ABSTRACT
The fluorocycline TP-271 was evaluated in mouse and nonhuman primate (NHP) models of inhalational anthrax. BALB/c mice were exposed by nose-only aerosol to Bacillus anthracis Ames spores at a level of 18 to 88 lethal doses sufficient to kill 50% of exposed individuals (LD50). When 21 days of once-daily dosing was initiated at 24 h postchallenge (the postexposure prophylaxis [PEP] study), the rates of survival for the groups treated with TP-271 at 3, 6, 12, and 18 mg/kg of body weight were 90%, 95%, 95%, and 84%, respectively. When 21 days of dosing was initiated at 48 h postchallenge (the treatment [Tx] study), the rates of survival for the groups treated with TP-271 at 6, 12, and 18 mg/kg TP-271 were 100%, 91%, and 81%, respectively. No deaths of TP-271-treated mice occurred during the 39-day posttreatment observation period. In the NHP model, cynomolgus macaques received an average dose of 197 LD50 of B. anthracis Ames spore equivalents using a head-only inhalation exposure chamber, and once-daily treatment of 1 mg/kg TP-271 lasting for 14 or 21 days was initiated within 3 h of detection of protective antigen (PA) in the blood. No (0/8) animals in the vehicle control-treated group survived, whereas all 8 infected macaques treated for 21 days and 4 of 6 macaques in the 14-day treatment group survived to the end of the study (56 days postchallenge). All survivors developed toxin-neutralizing and anti-PA IgG antibodies, indicating an immunologic response. On the basis of the results obtained with the mouse and NHP models, TP-271 shows promise as a countermeasure for the treatment of inhalational anthrax.
KEYWORDS: TP-271, fluorocycline, anthrax, Bacillus anthracis
INTRODUCTION
Anthrax is a serious disease caused by Bacillus anthracis, a Gram-positive, rod-shaped, facultatively anaerobic, spore-forming bacterium. Human disease is usually described as cutaneous, injectional, gastrointestinal, or inhalational, depending on the route of infection (1, 2). Inhalational anthrax is considered the most lethal form of anthrax and the form of disease that would cause the greatest number of fatalities resulting from an aerosolized release of spores in a bioterrorist mass attack (1, 3). Interest in new therapies for anthrax has increased within the research community due to the outbreak of inhalational anthrax resulting from the intentional release of B. anthracis during the 2001 civilian attacks in the United States (4). From extrapolation of data from studies with animals, the lethal dose sufficient to kill 50% of exposed individuals (LD50) is estimated to be 2,500 to 55,000 inhaled B. anthracis spores (1). Spores germinate in tissues at the site of infection and in lymph nodes, and vegetative bacteria produce a toxin, which leads to hemorrhage, edema, and necrosis. Mortality caused by B. anthracis is predominantly due to three plasmid-encoded virulence factors: a factor responsible for encapsulation (carried on plasmid pXO2) and factors responsible for the production of two toxins (carried on plasmid pXO1). The polyglutamate capsule that prevents phagocytosis of the bacterium and a tripartite toxin consisting of three polypeptides (protective antigen [PA], lethal factor [LF], and edema factor [EF]) interact to form two toxins that damage host cells (5). Both the toxin and the bacteria are able to disseminate into the systemic circulation and cause sepsis and septic shock (6). The incubation time for the onset of disease following exposure can range from 1 day to several weeks (2), and initial clinical symptoms and signs are nonspecific and similar to those of influenza and community-acquired pneumonia (malaise, headache, fever, nausea, and vomiting [1, 7]). These are followed by a sudden onset of respiratory distress with dyspnea, stridor, cyanosis, and chest pain. The onset of respiratory distress is followed by shock and death, with the mortality rate being close to 100%.
Antibiotic susceptibility testing of diverse isolate collections has shown that naturally occurring B. anthracis isolates are generally susceptible to most clinically used antibiotic classes (8–10); however, as with any other bacterial species, antibiotic resistance development can arise during antibiotic therapy (11) or it can be deliberately selected for or engineered (10). In retrospective analyses of patients with inhalational anthrax leading to systemic disease, antibiotic combination therapy was more successful than monotherapeutic regimens (3, 6). In the event of an anthrax mass casualty incident, the Centers for Disease Control and Prevention recommends triple intravenous (i.v.) antimicrobial therapy, when available, consisting of two bactericidal agents possessing good blood-brain barrier penetration (a fluoroquinolone and a β-lactam) plus a protein synthesis inhibitor (linezolid, clindamycin, chloramphenicol) or rifampin; if the possibility of meningitis can be excluded, only one of the bactericidal agents plus a protein synthesis inhibitor may be used (7). Since spores are not susceptible to antibiotics, treatment should start, ideally, soon after exposure and continue for at least 60 days to clear late-germinating B. anthracis spores and prevent a relapse of disease (12). Ciprofloxacin, levofloxacin, moxifloxacin, and doxycycline are FDA approved for use in postexposure prophylaxis for inhalational anthrax in adults. In addition to antibiotic therapy, an anthrax vaccine is approved for prevention and use postexposure in combination with antibiotics (13, 14). Antitoxin therapy, used in combination with appropriate antibiotics, is also approved for the treatment of inhalational anthrax (15–17).
The threat of deliberately engineered antibiotic-resistant biothreat pathogens warrants the development of novel agents that can serve as effective countermeasures against drug-resistant bioweapons. TP-271 is a novel fluorocycline antibiotic of the tetracycline class with potent activity against susceptible and antibiotic-resistant respiratory pathogens in vitro (18). Because of its broad-spectrum potency in vitro and the history of the effective use of tetracycline antibiotics in the treatment of serious infections due to inhalational exposure to biothreat pathogens, TP-271 is being considered for development as a countermeasure in the event of a bioterrorist attack. In the present study, TP-271 showed potent inhibition of B. anthracis in vitro. For the treatment of anthrax disease, similar to other antibiotics targeting translation, the antitranslational activity of TP-271 may also provide an additional benefit by inhibiting toxin production (18, 19). Since it is neither ethical nor feasible to run clinical studies to test TP-271 against human inhalational anthrax, efficacy against this respiratory infection must be assessed in animal models in which animals display an anthrax disease pathology similar to that in humans (20, 21). These studies assessed the nonclinical efficacy of TP-271 against inhalational anthrax as a proof of concept in models of anthrax in mice and cynomolgus monkeys (nonhuman primates [NHPs]). The NHP model of inhalational anthrax has been used in the pivotal studies for a recently approved vaccine and several new anthrax antitoxin therapies per guidance from the FDA Animal Rule (13, 15–17, 21). In the NHP model, approved antitoxin monotherapies produced survival rates of 31% to 70% when treatment was initiated in animals exhibiting disease symptoms including toxemia and bacteremia. In the studies with mouse and NHPs reported here, the TP-271 dosage was selected on the basis of the determination of tolerability and pharmacokinetic exposures from multiple-dose studies in uninfected animals (not shown).
RESULTS
In vitro susceptibility of the B. anthracis Ames strain.
