ABSTRACT
Limited literature is available assessing nephrotoxicity with prolonged β-lactam infusions. This study compared the incidence of acute kidney injury (AKI) associated with a prolonged β-lactam infusion or an intermittent infusion. This was a retrospective, matched-cohort study at an academic medical center from July 2006 to September 2015. Adult patients who received piperacillin-tazobactam (TZP), cefepime (FEP), or meropenem (MEM) for at least 48 h were evaluated. Patients were excluded for preexisting renal dysfunction or pregnancy. The primary outcome was difference in incidence of AKI evaluated using the RIFLE (risk, injury, failure, loss, and end-stage) criteria. Patients in the intermittent group were matched 3:1 to patients in the prolonged-infusion group based on the following: β-lactam agent, age, gender, Charlson comorbidity index, baseline creatinine clearance, hypotension, receipt of vancomycin, and treatment in an intensive care unit. A total of 2,390 patients were included in the matched analysis, with 1,700 receiving intermittent infusions and 690 receiving prolonged infusion. The incidence of AKI was similar in the prolonged-infusion group to that in the intermittent-infusion group (21.6% versus 18.6%; P = 0.1). After multivariate regression, prolonged infusion was not associated with increased odds of AKI (odds ratio [OR], 1.07; 95% confidence interval [95% CI], 0.83 to 1.39). Independent predictors of AKI included TZP therapy, concomitant nephrotoxins, hypotension, and heart failure. Although AKIs were numerically more common in patients receiving prolonged β-lactam infusions than those receiving intermittent infusions, prolonged infusion was not an independent risk factor for AKI.
KEYWORDS: acute kidney injury, β-lactams, extended infusion
INTRODUCTION
The β-Lactam antibiotics are an antimicrobial class with extensive clinical utility and are the mainstay of treatment in many Gram-positive and Gram-negative infections. These agents are bactericidal through inhibition of bacterial cell wall synthesis and exhibit time-dependent killing of microorganisms (1). Thus, the activity of β-lactam antibiotics depends on the free drug concentration time above the MIC of the targeted organism. β-Lactams are typically dosed intermittently and infused over 30 to 60 min. Prolonged β-lactam infusions are promoted as a strategy to optimize the percentage of the dosing interval that the concentration of free drug remains above the MIC. Enhancement of β-lactam pharmacodynamics is particularly useful in critically ill patients with altered pharmacokinetics and those who are at risk for resistant pathogens. Prolonged β-lactam infusions are associated with decreased mortality in patients diagnosed with pneumonia, as well as invasive Pseudomonas aeruginosa infections (2, 3).
While nephrotoxicity is a known adverse effect of β-lactam antibiotics, there is a low reported incidence of nephrotoxicity associated with traditional intermittent dosing. Mechanisms of nephrotoxicity reported with beta-lactams include primarily acute interstitial nephritis (AIN) and glomerulonephritis, although acute tubular necrosis (ATN) has been noted (4, 5). The prolonged-infusion literature available primarily focuses on efficacy rather than safety. Thus, there is limited information available regarding nephrotoxicity with prolonged infusion; the β-lactam evaluated in existing prolonged-infusion literature is piperacillin-tazobactam (TZP). Lau and colleagues reported drug-related adverse events in their evaluation of TZP administered via a continuous infusion over 24 h versus intermittent infusion given every 6 h (6). Renal failure was noted as a treatment-related adverse event in one patient treated with TZP continuous infusion. McCormick et al. completed a retrospective study of 200 patients and did not find a difference in acute kidney injuries (AKIs) between patients receiving intermittent TZP infusions versus those receiving extended infusions (11% versus 9%; P = 0.637) (7). The literature to date suggests similar rates of nephrotoxicity in patients receiving TZP and vancomycin (VAN) combination therapy, regardless of TZP infusion strategy (8, 9). No previous studies have reported the impact of prolonged infusions on AKI with β-lactams other than TZP. Therefore, this study was formulated to evaluate the incidence of AKIs based on TZP, cefepime (FEP), or meropenem (MEM) infusion strategy.
