ABSTRACT
Stenotrophomonas maltophilia is an emerging opportunistic pathogen, classified by the World Health Organization as one of the leading multidrug-resistant organisms in hospital settings. The need to discover novel compounds and/or combination therapies for S. maltophilia is urgent. We demonstrate the in vitro efficacy of aztreonam-avibactam (ATM-AVI) against S. maltophilia and kinetically characterize the inhibition of the L2 β-lactamase by avibactam. ATM-AVI overcomes aztreonam resistance in selected clinical strains of S. maltophilia, addressing an unmet medical need.
KEYWORDS: aztreonam, S. maltophilia, avibactam
TEXT
Stenotrophomonas maltophilia is a Gram-negative environmental bacillus and an emerging nosocomial pathogen (1). Primarily associated with respiratory tract infections, this bacterium is alarmingly increasing in prevalence among patients with cystic fibrosis, exacerbating an already-compromised respiratory function (2–5). S. maltophilia is intrinsically resistant to aminoglycosides, tetracycline, fosfomycin, and the majority of β-lactams. β-Lactam resistance is due to the expression of two β-lactamases: L1 is a B3 metallo-β-lactamase (MβL) that hydrolyzes all β-lactams with the exception of aztreonam (ATM), and it is resistant to all clinically available β-lactamase inhibitors (1, 6, 7); L2 is a clavulanate-susceptible class A cephalosporinase (1, 8, 9). Mimicking the AmpC cephalosporinases in Pseudomonas aeruginosa, L1 and L2 are inducible β-lactamases, and their expression is regulated by a similar mechanism (10, 11).
The growing challenge of treating infections caused by S. maltophilia is reflected in increasing reports of acquired resistance to historically effective drugs, like trimethoprim-sulfamethoxazole (SXT), ceftazidime (CAZ), ticarcillin-clavulanate (TIM), and fluoroquinolones, and the documented ability to develop high-level resistance during antibiotic treatment (8, 9, 12, 13). Recently, Mojica et al. described a clinical case in which the avibactam and ceftazidime combination coadministered with ATM (CZA) effectively treated a prolonged bacteremia caused by S. maltophilia (13). Notwithstanding the effectiveness of this triple combination, we anticipate that aztreonam-avibactam (ATM-AVI), the planned commercial preparation already in clinical development (ClinicalTrials.gov identifier NCT01689207), would be equally effective against S. maltophilia. This dual combination is highly active against Enterobacteriaceae producing MβLs (14). Accordingly, we asserted that AVI would inhibit L2, while ATM would bypass L1 to reach its likely target (penicillin-binding protein 3 [PBP-3]).
To test our assertion, we used the reference strain ATCC 51331 (A1) and 27 clinical isolates of S. maltophilia from University Hospitals (Cleveland, OH) and from the collection of the Burkholderia cepacia Research Laboratory and Repository at the University of Michigan (Ann Arbor, MI). The heterogeneity of these isolates was revealed by pulsed-field electrophoresis (data not shown). The susceptibilities of the isolates were determined by agar dilution MICs according to the Clinical and Laboratory Standards Institute (CLSI) guidelines, (15). Where breakpoints for S. maltophilia were not available, those for Pseudomonas aeruginosa were used. Table S1 in the supplemental material shows that the ATM-AVI combination fully restored ATM susceptibility in 23/28 (82%) isolates and lowered the MICs to intermediate in 3/5 of the remaining isolates.
Employing analytical isoelectric focusing (aIEF) with a nitrocefin overlay, β-lactamase induction was visualized on gels using crude extract of cells grown with and without 10 μg/ml imipenem, a known inducer (11). For this experiment, 10 isolates were selected based on their MICs to ATM-AVI (7 susceptible, 2 intermediates, and 1 highly resistant). Three of these 10 strains tested (A2, C1, and E1) were derepressed and demonstrated β-lactamase activity in the absence of imipenem (Fig. S1). We assert that these strains could possess alterations (e.g., amino acid substitutions) in one or more of the proteins involved in the β-lactamase regulatory pathway. However, a direct correlation between derepression of blaL1 or blaL2 and nonsusceptibility to ATM-AVI was not observed. Therefore, to test if ATM-AVI can induce β-lactamase expression, isolate A1 was grown in the presence of either 10 μg/ml imipenem, 4 μg/ml avibactam, 16 μg/ml aztreonam, or 4 μg/ml avibactam plus 0.5 μg/ml aztreonam. Interestingly, after 2 h of exposure, β-lactamase expression was induced by imipenem but not by ATM-AVI (Fig. S2). Since L1 activity was not detected by aIEF, even with the addition of 50 μM ZnSO4 to the nitrocefin overlay, we tested the specific activities of L1 and L2 in the crude lysates. In accordance with the aIEF assay, we found that the activities of both enzymes are enhanced only in lysates obtained after exposure to imipenem (Fig. S3). Taken together, these results suggest that neither ATM, AVI, nor the ATM-AVI combination induces β-lactamase expression.
