ABSTRACT
Lowering the gut exposure to antibiotics during treatments can prevent microbiota disruption. We evaluated the effects of an activated charcoal-based adsorbent, DAV131A, on the fecal free moxifloxacin concentration and mortality in a hamster model of moxifloxacin-induced Clostridium difficile infection. A total of 215 hamsters receiving moxifloxacin subcutaneously (day 1 [D1] to D5) were orally infected at D3 with C. difficile spores. They received various doses (0 to 1,800 mg/kg of body weight/day) and schedules (twice a day [BID] or three times a day [TID]) of DAV131A (D1 to D8). Moxifloxacin concentrations and C. difficile counts were determined at D3, and mortality was determined at D12. We compared mortality rates, moxifloxacin concentrations, and C. difficile counts according to DAV131A regimen and modeled the links between DAV131A regimen, moxifloxacin concentration, and mortality. All hamsters that received no DAV131A died, but none of those that received 1,800 mg/kg/day died. Significant dose-dependent relationships between DAV131A dose and (i) mortality, (ii) moxifloxacin concentration, and (iii) C. difficile count were evidenced. Mathematical modeling suggested that (i) lowering the moxifloxacin concentration at D3, which was 58 μg/g (95% confidence interval [CI] = 50 to 66 μg/g) without DAV131A, to 17 μg/g (14 to 21 μg/g) would reduce mortality by 90%; and (ii) this would be achieved with a daily DAV131A dose of 703 mg/kg (596 to 809 mg/kg). In this model of C. difficile infection, DAV131A reduced mortality in a dose-dependent manner by decreasing the fecal free moxifloxacin concentration.
KEYWORDS: Clostridium difficile infection, hamster animal model, mortality, prevention, moxifloxacin
INTRODUCTION
Clostridium difficile is a sporulating Gram-positive bacillus that can lead to mild to severe intestinal infections, including pseudomembranous colitis and toxic megacolon (1). With 500,000 cases and 29,000 deaths in 2011 in the United States (2), the burden of C. difficile infection on the U.S. health care system has reached $4.8 billion (3). C. difficile is the leading cause of health care-associated infections (4), and the Centers for Disease Control and Prevention consider it an immediate public health threat (5).
Antibiotics are the main risk factors for C. difficile infections because the gut microbiota is exposed to high concentrations of the drugs during oral or parenteral treatments, resulting in its disruption (6, 7). Reducing this exposure thus appears appealing for limiting the consequences of antibiotic treatments on the microbiota. Such an approach has been pioneered by administering β-lactamases together with β-lactam antibiotics. This prevents colonization by resistant bacteria in mice (8, 9) as well as in dogs (10) and humans (11). It also reduces antibiotic concentrations in the human gut (12, 13) during treatments, and it preserves the intestinal microbiota in humanized gnotobiotic pigs (12). However, this promising approach is limited to β-lactams, while many other antibiotics are also at risk of provoking C. difficile infection, particularly fluoroquinolones (14).
In rats, delivering activated charcoal to the intestine allowed removal of ciprofloxacin residues from the gut and decreased antibiotic exposure of the microbiota without affecting its plasma pharmacokinetics (15). Similarly, we showed previously that oral DAV131A, a charcoal-based adsorbent, decreased intestinal colonization by β-lactam-resistant Klebsiella pneumoniae in cefotaxime-treated mice (16).
Here we used the Syrian hamster model of C. difficile infection, which recapitulates many aspects of the human infection (17) and has been used widely to evaluate new therapies against C. difficile infection (18–20), to assess the protective effect of DAV131A. We also developed a mathematical model to analyze the relationships between DAV131A regimens, the fecal free moxifloxacin concentration, and hamster mortality.
(This work was partly presented at IDWeek 2016, New Orleans, LA, 26 to 30 October 2016 [21].)
RESULTS
Comparison of mortality rates, fecal free moxifloxacin concentrations, and C. difficile counts across DAV131A doses.
Values for the fecal free moxifloxacin concentration at day 3 (D3) were missing for only 3/215 hamsters (1.4%), and 1 value was below the limit of quantification (LOQ). Values for the C. difficile count at D3 were missing for only 9/215 hamsters (4.2%), but as many as 121/215 (56.3%) values were below the LOQ.
Descriptive statistics on fecal free moxifloxacin concentrations, C. difficile counts, and mortality for each group in each study are reported in Table 1.
TABLE 1.
