Abstract
Background: The specific metabolomic perturbations that occur in vitamin B-12 deficiency, and their associations with neurological function, are not well characterized.
Objective: We sought to characterize the human serum metabolome in subclinical vitamin B-12 deficiency and repletion.
Methods: A before-and-after treatment study provided 1 injection of 10 mg vitamin B-12 (with 100 mg pyridoxine and 100 mg thiamin) to 27 community-dwelling elderly Chileans (∼74 y old) with vitamin B-12 deficiency, as evaluated with serum vitamin B-12, total plasma homocysteine (tHcy), methylmalonic acid (MMA), and holotranscobalamin. The combined indicator of vitamin B-12 status (cB-12) was computed. Targeted metabolites [166 acylcarnitines, amino acids, sugars, glycerophospholipids, and sphingolipids (liquid chromatography–tandem mass spectrometry)], and untargeted metabolites [247 chemical entities (gas chromatography time-of-flight mass spectrometry)] were measured at baseline and 4 mo after treatment. A peripheral nerve score was developed. Differences before and after treatment were examined. For targeted metabolomics, the data from 18 individuals with adequate vitamin B-12 status (selected from the same population) were added to the before-and-after treatment data set. Network visualizations and metabolic pathways are illustrated.
Results: The injection increased serum vitamin B-12, holotranscobalamin, and cB-12 (P < 0.001), and reduced tHcy and serum MMA (P < 0.001). Metabolomic changes from before to after treatment included increases (P < 0.001) in acylcarnitines, plasmalogens, and other phospholipids, whereas proline and other intermediaries of one-carbon metabolism—that is, methionine and cysteine—were reduced (P < 0.001). Direct significant correlations (P < 0.05 after the false discovery rate procedure) were identified between acylcarnitines, plasmalogens, phospholipids, lyso-phospholipids, and sphingomyelins compared with vitamin B-12 status and nerve function. Multiple connections were identified with primary metabolites (e.g., an inverse relation between vitamin B-12 markers and tryptophan, tyrosine, and pyruvic, succinic, and citric acids, and a direct correlation between the nerve score and arginine).
Conclusions: The human serum metabolome in vitamin B-12 deficiency and the changes that occur after supplementation are characterized. Metabolomics revealed connections between vitamin B-12 status and serum metabolic markers of mitochondrial function, myelin integrity, oxidative stress, and peripheral nerve function, including some previously implicated in Alzheimer and Parkinson diseases. This trial was registered at www.controlled-trials.com as ISRCTN02694183.
Keywords: vitamin B-12, metabolomics, omics, nerve function, acylcarnitines, plasmalogens
Introduction
Vitamin B-12 is required for one-carbon and odd-chain FA metabolism (1). In the cytoplasm, vitamin B-12 participates in the metabolism of homocysteine to form methionine, a precursor of the universal methyl group donor, S-adenosylmethionine (SAM) (2). Vitamin B-12 is thus required for turnover of methyl groups during the synthesis of creatine, phospholipids, proteins, lipids, and neurotransmitters, as well as DNA and RNA (2). The vitamin is also a cofactor in the intramitochondrial conversion of methylmalonyl-CoA to succinyl-CoA catalyzed by methylmalonyl-CoA mutase (2).