The activity of TP-271 against a panel of 30 B. anthracis isolates was tested in vitro, and TP-271 showed MIC50 values and MIC90 values for the isolates of ≤0.008 μg/ml (Table 1). The activity of TP-271 was comparable to that of doxycycline and minocycline, and TP-271 was more potent than ciprofloxacin. The TP-271 and doxycycline MIC values for the B. anthracis Ames challenge isolates in the mouse studies were 0.008 μg/ml and 0.016 to 0.031 μg/ml, respectively, and in the NHP study, the TP-271 and doxycycline MIC values were 0.002 to 0.004 μg/ml and 0.008 μg/ml, respectively.
TABLE 1.
MIC50/MIC90 of TP-271 and comparators against B. anthracisa
| Antibiotic | MIC50/MIC90 (μg/ml) | MIC range (μg/ml) |
|---|---|---|
| TP-271 | ≤0.008/≤0.008 | ≤0.008–≤0.008 |
| Tetracycline | ≤0.008/0.03 | ≤0.008–0.06 |
| Doxycycline | ≤0.008/≤0.008 | ≤0.008–0.016 |
| Minocycline | ≤0.008/≤0.008 | ≤0.008–≤0.008 |
| Ciprofloxacin | 0.12/0.12 | 0.03–0.25 |
A panel of 30 B. anthracis isolates was tested.
TP-271 efficacy in mouse models of aerosolized B. anthracis infection.
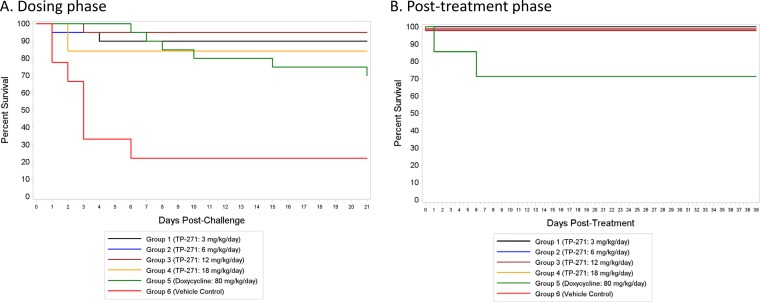
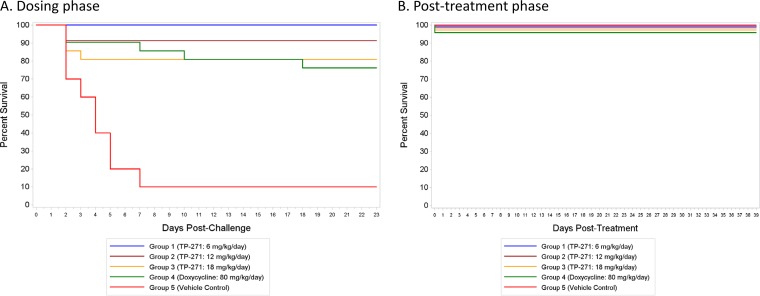
Female BALB/c mice were exposed by nose-only aerosol to B. anthracis Ames spores, and treatment was initiated at either 24 h postchallenge in the postexposure prophylaxis (PEP) study or 48 h postchallenge in the therapeutic treatment (Tx) study and was continued for 21 days. Single daily doses of TP-271 at 3 (PEP study only), 6, 12, and 18 mg/kg of body weight/day for 21 days were generally well tolerated by infected mice and improved the clinical signs in the treated animals. Cultures of blood from a satellite group of 4 to 6 untreated mice in each study, sacrificed at either 42 h (PEP study) or 48 h (Tx study) postinfection, confirmed that the infection progressed to bacteremia in the majority of untreated mice, as expected (22). The mean time to death for the vehicle control-treated groups in both studies was 2.7 to 3.8 days. The rates of survival at the end of dosing in the groups treated with TP-271 at 3, 6, 12, and 18 mg/kg/day in the PEP study were 90% (18/20 mice), 95% (19/20 mice), 95% (19/20 mice), and 84% (16/19 mice), respectively, and the rate of survival was 70% (14/20 mice) in the group treated with doxycycline at 80 mg/kg/day (Fig. 1A); the rate of survival was 22% (2/9) for vehicle control-treated animals in this study. At the end of dosing, similar rates of survival were seen in the groups treated with TP-271 at 6, 12, and 18 mg/kg/day in the Tx study: 100% (23/23 mice), 91% (21/23 mice), and 81% (17/21 mice), respectively. Seventy-six percent (16/21) of the mice in the doxycycline-treated group survived (Fig. 2A), and the rate of survival was 10% (1/10) for the vehicle control-treated animals in this study. During the 39-day posttreatment phase, no TP-271-treated mice in the PEP study (n = 9 to 10 per group) or Tx study (n = 9 to 13 per group) died; however, in the doxycycline group in the PEP study (n = 7), 1 mouse died on day 22 postchallenge and a second mouse died on day 26 postchallenge (Fig. 1B and 2B). All eight mice in the doxycycline group survived during the posttreatment phase of the Tx study.
FIG 1.
Efficacy of TP-271 in a postexposure prophylaxis (PEP) model of inhalational anthrax in mice. Antibiotic and vehicle control dosing was initiated at 24 h postinfection. Survival plots for the 21-day dosing phase (A) and the 39-day posttreatment phase (B) are shown. The lines at 100% survival are drawn staggered for visibility.
FIG 2.
Efficacy of TP-271 in a treatment (Tx) model of inhalational anthrax in mice. Antibiotic and vehicle control dosing was initiated at 48 h postinfection. Survival plots for the 21-day dosing phase (A) and the 39-day posttreatment phase (B) are shown. The lines at 100% survival are drawn staggered for visibility.
For the PEP study, the overall rates of survival for the groups treated with TP-271 at 3, 6, 12, and 18 mg/kg/day during the dosing phase were significantly greater than the rate for the vehicle control-treated group by Fisher's exact tests, with P values being 0.0091, 0.0025, 0.0025, and 0.0333, respectively. The P value for the doxycycline-treated group versus the vehicle control-treated group was 0.2496. A similar analysis of the results of the Tx study showed that the rates of survival for the groups treated with TP-271 at 6, 12, and 18 mg/kg/day and the doxycycline-treated group during the dosing phase were significantly greater than those for the mice in the vehicle control-treated group, with P values being <0.0001, 0.0001, 0.0024, and 0.0055, respectively. In both studies, there were no significant differences in the rates of survival between any of the TP-271- or doxycycline-treated groups at the 0.05 level. Time-to-death comparisons in conjunction with the overall rate of survival between groups in the dosing phase yielded the same significant results when the treated groups were compared to the vehicle control-treated group, demonstrating significant protection in the TP-271-treated groups. During the posttreatment phase, there were no significant differences in the rates of survival or the time to death between any pair of treatment groups (TP-271 or doxycycline) at the 0.05 level in the PEP study, and all of the mice that entered the posttreatment phase in the Tx study survived.