Descriptive statistics on fecal free moxifloxacin concentrations at D3, Clostridium difficile counts at D3, and mortality rates at D12 according to DAV131A daily dose for the three studies
| Study no. (n) | Group name | DAV131A administration |
Fecal free moxifloxacin concn at D3 (H0 to H12) (μg/g) |
C. difficile count at D3 (H12 to H24) (log10 CFU/g) |
Mortality at D12 (n [%]) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Daily dose (mg/kg/day) | Administration at D1H−10 | Dosing time at D1 | Dosing time at D2 to D8 | n | n | Median | Minimum | Maximum | n | Median | Minimum | Maximum | |||
| 1 (90) | 1A | 0 (no placebo) | No | 10 | 10 | 46.4 | 24.5 | 136.5 | 9 | 4.9 | 3.5 | 6.0 | 10 (100) | ||
| 1B | 200 (100 BID) | Yes | H−4, H1 | H−4, H1 | 10 | 10 | 47.5 | 24.5 | 463.4 | 8 | 4.8 | <3.3 | 5.8 | 10 (100) | |
| 1C | 200 (100 BID) | No | H−4, H1 | H−4, H1 | 10 | 8 | 34.6 | 24.8 | 62.9 | 7 | 4.4 | 4 | 5.5 | 10 (100) | |
| 1D | 600 (300 BID) | Yes | H−4, H1 | H−4, H1 | 10 | 10 | 14.8 | 8.2 | 30.4 | 10 | <3.3 | <3.3 | <3.3 | 0 (0) | |
| 1E | 600 (300 BID) | No | H−4, H1 | H−4, H1 | 10 | 10 | 12.3 | 9.1 | 27.8 | 10 | <3.3 | <3.3 | <3.3 | 2 (20) | |
| 1F | 1,200 (600 BID) | Yes | H−4, H1 | H−4, H1 | 10 | 10 | 7.1 | 3.0 | 10.8 | 10 | <3.3 | <3.3 | <3.3 | 0 (0) | |
| 1G | 1,200 (600 BID) | No | H−4, H1 | H−4, H1 | 10 | 10 | 6.6 | 5.1 | 27 | 10 | <3.3 | <3.3 | <3.3 | 0 (0) | |
| 1H | 1,800 (900 BID) | Yes | H−4, H1 | H−4, H1 | 10 | 10 | 1.5 | 0.2 | 4.9 | 10 | <3.3 | <3.3 | <3.3 | 0 (0) | |
| 1I | 1,800 (900 BID) | No | H−4, H1 | H−4, H1 | 10 | 10 | 5.2 | 1.6 | 20.3 | 10 | <3.3 | <3.3 | <3.3 | 0 (0) | |
| 2 (45) | 2A | 0 (no placebo) | No | 15 | 14 | 42.0 | 27.7 | 56.2 | 12 | 7.3 | 5.3 | 7.8 | 15 (100) | ||
| 2B | 1,800 (600 TID) | Yes | H−4, H1, H6 | H−4, H1, H6 | 15 | 15 | 1.6 | 1.0 | 2.0 | 15 | 4.6 | 3.4 | 5.4 | 0 (0) | |
| 2C | 1,800 (900 BID) | Yes | H−4, H1 | H−4, H1 | 15 | 15 | 1.9 | 0.9 | 3.8 | 15 | 4.6 | 3.7 | 5.9 | 0 (0) | |
| 3 (80) | 3A | 0 (placebo) | No | 10 | 10 | 90 | 75.5 | 211.3 | 10 | 5.7 | <3.3 | 7.1 | 10 (100) | ||
| 3B | 600 (300 BID) | No | H−4, H1 | H−4, H1 | 10 | 10 | 19.6 | 10 | 24.1 | 10 | <3.3 | <3.3 | 4.0 | 1 (10) | |
| 3C | 600 (300 BID) | No | H0, H5 | H−4, H1 | 10 | 10 | 23.4 | 17.3 | 42.9 | 10 | <3.3 | <3.3 | 3.7 | 0 (0) | |
| 3D | 600 (300 BID) | No | H2, H7 | H−4, H1 | 10 | 10 | 15.7 | 11.3 | 38.9 | 10 | <3.3 | <3.3 | <3.3 | 2 (20) | |
| 3E | 1,200 (600 BID) | No | H−4, H1 | H−4, H1 | 10 | 10 | 8.9 | 5.6 | 15.7 | 10 | <3.3 | <3.3 | <3.3 | 0 (0) | |
| 3F | 1,200 (600 BID) | No | H0, H5 | H−4, H1 | 10 | 10 | 5 | 3.6 | 14 | 10 | <3.3 | <3.3 | <3.3 | 0 (0) | |
| 3G | 1,200 (600 BID) | No | H2, H7 | H−4, H1 | 10 | 10 | 7.3 | 3.0 | 29.6 | 10 | <3.3 | <3.3 | 3.7 | 0 (0) | |
| 3H | 1,800 (900 BID) | No | H2, H7 | H−4, H1 | 10 | 10 | 2.4 | 0.7 | 16.3 | 10 | <3.3 | <3.3 | 4.9 | 0 (0) | |
| All groups | 215 | 212 | 11.6 | 0.2 | 463.4 | 206 | <3.3 | <3.3 | 7.8 | 60 (27.9) | |||||
All (100%; 95% confidence interval [CI] = 90.0% to 100%) 35 hamsters from the control groups that received moxifloxacin but no DAV131A (groups 1A, 2A, and 3A) died; they had a median fecal free moxifloxacin concentration of 53.8 μg/g (range, 24.5 to 211.3 μg/g) at D3, at the time of C. difficile inoculation. Conversely, none of the 60 animals receiving a daily dose of DAV131A of 1,800 mg/kg of body weight (groups 1H, 1I, 2B, 2C, and 3H) died (0%; 95% CI = 0.0% to 6.0%); they had a median fecal free moxifloxacin concentration of only 1.8 μg/g (range, 0.0 to 20.3 μg/g). These animals showed no signs of disease or inflection in their weight gain (data not shown). Also, the median count of C. difficile was 6.0 log10 CFU/g (range, <3.3 to 7.8 log10 CFU/g) for the control groups that received moxifloxacin but no DAV131A, which is much higher than the 3.8 log10 CFU/g (range, <3.3 to 5.9 log10 CFU/g) observed for the moxifloxacin- and DAV131A (1,800 mg/kg/day)-treated group.
