ABSTRACT
The combination of trimethoprim and sulfamethoxazole (TMP-SMX) is the most effective regimen for therapy of Pneumocystis pneumonia (PCP). As many patients with PCP are allergic or do not respond to it, efforts have been devoted to develop alternative therapies for PCP. We have found that the combination of vitamin D3 (VitD3) (300 IU/kg/day) and primaquine (PMQ) (5 mg/kg/day) was as effective as TMP-SMX for therapy of PCP. In this study, we investigated the mechanisms by which vitamin D enhances the efficacy of PMQ. C57BL/6 mice were immunosuppressed by CD4+ cell depletion, infected with Pneumocystis murina for 8 weeks, and then treated for 9 days with the combination of VitD3 and PMQ (VitD3-PMQ) or with TMP-SMX or PMQ to serve as controls. The results showed that vitamin D supplementation increased the number of CD11c+ cells, suppressed the production of proinflammatory cytokines (tumor necrosis factor alpha [TNF-α], gamma interferon [IFN-γ], and interleukin-6 [IL-6]) and inducible nitric oxide synthase (iNOS), and enhanced the expression of genes related to antioxidation (glutathione reductase and glutamate-cysteine ligase modifier subunit), antimicrobial peptides (cathelicidin), and autophagy (ATG5 and beclin-1). These results suggest that the main action of vitamin D is enhancing the ability of the host to defend against Pneumocystis infection.
KEYWORDS: alveolar macrophages, Pneumocystis pneumonia, vitamin D3
INTRODUCTION
Pneumocystis jirovecii is the causative organism of Pneumocystis pneumonia (PCP) in humans. The mortality rate of PCP is 5 to 40% in patients with treatment and close to 100% in those without treatment (1). Pneumocystis organisms also infect other mammalian species. Pneumocystis infection is very species specific. The organism that infects mice is P. murina.
Currently, the most effective drug for treatment of PCP is the combination of trimethoprim and sulfamethoxazole (TMP-SMX). Unfortunately, TMP-SMX has adverse effects such as rash, fever, neutropenia, thrombocytopenia, or transaminase elevation in some people (2), and many AIDS patients with PCP do not respond to therapy with TMP-SMX (3). We have previously found that myeloid-derived suppressor cells (MDSCs) accumulate in the lungs of mice and rats with PCP (4). Treatment of P. murina-infected mice and rats with all-trans-retinoic acid (ATRA) was found to clear the infection in 5 weeks, with the disappearance of MDSCs and increased number of alveolar macrophages (AMs) in the lungs (4). These findings suggest that ATRA treatment stimulates MDSCs to differentiate to AMs, allowing the host to effectively defend the infection. These results also suggest that ATRA can be used as a supplemental therapy for PCP. To test this hypothesis, we treated mice having PCP with ATRA in combination with 2 mg/kg/day of primaquine (PMQ) and found that this ATRA-PMQ combination was as effective as TMP-SMX for PCP therapy and cleared the infection in 2 weeks (5).
Since ATRA also has significant adverse effects (6), we sought alternatives to replace it and found that vitamin D3 (VitD3) (300 IU/kg/day) had a synergistic effect with a higher dose of PMQ (5 mg/kg/day) for therapy of PCP (7). This VitD3-PMQ combination was found to clear P. murina infection, reduce the severity of inflammation, and increase the CD11blow CD11chigh alveolar macrophage population in the lungs of mice with PCP within 3 weeks. However, those studies were performed on mice with mild PCP, i.e., mice infected with P. murina for 4 weeks. In this study, we investigated the efficacy of VitD3-PMQ for treatment of mice with severe PCP. The mechanisms of action of VitD3 as supplemental therapy for PCP were also investigated. We hypothesized that VitD3 treatment reduces lung inflammation and enhances host innate immunity by affecting the expression of some of its target genes.
RESULTS
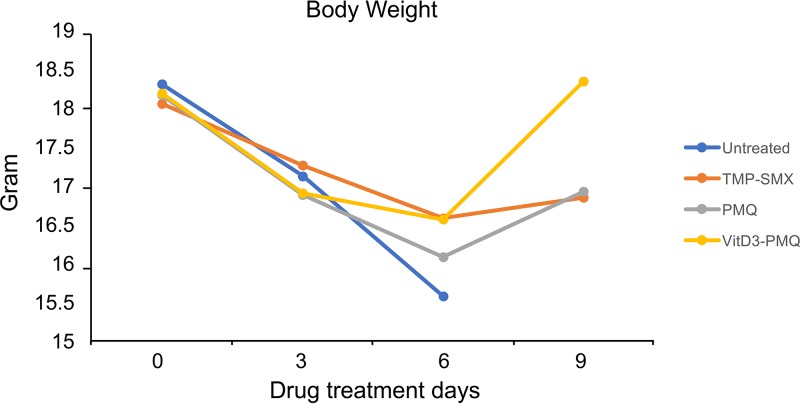
VitD3-PMQ therapy restores mouse body weight.
Infected mice started to show signs of PCP such as labored breathing at 3 to 4 weeks after infection, and weight loss was noticeable at 6 to 7 weeks of infection. At 8 weeks after infection, labored breathing became very severe, bony structures were easily palpated, and hunched posture was observed. Before treatment, the average body weight of mice that had been infected with P. murina for 8 weeks was 18.1 ± 1.23 g. At the 3-day time point, the average body weight of these mice was in the range of 16.88 ± 1.4 g to 17.26 ± 1.44 g, and there was no significant difference (P = 0.36) in body weight among untreated PCP mice and those treated with TMP-SMX, PMQ, or VitD3-PMQ. At the 6-day time point, the average body weights of infected mice treated with TMP-SMX or VitD3-SMQ were 16.58 ± 1.6 g and 16.56 ± 1.7 g, respectively, while the average body weight of mice treated with PMQ was 16.08 ± 1.89 g and that of untreated mice was 15.58 ± 0.93 g. At the 9-day time point, the average body weights of mice treated with TMP-SMX or PMQ were 16.84 ± 1.48 g and 16.92 ± 1.63 g, respectively, whereas the body weight of those treated with VitD3-PMQ was increased to 18.34 ± 1.99 g (P = 0.02). These results indicated that VitD3-PMQ treatment allowed the severely infected mice to regain body weight to approximately 1.5 g above those treated with PMQ or with the standard regimen (TMP-SMX) (Fig. 1).
FIG 1.
Changes in body weight during therapy. Mice infected with P. murina for 8 weeks were treated with TMP-SMX, PMQ, or VitD3-PMQ. Each mouse was weighed before and every 3 days after initiation of treatment for 9 days. Each dot represents the average body weight of 5 mice in various treatment groups.
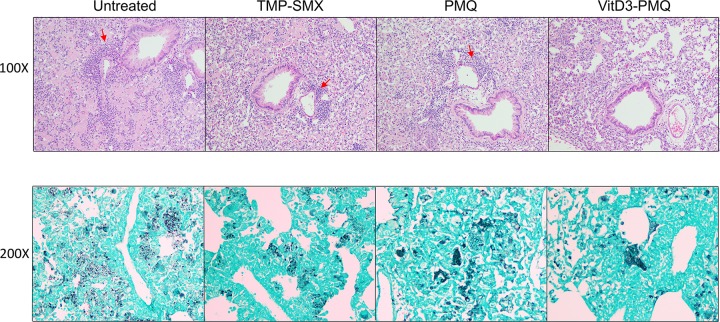
VitD3-PMQ therapy reduces lung inflammation.
Examination of methenamine silver-stained lung sections of mice revealed that those of untreated mice had a very heavy P. murina organism load. Hematoxylin and eosin (H&E)-stained lung sections showed signs of severe inflammation, i.e., infiltration of numerous inflammatory cells. TMP-SMX or PMQ treatment reduced the severity of inflammation to about the same level. The organism load of mice treated with PMQ was slightly decreased, while that of mice treated with TMP-SMX or VitD3-PMQ was significantly reduced. The severity of lung inflammation in mice treated with VitD3-PMQ was much less than that in mice treated with TMP-SMX (Fig. 2).