Blood, lung, spleen, and liver samples from approximately half of the surviving mice from the TP-271 and doxycycline treatment groups were assessed for bacteremia (blood) or bacterial burden (tissue samples) after the end of treatment as well as after the posttreatment period. In mice sacrificed at the end of treatment in the PEP study, B. anthracis was found in the lungs of 7/9 (3 mg/kg/day), 8/9 (6 mg/kg/day), 5/9 (12 mg/kg/day), and 3/8 (18 mg/kg/day) mice treated with TP-271 and 6/7 mice treated with doxycycline. Similarly, in the Tx study, B. anthracis was cultured from the lungs of all treated mice (n = 8 to 10 per group) sacrificed at the end of treatment. These findings for the lungs of antibiotic-treated surviving mice are consistent with results obtained by Heine et al. (22). No B. anthracis bacteria were found in the spleen, liver, or blood of treated mice at the end of dosing in either study. At the end of the posttreatment period in both studies, no B. anthracis bacteria were cultured from the lungs, spleen, liver, or blood of any of the surviving animals, except for one animal in the group treated with at TP-271 18 mg/kg/day group in the PEP study, where B. anthracis was found in liver tissue. The TP-271 MIC values for lung B. anthracis isolates from animals that were treated with TP-271 and that died prior to their scheduled necropsy (four animals from the PEP study and six animals from the Tx study) were determined. These B. anthracis isolates exhibited MIC values similar to the MIC for the challenge strain, supporting the suggestion that TP-271 did not select for resistance in B. anthracis during the course of treatment in this study. The levels of TP-271 exposure in infected mice at 24 h and 48 h were similar and comparable to the levels of exposure obtained with similar doses in uninfected mice (data not shown) and in prior efficacy studies (23), indicating that B. anthracis infection did not significantly affect the pharmacokinetics of TP-271 in mice (Table 2). The pharmacokinetics of doxycycline have been reported previously, and the dose of 40 mg/kg administered twice daily was predicted to be efficacious on the basis of successful outcomes in prior infection models (23, 24).
TABLE 2.
Mean composite TP-271 exposure in infected mice given a single i.p. dose of TP-271a
| TP-271 dose (mg/kg/day) |
Cmax (μg/ml) |
AUC0–24 (μg · h/ml) |
||
|---|---|---|---|---|
| PEP study | Tx study | PEP study | Tx study | |
| 3 | 0.83 | NA | 7.18 | NA |
| 6 | 1.09 | 1.65 | 8.91 | 16.90 |
| 12 | 1.98 | 1.57 | 20.3 | 25.70 |
| 18 | 2.75 | 1.83 | 28.2 | 28.40 |
Data from the PEP study are from 24 h postinfection, and data from the Tx study are from 48 h postinfection. NA, not applicable.
Efficacy of TP-271 in an NHP model of aerosolized B. anthracis infection.
The group median times to a positive result by a electrochemiluminescence assay (ECL) for protective antigen (PA) (PA-ECL), which triggered treatment initiation, ranged from approximately 35 to 43.5 h, and all animals except for one animal in the vehicle control-treated group (group 3) had a positive PA-ECL result prior to treatment initiation. Additionally, all animals had positive blood culture results prior to treatment, and all vehicle control-treated animals were bacteremic at the terminal time point.
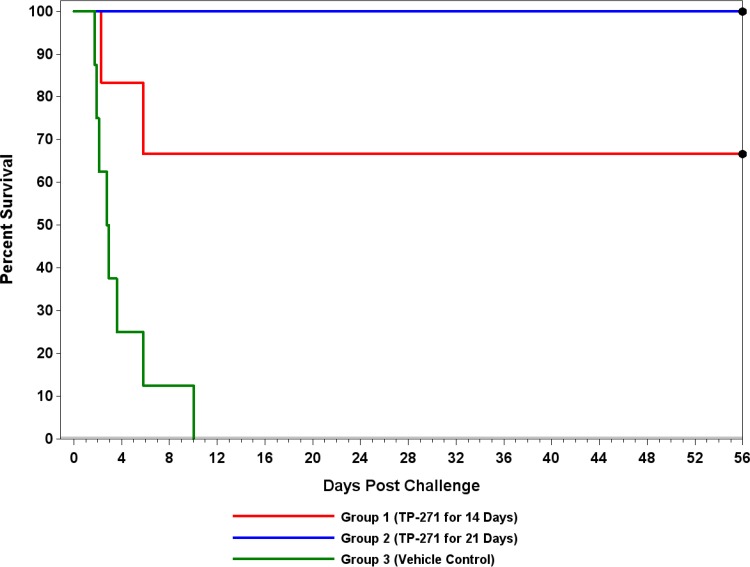
Macaques treated with TP-271 at doses of 1 mg/kg for 14 consecutive days (group 1) and 1 mg/kg for 21 consecutive days (group 2) demonstrated survival rates of 67% (4/6) and 100% (8/8), respectively, whereas none (0/8) of the control animals receiving the vehicle control (group 3) after detection of circulating PA survived inhalational exposure to B. anthracis (Fig. 3). Both deaths in the 14-day treatment group (group 1) occurred during the treatment phase of the study (day 2 and day 6 postchallenge). The survival proportions in each of the treatment groups (groups 1 and 2) were significantly greater than the survival proportion in the control group (group 3), determined using the unadjusted P values (P = 0.0150 and <0.0001, respectively), as well as the Bonferroni-Holm multiple-comparison-adjusted P values (P = 0.0300 and 0.0002, respectively). Differences in the survival rates between group 1 and group 2 were not significantly different at the 0.05 level. The times to death in each of the treatment groups (groups 1 and 2) were significantly greater than the time to death in the control group (group 3), using the unadjusted P values (P = 0.0125 and <0.0001, respectively), as well as the Bonferroni-Holm multiple-comparison-adjusted P values (P = 0.0249 and 0.0001, respectively). Differences between the times to death in group 1 and group 2 were not significantly different at the 0.05 level.
FIG 3.
Efficacy of TP-271 in a treatment (Tx) model of inhalational anthrax in NHPs. TP-271 or vehicle treatment was initiated within 3 h of a positive result for PA by ECL, as described in Materials and Methods.
Gross lesions typical of anthrax were present in animals that died or were euthanized due to their moribund condition, and their deaths were attributed to sepsis secondary to B. anthracis infection. Histologically, the lesions seen in animals with an early death on study were consistent with B. anthracis infection. Culturable B. anthracis was detected at necropsy in the brain, lungs, spleen, and mediastinal lymph node from all eight vehicle control animals. In contrast, B. anthracis was not detected in the brain or mediastinal lymph node from any of the TP-271-treated animals (survivors and nonsurvivors). B. anthracis was detected but was present at levels below the lower limit of quantitation (LLOQ) in the lungs of three surviving group 1 animals (treated with TP-271 for 14 days) and two surviving group 2 animals (treated with TP-271 for 21 days) at the end of the study and in the culture of spleen tissue from one surviving group 2 animal at the end of the study (but the level was below the LLOQ). Quantifiable levels of B. anthracis were detected in the lungs of the two TP-271-treated animals (group 1, treated with TP-271 for 14 days) that succumbed during the study. For one of these animals, isolates were not recoverable from frozen storage, and susceptibility testing of isolates from the other animal showed that the TP-271 MIC for the isolates was the same as the MIC for the challenge strain. Therefore, TP-271 did not select for reduced susceptibility in this animal that died before the end of the study.