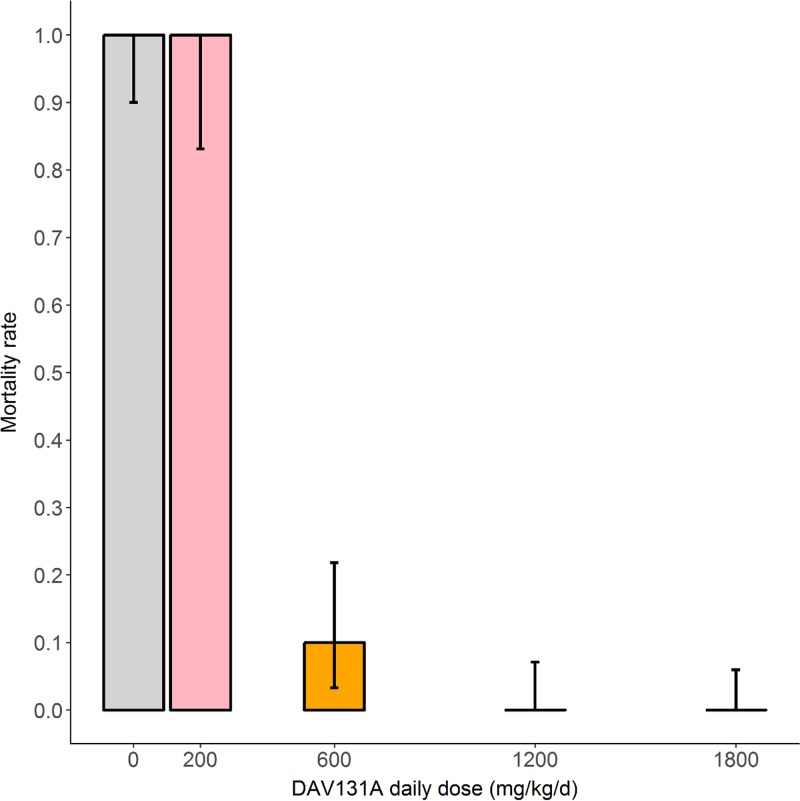
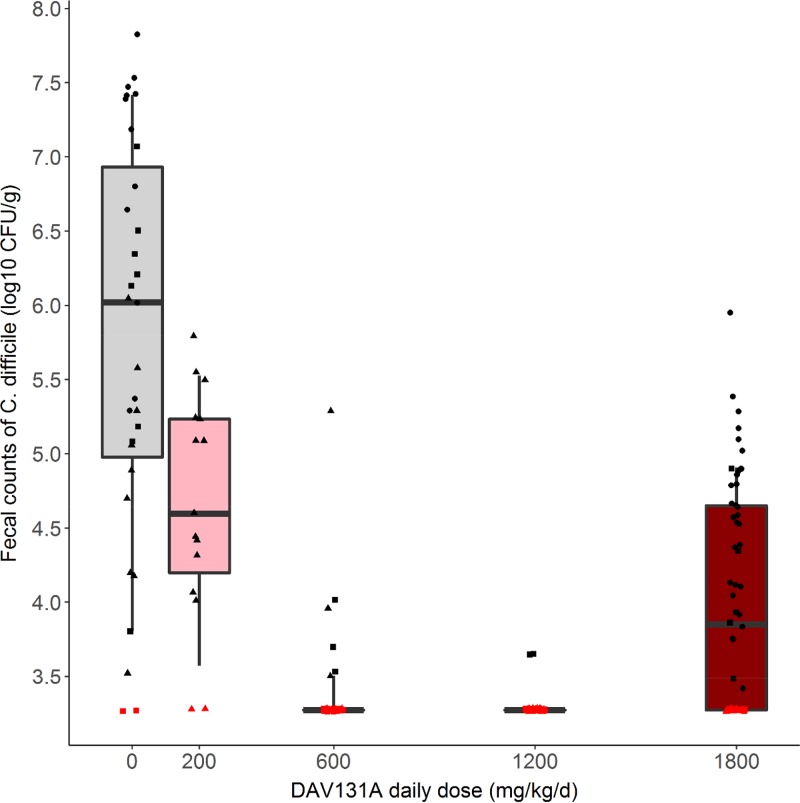
We observed a highly significant decrease in mortality when the DAV131A daily dose administered to hamsters increased (P < 10−15) (Fig. 1). A significant association was also evidenced between the DAV131A daily dose and the fecal free moxifloxacin concentration (Spearman rank correlation coefficient [s] = −0.9; P < 10−15) (Fig. 2) as well as between the DAV131A daily dose and the fecal count of C. difficile (s = −0.3; P < 10−4) (Fig. 3). Approximately 60% of the moxifloxacin excreted during D3 was retrieved from the fecal pellets collected during the period from 0 h (H0) to H12.
FIG 1.
Mortality rates at D12 according to DAV131A daily dose administered for the 215 hamsters in the 3 pooled studies. Bars represent the exact 95% confidence intervals of observed proportions.
FIG 2.