RESULTS
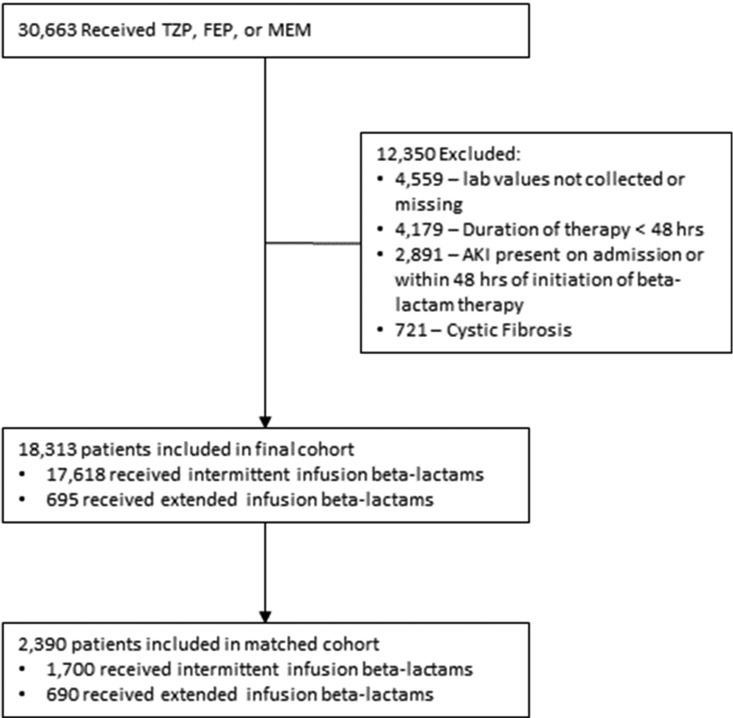
In total, 2,390 patients of the 18,313 evaluated met criteria to be included in our matched analysis (Fig. 1), with 1,700 receiving intermittent infusions and 690 receiving prolonged infusions.
FIG 1.
Patient selection diagram.
The baseline characteristics of the matched cohort are displayed in Table 1. The majority of baseline factors were well balanced between the prolonged-infusion and intermittent-infusion groups, with the exception of exposure to aminoglycosides, calcineurin inhibitors, intravenous (i.v.) contrast dye, loop diuretics, and vasopressors. TZP was the most common β-lactam utilized, with 37.0% of patients in the prolonged-infusion group and 46.4% in the intermittent-infusion group receiving this antimicrobial. FEP was administered in the prolonged-infusion group to 31.0% of patients and 36.6% in the intermittent-infusion group. More patients in the prolonged-infusion group received MEM (32.0% versus 16.9%; P < 0.0001). Length of hospital stay was significantly longer in the prolonged-infusion cohort (median of 12 days [range, 7 to 21 days] versus 10 days [5 to 18 days]; P < 0.0001).
TABLE 1.
