ABSTRACT
Linezolid serum trough (Cmin) and peak (Cmax) levels were determined prospectively in 90 patients. Adequate exposure was defined as a Cmin of 2 to 8 mg/liter. Therapy was empirical (73.3%) or targeted (26.7%). Wide interindividual variability in linezolid Cmin levels was recorded (0.1 to 25.2 μg/ml). Overall, 65.5% of the patients had out-of-range, 41.1% had subtherapeutic, and 24.4% had supratherapeutic trough levels. We did not find a correlation between abnormal levels and adverse events, in-hospital mortality, or overall poor outcome.
KEYWORDS: linezolid, therapeutic drug monitoring, drugs for Gram-positive bacteria
TEXT
Linezolid has become increasingly important for the treatment of multidrug-resistant infections caused by Gram-positive microorganisms (1, 2). Pivotal studies reported that 600 mg/12 h was the standard dose of linezolid for patients aged >12 years (3), and current guidelines do not recommend therapeutic drug monitoring (TDM). However, different studies reported significant variations in the serum levels of linezolid in specific situations and in patients taking concomitant medications, such as phenobarbital, dexamethasone, rifampin, proton pump inhibitors, calcium channel antagonists, and amiodarone (4–12). We questioned the need for linezolid TDM, as suggested by several authors (1, 8, 13–16).
We performed a prospective study of inpatients receiving linezolid for empirical or targeted treatment at a tertiary hospital. The patients agreed to participate and gave their written informed consent. The dosage of linezolid was standard and in accordance with guidelines. The study was approved by the Ethics Committee of Hospital General Universitario Gregorio Marañón, Madrid, Spain (study number MICRO.HGUGM.2016-014). Two blood samples were drawn from each patient at least 3 days after initiation of treatment. Trough levels (Cmin) were obtained within 30 min of drug administration, and peak levels (Cmax) were obtained 1 h after intravenous (IV) infusion or 2 h after oral administration. Trough levels of 2 to 8 μg/ml and peak levels of 10 to 20 μg/ml were considered normal (3).
Linezolid serum levels were detected by a validated high-performance liquid chromatograph method (17). The results were reported to the physician responsible for the patient without further recommendations. Clinical outcome was classified, prospectively, as favorable when there was a clinical improvement or cure and no evidence of adverse events (thrombocytopenia, anemia) and as poor when there was no clinical response, infection recurrence, related mortality, or adverse events. Episodes of thrombocytopenia and anemia during treatment were defined as a reduction of >30% in the platelet count or hemoglobin level from baseline, respectively (5).
Ninety patients were included in the study (68.9% male). Patient characteristics and underlying diseases are shown in Table 1. Linezolid was prescribed as empirical treatment in 73.3% and as targeted therapy in 26.7% of the patients (Table 2). The median dose of linezolid was 8.8 mg/kg (interquartile range [IQR], 7.5 to 10.0 mg/kg), and the median duration of linezolid treatment was 8 days (IQR, 5.0 to 13.2 days). The main reasons for linezolid therapy, accounting for 74.5% of the total, were pneumonia, complicated skin and soft tissue infections, and undocumented febrile episodes. Seventy-seven percent of the patients were treated with proton pump inhibitors (Table 2). Infection, caused mainly by Staphylococcus aureus or coagulase-negative Staphylococcus, was confirmed microbiologically in 30.0% of the patients. All isolates were susceptible to linezolid. Attending physicians adjusted the linezolid dose in only 3 patients based on serum levels.
TABLE 1.