FIG 2.
Lung histopathology. H&E- and methenamine silver-stained lung sections of the following mouse groups are shown: immunosuppressed mice with PCP for 8 weeks (Untreated) and mice with PCP for 8 weeks treated with TMP-SMX, PMQ, or VitD3-PMQ for 9 days. (A) H&E-stained lung sections (magnification, ×100). Arrows indicate infiltrated inflammatory cells. (B) Methenamine silver-stained lung sections (magnification, ×200). Pneumocystis cysts are seen as black dots or clusters.
VitD3-PMQ therapy decreases the levels of proinflammatory cytokines.
To investigate the mechanism of action of VitD3-PMQ, levels of proinflammatory cytokines, including tumor necrosis factor alpha (TNF-α,) gamma interferon (IFN-γ), and interleukin-6 (IL-6), in bronchoalveolar lavage (BAL) fluids of various groups of mice were measured by enzyme-linked immunosorbent assay (ELISA). The TNF-α level in BAL fluids of uninfected mice was very low (0.36 ± 0.6 pg/ml), but it was greatly increased (45 ± 3.7 pg/ml) in those of untreated mice with PCP, indicating that P. murina infection profoundly induced the production of TNF-α in the lung. Treatment of P. murina-infected mice with TMP-SMX reduced TNF-α levels to 30 ± 7.2 pg/ml. PMQ was more effective than TMP-SMX in reducing TNF-α levels (15.1 ± 4.0 pg/ml). VitD3-PMQ treatment was even more effective and reduced TNF-α levels to 2.8 ± 2.1 pg/ml, very close to those of uninfected mice (Fig. 3).
FIG 3.
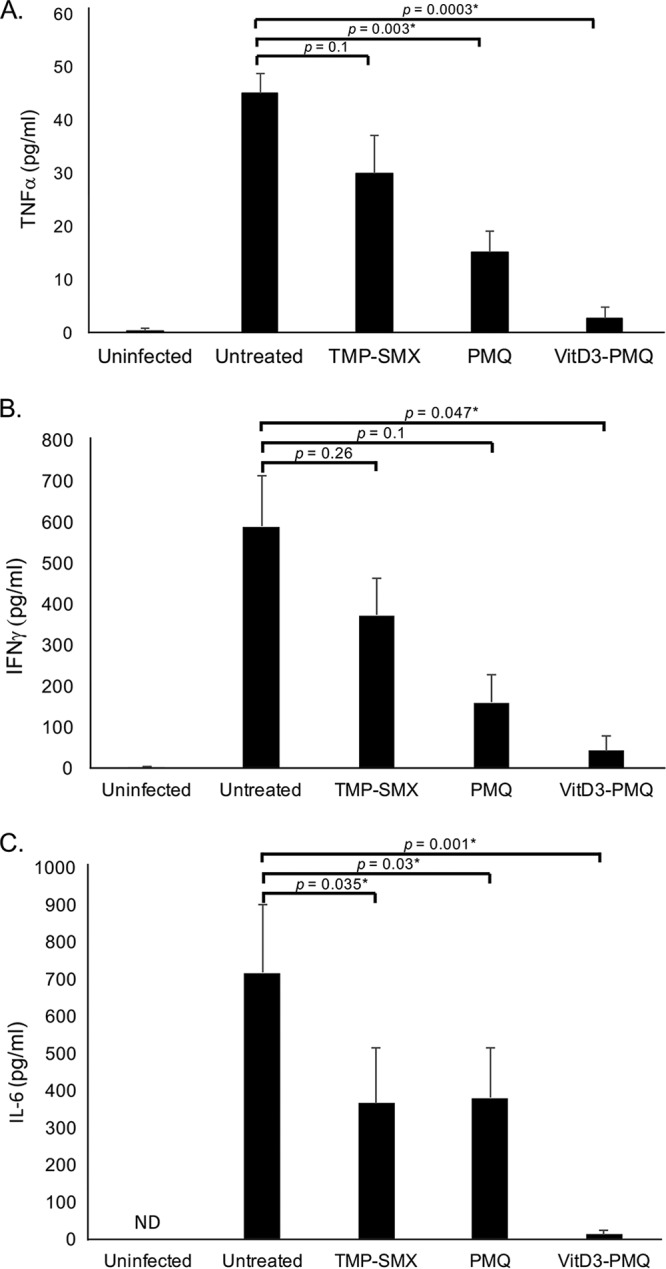
Cytokine levels in BAL fluids. The first 2 ml of BAL fluid was used for ELISA. (A) TNF-α levels in BAL fluids of immunosuppressed uninfected mice, mice with PCP, and mice with PCP treated with TMP-SMX, PMQ, or VitD3-PMQ. (B) IFN-γ levels in BAL fluids of various mouse groups. (C) IL-6 levels in BAL fluids of various mouse groups. ND, not detected. *, P < 0.05.
A similar pattern was observed for IFN-γ levels. P. murina infection caused an increase in IFN-γ levels in BAL fluids of more than 160-fold (from 3.6 ± 1.0 to 588.5 ± 125.0 pg/ml). TMP-SMX, PMQ, and VitD3-PMQ treatments reduced IFN-γ levels to 372.6 ± 90.0 pg/ml, 160.0 ± 68.5 pg/ml, and 42.6 ± 36.6 pg/ml, respectively.
IL-6 was not detectable in BAL fluids of uninfected mice. P. murina infection caused a dramatic increase in IL-6 levels in BAL fluids (716.4 ± 183.5 pg/ml). TMP-SMX and PMQ were equally effective in reducing IL-6 levels (366.6 ± 150.2 pg/ml and 380.6 ± 134.4 pg/ml, respectively). VitD3-PMQ treatment greatly reduced IL-6 levels (13.7 ± 10.1 pg/ml). These data indicated that VitD3-PMQ was much more effective than TMP-SMX and PMQ in reducing the levels of proinflammatory cytokines in the lungs of P. murina-infected mice.
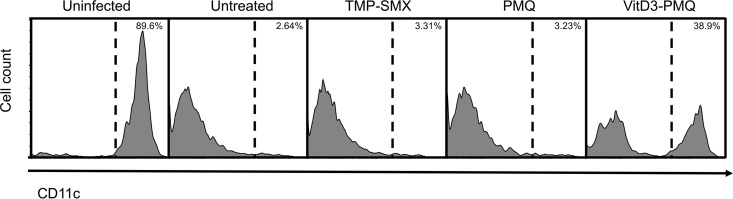
VitD3-PMQ therapy increases the number of AMs.
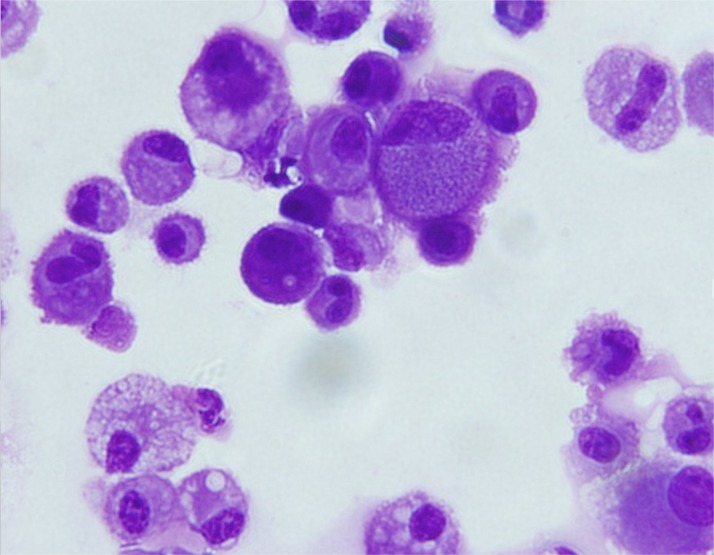
We have previously shown that P. murina infection causes a decrease in the number of alveolar macrophages (AMs) (8). As AMs are CD11c+, changes in the number of CD11c+ cells in BAL fluids before and after treatment were measured by flow cytometry. The results showed that 88.9% ± 4.61% of cells in the BAL fluids of uninfected mice were CD11c+ cells. Very few CD11c+ cells were detected in the BAL fluids of untreated mice with PCP and in those treated with TMP-SMX or PMQ (3.22% ± 0.72%, 2.75% ± 1.36%, and 3.36% ± 0.32%, respectively). However, a significant increase in the number of CD11c+ cells (39.6% ± 2.79%) was observed in BAL fluids of VitD3-PMQ treated mice (Fig. 4). Since dendritic cells are also CD11c+, CD11c+ cells in BAL fluids were isolated using biotin–anti-mouse CD11c antibody and antibiotin magnetic sorting microbeads and examined by light microscopy. The results showed that the great majority of the isolated cells had a typical morphology of macrophages (Fig. 5). These results suggest that VitD3-PMQ treatment caused an increase in the number of alveolar macrophages.