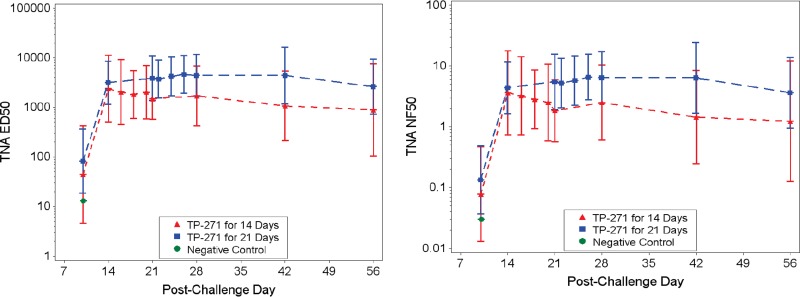
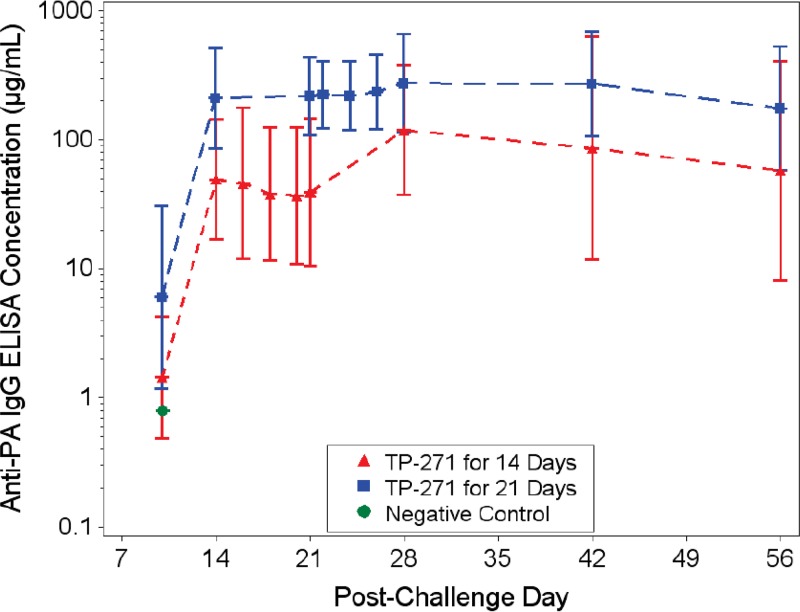
Quantification of PA by enzyme-linked immunosorbent assay (ELISA) showed that at all time points after the initiation of treatment, PA was undetectable in all TP-271-treated animals, except for one animal in group 2 (which received 21 days of treatment) on day 7; the PA levels in this animal were undetectable at all subsequent time points (data not shown). As expected, the 50% effective dilution (ED50) and the 50% neutralization factor (NF50) values obtained by the toxin-neutralizing assay (TNA) were below the limit of detection (LOD) for all animals assessed prior to aerosol challenge (the day −7 time point). The ED50 is the reciprocal of the dilution of a serum sample that results in 50% neutralization of anthrax lethal toxin, and it is defined as the reciprocal of the dilution corresponding to the inflection point (the c parameter) of a 4-parameter logistic log fit of the curve. The NF50 is the quotient of the ED50 of the test sample and the ED50 of the reference serum sample. The NF50 serves as a relative measure of toxin neutralization. By day 14 postchallenge, all survivors for which a sample was analyzed had developed measurable TNA ED50 and NF50 titers, indicating an immunologic response (Fig. 4). At the baseline time point, the anti-PA IgG titer was below the LOD or limit of quantitation (LOQ) for all animals; however, by day 14 postchallenge, all survivors had developed an anti-PA IgG titer (Fig. 5). A regression analysis demonstrated a strong significant linear correlation between the concentrations obtained by the anti-PA ELISA and both the ED50 and NF50 endpoints at each time point postchallenge, especially starting on day 21 or 28 postchallenge, showing a high level of agreement between the TNA and anti-PA ELISA measures of the immune response developing at these later time points (not shown).
FIG 4.
Relative quantification of toxin-neutralizing antibody in the serum of B. anthracis-infected NHPs by the 50% effective dilution (ED50) and the 50% neutralization factor (NF50). Samples were taken on days 10, 14, 21, 28, 42, and 56 and on each of the 3 days after the last treatment and analyzed for toxin-neutralizing antibody (left) and neutralization factor (right). Group geometric mean values with 95% confidence intervals are presented for group 1 (TP-271 for 14 days) and group 2 (TP-271 for 21 days).
FIG 5.
Quantification of anti-PA IgG titers in the serum of B. anthracis-infected NHPs by ELISA. Samples were taken on days 10, 14, 21, 28, 42, and 56 and on each of the 3 days after the last treatment and analyzed for anti-PA IgG titers. Group geometric mean values with 95% confidence intervals are presented for group 1 (TP-271 for 14 days) and group 2 (TP-271 for 21 days).
Animals that succumbed to disease demonstrated a progression of signs that generally followed a hallmark progression from appearing normal to displaying lethargy or a hunched posture, followed by respiratory abnormalities and, finally, moribundity. Animals that survived until the end of the study were most notably documented as having a hunched posture or lethargy within the first few days postchallenge, but most animals returned to normal between 5 and 15 days postchallenge. The mean body weights for the survivors in the TP-271-treated groups remained above the day 0 body weights throughout the postchallenge period.
The values of the pharmacokinetic parameters for TP-271 (Table 3) correlated with the levels of TP-271 exposure in uninfected animals (data not shown) and what was observed in prior efficacy studies (23). After a 14-day administration of TP-271 by intravenous infusion, the mean maximum concentration (Cmax) increased from 0.358 μg/ml (day 1) to 0.813 μg/ml (day 14), a 2.3-fold accumulation. The area under the concentration-time curve from 0 to 24 h (AUC0–24) increased from 1.87 to 3.89 μg · h/ml, a 2.1-fold increase. Cmax increased from 0.265 to 0.426 μg/ml, a 1.6-fold accumulation after 21 daily i.v. infusions. AUC0–24 increased from 1.37 to 2.50 μg · h/ml (day 21), a 1.8-fold accumulation. Minimal to modest accumulation has been reported for TP-271 in prior studies (23).
TABLE 3.
TP-271 exposure in infected cynomolgus macaques given a single i.v. infusion of 1 mg/kg TP-271 on day 1 or after multiple, once-daily i.v. infusions for 14 or 21 days
| Group | Day | Cmaxa (μg/ml) | AUC0–24a (μg · h/ml) |
|---|---|---|---|
| 1 | 1 | 0.358 (0.064) | 1.87 (0.494) |
| 14 | 0.813 (0.811) | 3.89 (2.42) | |
| 2 | 1 | 0.265 (0.059) | 1.37 (0.218) |
| 21 | 0.426 (0.091) | 2.50 (0.510) |
The data represent means (standard deviations).