Vitamin B-12 deficiency is expected to result in low serum or plasma concentrations of total vitamin B-12 and holotranscobalamin, accompanied by high methylmalonic acid (MMA) and total plasma homocysteine (tHcy) (1). Severe vitamin B-12 deficiency is characterized clinically by megaloblastic anemia and neurodegenerative changes of the central and peripheral systems (3). Neurological effects may include cognitive impairment, peripheral neuropathy, subacute combined degeneration of the spinal cord, and psychiatric disorders (3, 4). Poor vitamin B-12 status occurs more frequently in older adults because of age-related gastric atrophy; it is often asymptomatic and therefore left untreated (5). We previously described subclinical neurological manifestations of vitamin B-12 insufficiency in Chilean elderly (i.e., slower nerve conduction in myelinated peripheral nerves) (6) and postulated that vitamin B-12 status might have an influence on metabolic pathways associated with myelin integrity and mitochondrial function. Here we used serum metabolomic analyses to explore this postulate and identify metabolic pathways associated with subclinical functional impairment and responses to supplementation, which could potentially reveal new biomarkers of vitamin B-12 status and provide novel insights into the mechanisms underlying the pathobiology of vitamin B-12 deficiency. Vitamin B-12 status was described by the combined indicator of vitamin B-12 status (cB-12), a recently designed discriminant function that merges serum vitamin B-12, holotranscobalamin, MMA, and tHcy into a more robust single status indicator (7). We analyzed targeted and untargeted metabolomic profiles in blood samples from Chilean older adults with subclinical borderline vitamin B-12 deficiency. Based on the finding of low vitamin B-12 status (serum vitamin B-12 <120 pmol/L) at screening, peripheral nerve conduction was assessed in these patients, and they were immediately treated with a high-dose intramuscular vitamin B-12 injection (6). In addition, targeted metabolomics was performed in a group of asymptomatic elderly with adequate vitamin B-12 status to better define the range of vitamin B-12 status indicators in a correlation analysis. This study characterizes the human metabolome of subclinical vitamin B-12 deficiency in the elderly.
Methods
Study participants
A total of 51 elderly people (>70 y) from Santiago, Chile, were excluded for ethical reasons from a randomized controlled trial of vitamin B-12 supplementation (8) because they failed to meet the selection requirement of normal vitamin B-12 status (serum B-12 >120 pmol/L) at baseline. Vitamin B-12 status was remeasured and metabolomic analyses were performed in 27 participants who had poor vitamin B-12 status, which was confirmed by abnormal values of ≥3 of 4 biomarkers (serum vitamin B-12 <148 pmol/L, holotranscobalamin <35 pmol/L, tHcy >15 μmol/L, or MMA >271 nmol/L). All participants were “apparently healthy,” free-living individuals and beneficiaries of PACAM (Chile’s national nutritional supplementation program for the elderly). Since 2005, this program has provided a multiple-micronutrient food supplement that delivers daily ∼1.7 μg of vitamin B-12 (equivalent to ∼71% of the RDA of vitamin B-12) to individuals aged ≥70 y registered at primary health centers in Chile (8). The fieldwork was conducted between October 2007 and May 2008. The research protocol was approved by the Ethics Committee of the Institute of Nutrition and Food Technology at the University of Chile (record no. 19, July 19, 2006). Written informed consent was obtained from each volunteer before they entered the study and after they received an explanation that all participants would be treated and followed to assess their outcomes, and an explanation of the risks and benefits, confidentiality, and their right to withdraw at any time.
Eligibility criteria
Participants were selected based on exclusion criteria predefined in the randomized controlled trial from which they were selected (8). Exclusion criteria, based on surveys and other tools applied by trained personnel in primary health centers and by laboratory analyses conducted at the Institute of Nutrition and Food Technology, University of Chile, were severe cognitive impairment (Mini Mental State Examination score <19), diabetes mellitus (fasting glucose ≥126 mg/dL or receiving insulin or other antidiabetic medications), advanced renal impairment (creatinine clearance ≥30 g/L), hypothyroidism (plasma thyroid-stimulating hormone >6 mlU/L), gastrointestinal surgery, alcohol abuse based on reported daily alcohol consumption in the past 3 mo, and clinical history of stroke.
Design
Before-and-after treatment study.
Based on their low serum vitamin B-12 concentration (<120 pmol/L) at baseline, the 27 asymptomatic elderly participants were considered to need vitamin B-12 treatment. They received a single intramuscular injection of the commercially available combination of 10 mg cyanocobalamin, 100 mg pyridoxine, and 100 mg thiamin (Neurobion; Merck) as part of a “before-and-after treatment” study design. We assessed vitamin B-12 and folate status, peripheral nerve conductivity, and targeted and untargeted metabolomics profiles at baseline and 4 mo after injection.