FIG 4.
Histograms of CD11c+ cells from BAL fluids. Immunosuppressed mice were infected with P. murina for 8 weeks and then treated with TMP-SMX, PMQ, or VitD3-PMQ for 9 days. BAL fluid cells of mice in various groups were collected, stained with Alexa Fluor 647-labeled anti-CD11c antibodies, and then analyzed by flow cytometry. The percentage of CD11c+ cells in each group of mice is indicated.
FIG 5.
Morphology of isolated CD11c+ cells. BAL fluid cells from mice with PCP treated with VitD3-PMQ for 9 days were collected. CD11c+ cells were isolated using biotin–anti-mouse CD11c antibody and antibiotin magnetic microbeads. Isolated cells were cytospun on slides, stained with Giemsa stain, and examined by light microscopy.
VitD3-PMQ therapy affects the expression of stress-related genes.
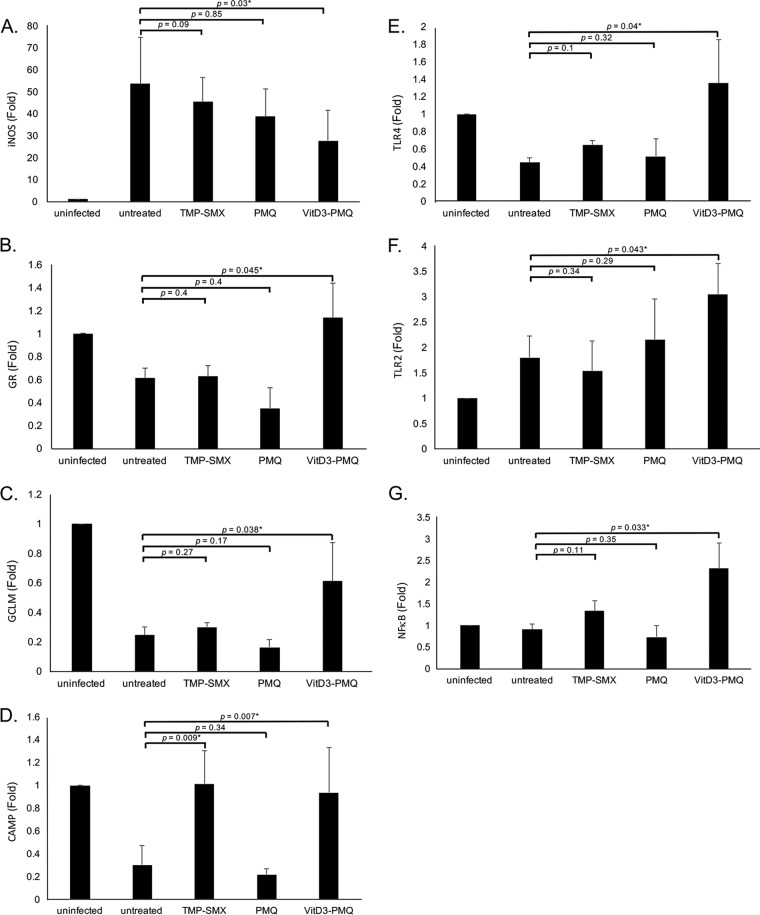
Since P. murina infection is a stress to cells, mRNA levels of several stress-related genes, including those encoding inducible nitric oxide synthase (iNOS), glutathione reductase (GR), glutamate-cysteine ligase regulatory subunit (GCLM), cathelicidin antimicrobial peptide (CAMP), Toll-like receptor 4 (TLR4), TLR2, and NF-κB, in lung cells were determined by SYBR green quantitative real-time PCR (qRT-PCR) to determine whether VitD3-PMQ treatment relieves the stress.
Compared to the control (uninfected mice), P. murina infection caused a 50-fold increase in iNOS mRNA levels in lung cells. Both TMP-SMX and PMQ treatments slightly decreased iNOS mRNA levels (20% and 24% decreases, respectively). VitD3-PMQ treatment decreased iNOS mRNA levels by 50% compared to those in untreated mice with PCP (Fig. 6A).
FIG 6.
Expression levels of stress genes. Thirty milligrams of lung tissue from each mouse in various treatment groups was placed in 600 μl RLT buffer (RNeasy minikit; Qiagen) and homogenized. Total RNA was isolated and reverse transcribed to cDNA. mRNA levels of iNOS (A), GR (B), GCLM (C), CAMP (D), TLR2 (E), TLR4 (F), and NF-κB (G) were determined by SYBR green qRT-PCR. Data are presented as fold change (mean ± SD). *, P < 0.05.
For glutathione reductase (GR), P. murina infection caused a 40% decrease in its mRNA levels compared to those in uninfected mice. TMP-SMX treatment did not decrease GR mRNA levels in lung cells of P. murina-infected mice. PMQ treatment caused a 70% decrease in GR mRNA levels, whereas VitD3-PMQ treatment increased its levels to those in uninfected mice (Fig. 6B).
P. murina infection was found to dramatically (80%) decrease the expression of glutamate-cysteine ligase regulatory subunit (GCLM). TMP-SMX treatment slightly increased and PMQ treatment slightly decreased its mRNA levels in lung cells of P. murina-infected mice. However, VitD3-PMQ treatment increased the levels by approximately 50% compared to those in untreated mice with PCP (Fig. 6C).
P. murina infection also dramatically (80%) decreased the expression of cathelicidin antimicrobial peptide (CAMP). PMQ treatment did not change its mRNA levels in lung cells of P. murina-infected mice. TMP-SMX treatment restored its expression to normal levels (uninfected), and VitD3-PMQ treatment increased its expression to very close (82%) to that in uninfected mice (Fig. 6D).
For TLR4, P. murina infection caused a 60% decrease in its expression. TMP-SMX treatment resulted in an approximately 20% increase in its expression. TLR4 mRNA levels in PMQ-treated mice were slightly higher than those in untreated mice. VitD3-PMQ treatment recovered TLR4 expression to a level approximately 10% higher than that in uninfected mice (Fig. 6E).
P. murina infection was found to increase TLR2 expression by about 35% compared to that in uninfected mice. TMP-SMX treatment resulted in an approximately 10% decrease in TLR2 expression compared to that in untreated mice with PCP. PMQ treatment had no effect on its expression, whereas VitD3-PMQ treatment increased TLR2 expression by 30% compared to the levels in untreated mice with PCP (Fig. 6F).
NF-κB mRNA levels in lung cells from uninfected and P. murina-infected mice were about the same. TMP-SMX treatment slightly (20%) increased its levels, whereas PMQ treatment decreased the levels by about 30% compared to the level in untreated mice. VitD3-PMQ treatment increased NF-κB mRNA levels by approximately 60% relative to the levels in untreated mice with PCP (Fig. 6G).
VitD3-PMQ therapy upregulates the expression of autophagy genes.