Demographic data, department of admission, comorbidities, and other clinical characteristics
| Characteristica | Value (n = 90) |
|---|---|
| Age (yr) (median [IQR]) | 69.3 (57.5–79.9) |
| Sex, male (no. [%]) | 62 (68.9) |
| Weight (kg) (median [IQR]) | 68.8 (60.0–82.0) |
| Body mass index (mg/kg2) (median [IQR]) | 25.1 (22.2–29.2) |
| Race (no. [%]) | |
| White | 85 (94.4) |
| Black | 2 (2.2) |
| Other | 3 (3.3) |
| Department of admission (no. [%]) | |
| Medical | 43 (47.8) |
| Surgical | 13 (14.4) |
| ICU | 33 (36.7) |
| Pediatric | 1 (1.1) |
| Days in ICU (median [IQR]) | 15 (8.0–26.0) |
| Underlying disease (no. [%]) | |
| Cardiac disease | 29 (32.2) |
| Neurologic disease | 25 (27.8) |
| Diabetes mellitus | 22 (24.4) |
| Liver disease | 20 (22.2) |
| Solid tumor | 18 (20.0) |
| Chronic renal failure | 11 (12.2) |
| Psychiatric disease | 10 (11.1) |
| Chronic obstructive pulmonary disease | 9 (10.0) |
| HIV infection | 3 (3.3) |
| Hematologic neoplasia | 2 (2.2) |
| Solid organ transplantation | 1 (1.1) |
| Other | 3 (3.3) |
| Charlson comorbidity index (median [IQR]) | 3 (2–5) |
| McCabe index (no. [%]) | |
| Nonfatal | 56 (62.2) |
| Ultimately fatal | 27 (30.0) |
| Rapidly fatal | 7 (7.8) |
| Glomerular filtration rate (MDRD) (no. [%]) | |
| Normal (≥60 ml/min per 1.73 m2) | 61 (67.8) |
| Low (<60 ml/min per 1.73 m2) | 27 (30.0) |
| Extracorporeal membrane oxygenation (no. [%]) | 1 (1.1) |
| Hemodialysis (no. [%]) | 3 (3.3) |
ICU, intensive care unit; IQR, interquartile range; MDRD, modification of diet in renal disease.
TABLE 2.
Treatment characteristics; indications; microbiological isolates; dose, duration, and Cmin, and Cmax of linezolid; concomitant medications; and clinical outcome
| Characteristic | Value (%) (n = 90) |
|---|---|
| Type of treatment (no. [%]) | |
| Empirical | 66 (73.3) |
| Targeted | 24 (26.7) |
| Main indication for linezolid (no. [%]) | |
| Pneumonia | 50 (55.6) |
| Complicated skin and soft tissue infection | 10 (11.1) |
| Undocumented febrile episode | 7 (7.8) |
| Osteoarticular infection | 4 (4.4) |
| Mediastinitis | 4 (4.4) |
| Intra-abdominal infection | 4 (4.4) |
| CNSa infection | 3 (3.3) |
| Bacteremia | 2 (2.2) |
| Other | 6 (6.7) |
| Microbiological isolate (no. [%]) | |
| Coagulase-negative Staphylococcus | 13 (14.4) |
| Staphylococcus aureus | 9 (10) |
| Enterococcus faecalis | 2 (2.2) |
| Enterococcus faecium | 1 (1.1) |
| Enterococcus sp. | 1 (1.1) |
| Corynebacterium sp. | 1 (1.1) |
| Linezolid treatment | |
| Duration of treatment (days) (median [IQR]) | 8.0 (5.0–13.2) |
| Dose (mg/kg) (median [IQR]) | 8.8 (7.5–10.0) |
| Route of administration (no. IV/no. oral) | 61/29 |
| Cmin (mg/liter) (median [IQR]) | 2.9 (0.7–7.7) |
| Cmax (mg/liter) (median [IQR]) | 11.9 (7.8–17.9) |
| Concomitant medication (no. [%]) | |
| Phenobarbital | 0 (0) |
| Dexamethasone | 4 (4.4) |
| Rifampin | 1 (1.1) |
| Proton pump inhibitor | 70 (77.8) |
| Calcium channel antagonist | 11 (12.2) |
| Amiodarone | 5 (5.6) |
| Overall outcome (no. [%]) | |
| Favorable | 66 (73.3) |
| Poor | 24 (26.7) |
CNS, central nervous system.
The median Cmin in our study was 2.9 mg/liter, with a wide range of distribution (0.1 to 25.2 mg/liter) (Table 2). Cmin was below the therapeutic range (<2 mg/liter) in 41.1% and >8 mg/liter in 24.4% of patients. The median Cmax was 11.9 mg/liter and also had a wide range of distribution (1.7 to 36.8 mg/liter) (Table 2). Cmax was below the optimal range (<10 mg/liter) in 34.1% and >20 mg/liter in 20.4% of patients.