Steady-state kinetics and electrospray ionization mass spectrometry (ESI-MS) reveal that AVI competitively and reversibly inhibits L2 (Table 1). The carbamylation rates (k2/K) for L2 are comparable with those published for the AVI inactivation of another class A β-lactamase, KPC-2 (47,000 ± 131 M−1 · s−1 for L2 versus 13,000 ± 100 M−1 · s−1 for KPC-2 [16]). However, the inhibition of these two enzymes by AVI differs in the decarbamylation rate (koff). For KPC, this rate is 0.00014 · s−1, compared to 0.0015 ± 0.0001 · s−1 for L2. Thus, despite similar AVI carbamylation rates for KPC-2 and L2, the AVI koff rate of L2 is greater than that of KPC-2. Given the high on-rate of AVI for L2, this higher off-rate most likely will not be clinically significant. In other words, even when AVI decarbamylates off L2, the reformed active AVI will carbamylate L2 rapidly; this is supported by our ESI-MS data.
TABLE 1.
Steady-state kinetic parameters of L2 with NCF and AVIa
| Parameter | Value |
|---|---|
| NCF Km (μM) | 62 ± 4 |
| NCF kcat/Km (μM−1 · s−1) | 9.14 ± 0.01 |
| AVI Ki app (μM) | 0.66 ± 0.07 |
| AVI k2/K (M−1 · s−1) | 47,000 ± 131 |
| AVI koff (s−1) | 0.0015 ± 0.0001 |
| AVI koff t1/2 (min) | 4.0 ± 0.2 |
NCF, nitrocefin. Values reported are averages ± standard deviations from triplicate experiments.
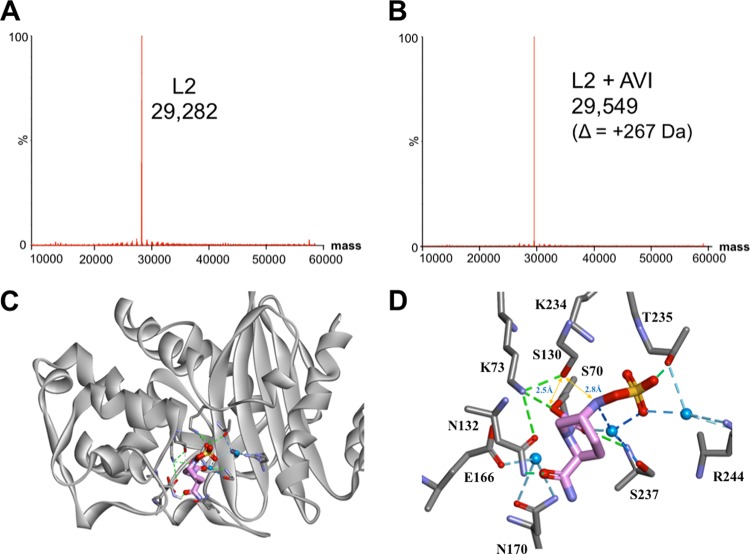
ESI-MS was used to determine if any intermediates (e.g., desulfation) were formed upon incubation of β-lactamases with AVI (16). Observations taken at several time points (i.e., 15 s, 1 min, and 1 h) reveal a mass of 29,549 ± 5 Da, which corresponds to the carbamylated enzyme (L2 [29,282 Da] plus AVI [265 Da]) (Fig. 1A and B). In addition, the AVI-L2 complex is stable for ≥24 h, without desulfation or hydrolysis observed. With L1 and AVI, only the apo-enzyme of L1 (29,282 Da) was detected by ESI-MS after a 24-h incubation with AVI, consistent with the lack of inhibition observed for L1-mediated nitrocefin hydrolysis in the presence of up to 500 μM AVI (data not shown).
FIG 1.
Avibactam is a potent inhibitor of L2. (A and B) ESI-MS spectrum of unreacted L2 (170 nM) (A) and 1:1 molar ratio of L2 and AVI (170 nM) (B) incubated at room temperature in 10 mM phosphate-buffered saline (PBS) (pH 7.4) for 15 s. Spectra obtained after 1-min, 15-min, 1-h, and 24-h incubations of L2 and AVI were identical to those in panel B. (C) Molecular docking of L2 and AVI. The L2 crystal structure (PDB no. 1N4O) is represented as a gray ribbon; AVI is displayed as sticks colored by heteroatoms (pink, carbon; red, oxygen; blue, nitrogen; yellow, sulfur). (D) Interactions of AVI with L2. Interacting residues are displayed in gray, while AVI is represented by pink. Heteroatoms are colored as described before. Interacting waters are shown in blue with their corresponding hydrogen bonds displayed as blue dashed lines; distances between important atoms are highlighted by yellow arrows; hydrogen bonds between residues are indicated by green dashed lines.