To determine whether P. murina infection and treatment of the infection affect normal cellular functions, mRNA levels of two representative autophagy genes, encoding ATG5 and BCLN1, were determined. P. murina infection was found to decrease mRNA levels of both genes in lung cells by approximately 50% compared to those in uninfected mice. TMP-SMX treatment slightly increased ATG5 gene expression but had no effect in BCLN1 gene expression in lung cells of P. murina-infected mice. PMQ treatment decreased the expression of ATG5 by approximately 30% and that of BCLN1 by 50%, whereas VitD3-PMQ treatment increased the expression of both the ATG5 and BCLN1 genes by approximately 25%, compared to those in untreated mice with PCP (Fig. 7A and B).
FIG 7.
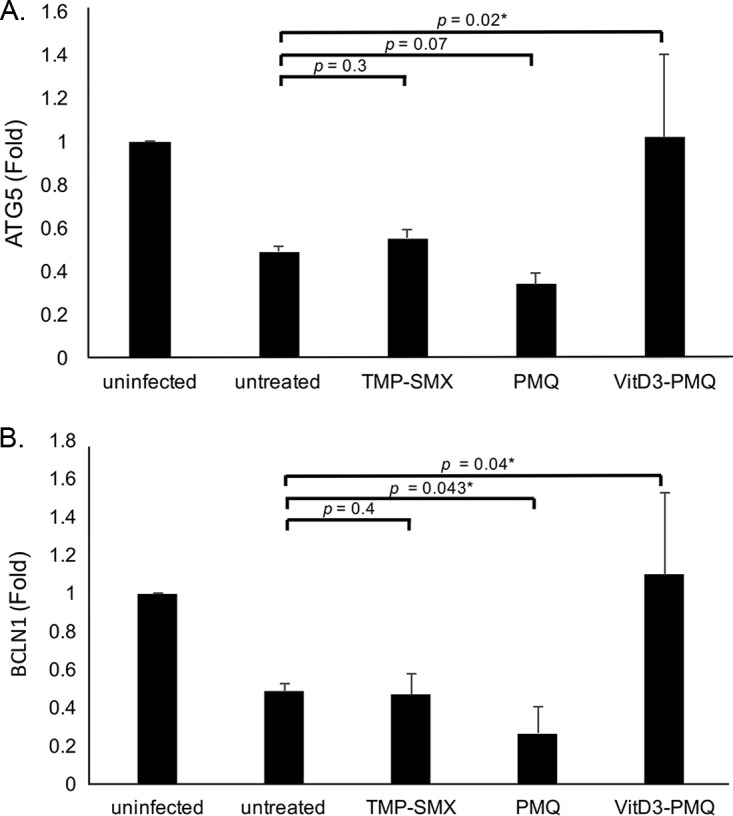
Expression levels of autophagy markers. The converted cDNA as described for Fig. 6 was used to determine mRNA levels of ATG5 (A) and BCN1 (B). Data are presented as fold change (mean ± SD). *, P < 0.05.
DISCUSSION
A major symptom of PCP is severe inflammation in the lung. Our observations that P. murina infection greatly increased the levels of proinflammatory cytokines (TNF-α, IFN-γ, and IL-6) and reduced the levels of antimicrobial peptides (cathelicidin) and proteins (GR and GCLM) related to antioxidation and autophagy (ATG5 and BCLN1) implicate these actions as mechanisms of pathogenesis of PCP. Therefore, clearing P. murina infection is critical in PCP therapy. We had previously found that PMQ alone was not completely effective in the clearance of P. murina infection, but the combination of VitD3 and PMQ (VitD3-PMQ) was as effective as TMP-SMX (7). In this study, we investigated the mechanisms by which VitD3 enhances the efficacy of PMQ in PCP therapy. Major efforts were devoted to comparing the effects of PMQ alone and the VitD3-PMQ combination in PCP therapy. The results showed that treatment of mice having PCP with VitD3-PMQ increased the number of alveolar macrophages, enhanced body weight recovery, and reduced P. murina organism load, levels of proinflammatory cytokines, and severity of inflammation in the lungs. VitD3-PMQ also decreased the expression of iNOS and increased the expression of GR, GCLM, CAMP, TLR4, TLR2, NF-κB, ATG5, and BCLN1 in lung cells of mice with PCP. Since these changes were not significantly observed in mice with PCP treated with PMQ alone, they were attributed to the action of vitamin D.
Vitamin D has been shown to reduce the expression of TNF-α by decreasing NF-κB-p65 mRNA expression and increasing IκB-α mRNA expression (9). In a recent study of 118 women, those who were deficient in vitamin D had higher serum levels of TNF-α (10). These observations indicate that vitamin D negatively regulates the expression of TNF-α, as we have observed in this study.
We also found that the IFN-γ level was very low in BAL fluids from uninfected mice but was very high in those from P. murina-infected mice (Fig. 3B). This increase in IFN-γ levels is likely a host defense mechanism against P. murina infection, as treatment of mice having PCP with TMP-SMX, PMQ, or VitD3-PMQ reduced their IFN-γ levels (Fig. 3B). Although IFN-γ can enhance the ability of alveolar macrophages to kill P. murina (11), excessive IFN-γ is harmful, as it is proinflammatory. Similar to what we observed in this study, vitamin D was found to significantly attenuate lipopolysaccharide (LPS)-induced elevation of IFN-γ in mice (12). Furthermore, treatment with vitamin D resulted in significant downregulation of IFN-γ in patients with recent-onset type 1 diabetes (13). Among the 3 regimens (TMP-SMX, PMQ, and VitD3-PMQ) tested, VitD3-PMQ was most effective in reducing IFN-γ levels (Fig. 3B).
IL-6 is another proinflammatory cytokine. Serum IL-6 levels were found to be higher in AIDS patients who died of PCP than in those who survived PCP (14). Non-AIDS patients with PCP also had significantly higher levels of IL-6 in their BAL fluids than non-PCP patients (15, 16). Vitamin D has been shown to decrease IL-6 production by human renal proximal tubular epithelial cells (17) and LPS-stimulated human periodontal ligament cells (18). These observations are consistent with our finding that treatment of mice having PCP with VitD3-PMQ greatly reduced the levels of IL-6 in their BAL fluids (Fig. 3C).
Another difference between PMQ alone and VitD3-PMQ in the treatment of PCP is the effect on the expression of iNOS. P. murina infection greatly increased iNOS mRNA levels in AMs (Fig. 6A). Shellito et al. found that both iNOS mRNA and protein levels are increased during PCP but that elevated iNOS levels do not result in clearance of P. murina infections (19). Based on this observation, they postulated that both iNOS and nitric oxide play no role in defense against P. murina infection. We had found that P. murina is susceptible to killing by nitric oxide. The reason why elevated iNOS levels do not correlate with P. murina clearance is that iNOS dimerization, which is required for production of a functional iNOS, is defective during PCP because of decreased levels of calmodulin in AMs (20). As a result, the production of nitric oxide is diminished. In this study, we found that treatment of mice having PCP with PMQ alone reduced iNOS mRNA levels in lung tissue and that VitD3-PMQ treatment caused a further reduction in iNOS mRNA levels (Fig. 6A). This is likely due to P. murina clearance by these treatments, thus eliminating the stimulation in iNOS expression.
Glutathione reductase (GR) converts glutathione disulfide (GSSG) to glutathione (GSH) (21), which scavenges hydroxyl radicals, reactive oxygen species (ROS), and various electrophiles, thus preventing oxidative stress in cells (22). GSH deficiency is implicated in many diseases, such as cardiovascular diseases, immune disorders, diseases related to aging, and diabetes (22–28). AIDS patients with PCP were found to have elevated levels of GSSG in BAL fluids (29), suggesting that patients with PCP suffer from ROS toxicity in their lungs. Our finding that VitD3-PMQ treatment resulted in greatly increased expression of GR is an indication that VitD3 promotes the conversion of GSSR to GSH, which in turn reduces the oxidative stress caused by P. murina infection. As PMQ alone did not affect GR expression (Fig. 6B), the GR upregulation seen in mice with PCP treated with VitD3-PMQ is very likely due to the action of VitD3. This result agrees with that of a previous study showing that vitamin D upregulates the expression of GR and decreases ROS levels in U937 cells (30).