Baseline patient characteristics of matched cohort
| Variablea | Result for infusion strategyb |
P value | |
|---|---|---|---|
| Prolonged (n = 690) | Intermittent (n = 1,700) | ||
| Age, mean (SD) yr | 53.47 (17.1) | 53.48 (16.9) | 0.982 |
| Male, no. (%) | 427 (61.9) | 1,028 (60.5) | 0.551 |
| Caucasian, no. (%) | 631 (91.4) | 1,562 (91.9) | 0.789 |
| Charlson comorbidity score, median (IQR) | 3 (1–5) | 3 (1–6) | 0.518 |
| Baseline CLCR, no. (%) | 0.908 | ||
| 30–59 ml/min | 129 (18.7) | 331 (19.5) | |
| 60–89 ml/min | 193 (28.0) | 469 (27.6) | |
| ≥90 ml/min | 368 (53.3) | 900 (52.9) | |
| ICU admission, no. (%) | 189 (27.4) | 431 (25.3) | 0.328 |
| Comorbidities, no. (%) | |||
| Diabetes mellitus | 159 (23.0) | 451 (26.5) | 0.0855 |
| Heart failure | 118 (17.1) | 241 (14.2) | 0.08 |
| Hypertension | 384 (55.6) | 905 (53.2) | 0.303 |
| Hypotension | 428 (62.0) | 1,002 (58.9) | 0.177 |
| β-Lactam received, no. (%) | <0.0001 | ||
| Piperacillin-tazobactam | 255 (37.0) | 789 (46.4) | |
| Cefepime | 214 (31.0) | 623 (36.6) | |
| Meropenem | 221 (32.0) | 288 (16.9) | |
| Concomitant nephrotoxins, no. (%) | |||
| Aminoglycoside | 154 (22.3) | 226 (13.3) | <0.0001 |
| Amphotericin B | 13 (1.9) | 37 (2.2) | 0.768 |
| ACE inhibitor | 118 (17.1) | 313 (18.4) | 0.486 |
| ARB | 24 (3.5) | 57 (3.3) | 0.977 |
| Calcineurin inhibitors | 91 (13.2) | 165 (9.7) | 0.0154 |
| i.v. contrast dye | 271 (39.3) | 553 (32.5) | 0.0019 |
| Loop diuretics | 134 (19.4) | 233 (13.7) | 0.0006 |
| NSAIDs | 33 (4.8) | 65 (3.8) | 0.338 |
| Vancomycin | 533 (77.2) | 1,327 (78.1) | 0.705 |
| Vasopressors | 136 (19.7) | 236 (13.9) | 0.0005 |
Abbreviations: IQR, interquartile range; CLCR, creatinine clearance; ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; NSAIDs, nonsteroidal anti-inflammatory drugs.
The values shown represent the number (percentage) of patients unless otherwise indicated.
AKI incidences were similar between the prolonged- and intermittent-infusion cohorts (21.6% versus 18.6%; P = 0.104), with no difference when stratified by RIFLE (risk, injury, failure, loss, and end-stage) criteria (Table 2). AKIs were more common among patients who received TZP (24.9%) compared to those receiving FEP or MEM (15.9% and 14.1%, respectively; P < 0.00001). After multiple regression, prolonged infusion was not associated with increased odds of AKI (OR, 1.07; 95% confidence interval [CI], 0.83 to 1.39). Several independent predictors of AKI were identified in the analysis (Table 3). Concomitant nephrotoxins associated with increased risk of AKI included aminoglycosides, amphotericin B, calcineurin inhibitors, loop diuretics, vancomycin, and vasopressors. Patients receiving TZP had 1.95 times the odds of AKI (95% CI, 1.50 to 2.52) compared to those receiving FEP. Patients experiencing hypotension had 1.43 times the odds of AKI (95% CI, 1.10 to 1.85). Heart failure at baseline was associated with a 1.78 multiplicative increase in AKI odds (95% CI, 1.31 to 2.43).
TABLE 2.
Incidence of nephrotoxicity by infusion strategy
| Variable | Result for infusion strategya |
P value | |
|---|---|---|---|
| Prolonged (n = 690) | Intermittent (n = 1,700) | ||
| AKI incidence, no. (%) | 149 (21.6) | 316 (18.6) | 0.104 |
| RIFLE criteria, no. (%) | |||
| Risk | 86 (12.5) | 186 (10.9) | |
| Injury | 39 (5.7) | 74 (4.4) | |
| Failure | 24 (3.5) | 56 (3.3) | |
The values shown represent the number (percentage) of patients.
TABLE 3.