We analyzed the variables that might influence linezolid serum levels. Multivariate analysis confirmed that patients with higher body weight had lower Cmin values and those with a higher age-adjusted Charlson comorbidity index value or lower glomerular filtration rate had higher Cmin levels (Table 3). The adjusted R2 of 0.404 proved that 40% of the variability in Cmin was related to these variables. Multivariate analysis confirmed that higher weight correlated with lower Cmax and that lower glomerular filtration rate correlated with increased Cmax (Table 3). The adjusted R2 of 0.234 proved that one-quarter of the variability in Cmax of linezolid among our population was related to these variables. Conversely, we did not identify a significant correlation between phenobarbital, dexamethasone, rifampin, proton pump inhibitors, calcium channel antagonists, and amiodarone and the Cmin and/or Cmax (Table 3) of linezolid in the univariate analysis.
TABLE 3.
Univariate and multivariate analyses of variables associated with Cmin and Cmax of linezolid
| Variable | Univariate analysis |
Multivariate analysis |
||
|---|---|---|---|---|
| Unstandardized β coefficient (95% CI) | P | Unstandardized β coefficient (95% CI) | P | |
| Cmina (n = 90) | ||||
| Age (yr) | 0.098 (0.015 to 0.181) | <0.01 | ||
| Sex | −0.224 (−0.515 to 0.068) | 0.131 | ||
| Weight (kg) | −0.012 (−0.018 to −0.007) | <0.01 | −0.013 (−0.018 to −0.008) | <0.01 |
| Body mass index (kg/m2) | −0.026 (−0.041 to −0.010) | <0.01 | ||
| Glomerular filtration rate (MDRDb) | 0.446 (0.161 to 0.731) | <0.01 | 0.364 (0.131 to 0.597) | <0.01 |
| Concomitant treatment | ||||
| Phenobarbital | NAc | |||
| Dexamethasone | −0.132 (−0.795 to 0.531) | 0.694 | ||
| Rifampin | 0.659 (−0.638 to 1.956) | 0.315 | ||
| Proton pump inhibitor | −0.158 (−0.485 to 0.169) | 0.340 | ||
| Calcium channel antagonist | 0.282 (−0.131 to 0.695) | 0.178 | ||
| Amiodarone | −0.393 (−0.984 to 0.198) | 0.190 | ||
| Charlson comorbidity index | 0.121 (0.063 to 0.180) | <0.01 | 0.100 (0.049 to 0.150) | <0.01 |
| McCabe index | 0.151 (−0.062 to 0.364) | 0.162 | ||
| Cmax (n = 88) | ||||
| Age (yr) | 0.098 (0.015 to 0.181) | 0.022 | ||
| Sex | 2.911 (−0.515 to 6.337) | 0.095 | ||
| Weight (kg) | −0.119 (−0.183 to −0.056) | <0.01 | −0.139 (−0.203 to −0.075) | <0.01 |
| Body mass index (kg/m2) | −0.266 (−0.449 to −0.084) | <0.01 | ||
| Glomerular filtration rate (MDRD) | 4.074 (0.625 to 7.522) | 0.021 | 3.725 (0.585 to 6.865) | 0.021 |
| Concomitant treatment | ||||
| Phenobarbital | NA | |||
| Dexamethasone | −2.368 (−10.061 to 5.324) | 0.542 | ||
| Rifampin | 2.589 (−12.552 to 17.729) | 0.735 | ||
| Proton pump inhibitor | −0.269 (−4.101 to 3.562) | 0.889 | ||
| Calcium channel antagonist | 2.583 (−2.447 to 7.612) | 0.310 | ||
| Amiodarone | −5.461 (−12.472 to 1.189) | 0.104 | ||
| Charlson comorbidity index | 0.773 (0.051 to 1.495) | 0.036 | ||
| McCabe index | 1.346 (−1.154 to 3.847) | 0.287 | ||
Trough levels were log10-transformed before being compared because of their non-normal distribution.