DISCUSSION
In nonclinical proof-of-concept efficacy studies in both mouse and NHP models of inhalational anthrax caused by B. anthracis Ames, TP-271 showed protection when it was given at relatively low, single daily doses for 14 days (NHP model only) or 21 days (mouse and NHP models). These findings are consistent with TP-271's potency against B. anthracis and the long-standing precedent of using tetracycline-class antibiotics to treat infections caused by B. anthracis. The Ames strain used in these studies is a relatively susceptible isolate; however, TP-271's demonstrated activity against public health pathogens expressing prevalent tetracycline-specific resistance mechanisms and resistance to other classes of antibiotics (18) suggests that TP-271 would also be efficacious against B. anthracis expressing a variety of antibiotic resistance mechanisms.
In the mouse model, TP-271 provided complete or nearly complete protection at the lowest doses tested: 3 mg/kg/day in a PEP model and 6 mg/kg/day in a Tx model. TP-271 was effective when it was administered at the onset of bacteremia. Protection from TP-271 was durable throughout the posttreatment observation period, showing no relapse and results exceeding those seen in the doxycycline treatment group in the PEP study. It was apparent that treatment for 14 or 21 days under the conditions of the NHP study resulted in adequate clearing of the bacteria and survival to the end of the study. When bacteria were recoverable from animals with unscheduled deaths in the mouse and the NHP models, the susceptibilities to TP-271 were unchanged from those of the challenge strains, supporting the suggestion that the deaths were not due to the emergence of TP-271-resistant isolates during treatment in either the mouse or the NHP model.
The virulence of B. anthracis is due to the production of a tripartite toxin and a poly-d-glutamate capsule expressed by two plasmids in pathogenic strains (5). In the NHP model, most animals had no detectable PA in their serum after the initiation of treatment; this may have been due in part to the antitranslational activity of TP-271 contributing to reduced toxin production, as has been seen in vitro with other translation inhibitors (19), in addition to a reduction in the bacterial load promoted by TP-271 and the immune system. Others have also shown that translation inhibitors may rapidly reduce the spore population during infection in vitro, keeping B. anthracis in an antibiotic-sensitive vegetative state (25). In the NHP model, not only did TP-271 treatment confer protection from overwhelming infection, but also animals that survived to the end of the study produced toxin-neutralizing antibodies, as reflected in the titers measured by TNA. Further, the vast majority of animals that survived to the end of the study had no detectable B. anthracis bacteria in the tissues examined in this study. These results suggest that TP-271 did not interfere with the host generation of a productive immune response.
Previous work showed that TP-271 was efficacious in mouse models of pneumonia in which the mice were challenged with tetracycline-resistant community-acquired bacterial respiratory pathogens (18). In addition to the proof-of-concept efficacy against inhalational anthrax presented here, TP-271 was previously shown to be efficacious in a mouse model of plague (26) and mouse and NHP models of tularemia (23); this supports the potential for the use of TP-271 as a broad-spectrum medical countermeasure if results in mouse and NHP models translate to efficacy against human disease.
Similar to observations made in prior multidose studies of the activity of TP-271 against tularemia (23), TP-271 was well tolerated in both mice and NHPs, improved the clinical signs in treated animals, and produced adequate plasma exposures for protection against inhalational anthrax. The duration of effective TP-271 dosing in these nonclinical studies was shorter than the recommended duration (60 days) for antibiotic therapy in human anthrax disease (12). TP-271 is currently in phase 1 clinical development, in which the safety and exposures in healthy volunteers receiving TP-271 i.v. and orally are being evaluated. Subsequent clinical evaluation will support the efficacy and safety during the treatment of bacterial respiratory infections in humans, and additional studies examining the efficacy of TP-271 against inhalational anthrax in NHPs per guidance from the FDA Animal Rule (21) will help to determine the optimal dose and treatment duration for TP-271 in the treatment of inhalational anthrax in humans.
MATERIALS AND METHODS
Antibiotics and formulation preparation.
For in vitro susceptibility testing, commercial-grade antibiotics were obtained from Sigma-Aldrich, (St. Louis, MO), and TP-271 was synthesized at Tetraphase Pharmaceuticals as described previously by Xiao et al. (27). Antibiotics for susceptibility testing were prepared in sterile deionized water and stored frozen at ≤−65°C. Dose formulations were prepared as described previously (23).
Susceptibility testing of B. anthracis.
All MIC assays were performed at CUBRC (Buffalo, NY) or at the United States Army Medical Research Institute of Infectious Diseases (USAMRIID; Frederick, MD) according to Clinical and Laboratory Standards Institute (CLSI) guidelines (28) under biosafety level 3 (BSL-3) laboratory conditions. The B. anthracis strains used in the MIC50/MIC90 panel were from the USAMRIID collection and were selected to represent the eight genotype clades identified by Keim et al. (29) and geographic diversity. Appropriate quality control organisms were obtained from the American Type Culture Collection (ATCC; Manassas, VA).
Animal infection models.
Mouse studies were performed at the IIT Research Institute, Chicago, IL, and the NHP study was performed at Battelle, Columbus OH, under BSL-3 conditions using approved protocols of the Institutional Animal Care and Use Committees of those institutions and conforming to the Office of Laboratory Animal Welfare standards.
Challenge material for studies. (i) Mouse study.
For the mouse studies, B. anthracis Ames stocks (sourced from the U.S. government), previously prepared using the medium and growth conditions of Leighton and Doi (30) and stored at approximately 2 to 8°C, were heat shocked for approximately 45 min at 60 to 65°C. After the heat shock step, the titer of the B. anthracis spores was determined by serial dilution with sterile American Society for Testing and Materials (ASTM) type 1 water (purified water with resistivity of 18.0 mΩ-cm or greater at 25°C), and the stocks were plated on Trypticase soy agar (TSA) and incubated at 37°C ± 2°C for approximately 18 to 22 h. The resulting colonies were approximately 2 to 4 mm in diameter with a cream, flat, and matte appearance with an irregular edge. Once the spore titer had been determined, the B. anthracis spores were prepared with sterile water for injection and to a concentration of 1.37 × 109 CFU/ml aerosolized administration.
(ii) NHP study.
NHPs were aerosol challenged with B. anthracis Ames strain spores according to previously described methods (31).
Animals and animal care. (i) Mice.
Female BALB/c mice from Charles River Breeding Laboratories (Raleigh, NC) were approximately 7 weeks old at the time of study initiation. They were received and held in quarantine for approximately 11 days prior to use in each study. The body weight range (taken 1 day after receipt) for a representative sample (≥10%) of mice was 12.7 to 19.9 g. All mice were housed at up to six per cage and given access to food and water ad libitum throughout the studies. Two sources of food were used for this study: Teklad certified global 18% protein rodent diet (2018C; Harlan Laboratories, Indianapolis, IN) was provided ad libitum throughout the study, and DietGel 76A food cups (Clear H2O, Portland, ME) were provided ad libitum after challenge. At 1 day prior to challenge, the mice were relocated into the IIT Research Institute BSL-3 facility.
(ii) Cynomolgus macaques (Macaca fascicularis).