Multivariate regression results postmatchinga
| Covariate | Bivariable regression |
Multivariable regression |
||||
|---|---|---|---|---|---|---|
| OR | CI | P value | aOR | CI | P value | |
| Treatment group | ||||||
| Intermittent infusion (reference) | ||||||
| Prolonged infusion | 1.21 | 0.97–1.50 | 0.093 | 1.07 | 0.83–1.39 | 0.584 |
| Age group | ||||||
| 18–44 yr (reference) | ||||||
| 45–64 yr | 1.21 | 0.95–1.55 | 0.12 | 1.11 | 0.83–1.48 | 0.474 |
| 65–79 yr | 1.19 | 0.89–1.58 | 0.25 | 1.23 | 0.85–1.78 | 0.266 |
| ≥80 yr | 0.80 | 0.49–1.28 | 0.376 | 1.10 | 0.61–2.00 | 0.745 |
| Male | 1.06 | 0.86–1.30 | 0.603 | |||
| Caucasian | 0.78 | 0.55–1.11 | 0.15 | 0.70 | 0.47–1.03 | 0.069 |
| Charlson comorbidity score | 1.02 | 1.00–1.05 | 0.099 | 1.00 | 0.97–1.04 | 0.856 |
| Baseline CLCR of: | ||||||
| 30–59 ml/min (reference) | ||||||
| 60–89 ml/min | 0.86 | 0.63–1.19 | 0.362 | 1.13 | 0.78–1.65 | 0.51 |
| ≥90 ml/min | 1.33 | 1.02–1.76 | 0.04 | 2.42 | 1.67–3.50 | <0.001 |
| Comorbidities | ||||||
| Diabetes | 1.09 | 0.87–1.37 | 0.454 | |||
| Heart failure | 2.74 | 2.14–3.50 | <0.001 | 1.78 | 1.31–2.43 | <0.001 |
| ICU admission | 1.35 | 1.08–1.68 | 0.009 | 0.99 | 0.75–1.29 | 0.918 |
| Hypotension | 2.17 | 1.73–2.72 | <0.001 | 1.43 | 1.10–1.85 | 0.007 |
| Hypertension | 1.13 | 0.92–1.38 | 0.245 | |||
| β-Lactam | ||||||
| Cefepime (reference) | ||||||
| Meropenem | 0.87 | 0.64–1.19 | 0.388 | 1.04 | 0.73–1.48 | 0.831 |
| Piperacillin-tazobactam | 1.76 | 1.39–2.22 | <0.001 | 1.95 | 1.50–2.52 | <0.001 |
| Concomitant nephrotoxins | ||||||
| Aminoglycoside | 2.41 | 1.88–3.07 | <0.001 | 1.71 | 1.29–2.27 | <0.001 |
| Amphotericin B | 2.84 | 1.57–5.01 | <0.001 | 2.13 | 1.03–4.43 | 0.042 |
| ACE inhibitor | 1.23 | 0.95–1.59 | 0.103 | 1.04 | 0.76–1.41 | 0.825 |
| ARB | 0.94 | 0.51–1.61 | 0.828 | 0.97 | 0.50–1.88 | 0.935 |
| i.v. contrast dye | 0.79 | 0.55–1.11 | 0.193 | 0.84 | 0.57–1.24 | 0.386 |
| Calcineurin inhibitor | 2.19 | 1.40–3.34 | <0.001 | 2.13 | 1.27–3.57 | 0.004 |
| Loop diuretic | 3.75 | 3.05–4.64 | <0.001 | 2.74 | 2.13–3.53 | <0.001 |
| NSAIDs | 0.80 | 0.59–1.07 | 0.137 | 0.70 | 0.50–0.99 | 0.041 |
| Vancomycin | 2.39 | 1.79–3.24 | <0.001 | 1.60 | 1.16–2.21 | 0.004 |
| Vasopressor | 3.34 | 2.62–4.24 | <0.001 | 1.98 | 1.45–2.72 | <0.001 |
OR, odds ratio; CI, confidence interval; aOR, adjusted odds ratio; CLCR, creatinine clearance; ICU, intensive care unit; ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; NSAIDs, nonsteroidal anti-inflammatory drugs.
DISCUSSION
In this single-center, retrospective matched-cohort study, we compared rates of nephrotoxicity in patients who received prolonged infusion of β-lactams versus those with intermittent infusion. The incidence of AKI according to RIFLE criteria was 21.6% with a prolonged-infusion strategy compared to 18.6% with an intermittent-infusion strategy. Our observed nephrotoxicity rates were higher than the 9 to 11% reported by McCormick et al. (7). This variance may be explained by differences in patient populations and AKI definition; while we defined AKI with RIFLE criteria, McCormick and colleagues defined acute renal injury by an increase in serum creatinine of 0.5 mg/dl within 24 h or a serum creatinine level of 2 times the patient's baseline value.