Box plots of fecal free moxifloxacin concentrations measured at D3 according to DAV131A daily dose administered for the 212 hamsters in the 3 pooled studies. Triangles, dots, and squares represent the observed concentrations in studies 1, 2, and 3, respectively. Whiskers represent 10th and 90th percentiles. The red symbol represents a point below the limit of quantification.
FIG 3.
Box plots of the counts of Clostridium difficile measured at D3 according to the DAV131A daily dose administered in the 206 hamsters of the three pooled studies. Triangles, dots, and squares represent the observed concentrations in studies 1, 2, and 3, respectively. Whiskers represent 10th and 90th percentiles. Red symbols represent data below the limit of quantification.
Comparison of fecal free moxifloxacin concentrations and C. difficile counts according to vital status.
The median fecal concentration of free moxifloxacin was 46.0 μg/g (range, 12.3 to 463.4 μg/g) in hamsters in which death occurred by D12 and 6.8 μg/g (range, 0.28 to 42.9 μg/g) in hamsters that survived (P < 10−15). Similarly, C. difficile counts were higher in hamsters in which death occurred than in survivors (5.2 log10 CFU/g [range, <3.3 to 7.8 log10 CFU/g] versus <3.3 log10 CFU/g [range, <3.3 to 5.9 log10 CFU/g]) (P < 10−15). The areas under the receiver operating characteristic (ROC) curves for the fecal concentration of free moxifloxacin and for C. difficile counts for prediction of death were 0.97 (95% CI = 0.95 to 0.99) and 0.86 (95% CI = 0.79 to 0.92), respectively.
Influence of DAV131A dosing schedule on fecal free moxifloxacin concentration.
Among the 180 hamsters treated with DAV131A (all groups except 1A, 2A, and 3A), the administration of an additional initial dose of DAV131A at D1H−10 was significantly associated with a lower fecal free moxifloxacin concentration (median [range], 3.1 μg/g [0.0 to 463.4 μg/g] versus 11.7 μg/g [0.7 to 62.9 μg/g]) (P < 10−5) (see Fig. S1 in the supplemental material).
For the 40 hamsters treated with a daily dose of 1,800 mg/kg DAV131A and receiving an additional initial dose of DAV131A at D1H−10 (groups 1H, 2B, and 2C), fecal free moxifloxacin concentrations were not significantly different between hamsters treated with DAV131A twice a day (BID) and those treated three times a day (TID) (1.6 μg/g [0.0 to 4.9 μg/g] versus 1.9 μg/g [0.9 to 3.8 μg/g]) (P = 0.2) (Fig. S2).
Among the 80 hamsters treated with a daily dose of 600 or 1,200 mg/kg DAV131A BID and not receiving an additional initial dose of DAV131A at D1H−10 (groups 1E, 1G, 3B, 3C, 3D, 3E, 3F, and 3G), there was no significant difference in fecal free moxifloxacin concentration whether the first dose of DAV131A was administered 4 h before (12.1 μg/g [5.1 to 27.8 μg/g]), concomitant with (15.6 μg/g [3.6 to 42.9 μg/g]), or 2 h after (12.8 μg/g [3.0 to 38.9 μg/g]) the first administration of moxifloxacin (P = 0.7) (Fig. S3).
DISCUSSION
Our most important result was that DAV131A provided a dose-dependent reduction of mortality in a hamster model of moxifloxacin-induced C. difficile infection. A fecal free moxifloxacin concentration of 53.8 μg/g (range, 24.5 to 211.3 μg/g), a C. difficile count of 6.0 log10 CFU/g (range, 3.1 to 7.8 log10 CFU/g), and a 100% mortality rate were observed for animals that did not receive DAV131A treatment, while the fecal free moxifloxacin concentration, C. difficile count, and mortality were decreased to 7.3 μg/g (range, 3.0 to 29.6 μg/g), 3.8 log10 CFU/g (range, 3.1 to 5.9 log10 CFU/g), and 0%, respectively, with doses of 1,800 mg/kg/day. DAV131A is the first product to exhibit such a level of protection against mortality from antibiotic-induced C. difficile infection in hamsters. Indeed, a polymeric toxin-binding compound had been shown to protect only 70% to 90% of hamsters in an animal model of clindamycin-induced C. difficile infection (22); however, 20% to 40% of animals from the toxin-binding treatment group still had diarrhea 15 days after cessation of therapy, whereas protected animals in the experiments reported here had no signs of disease (data not shown).
The schedule of DAV131A administration (4 h before, concomitant with, or 2 h after the first moxifloxacin administration) did not significantly affect the protective effect as assessed by survival, fecal free moxifloxacin concentration, and C. difficile count in feces (Table 1; see Fig. S3 in the supplemental material). In comparing administration schedules for the same total daily dose of DAV131A, BID was found not to be significantly less protective than TID. However, this result was drawn from analysis of only 15 hamsters that received DAV131A on a TID basis, all of which were treated with DAV131A at the highest dose (1,800 mg/kg/day) and had also received an additional initial dose of DAV131A at D1H−10.
Another important result of our work is that the modeling approach described in Text S1 allowed us to investigate the mechanism of action by which DAV131A reduced mortality in hamsters. The effect appeared to be mediated by the reduction of the fecal concentration of free moxifloxacin when the dose of DAV131A was increased, in a dose-dependent manner.