Correlation analyses.
Connections between targeted metabolomics, vitamin B-12 status, and nerve function were explored in an expansion of the before and after treatment groups with an additional 18 elderly participants who had adequate vitamin B-12 status. They were selected from the same population and were identified at baseline from the larger supplementation trial (9) with adequate status confirmed by ≥3 of 4 vitamin B-12 biomarkers (serum vitamin B-12 ≥148 pmol/L, holotranscobalamin ≥35 pmol/L, tHcy ≤15 μmol/L, or MMA ≤271 nmol/L). Vitamin B-12 and folate status and peripheral nerve conduction were assessed and included in complementary analyses in a correlation analysis. Health and other eligibility criteria were the same as those of the participants in the before-and-after treatment study.
Biochemical analyses.
Venous blood samples (13 mL) were obtained with and without added EDTA for biochemical and hematological analysis and shipped on dry ice to the USDA, Agricultural Research Service Western Human Nutrition Research Center in Davis, California. Serum vitamin B-12 and folate were assessed in duplicate (CV 15–20%) by the SimulTRAC-SNB radioassay kit (57Co/Folate125I) (MP Diagnostics). Serum MMA (CV ∼5%) was analyzed by ultra performance liquid chromatography–tandem mass spectrometry (10). Serum holotranscobalamin (CV ∼10%) was determined with the Axis-Shield HoloTC ELISA (Axis-Shield Diagnostics Ltd.), and tHcy (CV 10–15%) by HPLC with an Agilent 1200 fluorescence detector (6).
Metabolomic analyses.
Targeted metabolomics were measured using an AbsoluteIDQ p150 Kit (BIOCRATES Life Sciences AG) by sample direct infusion onto a 4000 QTRAP liquid chromatograph–tandem mass spectrometer (AB Sciex), according to the manufacturer’s instructions. This platform detects 163 moieties comprising 14 amino acids, 41 acylcarnitines (including hydroxyl and dicarboxyl acylcarnitines), hexoses, 15 sphingomyelins and sphingomyelin derivatives, 15 lyso-phosphatidylcholines, and 77 phosphatidylcholines (including phospholipids and plasmalogens). Untargeted metabolomics measured 138 known and 109 unknown unique chemical entities using gas chromatography time-of-flight mass spectrometry, as previously described (11).
Definitions of vitamin B-12 and folate status.
Low serum vitamin B-12 was screened initially using a cutoff of <120 pmol/L as indicative of probable vitamin B-12 deficiency. Vitamin B-12 status was remeasured and abnormal biomarkers were defined as <148 pmol/L for low serum vitamin B-12, between 148 and 221 pmol/L for marginal serum vitamin B-12, <35 pmol/L for low holotranscobalamin, >271 nmol/L for high MMA, and >15 μmol/L for high tHcy (6). Low serum folate was defined as <10 nmol/L (6). Vitamin B-12 status was defined based on the “combined indicator of vitamin B-12 status” (cB-12), a novel approach that combines serum vitamin B-12, serum holotranscobalamin, tHcy, and serum MMA into 1 parameter: cB-12 = log10[(holotranscobalamin · B-12)/(MMA · tHcy)] − (age in years) (7). The following scale was applied to categorize cB-12 values: deficient (cB-12 ≤−1.5), low (−1.5 < cB-12 ≤−0.5), and adequate (cB-12 >−0.5) (7).
Sensory nerve conductivity.
Sensory nerve conduction studies were performed antidromically (i.e., in the direction opposite to normal conductance) in both sural nerves and in the right median nerve, in both treatment groups and in the group with adequate vitamin B-12 status (Nicolet Viking Quest TM system; Nicolet Biomedical) (6). The sensory latency of the right (X1) and left (X2) sural nerves and the right median nerve (X3) was significantly improved after treatment (P < 0.001). A peripheral nerve conduction score (nerve score) that combined these sensory latencies was calculated as −log10(X1 · X2 · X3). Higher values correspond to better conduction (12).