MDRD, modification of diet in renal disease.
NA, not available.
Overall, 66 patients (73.3%) achieved a favorable outcome. As for adverse events, only 2 patients (2.2%) developed anemia during treatment. However, 12 out of 90 patients (13.3%) experienced a >30% reduction in platelet count. The median Cmin in the 14 patients was 4.6 mg/liter (range, 0.1 to 18.1 mg/liter). Discontinuation of linezolid was deemed necessary in only 1 of these patients.
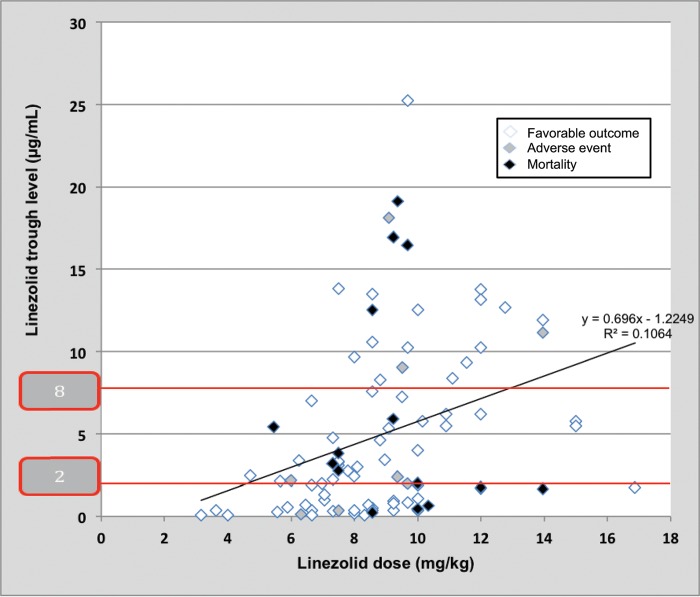
No clear correlation was observed between clinical outcome with the dose administered or abnormal trough levels of linezolid (subtherapeutic and supratherapeutic independently or out of range together) (Fig. 1). To further evaluate the potential relationship of linezolid levels with clinical outcome, we classified levels as normal or out of range. No differences were seen between normal and out-of-range levels with respect to adverse events (15.6% versus 15.5%, respectively; P = 0.9), in-hospital mortality (18.8% versus 15.5%, respectively; P = 0.7), or overall poor outcome (25.0% versus 27.6%, respectively; P = 0.8). Similarly, we were unable to demonstrate differences between normal and out-of-range peak levels or empirical and targeted therapy when they were analyzed separately.
FIG 1.
Correlation of administered doses of linezolid, obtained trough levels, and clinical outcomes (adverse events and mortality).
In our study, a high proportion of patients had inadequate linezolid levels, but we did not observe a clinical impact in patients who were outside the therapeutic range.
Different retrospective studies reported that 29% to 50% of patients had linezolid trough levels of <2 mg/liter (5, 7, 18), and trough levels of <1 mg/liter were observed in 50% of critically ill patients with sepsis (19).
Evaluation of potential factors affecting the Cmin of linezolid in the present study showed that part of the interpatient variability was due to weight, renal function, and the presence of comorbidities adjusted by age. The correlation between linezolid serum levels and weight and renal function has been reported by others (20–24). Age may also influence linezolid trough concentrations (25), since elderly patients with low body weight had a higher risk of accumulating linezolid (26). However, other studies did not find a correlation between age, weight, and renal function when evaluating the pharmacokinetics-pharmacodynamics of linezolid (5, 27, 28). Of note, relevant pharmacokinetic interactions of linezolid with several drugs have been reported in adult and pediatric patients (5, 7, 29, 30).
Our findings, with lack of correlation between levels and mortality or adverse events, do not support the need to assess linezolid serum trough levels. Note that none of the previous studies searched for a correlation between drug levels and clinical outcome, although patients with renal dysfunction showed a higher rate of adverse events due to accumulation of linezolid in some of the studies (8, 15, 16, 31, 32). Some authors suggest that the high frequency of low drug exposure with standard dosages of linezolid may explain the appearance of coagulase-negative staphylococci with resistance to linezolid (5).