Our results should, however, be tempered by the absence of bacteriology data from our modeling analysis. We did not include C. difficile counts in the model, as their ability to predict death was lower than that of fecal concentrations of free moxifloxacin. Furthermore, the symptoms of C. difficile infection are related to the action of toxins produced by pathogenic strains of C. difficile (1), whose presence and activity could not be assessed from fecal samples in our studies. We are currently developing new methods for measuring toxin production and activity in order to perform a more thorough analysis of the protective effect of DAV131A.
Altogether, our data provide encouraging prospects for the protection of the gut microbiota from perturbation during the use of antimicrobials, such as fluoroquinolones, which are widely used for therapeutic purposes and were associated with the rise of the hypervirulent epidemic C. difficile strains of the 027 ribotype (14). These results for hamsters suggest that the approach warrants further clinical development. Indeed, the hamster model of C. difficile infection is appropriate for reproducing the deleterious impact of antibiotic treatments on the gut microbiota that allows C. difficile spores to germinate and the disease to develop (23). Therefore, we believe that the efficacy of DAV131A obtained in this model is also relevant to the mechanism of action of the product. The modeling approach confirmed this by showing that the protective effect of DAV131A in the hamster model was mediated by the reduction of the antibiotic concentrations in the gut.
The interpolation of the dose of adsorbent between hamsters and humans constitutes a challenge because of the vast differences in gastrointestinal transit times and fecal excretion physiology between the two species. Additionally, they may also exhibit differences in antibiotic pharmacokinetics; however, the fact that the maximum fecal concentrations of free moxifloxacin measured in hamsters in the experiments reported here were within the range of what was found for humans treated with a clinical dose of moxifloxacin suggests similarities in the selective pressure exerted by antibiotics on the intestinal microbiota of hamsters in the model developed in this study and in humans (24). Even given the limitations discussed above in transposing results between animal models and human patients, in particular because gastrointestinal transit is much faster in hamsters than in humans, our results suggest that the product would not necessarily need to be administered before the antibiotic but could be given concomitantly or just after the first antibiotic intake. In cases where antimicrobial therapy can be programmed, a supplementary protection might be obtained by pretreating patients with DAV132, as suggested by the additional protection obtained in hamsters which received the first dose of DAV131A 10 h before the first injection of moxifloxacin. This should be investigated further in human studies.
It can thus be inferred from our results that DAV131A protected animals against C. difficile colonization and infection by preventing the disruption of the gut bacterial microbiota which is known to occur during fluoroquinolone treatments (6) and which constitutes the primary risk factor for C. difficile infection in humans (7). Therefore, treatment with DAV132, a human counterpart of DAV131A that was recently developed and tested in human volunteers (25), may represent a promising approach for the prevention of C. difficile infection during antibiotic treatments. The first phase 1 clinical trial in human volunteers recently showed that the active component of DAV131A contained in the human-directed product DAV132 could be targeted to the ileocecal region in humans and that its concomitant use with an orally administered antibiotic did not affect the plasma pharmacokinetics of the antibiotic (25). Studies to expand these results to patients and to establish the efficacy of DAV132 to prevent C. difficile infections following antibiotic treatments are under way.
MATERIALS AND METHODS
DAV131A.
DAV131A is an activated charcoal-based adsorbent with a high adsorption capacity (16). It was administered to hamsters by oral gavage after mixing with 0.25% (wt/vol) Natrosol 250 hydroxyethylcellulose. Hamsters from placebo groups received Natrosol alone.
Hamster model of moxifloxacin-induced C. difficile infection.
A previously developed hamster model of antibiotic-induced C. difficile infection was adapted for use with moxifloxacin (23). Male Golden Syrian hamsters (80 to 120 g) received 30 mg/kg of moxifloxacin by the subcutaneous route, at a time designated H0, once a day from day 1 (D1) to day 5 (D5). This dose was chosen as the lowest dose resulting in a 100% mortality rate in treated hamsters infected with C. difficile spores. It is not expected to cause any toxicity in hamsters, since the minimal lethal intravenous dose reported for mice and rats is 100 mg/kg (26).
Animals were infected orally on day 3 (D3), 4 h after moxifloxacin administration (H4), with 104 spores of the nonepidemic C. difficile strain UNT103-1 (VA-11, REA J strain; TcdA+ TcdB+ CdtB−; vancomycin MIC = 2 μg/ml, moxifloxacin MIC = 16 μg/ml, clindamycin MIC > 256 μg/ml, and ceftriaxone MIC = 128 μg/ml), obtained from Curtis Donskey, Ohio VA Medical Centre. All surviving hamsters were euthanized at day 12 (D12). Animals were housed in conformity with NIH guidelines (27). All procedures were conducted at the University of North Texas Health Science Center (Fort Worth, TX, USA) in accordance with protocol 2012/13-21-A06, approved by the local Institutional Animal Care and Use Committee.
Studies.
Three studies were conducted in order to test the protection afforded by DAV131A from lethal moxifloxacin-induced C. difficile infection. Their designs are summarized in Table 1. All hamsters received moxifloxacin and were inoculated with C. difficile spores as described above. DAV131A was administered from D1 to D8.