The glutamate-cysteine ligase (GCL) also plays a key role in the production of GSH. It consists of two subunits, a catalytic subunit and a modifier subunit. The GCLM gene encodes the modifier subunit of GCL. GCL condenses l-glutamate and l-cysteine to become gamma-glutamyl cysteine, which is the precursor of glutathione. The effect of P. murina infection on the expression of GCL or the production of gamma-glutamyl cysteine has never been reported. In this study, we discovered that P. murina infection dramatically decreased the levels of GCLM. Treatment of PCP with TMP-SMX slightly increased its levels. PMQ had no effect, but VitD3-PMQ treatment returned GCLM levels close to those in uninfected mice (Fig. 6C).
A major function of vitamin D is inducing the production of antimicrobial peptides such as β-defensin and cathelicidin (31, 32). Patients with tuberculosis (TB) infection who are deficient in vitamin D produce very low levels of cathelicidin LL37 (33), and Camp gene knockout is found to block the antimicrobial effect of vitamin D (34). In this study, we found that P. murina infection greatly suppressed the expression of cathelicidin and that treatment with TMP-SMX or VitD3-PMQ restored its expression (Fig. 6D).
It has been shown that impaired recognition through TLR4 is responsible for exacerbated PCP (35). We had previously shown that mouse AMs respond to P. murina organisms through TLR2, leading to nuclear translocation of NF-κB and production of proinflammatory cytokines TNF-α and MIP-2 (36). In this study, we found that VitD3-PMQ, but not TMP-SMX or PMQ, significantly increased the expression of both TLR2 and TLR4. Reports on the effect of vitamin D on the expression of TLR4 have been variable. Some studies showed that vitamin D suppresses the expression of TLR4 and thus alleviates inflammation (37–41), but others demonstrated that vitamin D has no effect on the expression of TLR4 (42, 43) as it is normally constitutively expressed (44).
In this study, we found for the first time that P. murina infection caused a dramatic decrease in the expression of ATG5 and BCLN1 (Fig. 7). These two proteins play a major role in autophagy, which is a critical process in cell metabolism. Autophagy is mediated by a membrane trafficking pathway in which autophagosomes engulf cytoplasmic materials to form vesicles that are then transported to the lysosome for degradation (45). ATG5 is involved in the early stages of autophagosome formation (46), and BCLN1 functions as a scaffolding protein and interacts with the PI3KC3/VPS34 lipid kinase. This kinase complex produces phosphatidylinositol-3-phosphate (PI3P), which is a critical regulator for autophagy induction and membrane trafficking (47).
PMQ almost completely suppressed the expression of both ATG5 and BCLN1. VitD3-PMQ treatment restored their expression in mice with PCP to a level very close to that in uninfected mice. TMP-SMX treatment had no significant effect on restoring the expression of these two proteins (Fig. 7). These results suggest that restoration of the expression of these two proteins in mice with PCP treated with VitD3-PMQ was due not to the clearance of P. murina organisms but to the action of vitamin D. This hypothesis is supported by previous studies showing that vitamin D upregulates the expression of both ATG5 and BCN1 in monocytes and macrophages from healthy individuals or patients with tuberculosis (48). This action was found to be mediated by cathelicidin (49, 50). Vitamin D has also been shown to induce autophagy in human macrophages through a phosphatidylinositol-3-kinase-, ATG5-, and beclin-1-dependent mechanism and significantly inhibits HIV-1 replication in a dose-dependent manner (51, 52).
Based on the results of this study, we postulate that the action of vitamin D is centered on enhancing self-protection of the host. Increased production of cathelicidin and recovery of AMs would lead to a decreased P. murina organism load. Cathelicidin also restores the production of ATG5 and BCLN1, thus activating autophagy to recycle cytoplasmic materials. Decreased expression of iNOS would lead to reduced production of nitric oxide, and the recovery of the expression of GCLM and GR would decrease the levels of reactive oxygen species (ROS); these two actions would reduce cell damage. Although increased expression of TLR2 and TLR4 may trigger inflammation, the diminished production of proinflammatory cytokines would alleviate lung damage. We believe that the combination of these functions makes vitamin D an effective supplementary therapy for PCP.
It should be noted that mice used in this study had very severe PCP (they were infected by P. murina for 8 weeks and had body weights less than 20 g). Most regimens, including TMP-SMX, often fail, but VitD3-PMQ effectively cured them. It has been predicted that vitamin D can affect the expression of more than 200 genes (53). Whether genes other than those investigated in this study also contribute to host protection during PCP remains to be investigated. Although we have clearly demonstrated anti-infective and anti-inflammatory functions of vitamin D in mice, it is not known whether similar effects would occur in humans with PCP. It has been reported that 65% (63 of 97) of HIV-infected patients are deficient in vitamin D and that vitamin D supplementation for 24 weeks significantly increased the number of CD4+ cells in these patients (54). This observation suggests that vitamin D supplementation would help resolve PCP in humans. To translate the findings of this study to clinical applications, an epidemiological study to determine whether there is a correlation between serum vitamin D levels and the severity of PCP in humans should be conducted. The dose of vitamin D3 required for adjunctive therapy of PCP also needs to be determined. We used 300 IU/kg/day for mice, but it is unknown whether this dose is appropriate for humans. Although the major action of vitamin D in PCP supplemental therapy is anti-inflammation, it is unknown whether vitamin D can replace a corticosteroid, which is usually administered to reduce lung inflammation. PMQ in combination with clindamycin has been used to treat patients with mild to moderately severe PCP (55). As clindamycin caused severe gastrointestinal problems in mice when it was administered orally, we were unable to compare the effectiveness of VitD3-PMQ to that of clindamycin-PMQ in PCP therapy. Whether VitD3-PMQ is superior to clindamycin-PMQ for treatment of PCP patients is also unknown. All of these questions must be answered before the VitD3-PMQ combination can be tried in patients with PCP.
MATERIALS AND METHODS
Mouse model of PCP.
Female C57BL/6 mice (approximately 20 g each) obtained from Envigo (Indianapolis, IN) were used. The use of animals in this study was approved by the Indiana University Animal Care and Use Committee. Mice were immunosuppressed by intraperitoneal injection of 0.3 mg anti-L3T4 monoclonal antibody (MAb) (clone GK1.5; Envigo, Indianapolis, IN) once a week for the entire period of the study to deplete CD4+ cells. A single injection of 0.3 mg anti-L3T4 MAb had been shown to reduce the number of CD4+ cells by 95% in 5 days (56). Immunosuppressed mice were inoculated with 2 × 106 P. murina organisms in 30 μl phosphate-buffered saline (PBS) by transtracheal injection 1 week after the initiation of immunosuppression as previously described (8). Tetracycline (0.74 g/liter) was added to their drinking water to prevent bacterial infections.
Drug treatment.
A total of 40 C57BL/6 mice were used. These mice were divided into the following 5 groups (8 mice per group): immunosuppressed and uninfected, P. murina infected and untreated, P. murina infected and TMP-SMX treated, P. murina infected and PMQ treated, and P. murina infected and VitD3-PMQ treated. To investigate the efficacy of VitD3-PMQ for severe PCP, immunosuppressed mice were infected with P. murina for 8 weeks before they were treated with TMP-SMX (TMP, 50 mg/kg/day; SMX, 250 mg/kg/day), PMQ (5 mg/kg/day), or VitD3-PMQ (VitD3, 300 IU/kg/day; PMQ, 5 mg/kg/day). TMP-SMX (Septra) was acquired from Hi-Tech Pharmacal (Amityville, NY). VitD3 (cholecalciferol) and PMQ were purchased from Sigma-Aldrich (St. Louis, MO).
BAL, examination of AMs, and preparation of lung sections.