Our study is based on real-life data in a tertiary hospital. However, the data were subject to limitations. The small sample size, heterogeneity of the cases, and fact that most of the patients were receiving linezolid as empirical therapy during short periods are the most relevant. In addition, dose adjustments were made in only 3 patients at the discretion of the attending physicians, and we did not intervene directly with that decision.
We conclude that a high proportion of linezolid levels were off target at initiation of antibiotic therapy. Despite the significant influence of weight, renal function, and comorbidities in trough and peak serum linezolid levels, we found no correlation between abnormal levels and clinical outcome.
ACKNOWLEDGMENT
We have no conflicts of interest to declare, and no funding was received for this study.
REFERENCES
- 1.Pea F, Viale P, Cojutti P, Del Pin B, Zamparini E, Furlanut M. 2012. Therapeutic drug monitoring may improve safety outcomes of long-term treatment with linezolid in adult patients. J Antimicrob Chemother 67:2034–2042. doi: 10.1093/jac/dks153. [DOI] [PubMed] [Google Scholar]
- 2.Woodford N, Livermore DM. 2009. Infections caused by Gram-positive bacteria: a review of the global challenge. J Infect 59(Suppl 1):S4–S16. doi: 10.1016/S0163-4453(09)60003-7. [DOI] [PubMed] [Google Scholar]
- 3.Cattaneo D, Alffenaar JW, Neely M. 2016. Drug monitoring and individual dose optimization of antimicrobial drugs: oxazolidinones. Expert Opin Drug Metab Toxicol 12:533–544. doi: 10.1517/17425255.2016.1166204. [DOI] [PubMed] [Google Scholar]
- 4.Bolhuis MS, van Altena R, Uges DR, van der Werf TS, Kosterink JG, Alffenaar JW. 2010. Clarithromycin significantly increases linezolid serum concentrations. Antimicrob Agents Chemother 54:5418–5419. doi: 10.1128/AAC.00757-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cojutti P, Maximova N, Crichiutti G, Isola M, Pea F. 2015. Pharmacokinetic/pharmacodynamic evaluation of linezolid in hospitalized paediatric patients: a step toward dose optimization by means of therapeutic drug monitoring and Monte Carlo simulation. J Antimicrob Chemother 70:198–206. doi: 10.1093/jac/dku337. [DOI] [PubMed] [Google Scholar]
- 6.Gandelman K, Zhu T, Fahmi OA, Glue P, Lian K, Obach RS, Damle B. 2011. Unexpected effect of rifampin on the pharmacokinetics of linezolid: in silico and in vitro approaches to explain its mechanism. J Clin Pharmacol 51:229–236. doi: 10.1177/0091270010366445. [DOI] [PubMed] [Google Scholar]
- 7.Pea F, Furlanut M, Cojutti P, Cristini F, Zamparini E, Franceschi L, Viale P. 2010. Therapeutic drug monitoring of linezolid: a retrospective monocentric analysis. Antimicrob Agents Chemother 54:4605–4610. doi: 10.1128/AAC.00177-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cattaneo D, Orlando G, Cozzi V, Cordier L, Baldelli S, Merli S, Fucile S, Gulisano C, Rizzardini G, Clementi E. 2013. Linezolid plasma concentrations and occurrence of drug-related haematological toxicity in patients with Gram-positive infections. Int J Antimicrob Agents 41:586–589. doi: 10.1016/j.ijantimicag.2013.02.020. [DOI] [PubMed] [Google Scholar]