Twenty-four cynomolgus macaques were procured from a USDA-licensed facility. The animals used in this study were ≥3 years of age and weighed between 3.1 and 5.6 kg at the time of exposure to B. anthracis. The macaques were in good health and free of malformations or clinical signs of disease. The animals were prescreened for infection with Mycobacterium tuberculosis, simian immunodeficiency virus, simian T-lymphotropic virus 1, macacine herpesvirus 1, simian retrovirus, Salmonella, Shigella, intestinal parasites, Trypanosoma cruzi, and Klebsiella pneumoniae. Animals were confirmed to be negative for previous exposure to B. anthracis protective antigen (by TNA; see the description of the assay below). Animals were quarantined for approximately 5 weeks prior to implantation of two venous access ports (VAP; in the right and left jugular veins; CATH in CATH 2 ALAT-6; AVA Biomedical, Wilmette, IL). Animals were transferred to the Battelle BSL-3 facility a minimum of 14 days prior to aerosol challenge for acclimation. The animals were not administered any antibiotics or antiparasitic agent within 14 days prior to B. anthracis challenge and underwent a complete health examination by a staff veterinarian prior to challenge. Water was available ad libitum during the entire study, and food was provided per standard operating procedures at Battelle.
Mouse model of anthrax.
Mice were randomized into the experimental groups and exposed by nose-only aerosol to B. anthracis Ames spores. The target exposure was 50 LD50; the approximate BALB/c mouse LD50 determinations for spore preparations were 3.16 × 104 CFU (PEP study) and 6.03 × 104 CFU (Tx study), as determined in prior studies at the IIT Research Institute (data not shown). Each challenge session utilized six Pari LC Plus nebulizers (Pari GmbH, Starnberg, Germany) operating simultaneously at approximately 20 lb/in2 gauge (psig) of house air. The mean exposures per mouse were determined to be 5.70 × 105 CFU (18 LD50) in the PEP study and 5.33 × 106 CFU (88 LD50) in the Tx study. Mice designated for the efficacy arms were challenged in two separate aerosol sessions, and mice designated for the pharmacokinetic arm were challenged in a third aerosol session due to the limited capacity of the aerosolization apparatus. Infected mice from each of the two aerosol sessions in the efficacy arms were distributed across groups to allow relative uniformity of aerosolized exposure between groups. Confirmation of the number of CFU in each challenge inoculum was performed by plating serial dilutions of the culture on TSA and quantifying the number of CFU after approximately 18 to 22 h of incubation at 37°C ± 2°C.
(i) Determination of TP-271 efficacy.
In the PEP efficacy study, the treated groups contained 19 to 20 infected mice each and the vehicle control-treated group contained 9 infected mice. In the Tx efficacy study, the treated groups contained 21 to 23 infected mice in each group and the vehicle control-treated group contained 10 infected mice. For each efficacy study, a separate group of 6 infected mice was sacrificed at 42 h (PEP study) or 48 h (Tx study), and blood was plated on TSA to confirm the progression of the untreated infection to bacteremia. TP-271 dosages were selected on the basis of the determination of tolerability and pharmacokinetic exposures from multiple-dose studies in uninfected mice (data not shown). At 24 h (PEP study) or 48 h (Tx study) postinfection, TP-271 dissolved in pH 6.5 saline was dosed intraperitoneally (i.p.) once daily at 3, 6, 12, or 18 mg/kg/day for 21 days. The positive comparator, doxycycline, was given i.p. every 12 h at 40 mg/kg/dose (total dose, 80 mg/kg/day) for 21 days starting at either 24 h (PEP study) or 48 h (Tx study) postinfection. The dose volume was 15 ml/kg, with a maximum volume of 0.5 ml being administered to each mouse. On day 22 after the initiation of dosing, at 24 h after the final dose of TP-271 or doxycycline, half of the survivors in each group were sacrificed for bacterial burden enumeration, and the survival of the remaining half was monitored for 14 days. Bacterial bioburdens were determined from homogenized blood and lung, liver, and spleen tissue samples collected from the animals scheduled to be sacrificed at the end of dosing and the end of the relapse observation period (excluding the vehicle-treated controls). All unscheduled deaths were qualitatively investigated by homogenizing lung tissue samples and plating the homogenates on TSA for confirmation of the presence of B. anthracis colonies.
(ii) Determination of TP-271 pharmacokinetics in infected mice.
The values of the pharmacokinetic parameters for TP-271 were determined for a separate set of infected mice on the first day of dosing in the PEP and Tx studies as previously described (23). The verified method for bioanalysis of mouse plasma used in this study distinguished TP-271 from its C-4 epimer and had a dynamic range of 2 to 3,000 ng/ml; the quality control intrarun accuracy (percentage of the nominal value) was 102.2 to 113.9%, and the precision (percent coefficient of variation [CV]) was 1.4 to 6.9%.
NHP model of anthrax.
NHPs were randomized by weight (prior to challenge) into one of three groups, followed by randomization by challenge cohort and challenge order. Animals anesthetized with tiletamine-zolazepam (Telazol; approximately 4 mg/kg intramuscularly) were aerosol challenged via a head-only inhalation exposure chamber with a targeted exposure of 200 LD50 (1.24 × 107 B. anthracis Ames spores), presented using a Collison nebulizer; the LD50 of 61,800 CFU was previously published by USAMRIID (31). The average calculated aerosol exposure for all animals was 197 LD50. Treatment was initiated on the basis of the trigger-to-treat PA-ECL assay (rapid protective antigen screening assay; MesoScale Discovery [MSD], Gaithersburg, MD). Serum samples were collected and processed for detection by PA-ECL starting at 24 h postchallenge and then every 6 h until 72 h, as previously described (32). TP-271 or vehicle treatment was initiated within 3 h of a blood collection producing a positive assay result for PA by ECL versus the result for the positive and negative controls. Groups 1 and 2 received TP-271 (1 mg/kg) for a maximum of 14 and 21 days, respectively, and group 3 received the negative-control article (saline for injection, pH 6.0) via a once-daily 90-min infusion through the VAP not used for blood collection. All dose volumes through day 7 postchallenge were based on the body weights measured on day 0. Dose volumes after day 7 postchallenge were modified on the basis of the most recent body weight measurement. Animals were monitored daily for clinical signs and weekly for weight during dosing and after treatment until the end of the study, which was at 56 days postchallenge. Bacteremia was monitored postchallenge by culture of blood starting at 24 h, then every 6 h until 72 h, and then on days 7, 10, 14, 21, 28, 35, 42, and 56, as previously described (32). In addition to the ECL method used to determine the treatment trigger, a PA ELISA directly quantifying the PA in serum was performed retrospectively on frozen serum samples for the quantification of circulating PA on days 1, 2, and 3; immediately pretreatment; and on days 7, 14, 28, and 56, as previously described (32). Toxin-neutralizing antibodies and anti-PA IgG titers were monitored by the TNA and the ELISA, respectively, on days 10, 14, 21, 28, 42, and 56 and on each of the 3 days after the last treatment, as previously described (33, 34).
A complete necropsy was conducted on all animals under the supervision of a veterinary pathologist board certified by the American College of Veterinary Pathologists. The bacterial burden in the lung, liver, mediastinal lymph nodes, and spleen was determined by plating homogenized ∼1-cm3 tissue samples onto blood agar.