At 10 days after initiation of treatment, all mice were sacrificed and lavaged to obtain bronchoalveolar lavage (BAL) fluids. Each mouse was lavaged with 1 ml sterile saline at a time through an intratracheal catheter after it had been anesthetized by intramuscular injection of 100 μl ketamine cocktail (ketamine, 17.2 mg/ml; xylazine, 0.475 mg/ml; acepromazine, 0.238 g/ml) as described previously (8). As approximately 0.7 ml of BAL fluid was recovered from 1 ml lavage, a total of 15 lavages were performed to obtain 10 ml of BAL fluid from each mouse. BAL fluid from each mouse was treated as an individual sample. The first 2 ml of the BAL fluid was placed in one tube, and the remaining 8 ml was saved in a separate tube. Cells in the two tubes were pelleted by centrifugation at 300 × g for 10 min and then combined by resuspending them in 1 ml PBS containing 0.5% bovine serum albumin (BSA). CD11c+ AMs were isolated from this cell suspension using biotin–anti-mouse CD11c antibody (130-101-929; Miltenyi Biotech) and antibiotin magnetic sorting microbeads (130-090-485; Miltenyi Biotech) as described previously (57). A portion of the lung was formalin fixed, paraffin embedded, and sectioned. Lung sections were stained with H&E and methenamine silver as described previously (5, 7, 57). As the focus of this study was effect of VitD3-PMQ on lung inflammation and organism load, other markers, such as serum levels of 1,3-beta-d-glucan, were not evaluated.
qRT-PCR for analysis of gene expression.
Total RNA was isolated from the lung tissue of each mouse using the RNeasy minikit (Qiagen) according to the manufacturer's instructions. RNA concentration and purity were determined by spectrophotometry. cDNA was synthesized from 200 ng of each total RNA sample using the iScript cDNA synthesis kit (Bio-Rad) in a total reaction volume of 20 μl at 25°C for 5 min, 46°C for 20 min, and 95°C for 2 min. Two microliters of each cDNA product was used for quantitative real-time PCR (qRT-PCR), which was performed in Rotor-Gene Q (Qiagen) with the Rotor-Gene SYBR green PCR kit (204074; Qiagen) under the following conditions: 95°C for 5 min followed by 40 cycles of 15 s at 95°C and 30 s at 60°C. PCR primers used for amplification of genes encoding iNOS, GR, GCLM, CAMP, TLR2, TLR4, NF-κB, ATG5, and BCLN1 are listed in Table 1. These genes were examined because they are involved in antimicrobial, inflammatory, antioxidation, or autophagy responses. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNAs were assayed in an identical manner to serve as the internal control. Data are shown as fold change relative to the control group.
TABLE 1.
PCR primer sets used in this study
| Namea | Sequence or designation | Amplicon size (bp) |
|---|---|---|
| GAPDH For | 5′-TGTGTCCGTCGTGGATCTGA-3′ | 161 |
| GAPDH Rev | 5′-CCTGCTTCACCACCTTCTTGA-3′ | |
| INOS For | 5′-TGAACTTGAGCGAGGAGCA-3′ | 68 |
| INOS Rev | 5′-TTCATGATAACGTTTCTGGCTCT-3′ | |
| GR primer mix | QuantiTech primer assay no. QT00162344 | |
| GCLM primer mix | QuantiTech primer assay no. QT00174300 | |
| CAMP For | 5′-GCTGTGGCGGTCACTATCAC-3′ | 116 |
| CAMP Rev | 5′-TGTCTAGGGACTGCTGGTTGA-3′ | |
| TLR2 For | 5′-GCAAACGCTGTTCTGCTCAG-3′ | 231 |
| TLR2 Rev | 5′-AGGCGTCTCCCTCTATTGTATT-3′ | |
| TLR4 For | 5′-CGCTTTCACCTCTGCCTTCACTACAG-3′ | 109 |
| TLR4 Rev | 5′-ACACTACCACAATAACCTTCCGGCTC-3′ | |
| NFκB For | 5′-TCCCCACAGTTGCCTTCAC-3′ | 222 |
| NFκB Rev | 5′-GAGCGGCGTCTTGCCTTTA-3′ | |
| ATG5 For | 5′-AGCCAGGTGATGATTCACGG-3′ | 113 |
| ATG5 Rev | 5′-GGCTGGGGGACAATGCTAA-3′ | |
| BCLN1 For | 5′-ATGGAGGGGTCTAAGGCGTC-3′ | 197 |
| BCLN1 Rev | 5′-TCCTCTCCTGAGTTAGCCTCT-3′ |
For: forward; Rev: reverse.
ELISA for mouse TNF-α, IL-6, and IFN-γ in BAL fluid.
The supernatant of the first 2 ml of each BAL fluid was filtered through a 0.2-μm syringe filter (431224; Corning) and analyzed for TNF-α, IL-6, and IFN-γ levels using the following ELISA kits from BioLegend according to the manufacturer's instructions: TNF-α, catalog no. 430907; IL-6, catalog no. 431307; and IFN-γ, catalog no. 430807. ELISA plates were read using the Teacan Infinite 200Pro plate reader.
Statistical analysis.
An unpaired two-tailed Student t test was performed to compare the difference between treatment and control (untreated) groups or the efficacy of VitD3-PMQ to that of TMP-SMX or PMQ alone in PCP therapy. Data presented are means ± standard deviation (SD). A P value of <0.05 is considered significant.
ACKNOWLEDGMENT
This study was supported by grant R21AI122837 from the National Institutes of Health.
REFERENCES
- 1.CDC. 2014. Pneumocystis pneumonia statistics. http://www.cdc.gov/fungal/diseases/pneumocystis-pneumonia/statistics.html.