- 9.Di Paolo A, Malacarne P, Guidotti E, Danesi R, Del Tacca M. 2010. Pharmacological issues of linezolid: an updated critical review. Clin Pharmacokinet 49:439–447. doi: 10.2165/11319960-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 10.Hallam MJ, Allen JM, James SE, Donaldson PM, Davies JG, Hanlon GW, Dheansa BS. 2010. Potential subtherapeutic linezolid and meropenem antibiotic concentrations in a patient with severe burns and sepsis. J Burn Care Res 31:207–209. doi: 10.1097/BCR.0b013e3181c89ee3. [DOI] [PubMed] [Google Scholar]
- 11.Lovering AM, Le Floch R, Hovsepian L, Stephanazzi J, Bret P, Birraux G, Vinsonneau C. 2009. Pharmacokinetic evaluation of linezolid in patients with major thermal injuries. J Antimicrob Chemother 63:553–559. doi: 10.1093/jac/dkn541. [DOI] [PubMed] [Google Scholar]
- 12.Zoller M, Maier B, Hornuss C, Neugebauer C, Dobbeler G, Nagel D, Holdt LM, Bruegel M, Weig T, Grabein B, Frey L, Teupser D, Vogeser M, Zander J. 2014. Variability of linezolid concentrations after standard dosing in critically ill patients: a prospective observational study. Crit Care 18:R148. doi: 10.1186/cc13984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dong H, Xie J, Chen L, Wang T, Sun J, Zhao Y, Dong Y. 2014. Developments in the pharmacokinetic/pharmacodynamic index of linezolid: a step toward dose optimization using Monte Carlo simulation in critically ill patients. Int J Infect Dis 22:35–40. doi: 10.1016/j.ijid.2014.01.016. [DOI] [PubMed] [Google Scholar]
- 14.Ichie T, Suzuki D, Yasui K, Takahashi H, Matsuda M, Hayashi H, Sugiura Y, Sugiyama T. 2015. The association between risk factors and time of onset for thrombocytopenia in Japanese patients receiving linezolid therapy: a retrospective analysis. J Clin Pharm Ther 40:279–284. doi: 10.1111/jcpt.12260. [DOI] [PubMed] [Google Scholar]
- 15.Matsumoto K, Shigemi A, Takeshita A, Watanabe E, Yokoyama Y, Ikawa K, Morikawa N, Takeda Y. 2014. Analysis of thrombocytopenic effects and population pharmacokinetics of linezolid: a dosage strategy according to the trough concentration target and renal function in adult patients. Int J Antimicrob Agents 44:242–247. doi: 10.1016/j.ijantimicag.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 16.Nukui Y, Hatakeyama S, Okamoto K, Yamamoto T, Hisaka A, Suzuki H, Yata N, Yotsuyanagi H, Moriya K. 2013. High plasma linezolid concentration and impaired renal function affect development of linezolid-induced thrombocytopenia. J Antimicrob Chemother 68:2128–2133. doi: 10.1093/jac/dkt133. [DOI] [PubMed] [Google Scholar]
- 17.Foj Capell L, Soriano Viladomiu A, Brunet Serra M, López Galera R. 2011. Determinación de linezolid en plasma mediante cromatografía líquida de alta resolución para la monitorización terapéutica en pacientes. Rev Lab Clin 4:207–213. [Google Scholar]
- 18.Morata L, Cuesta M, Rojas JF, Rodriguez S, Brunet M, Casals G, Cobos N, Hernandez C, Martinez JA, Mensa J, Soriano A. 2013. Risk factors for a low linezolid trough plasma concentration in acute infections. Antimicrob Agents Chemother 57:1913–1917. doi: 10.1128/AAC.01694-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adembri C, Fallani S, Cassetta MI, Arrigucci S, Ottaviano A, Pecile P, Mazzei T, De Gaudio R, Novelli A. 2008. Linezolid pharmacokinetic/pharmacodynamic profile in critically ill septic patients: intermittent versus continuous infusion. Int J Antimicrob Agents 31:122–129. doi: 10.1016/j.ijantimicag.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 20.Hamilton R, Thai XC, Ameri D, Pai MP. 2013. Oral bioavailability of linezolid before and after Roux-en-Y gastric bypass surgery: is dose modification necessary in obese subjects? J Antimicrob Chemother 68:666–673. doi: 10.1093/jac/dks431. [DOI] [PubMed] [Google Scholar]