Blood samples for plasma isolation were collected predosing, at the end of infusion, and at 2, 4, 8, and 24 h postdosing on dosing day 1 and day 14 (group 1) or day 21 (group 2), and the values of the pharmacokinetic parameters were determined as previously described (23). The validated method for the bioanalysis of cynomolgus monkey plasma used in this study distinguished TP-271 from its C-4 epimer and had a dynamic range of 2 to 3,000 ng/ml; the quality control intrarun accuracy (percentage of the nominal value) was 93.6 to 104.6%, and the precision (percent CV) was 3.8 to 10.2%.
Statistical analysis of survival data.
In a primary analysis for each group, survival rates and the corresponding 95% exact binomial confidence intervals were calculated and are presented. One-sided Fisher's exact tests were used to compare the survival rates for each of the treated groups to the survival rate for the vehicle control-treated group. In addition, a similar test was conducted to compare the survival rates between the treatment groups. Because multiple tests were being run on the same data, a Bonferroni-Holm adjustment was used to maintain an overall 0.05 level of significance for these tests. In a secondary analysis taking into account survival time as well as survival rates, a time-to-death analysis was also performed on these data to determine if there were differences in protection for the different groups on the basis of a length-of-survival model. The Kaplan-Meier estimates of survival probabilities were plotted to visually represent the survival rates and the times to death for each group. Pairwise log-rank tests were used to determine if there were significant differences between the groups when time-to-death data were combined with survival data and, if so, which groups had significantly different data. The Bonferroni-Holm adjustment was used to maintain an overall 0.05 level of significance.
For each animal and each of six comparable time points (days 10, 14, 21, 28, 42, and 56 postchallenge), the anti-PA ELISA reportable value was matched with both the corresponding TNA ED50 and the TNA NF50 endpoints. Linear regression models were fitted to each of the log10 TNA ED50 and log10 TNA NF50 endpoints as functions of the log10 anti-PA ELISA values separately for each day postchallenge. Data were included in the regressions only for those matched pairs of values where both the TNA and anti-PA ELISA values were above their LOD and LLOQ, respectively. Available measurable data from both treatment groups were included together in all regressions. Estimated regression coefficients with the slope of P values were compiled, and the correlation coefficient was estimated from each linear model as the square root of the R2 value.
ACKNOWLEDGMENTS
These studies were funded by NIAID partnership grant number 1R01AI093484-01 and NIAID contract number HHSN272201100028C, awarded to CUBRC and Tetraphase Pharmaceuticals.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
We acknowledge the contributions from the Tetraphase Chemistry Department for the synthesis of TP-271 for these studies and appreciate the support of Katie Edwards at CUBRC.
REFERENCES
- 1.Inglesby TV, O'Toole T, Henderson DA, Bartlett JG, Ascher MS, Eitzen E, Friedlander AM, Gerberding J, Hauer J, Hughes J, McDade J, Osterholm MT, Parker G, Perl TM, Russell PK, Tonat K. 2002. Anthrax as a biological weapon, 2002: updated recommendations for management. JAMA 287:2236–2252. doi: 10.1001/jama.287.17.2236. [DOI] [PubMed] [Google Scholar]
- 2.Adalja AA, Toner E, Inglesby TV. 2015. Clinical management of potential bioterrorism-related conditions. N Engl J Med 372:954–962. doi: 10.1056/NEJMra1409755. [DOI] [PubMed] [Google Scholar]
- 3.Holty JE, Bravata DM, Liu H, Olshen RA, McDonald KM, Owens DK. 2006. Systematic review: a century of inhalational anthrax cases from 1900 to 2005. Ann Intern Med 144:270–280. doi: 10.7326/0003-4819-144-4-200602210-00009. [DOI] [PubMed] [Google Scholar]
- 4.Jernigan JA, Stephens DS, Ashford DA, Omenaca C, Topiel MS, Galbraith M, Tapper M, Fisk TL, Zaki S, Popovic T, Meyer RF, Quinn CP, Harper SA, Fridkin SK, Sejvar JJ, Shepard CW, McConnell M, Guarner J, Shieh WJ, Malecki JM, Gerberding JL, Hughes JM, Perkins BA. 2001. Bioterrorism-related inhalational anthrax: the first 10 cases reported in the United States. Emerg Infect Dis 7:933–944. doi: 10.3201/eid0706.010604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goel AK. 2015. Anthrax: a disease of biowarfare and public health importance. World J Clin Cases 3:20–33. doi: 10.12998/wjcc.v3.i1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pillai SK, Huang E, Guarnizo JT, Hoyle JD, Katharios-Lanwermeyer S, Turski TK, Bower WA, Hendricks KA, Meaney-Delman D. 2015. Antimicrobial treatment for systemic anthrax: analysis of cases from 1945 to 2014 identified through a systematic literature review. Health Secur 13:355–364. doi: 10.1089/hs.2015.0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bower WA, Hendricks K, Pillai S, Guarnizo J, Meaney-Delman D, Centers for Disease Control and Prevention. 2015. Clinical framework and medical countermeasure use during an anthrax mass-casualty incident. Morb Mortal Wkly Rep 64:1–22. [DOI] [PubMed] [Google Scholar]
- 8.Jones ME, Goguen J, Critchley IA, Draghi DC, Karlowsky JA, Sahm DF, Porschen R, Patra G, DelVecchio VG. 2003. Antibiotic susceptibility of isolates of Bacillus anthracis, a bacterial pathogen with the potential to be used in biowarfare. Clin Microbiol Infect 9:984–986. doi: 10.1046/j.1469-0691.2003.00775.x. [DOI] [PubMed] [Google Scholar]
- 9.Mohammed MJ, Marston CK, Popovic T, Weyant RS, Tenover FC. 2002. Antimicrobial susceptibility testing of Bacillus anthracis: comparison of results obtained by using the National Committee for Clinical Laboratory Standards broth microdilution reference and Etest agar gradient diffusion methods. J Clin Microbiol 40:1902–1907. doi: 10.1128/JCM.40.6.1902-1907.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Athamna A, Athamna M, Abu-Rashed N, Medlej B, Bast DJ, Rubinstein E. 2004. Selection of Bacillus anthracis isolates resistant to antibiotics. J Antimicrob Chemother 54:424–428. doi: 10.1093/jac/dkh258. [DOI] [PubMed] [Google Scholar]
- 11.Brouillard JE, Terriff CM, Tofan A, Garrison MW. 2006. Antibiotic selection and resistance issues with fluoroquinolones and doxycycline against bioterrorism agents. Pharmacotherapy 26:3–14. doi: 10.1592/phco.2006.26.1.3. [DOI] [PubMed] [Google Scholar]