The in silico molecular model of AVI covalently bound with L2 (Fig. 1C and D) suggests that AVI preserves key interactions with conserved residues in class A β-lactamases (16–18). We propose that the C-7 carbamoyl of AVI is present in the oxyanion hole and forms hydrogen bonds (H-bonds) with S70:N and S237:N. Moreover, the carbonyl group of the AVI carboxamide makes H-bonds with N132. The highly polar SO4 is proposed to interact with T235, R244, and S237 (forming water-mediated interactions), and possibly with K234 (distance of ≈3.1 Å). These interactions result in a highly stable complex, as observed via ESI-MS.
Given the higher koff value (compared to KPC-2), we further assessed our model to identify potential pathways that could lead to recyclization. Recyclization is predicted to be initiated by K73 and S130 via a proton shuttle, resulting in a nucleophilic attack on the carbamate bond by the N-6 of AVI, thus reforming active AVI. In our model, AVI's N-6 is positioned at 2.8 Å away from S130:O and its C-7 is at H-bond distance from a water molecule (close enough for S130 to donate a proton to N-6). Moreover, K73 is within H-bond distance to S70 (≈2.4 Å from S70:Oδ), and S130 is ≈2.5 Å away from S70:O. Thus, a possible proton shuttle pathway for recyclization is present. In addition, the positioning of key catalytic residues in the active site may contribute to the faster recyclization of AVI observed with the L2 enzyme (Fig. S4).
In summary, AVI is a potent inhibitor of L2, and AZT-AVI is an effective in vitro combination against multidrug-resistant (MDR) S. maltophilia. This combination suggests that a potential therapeutic option can be tested against this MDR threat.
Supplementary Material
ACKNOWLEDGMENTS
We thank Allergan for supplying avibactam powder for this work through an investigator-initiated trial.
The research reported in this publication was supported in part by funds and/or facilities provided by the Cleveland Department of Veterans Affairs, the Veterans Affairs Merit Review Program BX002872 (K.M.P.-W.) and BX001974 (R.A.B.) from the U.S. Department of Veterans Affairs Biomedical Laboratory Research and Development Service of the VA Office of Research and Development, the Geriatric Research Education and Clinical Center VISN 10 (R.A.B.), and the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under awards R21AI114508, R01AI100560, R01AI063517, and R01AI072219 (R.A.B.). This work was also supported by funding from the Cystic Fibrosis Foundation (J.J.L.).
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, the U.S. Department of Veterans Affairs, or the U.S. government.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.00777-17.
REFERENCES
- 1.Brooke JS. 2012. Stenotrophomonas maltophilia: an emerging global opportunistic pathogen. Clin Microbiol Rev 25:2–41. doi: 10.1128/CMR.00019-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pompilio A, Crocetta V, Ghosh D, Chakrabarti M, Gherardi G, Vitali LA, Fiscarelli E, Di Bonaventura G. 2016. Stenotrophomonas maltophilia phenotypic and genotypic diversity during a 10-year colonization in the lungs of a cystic fibrosis patient. Front Microbiol 7:1551. doi: 10.3389/fmicb.2016.01551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Salsgiver EL, Fink AK, Knapp EA, LiPuma JJ, Olivier KN, Marshall BC, Saiman L. 2016. Changing epidemiology of the respiratory bacteriology of patients with cystic fibrosis. Chest 149:390–400. doi: 10.1378/chest.15-0676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Waters V, Yau Y, Prasad S, Lu A, Atenafu E, Crandall I, Tom S, Tullis E, Ratjen F. 2011. Stenotrophomonas maltophilia in cystic fibrosis: serologic response and effect on lung disease. Am J Respir Crit Care Med 183:635–640. doi: 10.1164/rccm.201009-1392OC. [DOI] [PubMed] [Google Scholar]