Dietary intake estimation.
Usual dietary intake of vitamin B-12 was estimated with a semiquantitative questionnaire that focused on amounts of animal source foods and other potential sources of vitamin B-12, including fortified cereals and supplements, consumed during the previous month. We used the estimated average requirement (EAR) and RDA for older adults (>50 y) in the United States and Canada to categorize the adequacy of vitamin B-12 (EAR: 2.0 μg; RDA: 2.4 μg), and folate (EAR: 320 μg; RDA: 400 μg) intake, expressed as dietary folate equivalents (9).
Statistical analyses
Variables were expressed as means ± SDs (unless otherwise stated). Differences between values before and after treatment were examined by a paired t test and the Wilcoxon nonparametric signed rank test. The Benjamini-Hochberg false discovery rate (FDR) procedure (13) was used to correct 2-sided P values for multiple comparisons across targeted or untargeted metabolites, controlling FDR at α = 0.05. We used ratios (levels after to levels before treatment) to illustrate the relative changes in metabolites found to be significantly different between the before- and after-treatment assessments for both the t test and the Wilcoxon test. A data set was constructed randomly by selecting 14 (of 27) individuals before treatment; the data from the remaining 13 subjects were used only after treatment (to ensure independence between groups). For targeted metabolomics, the data from the 18 individuals with adequate vitamin B-12 status (mentioned above) was added to the before- and after-treatment data set. Adding this group allowed us to expand the range of vitamin B-12 status. Spearman rank correlations were calculated between vitamin B-12 biomarkers, cB-12, the nerve score, and metabolomic variables in the correlation analyses. Data were analyzed and plotted with R software version 3.1.0 (R Foundation for Statistical Computing) (14). The network of the correlation analyses was visualized through the use of Cytoscape software version 2.8.2 (Cytoscape Consortium) (15). Metabolic pathways related to the significantly different metabolites were plotted using CMAp software version 1.01 (Florida Institute of Human and Machine Cognition) (16).
Results
General characteristics of the participants
Table 1 summarizes general characteristics of the before-and-after treatment group and the group with adequate vitamin B-12 status. Before treatment, the group with poor vitamin B-12 status had a mean age of 74 y (range: 70–78 y), and 63% of the participants were female. Of these participants, ∼60% reported regular consumption of foods provided by PACAM. More than one-third did not consume enough vitamin B-12 to meet the EAR. All participants consumed bread fortified with folic acid on a daily basis. Almost all of the participants (∼90%) met the folate EAR just by consuming bread. None of them presented with macrocytosis, and only one had microcytosis (Table 1). The group with adequate vitamin B-12 mainly comprised women (∼83%). Although >90% of them reported regular consumption of food provided by PACAM, ∼33% did not meet the EAR for vitamin B-12, and almost 40% did not meet the EAR for folate just by consuming bread.
TABLE 1.
General characteristics of elderly Chilean participants with deficient or adequate vitamin B-12 status1
| Deficient2 | Adequate | |
| Participants, n | 27 | 18 |
| Age, y | 74 ± 3 | 73 ± 2 |
| Women, % | 63 | 83.3 |
| Currently receiving supplementation from PACAM, % | 61.5 | 94.4 |
| Vitamin B-12 dietary intake, μg/d | 2.3 ± 1.1 | 2.8 ± 1.4 |
| Vitamin B-12 intake <EAR,3 % | 38.4 | 33.3 |
| Currently consuming bread fortified with folic acid, % | 100 | 94.4 |
| Bread consumption, g/d | 206 ± 74 | 163 ± 71 |
| Folic acid intake from bread, μg/d | 370 ± 133 | 293 ± 128 |
| Folic acid intake from bread, μg DFE/d | 617 ± 221 | 487.5 ± 213 |
| Folate intake from bread <EAR,4 % | 11.5 | 38.8 |
| Hemoglobin, g/L | 13.1 ± 1.4 | 13.4 ± 0.9 |
| Mild anemia,5 % | 11.1 | 11.1 |
| MCV, fL | 89 ± 4 | 88 ± 4 |
| Macrocytosis,6 % | 0 | 0 |
| Microcytosis,7 % | 3.7 | 0 |
| Mini Mental State Examination score | 27 ± 3 | 27 ± 2 |
| BMI, kg/m2 | 29.1 ± 3.4 | 27.9 ± 5.0 |
Values are means ± SDs unless otherwise indicated. DFE, daily folate equivalent; EAR, estimated average requirement; MCV, mean corpuscular volume; PACAM, Chile's national nutritional supplementation program for the elderly.