- 21.Stein GE, Schooley SL, Peloquin CA, Kak V, Havlichek DH, Citron DM, Tyrrell KL, Goldstein EJ. 2005. Pharmacokinetics and pharmacodynamics of linezolid in obese patients with cellulitis. Ann Pharmacother 39:427–432. doi: 10.1345/aph.1E484. [DOI] [PubMed] [Google Scholar]
- 22.Tsuji Y, Hiraki Y, Matsumoto K, Mizoguchi A, Sadoh S, Kobayashi T, Sakamoto S, Morita K, Yukawa E, Kamimura H, Karube Y. 2012. Evaluation of the pharmacokinetics of linezolid in an obese Japanese patient. Scand J Infect Dis 44:626–629. doi: 10.3109/00365548.2011.652164. [DOI] [PubMed] [Google Scholar]
- 23.Corcione S, Pagani N, Baietto L, Fanelli V, Urbino R, Ranieri VM, Di Perri G, D'Avolio A, De Rosa FG. 2015. Pharmacokinetics of high dosage of linezolid in two morbidly obese patients. J Antimicrob Chemother 70:2925. doi: 10.1093/jac/dkv238. [DOI] [PubMed] [Google Scholar]
- 24.Matsumoto K, Takeshita A, Ikawa K, Shigemi A, Yaji K, Shimodozono Y, Morikawa N, Takeda Y, Yamada K. 2010. Higher linezolid exposure and higher frequency of thrombocytopenia in patients with renal dysfunction. Int J Antimicrob Agents 36:179–181. doi: 10.1016/j.ijantimicag.2010.02.019. [DOI] [PubMed] [Google Scholar]
- 25.Cattaneo D, Gervasoni C, Cozzi V, Castoldi S, Baldelli S, Clementi E. 2016. Therapeutic drug management of linezolid: a missed opportunity for clinicians? Int J Antimicrob Agents 48:728–731. doi: 10.1016/j.ijantimicag.2016.08.023. [DOI] [PubMed] [Google Scholar]
- 26.Abe S, Chiba K, Cirincione B, Grasela TH, Ito K, Suwa T. 2009. Population pharmacokinetic analysis of linezolid in patients with infectious disease: application to lower body weight and elderly patients. J Clin Pharmacol 49:1071–1078. doi: 10.1177/0091270009337947. [DOI] [PubMed] [Google Scholar]
- 27.Sisson TL, Jungbluth GL, Hopkins NK. 2002. Age and sex effects on the pharmacokinetics of linezolid. Eur J Clin Pharmacol 57:793–797. doi: 10.1007/s00228-001-0380-y. [DOI] [PubMed] [Google Scholar]
- 28.Bhalodi AA, Papasavas PK, Tishler DS, Nicolau DP, Kuti JL. 2013. Pharmacokinetics of intravenous linezolid in moderately to morbidly obese adults. Antimicrob Agents Chemother 57:1144–1149. doi: 10.1128/AAC.01453-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Egle H, Trittler R, Kummerer K, Lemmen SW. 2005. Linezolid and rifampin: drug interaction contrary to expectations? Clin Pharmacol Ther 77:451–453. doi: 10.1016/j.clpt.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 30.Matheny CJ, Lamb MW, Brouwer KR, Pollack GM. 2001. Pharmacokinetic and pharmacodynamic implications of P-glycoprotein modulation. Pharmacotherapy 21:778–796. doi: 10.1592/phco.21.9.778.34558. [DOI] [PubMed] [Google Scholar]
- 31.Tsuji Y, Hiraki Y, Matsumoto K, Mizoguchi A, Kobayashi T, Sadoh S, Morita K, Kamimura H, Karube Y. 2011. Thrombocytopenia and anemia caused by a persistent high linezolid concentration in patients with renal dysfunction. J Infect Chemother 17:70–75. doi: 10.1007/s10156-010-0080-6. [DOI] [PubMed] [Google Scholar]
- 32.Tsuji Y, Yukawa E, Hiraki Y, Matsumoto K, Mizoguchi A, Morita K, Kamimura H, Karube Y, To H. 2013. Population pharmacokinetic analysis of linezolid in low body weight patients with renal dysfunction. J Clin Pharmacol 53:967–973. doi: 10.1002/jcph.133. [DOI] [PubMed] [Google Scholar]