Although the prolonged-infusion group had a numerically higher incidence of AKI than the intermittent-infusion group, multiple regression analysis revealed that prolonged infusion was not independently associated with an increased risk of AKI.
Many of the variables that were independent risk factors of AKI in multiple regression analysis are anticipated, including administration of concomitant nephrotoxins, hypotension, and receipt of TZP. Recent literature continues to suggest that TZP is associated with a higher incidence of AKI than other intravenous β-lactams when used concomitantly with VAN (10, 11). Burgess and Drew reported that patients receiving VAN combined with TZP were more likely to develop nephrotoxicity than patients who received VAN alone (8.1% versus 16.3%; P < 0.041) (10). In a study by Gomes and colleagues, the incidence of AKI in patients receiving TZP and VAN was significantly higher than that in patients receiving FEP and VAN (36.4% versus 10.9%; P = 0.003) in the matched analysis, and the investigators concluded that the combination of TZP and VAN was an independent predictor of AKI (11). The concern for AKI induced with use of TZP and VAN combination therapy raised a new clinical question—does the infusion strategy of TZP affect the incidence of AKI when used concomitantly with VAN? Documented nephrotoxicity rates in patients receiving TZP and vancomycin (VAN) combination therapy are similar, regardless of TZP infusion strategy (8, 9). Results of a study including 320 patients performed by Karino and colleagues suggest comparable rates of AKI (32.5% versus 33.1%; P = 1.0) in the prolonged- and intermittent-infusion groups, respectively (8). Mousavi et al. had analogous findings in their study of 280 patients receiving combination TZP and VAN; the rates of nephrotoxicity did not differ based on TZP infusion strategy (prolonged infusion, 17.9%, versus intermittent, 17.1%; P > 0.99) (9). The TZP nephrotoxicity rate in our study is similar to what has been reported in the literature to date; however, unlike previous studies, not all patients received vancomycin in combination with a β-lactam in our analysis (77.2% in the prolonged-infusion group versus 78.1% in the intermittent-infusion group). Additionally, no previous studies have reported the impact of prolonged infusion on AKI with β-lactams other than TZP.
This study had several limitations, specifically the single-center, retrospective design. An attempt to reduce bias and control for confounders was made through propensity score matching and multiple logistic regression analysis. During the study period, there was no protocol in place at our institution for use of extended-infusion β-lactams. Due to the retrospective nature of the study, evaluation of nursing compliance with the prolonged-infusion order was not possible. Additionally, we acknowledge that the potentials for nephrotoxicity were assumed to be equal among the concomitant nephrotoxins analyzed. Exclusion of patients with a preexisting need for renal replacement and creatinine clearance (CLCR) of <30 ml/min will limit the generalizability of our results. Despite these limitations, this study currently provides the largest set of data available assessing nephrotoxicity of prolonged-infusion β-lactams.
In conclusion, prolonged infusion was not independently associated with increased AKI risk compared to intermittent infusion. The results of this large retrospective study lessen any concern that the use of prolonged infusion will cause increased incidence of AKI and suggest it is safe for clinicians to prolong β-lactam infusions to optimize efficacy of treatment in their patients.
MATERIALS AND METHODS
Study design and setting.
This retrospective matched-cohort study was conducted at University of Kentucky HealthCare (UKHC) in Lexington, KY, which is comprised of two hospitals, the Albert B. Chandler Medical Center and Good Samaritan Hospital. The study was approved by the investigational review board with a waiver of consent. Adult patients admitted from July 2006 to September 2015 who received TZP, FEP, or MEM for at least 48 h were evaluated. Patients were excluded for preexisting renal dysfunction, cystic fibrosis, or pregnancy. Preexisting renal dysfunction was defined as the need for renal replacement therapy or creatinine clearance (CLCR) of <30 ml/min at presentation.
Data source and collection.