Data were collected from the vitamin B-12–deficient participants before the vitamin B-12 treatment was administered.
In the elderly, EAR for vitamin B-12 is 2 μg/d.
EAR for folate = 320 μg DFE (9).
Hemoglobin <130 g/L for men, <120 g/L for women.
MCV >100 fL.
MCV <80 fL.
Changes before and after treatment
Vitamin B-12 and folate status.
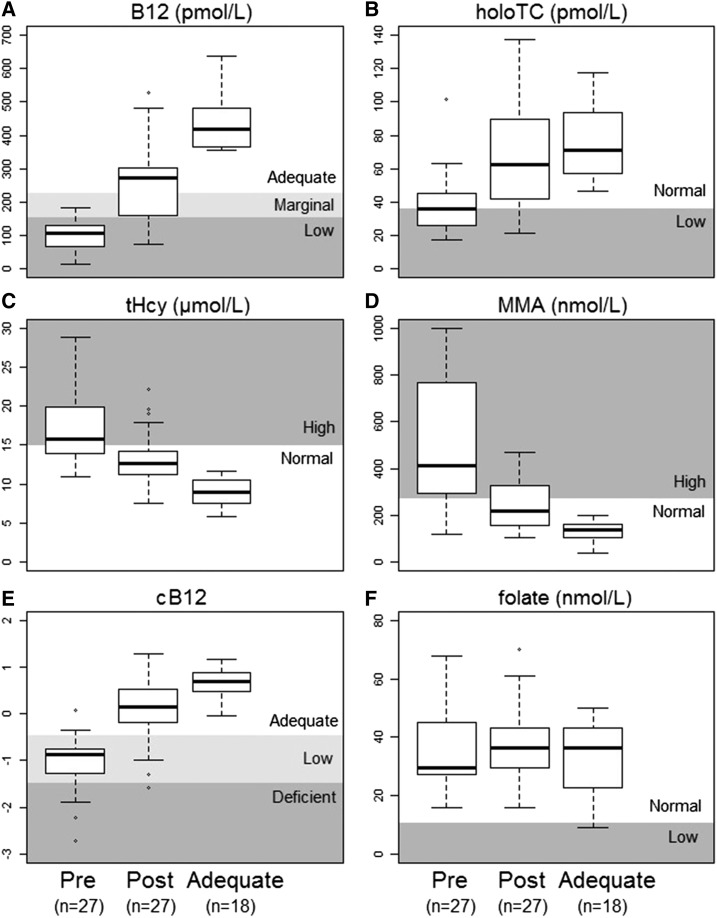
Serum vitamin B-12, holotranscobalamin, and cB-12 increased significantly (P < 0.0001) after treatment (Figure 1). tHcy and MMA were significantly reduced after treatment (P < 0.0001). No change was observed in serum folate (P = 0.50). Only one participant had low serum folate (<10 nmol/L) at baseline.
FIGURE 1.
Vitamin B-12 and folate status in the groups of Chilean elderly adults before and after treatment (n = 27) and in those with adequate vitamin B-12 status (n = 18). Values are medians (IQRs). The treatment was a single injection of 10 mg vitamin B-12 with 100 mg pyridoxine and 100 mg thiamin. Asterisks (*) represent individual values below or above the 25th and 75th percentiles. B12, vitamin B-12; cB12, combined indicator of vitamin B-12 status; holoTC, holotranscobalamin; MMA, methylmalonic acid; post, after; pre, before; tHcy, total plasma homocysteine.