- 12.Hendricks KA, Wright ME, Shadomy SV, Bradley JS, Morrow MG, Pavia AT, Rubinstein E, Holty JE, Messonnier NE, Smith TL, Pesik N, Treadwell TA, Bower WA. 2014. Centers for Disease Control and Prevention expert panel meetings on prevention and treatment of anthrax in adults. Emerg Infect Dis 20:130687. doi: 10.3201/eid2002.130687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Emergent BioDefense Operations Lansing LLC. 2015. Biothrax suspension for intramuscular or subcutaneous injection, prescribing information. Emergent BioDefense Operations Lansing LLC, Lansing, MI. [Google Scholar]
- 14.Wright JG, Quinn CP, Shadomy S, Messonnier N, Centers for Disease Control and Prevention. 2010. Use of anthrax vaccine in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2009. MMWR Recommend Rep 59(RR-6):1–30. [PubMed] [Google Scholar]
- 15.Cangene Corporation. 2015. Prescribing information for Anthrasil injection for intravenous use. Cangene Corporation, Emergent BioSolutions, Winnipeg, MB, Canada. [Google Scholar]
- 16.GlaxoSmithKline. 2016. Prescribing information for Raxibacumab injection for intravenous use. GlaxoSmithKline, Research Triangle Park, NC. [Google Scholar]
- 17.Elusys Therapeutics, Inc. 2016. Prescribing information for Anthim injection for intravenous use. Elusys Therapeutics, Inc, Pine Brook, NJ. [Google Scholar]
- 18.Grossman TH, Fyfe C, O'Brien W, Hackel M, Minyard MB, Waites KB, Dubois J, Murphy TM, Slee AM, Weiss WJ, Sutcliffe JA. 2017. Fluorocycline TP-271 is potent against complicated community-acquired bacterial pneumonia pathogens. mSphere 2:e00004-17. doi: 10.1128/mSphere.00004-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Louie A, Vanscoy BD, Heine HS III, Liu W, Abshire T, Holman K, Kulawy R, Brown DL, Drusano GL. 2012. Differential effects of linezolid and ciprofloxacin on toxin production by Bacillus anthracis in an in vitro pharmacodynamic system. Antimicrob Agents Chemother 56:513–517. doi: 10.1128/AAC.05724-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Twenhafel NA. 2010. Pathology of inhalational anthrax animal models. Vet Pathol 47:819–830. doi: 10.1177/0300985810378112. [DOI] [PubMed] [Google Scholar]
- 21.FDA. 2015. Product development under the Animal Rule—guidance for industry. CBER, CDER, FDA, U.S. Department of Health and Human Services. [Google Scholar]
- 22.Heine HS, Bassett J, Miller L, Hartings JM, Ivins BE, Pitt ML, Fritz D, Norris SL, Byrne WR. 2007. Determination of antibiotic efficacy against Bacillus anthracis in a mouse aerosol challenge model. Antimicrob Agents Chemother 51:1373–1379. doi: 10.1128/AAC.01050-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grossman TH, Anderson MS, Christ D, Gooldy M, Henning LN, Heine HS, Kindt MV, Lin W, Siefkas-Patterson K, Radcliff AK, Tam VH, Sutcliffe JA. 2017. The fluorocycline TP-271 is efficacious in models of aerosolized Francisella tularensis SCHU S4 infection in BALB/c mice and cynomolgus macaques. Antimicrob Agents Chemother 61:e00448-17. doi: 10.1128/AAC.00448-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heine HS, Louie A, Sorgel F, Bassett J, Miller L, Sullivan LJ, Kinzig-Schippers M, Drusano GL. 2007. Comparison of 2 antibiotics that inhibit protein synthesis for the treatment of infection with Yersinia pestis delivered by aerosol in a mouse model of pneumonic plague. J Infect Dis 196:782–787. doi: 10.1086/520547. [DOI] [PubMed] [Google Scholar]
- 25.Louie A, VanScoy BD, Brown DL, Kulawy RW, Heine HS, Drusano GL. 2012. Impact of spores on the comparative efficacies of five antibiotics for treatment of Bacillus anthracis in an in vitro hollow fiber pharmacodynamic model. Antimicrob Agents Chemother 56:1229–1239. doi: 10.1128/AAC.01109-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grossman TH, Lin W, Siefkas-Patterson K, Gooldy M, Sutcliffe JA. 2014. TP-271, a novel fluorocycline, is efficacious in a post-exposure prophylaxis (PEP) model of aerosolized Yersinia pestis infection in BALB/c mice, abstr F-1599. Abstr 54th Intersci Conf Antimicrob Agents Chemother. American Society for Microbiology, Washington, DC. [Google Scholar]
- 27.Xiao XY, Hunt DK, Zhou J, Clark RB, Dunwoody N, Fyfe C, Grossman TH, O'Brien WJ, Plamondon L, Ronn M, Sun C, Zhang WY, Sutcliffe JA. 2012. Fluorocyclines. 1. 7-Fluoro-9-pyrrolidinoacetamido-6-demethyl-6-deoxytetracycline: a potent, broad spectrum antibacterial agent. J Med Chem 55:597–605. doi: 10.1021/jm201465w. [DOI] [PubMed] [Google Scholar]
- 28.Clinical and Laboratory Standards Institute. 2015. Methods for antimicrobial dilution and disk susceptibility testing of infrequently isolated or fastidious bacteria. CLSI document M45, 3rd ed. Clinical and Laboratory Standards Institute, Wayne, PA. [DOI] [PubMed] [Google Scholar]
- 29.Keim P, Price LB, Klevytska AM, Smith KL, Schupp JM, Okinaka R, Jackson PJ, Hugh-Jones ME. 2000. Multiple-locus variable-number tandem repeat analysis reveals genetic relationships within Bacillus anthracis. J Bacteriol 182:2928–2936. doi: 10.1128/JB.182.10.2928-2936.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leighton TJ, Doi RH. 1971. The stability of messenger ribonucleic acid during sporulation in Bacillus subtilis. J Biol Chem 246:3189–3195. [PubMed] [Google Scholar]
- 31.Vasconcelos D, Barnewall R, Babin M, Hunt R, Estep J, Nielsen C, Carnes R, Carney J. 2003. Pathology of inhalation anthrax in cynomolgus monkeys (Macaca fascicularis). Lab Invest 83:1201–1209. doi: 10.1097/01.LAB.0000080599.43791.01. [DOI] [PubMed] [Google Scholar]
- 32.Henning LN, Comer JE, Stark GV, Ray BD, Tordoff KP, Knostman KA, Meister GT. 2012. Development of an inhalational Bacillus anthracis exposure therapeutic model in cynomolgus macaques. Clin Vaccine Immunol 19:1765–1775. doi: 10.1128/CVI.00288-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ionin B, Hopkins RJ, Pleune B, Sivko GS, Reid FM, Clement KH, Rudge TL Jr, Stark GV, Innes A, Sari S, Guina T, Howard C, Smith J, Swoboda ML, Vert-Wong E, Johnson V, Nabors GS, Skiadopoulos MH. 2013. Evaluation of immunogenicity and efficacy of anthrax vaccine adsorbed for postexposure prophylaxis. Clin Vaccine Immunol 20:1016–1026. doi: 10.1128/CVI.00099-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stark GV, Sivko GS, VanRaden M, Schiffer J, Taylor KL, Hewitt JA, Quinn CP, Nuzum EO. 2016. Cross-species prediction of human survival probabilities for accelerated anthrax vaccine absorbed (AVA) regimens and the potential for vaccine and antibiotic dose sparing. Vaccine 34:6512–6517. doi: 10.1016/j.vaccine.2016.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]