Clinical data were extracted from the University of Kentucky Center for Clinical and Translational Science Enterprise Data Trust (EDT). Data stored in the EDT include demographics, financial classification (Medicare, Medicaid, private insurance), medical diagnoses (International Classification of Diseases 9 [ICD-9]), medical procedures (Current Procedural Terminology [CPT] codes), laboratory results, vital signs, and visit details (length of stay, medical providers, etc.).
Data collected included demographics, hospital visit data (length of stay, admitting and primary diagnosis codes), laboratory values and vital signs obtained throughout admission, immunosuppression status, receipt of concomitant nephrotoxins (listed in Table 1), severity of underlying comorbidity as defined by the Charlson comorbidity index (12), and mortality information. Baseline creatinine clearance was calculated with the adjusted Cockcroft-Gault equation from the first documented serum creatinine level measured during admission (13). Concomitant nephrotoxins were defined as receipt of at least 1 dose of a nephrotoxic agent during or within 24 h of treatment with β-lactams. Hypotension was defined as diagnosis of hypotension by ICD-9 codes, mean arterial pressure of <60 mm Hg, or receipt of vasopressors during or within 24 h of treatment (14). Antibiotic information obtained included dosing regimen and days of antibiotic therapy, defined as receipt of at least one dose of antibiotic per day.
Study outcome.
The primary objective was to evaluate the incidence of AKI in patients receiving prolonged infusion β-lactam therapy compared to intermittent β-lactam therapy defined by the RIFLE (risk, injury, failure, loss, and end-stage) criteria (15). Prolonged infusion in this study includes both extended infusions and infusions administered continuously over 24 h. β-Lactam infusion strategy was determined by the individual prescriber. Glomerular filtration rate (GFR) was estimated using the maximum serum creatinine level between 48 h of initiation of the β-lactam regimen through 7 days after the last dose of antibiotics. The GFR estimation for use in the RIFLE criteria was completed via the adjusted Cockcroft-Gault equation (13).
Patient matching.
Propensity score matching was utilized to reduce potential bias and mimic randomization. Patients in the intermittent group were matched 3:1 to patients in the prolonged-infusion group based on the logistic regression model with the following variables included: β-lactam agent, age (as a categorical variable: 18 to 44, 45 to 64, 65 to 79, or ≥80 years of age), gender, Charlson comorbidity index, baseline creatinine clearance, hypotension, receipt of vancomycin, and treatment in the intensive care unit. A greedy, nearest-neighbor matching algorithm with a caliper of 0.2 was utilized to ensure adequate matches.
Statistical analysis.
Descriptive statistics were performed for the study population. Baseline characteristics for each group were compared using a 2-tailed Student's t test or Wilcoxon rank sum test for continuous variables and chi-square or Fisher's exact test for categorical variables. Following propensity score matching, bivariable logistic regression models were generated to describe relationships between the variables and outcomes. The final multiple regression model was created using covariates associated with AKI from the bivariable models at a P value of <0.2. Significance was defined at an α of 0.05. Goodness of fit of the multivariate logistic regression was evaluated with the standardized Hosmer-Lemeshow test (16).
ACKNOWLEDGMENTS
This project was supported by the NIH National Center for Advancing Translational Sciences through grant no. UL1TR001998.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The authors have no conflicts of interest in relation to the study presented to disclose.