- 2.Hemstreet BA, Page RL II. 2006. Sulfonamide allergies and outcomes related to use of potentially cross-reactive drugs in hospitalized patients. Pharmacotherapy 26:551–557. doi: 10.1592/phco.26.4.551. [DOI] [PubMed] [Google Scholar]
- 3.Gordin FM, Simon GL, Wofsy CB, Mills J. 1984. Adverse reactions to trimethoprim-sulfamethoxazole in patients with the acquired immunodeficiency syndrome. Ann Intern Med 100:495–499. doi: 10.7326/0003-4819-100-4-495. [DOI] [PubMed] [Google Scholar]
- 4.Zhang C, Lei GS, Shao S, Jung HW, Durant PJ, Lee CH. 2012. Accumulation of myeloid-derived suppressor cells in the lungs during Pneumocystis pneumonia. Infect Immun 80:3634–3641. doi: 10.1128/IAI.00668-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lei GS, Zhang C, Shao S, Jung HW, Durant PJ, Lee CH. 2013. All-trans retinoic acid in combination with primaquine clears pneumocystis infection. PLoS One 8:e53479. doi: 10.1371/journal.pone.0053479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee JS, Newman RA, Lippman SM, Huber MH, Minor T, Raber MN, Krakoff IH, Hong WK. 1993. Phase I evaluation of all-trans-retinoic acid in adults with solid tumors. J Clin Oncol 11:959–966. doi: 10.1200/JCO.1993.11.5.959. [DOI] [PubMed] [Google Scholar]
- 7.Lei GS, Zhang C, Zimmerman MK, Lee CH. 2015. Vitamin D as supplemental therapy for Pneumocystis pneumonia. Antimicrob Agents Chemother 60:1289–1297. doi: 10.1128/AAC.02607-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lasbury ME, Durant PJ, Bartlett MS, Smith JW, Lee CH. 2003. Correlation of organism burden and alveolar macrophage counts during infection with Pneumocystis carinii and recovery. Clin Diagn Lab Immunol 10:293–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Al-Rasheed NM, Al-Rasheed NM, Bassiouni YA, Hasan IH, Al-Amin MA, Al-Ajmi HN, Mohamad RA. 2015. Vitamin D attenuates pro-inflammatory TNF-alpha cytokine expression by inhibiting NF-kB/p65 signaling in hypertrophied rat hearts. J Physiol Biochem 71:289–299. doi: 10.1007/s13105-015-0412-1. [DOI] [PubMed] [Google Scholar]
- 10.Azizieh F, Alyahya KO, Raghupathy R. 2016. Association between levels of vitamin D and inflammatory markers in healthy women. J Inflamm Res 9:51–57. doi: 10.2147/JIR.S103298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Downing JF, Kachel DL, Pasula R, Martin WJ II. 1999. Gamma interferon stimulates rat alveolar macrophages to kill Pneumocystis carinii by l-arginine- and tumor necrosis factor-dependent mechanisms. Infect Immun 67:1347–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou Y, Chen YH, Fu L, Yu Z, Xia MZ, Hu XG, Wang H, Xu DX. 3 January 2017. Vitamin D3 pretreatment protects against lipopolysaccharide-induced early embryo loss through its anti-inflammatory effects. Am J Reprod Immunol doi: 10.1111/aji.12620. [DOI] [PubMed] [Google Scholar]
- 13.Ysmail-Dahlouk L, Nouari W, Aribi M. 2016. 1,25-dihydroxyvitamin D3 down-modulates the production of proinflammatory cytokines and nitric oxide and enhances the phosphorylation of monocyte-expressed STAT6 at the recent-onset type 1 diabetes. Immunol Lett 179:122–130. doi: 10.1016/j.imlet.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 14.Sun J, Su J, Xie Y, Yin MT, Huang Y, Xu L, Zhou Q, Zhu B. 2016. Plasma IL-6/IL-10 ratio and IL-8, LDH, and HBDH level predict the severity and the risk of death in AIDS patients with Pneumocystis pneumonia. J Immunol Res 2016:1583951. doi: 10.1155/2016/1583951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chou CW, Lin FC, Tsai HC, Chang SC. 2013. The importance of pro-inflammatory and anti-inflammatory cytokines in Pneumocystis jirovecii pneumonia. Med Mycol 51:704–712. doi: 10.3109/13693786.2013.772689. [DOI] [PubMed] [Google Scholar]
- 16.Iriart X, Witkowski B, Courtais C, Abbes S, Tkaczuk J, Courtade M, Cassaing S, Fillaux J, Blancher A, Magnaval JF, Pipy B, Berry A. 2010. Cellular and cytokine changes in the alveolar environment among immunocompromised patients during Pneumocystis jirovecii infection. Med Mycol 48:1075–1087. doi: 10.3109/13693786.2010.484027. [DOI] [PubMed] [Google Scholar]
- 17.Chung BH, Kim BM, Doh KC, Cho ML, Kim KW, Yang CW. 2017. Protective effect of 1α,25-dihydroxyvitamin D3 on effector CD4+ T cell induced injury in human renal proximal tubular epithelial cells. PLoS One 12:e0172536. doi: 10.1371/journal.pone.0172536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nebel D, Svensson D, Arosenius K, Larsson E, Jonsson D, Nilsson BO. 2015. 1α,25-Dihydroxyvitamin D3 promotes osteogenic activity and downregulates proinflammatory cytokine expression in human periodontal ligament cells. J Periodont Res 50:666–673. doi: 10.1111/jre.12249. [DOI] [PubMed] [Google Scholar]
- 19.Shellito JE, Kolls JK, Olariu R, Beck JM. 1996. Nitric oxide and host defense against Pneumocystis carinii infection in a mouse model. J Infect Dis 173:432–439. doi: 10.1093/infdis/173.2.432. [DOI] [PubMed] [Google Scholar]
- 20.Lasbury ME, Liao CP, Hage CA, Durant PJ, Tschang D, Wang SH, Zhang C, Lee CH. 2011. Defective nitric oxide production by alveolar macrophages during Pneumocystis pneumonia. Am J Respir Cell Mol Biol 44:540–547. doi: 10.1165/rcmb.2009-0367OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deponte M. 2013. Glutathione catalysis and the reaction mechanisms of glutathione-dependent enzymes. Biochim Biophys Acta 1830:3217–3266. doi: 10.1016/j.bbagen.2012.09.018. [DOI] [PubMed] [Google Scholar]
- 22.Townsend DM, Tew KD, Tapiero H. 2003. The importance of glutathione in human disease. Biomed Pharmacother 57:145–155. doi: 10.1016/S0753-3322(03)00043-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qin L, Bartlett H, Griffiths HR, Eperjesi F, Armstrong RA, Gherghel D. 2011. Macular pigment optical density is related to blood glutathione levels in healthy individuals. Invest Ophthalmol Vis Sci 52:5029–5033. doi: 10.1167/iovs.11-7240. [DOI] [PubMed] [Google Scholar]
- 24.Zampagni M, Wright D, Cascella R, D'Adamio G, Casamenti F, Evangelisti E, Cardona F, Goti A, Nacmias B, Sorbi S, Liguri G, Cecchi C. 2012. Novel S-acyl glutathione derivatives prevent amyloid oxidative stress and cholinergic dysfunction in Alzheimer disease models. Free Radic Biol Med 52:1362–1371. doi: 10.1016/j.freeradbiomed.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 25.Droge W. 2005. Oxidative stress and ageing: is ageing a cysteine deficiency syndrome? Philos Trans R Soc Lond B Biol Sci 360:2355–2372. doi: 10.1098/rstb.2005.1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sekhar RV, McKay SV, Patel SG, Guthikonda AP, Reddy VT, Balasubramanyam A, Jahoor F. 2011. Glutathione synthesis is diminished in patients with uncontrolled diabetes and restored by dietary supplementation with cysteine and glycine. Diabetes Care 34:162–167. doi: 10.2337/dc10-1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morris D, Khurasany M, Nguyen T, Kim J, Guilford F, Mehta R, Gray D, Saviola B, Venketaraman V. 2013. Glutathione and infection. Biochim Biophys Acta 1830:3329–3349. doi: 10.1016/j.bbagen.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 28.Darmaun D, Smith SD, Sweeten S, Hartman BK, Welch S, Mauras N. 2008. Poorly controlled type 1 diabetes is associated with altered glutathione homeostasis in adolescents: apparent resistance to N-acetylcysteine supplementation. Pediatr Diabetes 9:577–582. doi: 10.1111/j.1399-5448.2008.00436.x. [DOI] [PubMed] [Google Scholar]
- 29.Adams JD, Jaresko GS, Louie SG, Klaidman LK, Kennedy D, Sharma O, Boylen CT. 1993. Pneumocystis carinii pneumonia in HIV infected patients: effects of the diseases on glutathione and glutathione disulfide. J Med 24:337–352. [PubMed] [Google Scholar]