- 5.Junge S, Görlich D, den Reijer M, Wiedemann B, Tümmler B, Ellemunter H, Dübbers A, Küster P, Ballmann M, Koerner-Rettberg C, Große-Onnebrink J, Heuer E, Sextro W, Mainz JG, Hammermann J, Riethmüller J, Graepler-Mainka U, Staab D, Wollschläger B, Szczepanski R, Schuster A, Tegtmeyer FK, Sutharsan S, Wald A, Nofer JR, van Wamel W, Becker K, Peters G, Kahl BC. 2016. Factors associated with worse lung function in cystic fibrosis patients with persistent Staphylococcus aureus. PloS One 11:e0166220. doi: 10.1371/journal.pone.0166220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crossman LC, Gould VC, Dow JM, Vernikos GS, Okazaki A, Sebaihia M, Saunders D, Arrowsmith C, Carver T, Peters N, Adlem E, Kerhornou A, Lord A, Murphy L, Seeger K, Squares R, Rutter S, Quail MA, Rajandream MA, Harris D, Churcher C, Bentley SD, Parkhill J, Thomson NR, Avison MB. 2008. The complete genome, comparative and functional analysis of Stenotrophomonas maltophilia reveals an organism heavily shielded by drug resistance determinants. Genome Biol 9:R74. doi: 10.1186/gb-2008-9-4-r74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin C-W, Huang Y-W, Hu R-M, Chiang K-H, Yang T-C. 2009. The role of AmpR in regulation of L1 and L2 β-lactamases in Stenotrophomonas maltophilia. Res Microbiol 160:152–158. doi: 10.1016/j.resmic.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 8.Chang YT, Lin CY, Chen YH, Hsueh PR. 2015. Update on infections caused by Stenotrophomonas maltophilia with particular attention to resistance mechanisms and therapeutic options. Front Microbiol 6:693. doi: 10.3389/fmicb.2015.00893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perez F, Adachi J, Bonomo RA. 2014. Antibiotic-resistant Gram-negative bacterial infections in patients with cancer. Clin Infect Dis 59:S335–S339. doi: 10.1093/cid/ciu612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang Y-W, Wu C-J, Hu R-M, Lin Y-T, Yang T-C. 2015. Interplay among membrane-bound lytic transglycosylase D1, the CreBC two-component regulatory system, the AmpNG-AmpDI-NagZ-AmpR regulatory circuit, and L1/L2 β-lactamase expression in Stenotrophomonas maltophilia. Antimicrob Agents Chemother 59:6866–6872. doi: 10.1128/AAC.05179-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vadlamani G, Thomas MD, Patel TR, Donald LJ, Reeve TM, Stetefeld J, Standing KG, Vocadlo DJ, Mark BL. 2015. The β-lactamase gene regulator AmpR is a tetramer that recognizes and binds the d-Ala-d-Ala motif of its repressor UDP-N-acetylmuramic acid (MurNAc)-pentapeptide. J Biol Chem 290:2630–2643. doi: 10.1074/jbc.M114.618199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ryan RP, Monchy S, Cardinale M, Taghavi S, Crossman L, Avison MB, Berg G, van der Lelie D, Dow JM. 2009. The versatility and adaptation of bacteria from the genus Stenotrophomonas. Nat Rev Microbiol 7:514–525. doi: 10.1038/nrmicro2163. [DOI] [PubMed] [Google Scholar]
- 13.Mojica MF, Ouellette CP, Leber A, Becknell MB, Ardura MI, Perez F, Shimamura M, Bonomo RA, Aitken SL, Shelburne SA. 2016. Successful treatment of bloodstream infection due to metallo-β-lactamase-producing Stenotrophomonas maltophilia in a renal transplant patient. Antimicrob Agents Chemother 60:5130–5134. doi: 10.1128/AAC.00264-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Biedenbach DJ, Kazmierczak K, Bouchillon SK, Sahm DF, Bradford PA. 2015. In vitro activity of aztreonam-avibactam against a global collection of Gram-negative pathogens from 2012–2013. Antimicrob Agents Chemother 59:4239–4248. doi: 10.1128/AAC.00206-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.CLSI. 2012. Performance standards for antimicrobial susceptibility testing: 22nd informational supplement. CLSI document M100-S22. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 16.Ehmann DE, Jahić H, Ross PL, Gu R-F, Hu J, Durand-Réville TF, Lahiri S, Thresher J, Livchak S, Gao N, Palmer T, Walkup GK, Fisher SL. 2013. Kinetics of avibactam inhibition against class A, C, and D β-lactamases. J Biol Chem 288:27960–27971. doi: 10.1074/jbc.M113.485979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krishnan NP, Nguyen NQ, Papp-Wallace KM, Bonomo RA, van den Akker F. 2015. Inhibition of Klebsiella β-lactamases (SHV-1 and KPC-2) by avibactam: a structural study. PLoS One 10:e0136813. doi: 10.1371/journal.pone.0136813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lahiri SD, Mangani S, Durand-Reville T, Benvenuti M, De Luca F, Sanyal G, Docquier J-D. 2013. Structural insight into potent broad-spectrum inhibition with reversible recyclization mechanism: avibactam in complex with CTX-M-15 and Pseudomonas aeruginosa AmpC β-lactamases. Antimicrob Agents Chemother 57:2496–2505. doi: 10.1128/AAC.02247-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.