REFERENCES
- 1.MacVane SH, Kuti JL, Nicolau DP. 2014. Prolonging β-lactam infusion: a review of the rationale and evidence, and guidance for implementation. Int J Antimicrob Agents 43:105–113. doi: 10.1016/j.ijantimicag.2013.10.021. [DOI] [PubMed] [Google Scholar]
- 2.Bauer KA, West JE, O'Brien JM, Goff DA. 2013. Extended-infusion cefepime reduces mortality in patients with Pseudomonas aeruginosa infections. Antimicrob Agents Chemother 57:2907–2912. doi: 10.1128/AAC.02365-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Falagas ME, Tansarli GS, Ikawa K, Vardakas KZ. 2013. Clinical outcomes with extended or continuous versus short-term intravenous infusion of carbapenems and piperacillin/tazobactam: a systematic review and meta-analysis. Clin Infect Dis 56:272–282. doi: 10.1093/cid/cis857. [DOI] [PubMed] [Google Scholar]
- 4.Naughton CA. 2008. Drug-induced nephrotoxicity. Am Fam Physician 78:743–750. [PubMed] [Google Scholar]
- 5.Tune DM. 1997. Nephrotoxicity of β-lactam antibiotics: mechanisms and strategies for prevention. Pediatr Nephrol 11:768–772. doi: 10.1007/s004670050386. [DOI] [PubMed] [Google Scholar]
- 6.Lau WK, Mercer D, Itani KM, Nicolau DP, Kuti JL, Mansfield D, Dana A. 2006. Randomized, open-label, comparative study of pipercillin-tazobactam administered by continuous infusion versus intermittent infusion for treatment of hospitalized patients with complicated intra-abdominal infection. Antimicrob Agents Chemother 50:3556–3561. doi: 10.1128/AAC.00329-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McCormick H, Tomaka N, Baggett S, Heierman R, LaFosse J, Gilbert S, Imhof K. 2015. Comparison of acute renal injury associated with intermittent and extended infusion piperacillin/tazobactam. Am J Health Syst Pharm 72:S25–S30. doi: 10.2146/sp150007. [DOI] [PubMed] [Google Scholar]
- 8.Karino S, Kaye KS, Navalkele B, Nishan B, Salim M, Solanki S, Pervaiz A, Tashtoush N, Shaikh H, Koppula S, Martin ET, Mynatt RP, Murray KP, Rybak MJ, Pogue JM. 2016. Epidemiology of acute kidney injury among patients receiving concomitant vancomycin and piperacillin-tazobactam: opportunities for antimicrobial stewardship. Antimicrob Agents Chemother 60:3743–3750. doi: 10.1128/AAC.03011-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mousavi M, Zapolskaya T, Scipione MR, Louie E, Papadopoulos J, Dubrovskaya Y. 2017. Comparison of rates of nephrotoxicity associated with vancomycin in combination with piperacillin-tazobactam administered as an extended versus standard infusion. Pharmacotherapy 37:379–385. doi: 10.1002/phar.1901. [DOI] [PubMed] [Google Scholar]
- 10.Burgess LD, Drew RH. 2014. Comparison of the incidence of vancomycin-induced nephrotoxicity in hospitalized patients with and without concomitant piperacillin-tazobactam. Pharmacotherapy 34:670–676. doi: 10.1002/phar.1442. [DOI] [PubMed] [Google Scholar]
- 11.Gomes DM, Smotherman C, Birch A, Dupree L, Della Vecchia BJ, Kraemer DF, Jankowski CA. 2014. Comparison of acute kidney injury during treatment with vancomycin in combination with piperacillin-tazobactam or cefepime. Pharmacotherapy 34:662–669. doi: 10.1002/phar.1428. [DOI] [PubMed] [Google Scholar]
- 12.Quan H, Li B, Couris CM, Fushimi K, Graham P, Hider P, Januel J-M, Sundararajan V. 2011. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol 173:676–682. doi: 10.1093/aje/kwq433. [DOI] [PubMed] [Google Scholar]
- 13.Wilhelm SM, Kale-Pradhan PB. 2011. Estimating creatinine clearance: a meta-analysis. Pharmacotherapy 31:658–664. doi: 10.1592/phco.31.7.658. [DOI] [PubMed] [Google Scholar]
- 14.Elixhauser A, Steiner C, Palmer L. 2015. Clinical Classifications Software (CCS). Agency for Healthcare Research and Quality, Bethesda, MD: http://www.hcup-us.ahrq.gov/toolssoftware/ccs/ccs.jsp. [Google Scholar]
- 15.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P. 2004. Acute renal failure—definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conferences of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care 8:R204–R212. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paul P, Pennell ML, Lemeshow S. 2013. Standardizing the power of the Hosmer-Lemeshow goodness of fit test in large data sets. Stat Med 32:67–80. doi: 10.1002/sim.5525. [DOI] [PubMed] [Google Scholar]