In study 1, we aimed at analyzing the dose-response relationship between the DAV131A daily dose and survival. To that end, 4 groups of 10 hamsters each (groups 1C, 1E, 1G, and 1I) were treated with increasing daily doses of DAV131A (200, 600, 1,200, or 1,800 mg/kg/day) administered BID 4 h before (H−4) and 1 h after (H1) moxifloxacin injection. Four groups of 10 hamsters each (groups 1B, 1D, 1F, and 1H) received the same treatment plus an additional dose of DAV131A 10 h before the first administration of moxifloxacin, i.e., at D1H−10. A control group receiving moxifloxacin alone (group 1A) was included.
In study 2, we compared the effects on survival of BID and TID administrations of a high dose of DAV131A (1,800 mg/kg/day). Two groups of 15 hamsters each received DAV131A at a dose of 600 mg/kg TID (at H−4, i.e., 4 h before moxifloxacin administration, and at H1 and H6, i.e., 1 and 6 h after moxifloxacin administration, respectively) (group 2B) or at a dose of 900 mg/kg BID (at H−4 and H1) (group 2C). In addition, all these animals also received an additional initial dose of DAV131A at D1H−10. A control, untreated group (group 2A) was also included.
In study 3, we assessed the influence on survival of giving the first dose of DAV131A before (H−4), concomitant with, or after (H1) the first antibiotic administration. Seven groups of 10 hamsters each were included, all receiving DAV131A BID, at H−4 and H1 on D2 to D8 but at specific timings on D1. Two of these groups received 600 (group 3B) and 1,200 (group 3E) mg/kg/day of DAV131A at H−4 and H1 on D1; two other groups also received 600 (group 3C) and 1,200 (group 3F) mg/kg/day of DAV131A, but at H0 and H5 on D1. Three groups received 600 (group 3D), 1,200 (group 3G), and 1,800 (group 3H) mg/kg/day of DAV131A, respectively, at H2 and H7 on D1. The last group (group 3A) received placebo on the same schedule as that for the last 3 groups.
Feces collection and analysis.
We focused the analysis on D3, which bracketed C. difficile inoculation. Two pools of feces were collected daily from D2 to D4 in all studies. The first was made of all pellets emitted in the first 12 h after moxifloxacin administration (H0 to H12), and the second was made of all pellets emitted in the period between 12 and 24 h after moxifloxacin administration (H12 to H24). As it is a natural and physiological behavior of hamsters, coprophagy was not controlled.
The fecal free moxifloxacin concentration was determined at D3 for feces collected from H0 to H12. Fecal pools were stored at −80°C until we performed the assay. On the day of the assay, feces were weighed and homogenized in sterile saline, and debris was eliminated by centrifugation. The fecal free moxifloxacin concentration was measured by microbiological assay (with Bacillus subtilis ATCC 6633) after incubation at 37°C for 24 h (28), with a limit of quantification (LOQ) of 0.2 μg/g. Missing data were imputed according to the following algorithm: (i) if fecal free moxifloxacin concentrations were available for the period from H0 to H12 at D2 and D4, the missing value was imputed to the arithmetic mean of these 2 values; (ii) if the concentration was known for the period from H0 to H12 for D2 or D4 only, the missing value was imputed to this available value; and (iii) otherwise, the missing data were not imputed and the animal was excluded from analysis. Data below the LOQ were imputed to the LOQ.
Fecal counts of C. difficile were determined extemporaneously at D3 on the H12-to-H24 pool by plating serial dilutions of the samples on BBL C. difficile selective agar (BD). Counts were read after anaerobic incubation at 37°C for 48 h. Fecal counts below the LOQ (3.3 log10 CFU/g of feces) were imputed to the LOQ. Missing values were imputed using the counts at D4 (H12 to H24), if available. Otherwise, the missing counts were not imputed.
Statistical analysis.
We compared mortality rates at D12 for all hamsters according to DAV131A daily dose by using the nonparametric Fisher exact test. The links between the DAV131A daily dose and (i) the fecal free moxifloxacin concentration at D3 (H0 to H12) and (ii) the decimal logarithm of the C. difficile count in feces at D3 (H12 to H24) were studied using the Spearman rank correlation test. Exact 95% confidence intervals of the mortality rates were computed using the binomial distribution.
We compared fecal free moxifloxacin concentrations and C. difficile counts according to vital status at D12 by using the nonparametric Wilcoxon test. The abilities of fecal free moxifloxacin concentrations and C. difficile counts to predict death were assessed using the areas under the ROC curves and the 95% confidence interval, computed using 1,000 paired-bootstrap replicates (R functions roc and ci.auc).
For hamsters that received DAV131A treatment (all groups except 1A, 2A, and 3A), we compared fecal free moxifloxacin concentrations according to the administration, or not, of an additional initial dose of DAV131A at D1H−10 by using the nonparametric Wilcoxon test.
The impact of the BID versus TID DAV131A administrations on the fecal free moxifloxacin concentration was tested in hamsters receiving a daily dose of DAV131A of 1,800 mg/kg and also receiving a dose of DAV131A at D1H−10 (groups 1H, 2B, and 2C). The impact on fecal free moxifloxacin concentration of the timing of the first DAV131A administration (4 h before, with, or 2 h after the first moxifloxacin administration) was tested in hamsters receiving 600 or 1,200 mg/kg/day of DAV131A and not receiving DAV131A at D1H−10 (groups 1E, 1G, 3B, 3C, 3D, 3E, 3F, and 3G). Analyses were performed using nonparametric Wilcoxon or Kruskal-Wallis tests, as appropriate.