- 30.Jain SK, Micinski D. 2013. Vitamin D upregulates glutamate cysteine ligase and glutathione reductase, and GSH formation, and decreases ROS and MCP-1 and IL-8 secretion in high-glucose exposed U937 monocytes. Biochem Biophys Res Commun 437:7–11. doi: 10.1016/j.bbrc.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.White JH. 2010. Vitamin D as an inducer of cathelicidin antimicrobial peptide expression: past, present and future. J Steroid Biochem Mol Biol 121:234–238. doi: 10.1016/j.jsbmb.2010.03.034. [DOI] [PubMed] [Google Scholar]
- 32.Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, Ochoa MT, Schauber J, Wu K, Meinken C, Kamen DL, Wagner M, Bals R, Steinmeyer A, Zugel U, Gallo RL, Eisenberg D, Hewison M, Hollis BW, Adams JS, Bloom BR, Modlin RL. 2006. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science 311:1770–1773. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 33.Rahman S, Rehn A, Rahman J, Andersson J, Svensson M, Brighenti S. 2015. Pulmonary tuberculosis patients with a vitamin D deficiency demonstrate low local expression of the antimicrobial peptide LL-37 but enhanced FoxP3+ regulatory T cells and IgG-secreting cells. Clin Immunol 156:85–97. doi: 10.1016/j.clim.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 34.Liu PT, Stenger S, Tang DH, Modlin RL. 2007. Cutting edge: vitamin D-mediated human antimicrobial activity against Mycobacterium tuberculosis is dependent on the induction of cathelicidin. J Immunol 179:2060–2063. doi: 10.4049/jimmunol.179.4.2060. [DOI] [PubMed] [Google Scholar]
- 35.Ding K, Shibui A, Wang Y, Takamoto M, Matsuguchi T, Sugane K. 2005. Impaired recognition by Toll-like receptor 4 is responsible for exacerbated murine Pneumocystis pneumonia. Microbes Infect 7:195–203. doi: 10.1016/j.micinf.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 36.Zhang C, Wang SH, Lasbury ME, Tschang D, Liao CP, Durant PJ, Lee CH. 2006. Toll-like receptor 2 mediates alveolar macrophage response to Pneumocystis murina. Infect Immun 74:1857–1864. doi: 10.1128/IAI.74.3.1857-1864.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim MS, Lee S, Jung N, Lee K, Choi J, Kim SH, Jun J, Lee WM, Chang Y, Kim D. 2017. The vitamin D analogue paricalcitol attenuates hepatic ischemia/reperfusion injury through down-regulation of Toll-like receptor 4 signaling in rats. Arch Med Sci 13:459–469. doi: 10.5114/aoms.2016.60650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Qian L, Wang H, Wu F, Li M, Chen W, Lv L. 2015. Vitamin D3 alters Toll-like receptor 4 signaling in monocytes of pregnant women at risk for preeclampsia. Int J Clin Exp Med 8:18041–18049. [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 39.Chen Y, Li Q, Liu Y, Shu L, Wang N, Wu Y, Sun X, Wang L. 2015. Attenuation of hyperoxia-induced lung injury in neonatal rats by 1α,25-dihydroxyvitamin D3. Exp Lung Res 41:344–352. doi: 10.3109/01902148.2015.1039668. [DOI] [PubMed] [Google Scholar]
- 40.Wang H, Zhang Q, Chai Y, Liu Y, Li F, Wang B, Zhu C, Cui J, Qu H, Zhu M. 2015. 1,25(OH)2D3 downregulates the Toll-like receptor 4-mediated inflammatory pathway and ameliorates liver injury in diabetic rats. J Endocrinol Invest 38:1083–1091. doi: 10.1007/s40618-015-0287-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sadeghi K, Wessner B, Laggner U, Ploder M, Tamandl D, Friedl J, Zugel U, Steinmeyer A, Pollak A, Roth E, Boltz-Nitulescu G, Spittler A. 2006. Vitamin D3 down-regulates monocyte TLR expression and triggers hyporesponsiveness to pathogen-associated molecular patterns. Eur J Immunol 36:361–370. doi: 10.1002/eji.200425995. [DOI] [PubMed] [Google Scholar]
- 42.Yang M, Xu J, Yu J, Yang B, Gan H, Li S, Li X. 2015. Anti-inflammatory effects of 1,25-dihydroxyvitamin D3 in monocytes cultured in serum from patients with type 2 diabetes mellitus and diabetic nephropathy with uremia via Toll-like receptor 4 and nuclear factor-kappaB p65. Mol Med Rep 12:8215–8222. doi: 10.3892/mmr.2015.4482. [DOI] [PubMed] [Google Scholar]
- 43.Ojaimi S, Skinner NA, Strauss BJ, Sundararajan V, Woolley I, Visvanathan K. 2013. Vitamin D deficiency impacts on expression of Toll-like receptor-2 and cytokine profile: a pilot study. J Transl Med 11:176. doi: 10.1186/1479-5876-11-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li C, Wang Y, Gao L, Zhang J, Shao J, Wang S, Feng W, Wang X, Li M, Chang Z. 2002. Expression of Toll-like receptors 2 and 4 and CD14 during differentiation of HL-60 cells induced by phorbol 12-myristate 13-acetate and 1 alpha, 25-dihydroxy-vitamin D(3). Cell Growth Differ 13:27–38. [PubMed] [Google Scholar]
- 45.Gallagher LE, Williamson LE, Chan EY. 2016. Advances in autophagy regulatory mechanisms. Cells 5:E24. doi: 10.3390/cells5020024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Codogno P, Meijer AJ. 2006. Atg5: more than an autophagy factor. Nat Cell Biol 8:1045–1047. doi: 10.1038/ncb1006-1045. [DOI] [PubMed] [Google Scholar]
- 47.Zhu H, He L. 2015. Beclin 1 biology and its role in heart disease. Curr Cardiol Rev 11:229–237. doi: 10.2174/1573403X10666141106104606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Afsal K, Selvaraj P. 2016. Effect of 1,25-dihydroxyvitamin D3 on the expression of mannose receptor, DC-SIGN and autophagy genes in pulmonary tuberculosis. Tuberculosis (Edinb) 99:1–10. doi: 10.1016/j.tube.2016.03.010. [DOI] [PubMed] [Google Scholar]
- 49.Yuk JM, Shin DM, Lee HM, Yang CS, Jin HS, Kim KK, Lee ZW, Lee SH, Kim JM, Jo EK. 2009. Vitamin D3 induces autophagy in human monocytes/macrophages via cathelicidin. Cell Host Microbe 6:231–243. doi: 10.1016/j.chom.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 50.Sato E, Imafuku S, Ishii K, Itoh R, Chou B, Soejima T, Nakayama J, Hiromatsu K. 2013. Vitamin D-dependent cathelicidin inhibits Mycobacterium marinum infection in human monocytic cells. J Dermatol Sci 70:166–172. doi: 10.1016/j.jdermsci.2013.01.011. [DOI] [PubMed] [Google Scholar]
- 51.Campbell GR, Spector SA. 2011. Hormonally active vitamin D3 (1α,25-dihydroxycholecalciferol) triggers autophagy in human macrophages that inhibits HIV-1 infection. J Biol Chem 286:18890–18902. doi: 10.1074/jbc.M110.206110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Campbell GR, Spector SA. 2012. Vitamin D inhibits human immunodeficiency virus type 1 and Mycobacterium tuberculosis infection in macrophages through the induction of autophagy. PLoS Pathog 8:e1002689. doi: 10.1371/journal.ppat.1002689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hossein-nezhad A, Spira A, Holick MF. 2013. Influence of vitamin D status and vitamin D3 supplementation on genome wide expression of white blood cells: a randomized double-blind clinical trial. PLoS One 8:e58725. doi: 10.1371/journal.pone.0058725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Coelho L, Cardoso SW, Luz PM, Hoffman RM, Mendonca L, Veloso VG, Currier JS, Grinsztejn B, Lake JE. 2015. Vitamin D3 supplementation in HIV infection: effectiveness and associations with antiretroviral therapy. Nutr J 14:81. doi: 10.1186/s12937-015-0072-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Toma E, Thorne A, Singer J, Raboud J, Lemieux C, Trottier S, Bergeron MG, Tsoukas C, Falutz J, Lalonde R, Gaudreau C, Therrien R. 1998. Clindamycin with primaquine vs. trimethoprim-sulfamethoxazole therapy for mild and moderately severe Pneumocystis carinii pneumonia in patients with AIDS: a multicenter, double-blind, randomized trial (CTN 004). CTN-PCP Study Group. Clin Infect Dis 27:524–530. doi: 10.1086/514696. [DOI] [PubMed] [Google Scholar]
- 56.Lasbury ME, Merali S, Durant PJ, Tschang D, Ray CA, Lee CH. 2007. Polyamine-mediated apoptosis of alveolar macrophages during Pneumocystis pneumonia. J Biol Chem 282:11009–11020. doi: 10.1074/jbc.M611686200. [DOI] [PubMed] [Google Scholar]
- 57.Lei GS, Zhang C, Lee CH. 2015. Myeloid-derived suppressor cells impair alveolar macrophages through PD-1 receptor ligation during Pneumocystis pneumonia. Infect Immun 83:572–582. doi: 10.1128/IAI.02686-14. [DOI] [PMC free article] [PubMed] [Google Scholar]