceftazidime-nonsusceptible Pseudomonas aeruginosa isolates from U.S. hospitals. Antimicrob Agents Chemother 58:6844–6850. doi: 10.1128/AAC.03681-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lahiri SD, Johnstone MR, Ross PL, McLaughlin RE, Olivier NB, Alm RA. 2014. Avibactam and class C β-lactamases: mechanism of inhibition, conservation of the binding pocket, and implications for resistance. Antimicrob Agents Chemother 58:5704–5713. doi: 10.1128/AAC.03057-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bulik CC, Christensen H, Nicolau DP. 2010. In vitro potency of CXA-101, a novel cephalosporin, against Pseudomonas aeruginosa displaying various resistance phenotypes, including multidrug resistance. Antimicrob Agents Chemother 54:557–559. doi: 10.1128/AAC.00912-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takeda S, Ishii Y, Hatano K, Tateda K, Yamaguchi K. 2007. Stability of FR264205 against AmpC β-lactamase of Pseudomonas aeruginosa. Int J Antimicrob Agents 30:443–445. doi: 10.1016/j.ijantimicag.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 25.Clinical and Laboratory Standards Institute. 2016. Performance standards for antimicrobial susceptibility testing; twenty-fourth informational supplement. CLSI document M100-S24 U. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 26.Allergan, USA, Inc. 2015. Avycaz® (ceftazidime-avibactam) package insert. http://www.accessdata.fda.gov/drugsatfda_docs/label/2015/206494s000lbl.pdf Accessed 23 February 2017.