Finally, in order to identify independent features of the DAV131A dosing schedule associated with the reduction of fecal free moxifloxacin concentration and to link the DAV131A dosing regimen to the mortality rate, we performed a modeling analysis of the data. Full methods and results are presented in Text S1 in the supplemental material.
Data are presented as numbers of observations (n [%]) or medians (ranges). All tests were two-sided, with a type I error value of 0.05. All analyses were performed using R software v3.2.2.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by Da Volterra, Paris, France.
Charles Burdet and Thu Thuy Nguyen performed statistical work for the Da Volterra Company through a contract with INSERM UMR 1137. Nathalie Saint-Lu and Sakina Sayah-Jeanne are employees of the Da Volterra Company. Christine Miossec is a previous employee of the Da Volterra Company. Antoine Andremont is a scientific adviser of the Da Volterra Company within the framework of the French law on innovation and research. France Mentré and Jean de Gunzburg are consultants for the Da Volterra Company.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.00543-17.
REFERENCES
- 1.Leffler DA, Lamont JT. 2015. Clostridium difficile infection. N Engl J Med 372:1539–1548. doi: 10.1056/NEJMra1403772. [DOI] [PubMed] [Google Scholar]
- 2.Lessa FC, Mu Y, Bamberg WM, Beldavs ZG, Dumyati GK, Dunn JR, Farley MM, Holzbauer SM, Meek JI, Phipps EC, Wilson LE, Winston LG, Cohen JA, Limbago BM, Fridkin SK, Gerding DN, McDonald LC. 2015. Burden of Clostridium difficile infection in the United States. N Engl J Med 372:825–834. doi: 10.1056/NEJMoa1408913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dubberke ER, Olsen MA. 2012. Burden of Clostridium difficile on the healthcare system. Clin Infect Dis 55(Suppl 2):S88–S92. doi: 10.1093/cid/cis335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miller BA, Chen LF, Sexton DJ, Anderson DJ. 2011. Comparison of the burdens of hospital-onset, healthcare facility-associated Clostridium difficile infection and of healthcare-associated infection due to methicillin-resistant Staphylococcus aureus in community hospitals. Infect Control Hosp Epidemiol 32:387–390. doi: 10.1086/659156. [DOI] [PubMed] [Google Scholar]
- 5.CDC. 2013. Antibiotic resistance threats in the United States, 2013. www.cdc.gov/drugresistance/pdf/ar-threats-2013-508.pdf Accessed 6 July 2017.
- 6.Dethlefsen L, Relman DA. 2011. Incomplete recovery and individualized responses of the human distal gut microbiota to repeated antibiotic perturbation. Proc Natl Acad Sci U S A 108(Suppl 1):S4554–S4561. doi: 10.1073/pnas.1000087107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Theriot CM, Young VB. 2015. Interactions between the gastrointestinal microbiome and Clostridium difficile. Annu Rev Microbiol 69:445–461. doi: 10.1146/annurev-micro-091014-104115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leonard FC, Andremont AO, Tancrede CH. 1985. In vivo influence of three β-lactam antibiotics on the intestinal microflora of man. A preliminary study in gnotobiotic mice. Prog Clin Biol Res 181:279–282. [PubMed] [Google Scholar]
- 9.Stiefel U, Pultz NJ, Harmoinen J, Koski P, Lindevall K, Helfand MS, Donskey CJ. 2003. Oral administration of β-lactamase preserves colonization resistance of piperacillin-treated mice. J Infect Dis 188:1605–1609. doi: 10.1086/379153. [DOI] [PubMed] [Google Scholar]
- 10.Harmoinen J, Mentula S, Heikkila M, van der Rest M, Rajala-Schultz PJ, Donskey CJ, Frias R, Koski P, Wickstrand N, Jousimies-Somer H, Westermarck E, Lindevall K. 2004. Orally administered targeted recombinant β-lactamase prevents ampicillin-induced selective pressure on the gut microbiota: a novel approach to reducing antimicrobial resistance. Antimicrob Agents Chemother 48:75–79. doi: 10.1128/AAC.48.1.75-79.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tarkkanen AM, Heinonen T, Jogi R, Mentula S, van der Rest ME, Donskey CJ, Kemppainen T, Gurbanov K, Nord CE. 2009. P1A recombinant β-lactamase prevents emergence of antimicrobial resistance in gut microflora of healthy subjects during intravenous administration of ampicillin. Antimicrob Agents Chemother 53:2455–2462. doi: 10.1128/AAC.00853-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaleko M, Bristol JA, Hubert S, Parsley T, Widmer G, Tzipori S, Subramanian P, Hasan N, Koski P, Kokai-Kun J, Sliman J, Jones A, Connelly S. 2016. Development of SYN-004, an oral β-lactamase treatment to protect the gut microbiome from antibiotic-mediated damage and prevent Clostridium difficile infection. Anaerobe 41:58–67. doi: 10.1016/j.anaerobe.2016.05.015. [DOI] [PubMed] [Google Scholar]
- 13.Kokai-Kun JF, Roberts T, Coughlin O, Sicard E, Rufiange M, Fedorak R, Carter C, Adams MH, Longstreth J, Whalen H, Sliman J. 2017. The oral β-lactamase SYN-004 (ribaxamase) degrades ceftriaxone excreted into the intestine in phase 2a clinical studies. Antimicrob Agents Chemother 61:e02197-16. doi: 10.1128/AAC.02197-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pepin J, Saheb N, Coulombe MA, Alary ME, Corriveau MP, Authier S, Leblanc M, Rivard G, Bettez M, Primeau V, Nguyen M, Jacob CE, Lanthier L. 2005. Emergence of fluoroquinolones as the predominant risk factor for Clostridium difficile-associated diarrhea: a cohort study during an epidemic in Quebec. Clin Infect Dis 41:1254–1260. doi: 10.1086/496986. [DOI] [PubMed] [Google Scholar]
- 15.Khoder M, Tsapis N, Domergue-Dupont V, Gueutin C, Fattal E. 2010. Removal of residual colonic ciprofloxacin in the rat by activated charcoal entrapped within zinc-pectinate beads. Eur J Pharm Sci 41:281–288. doi: 10.1016/j.ejps.2010.06.018. [DOI] [PubMed] [Google Scholar]
- 16.Grall N, Massias L, Nguyen TT, Sayah-Jeanne S, Ducrot N, Chachaty E, de Gunzburg J, Andremont A. 2013. Oral DAV131, a charcoal-based adsorbent, inhibits intestinal colonization by β-lactam-resistant Klebsiella pneumoniae in cefotaxime-treated mice. Antimicrob Agents Chemother 57:5423–5425. doi: 10.1128/AAC.00039-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Price AB, Larson HE, Crow J. 1979. Morphology of experimental antibiotic-associated enterocolitis in the hamster: a model for human pseudomembranous colitis and antibiotic-associated diarrhoea. Gut 20:467–475. doi: 10.1136/gut.20.6.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Swanson RN, Hardy DJ, Shipkowitz NL, Hanson CW, Ramer NC, Fernandes PB, Clement JJ. 1991. In vitro and in vivo evaluation of tiacumicins B and C against Clostridium difficile. Antimicrob Agents Chemother 35:1108–1111. doi: 10.1128/AAC.35.6.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Babcock GJ, Broering TJ, Hernandez HJ, Mandell RB, Donahue K, Boatright N, Stack AM, Lowy I, Graziano R, Molrine D, Ambrosino DM, Thomas WD Jr. 2006. Human monoclonal antibodies directed against toxins A and B prevent Clostridium difficile-induced mortality in hamsters. Infect Immun 74:6339–6347. doi: 10.1128/IAI.00982-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barker RH Jr, Dagher R, Davidson DM, Marquis JK. 2006. Review article: tolevamer, a novel toxin-binding polymer: overview of preclinical pharmacology and physicochemical properties. Aliment Pharmacol Ther 24:1525–1534. doi: 10.1111/j.1365-2036.2006.03157.x. [DOI] [PubMed] [Google Scholar]
- 21.Burdet C, Nguyen TT, Saint-Lu N, Sayah-Jeanne S, Weiss W, Pulse M, Andremont A, Mentré F, de Gunzburg J. 2016. IDWeek 2016, New Orleans, LA, 26 to 30 October 2016, poster 2252. [Google Scholar]
- 22.Kurtz CB, Cannon EP, Brezzani A, Pitruzzello M, Dinardo C, Rinard E, Acheson DW, Fitzpatrick R, Kelly P, Shackett K, Papoulis AT, Goddard PJ, Barker RH Jr, Palace GP, Klinger JD. 2001. GT160-246, a toxin binding polymer for treatment of Clostridium difficile colitis. Antimicrob Agents Chemother 45:2340–2347. doi: 10.1128/AAC.45.8.2340-2347.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Phillips ST, Nagaro K, Sambol SP, Johnson S, Gerding DN. 2011. Susceptibility of hamsters to infection by historic and epidemic BI Clostridium difficile strains during daily administration of three fluoroquinolones. Anaerobe 17:166–169. doi: 10.1016/j.anaerobe.2011.03.015. [DOI] [PubMed] [Google Scholar]
- 24.Burkhardt O, Borner K, Stass H, Beyer G, Allewelt M, Nord CE, Lode H. 2002. Single- and multiple-dose pharmacokinetics of oral moxifloxacin and clarithromycin, and concentrations in serum, saliva and faeces. Scand J Infect Dis 34:898–903. doi: 10.1080/0036554021000026963. [DOI] [PubMed] [Google Scholar]
- 25.de Gunzburg J, Ducher A, Modess C, Wegner D, Oswald S, Dressman J, Augustin V, Feger C, Andremont A, Weitschies W, Siegmund W. 2015. Targeted adsorption of molecules in the colon with the novel adsorbent-based medicinal product, DAV132: a proof of concept study in healthy subjects. J Clin Pharmacol 55:10–16. doi: 10.1002/jcph.359. [DOI] [PubMed] [Google Scholar]
- 26.FDA. 2008. Avelox label. https://www.accessdata.fda.gov/drugsatfda_docs/label/2008/021085s039,021277s033lbl.pdf Accessed 6 July 2017.
- 27.National Research Council. 2011. Guide for the care and use of laboratory animals, 8th ed. National Academies Press, Washington, DC. [Google Scholar]
- 28.Kampougeris G, Antoniadou A, Kavouklis E, Chryssouli Z, Giamarellou H. 2005. Penetration of moxifloxacin into the human aqueous humour after oral administration. Br J Ophthalmol 89:628–631. doi: 10.1136/bjo.2004.050054. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.