Neurophysiological parameters.
After treatment, the sensory latency for both the left and right sural nerves and the right median nerve improved significantly (P < 0.001). The nerve score, based on a combination of these parameters, improved after treatment (P < 0.001).
Targeted metabolomics.
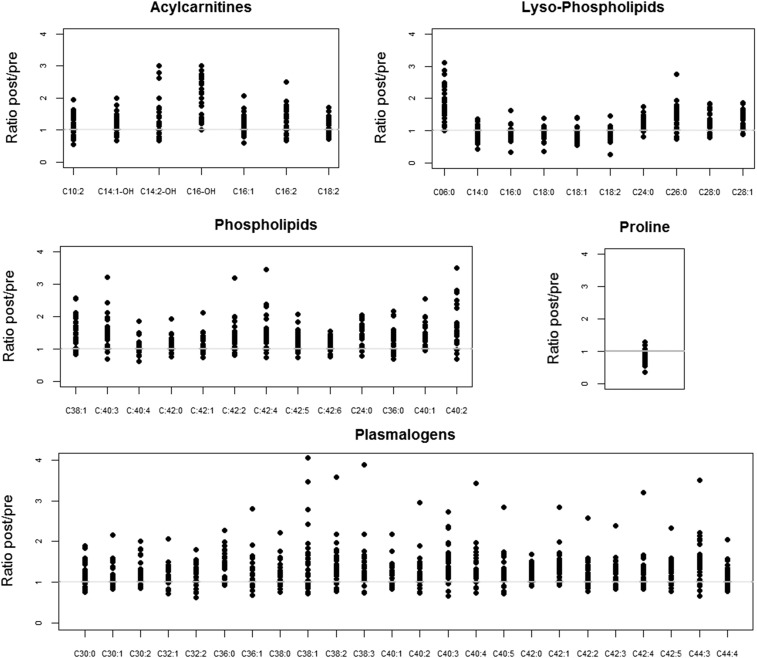
After treatment, acylcarnitines, plasmalogens, and other phospholipids increased (P < 0.05 after the FDR procedure). Proline was reduced (P < 0.05 after the FDR procedure), whereas some lyso-phospholipids increased (6:0, 24:0, 26:0, 28:0, 28:1) and others decreased (14:0, 16:0, 18:0, 18:1, 18:2) (P < 0.05 after the FDR procedure). We used ratios between the levels after treatment and those before treatment to illustrate the changes observed in targeted metabolites that were found to be significantly different in the paired analyses (Figure 2). (Supplemental Table 1 lists all targeted compounds measured and summarizes the metabolite levels at the before- and after-treatment assessments and the results of the paired analyses).
FIGURE 2.
Ratios for targeted metabolomics before and after vitamin B-12 treatment in vitamin B-12–deficient elderly Chilean adults. The treatment was a single injection of 10 mg vitamin B-12 with 100 mg pyridoxine and 100 mg thiamin. The horizontal lines at y = 1 indicate no change from before to after treatment. Post, after; pre, before.
Untargeted metabolomics.
Serum 2-hydroxyvaleric acid, maleimide, methionine sulfoxide, and N-acetylglycine increased after treatment (P < 0.05 after the FDR procedure), whereas serum cystine, cysteine, methionine, and oxalic acid decreased (P < 0.05 after the FDR procedure). Significant (P < 0.05 after FDR) changes were observed in additional unidentified serum metabolites (BinBase identifiers: 223548, 223618, 225446, 309642, 337230, and 425836) (O Fiehn, NIH West Coast Metabolomics Center, University of California, Davis, 2016). (Supplemental Table 2 lists all untargeted moieties measured and summarizes levels at the before- and after-treatment assessments and results of the paired analyses).
Correlation analyses
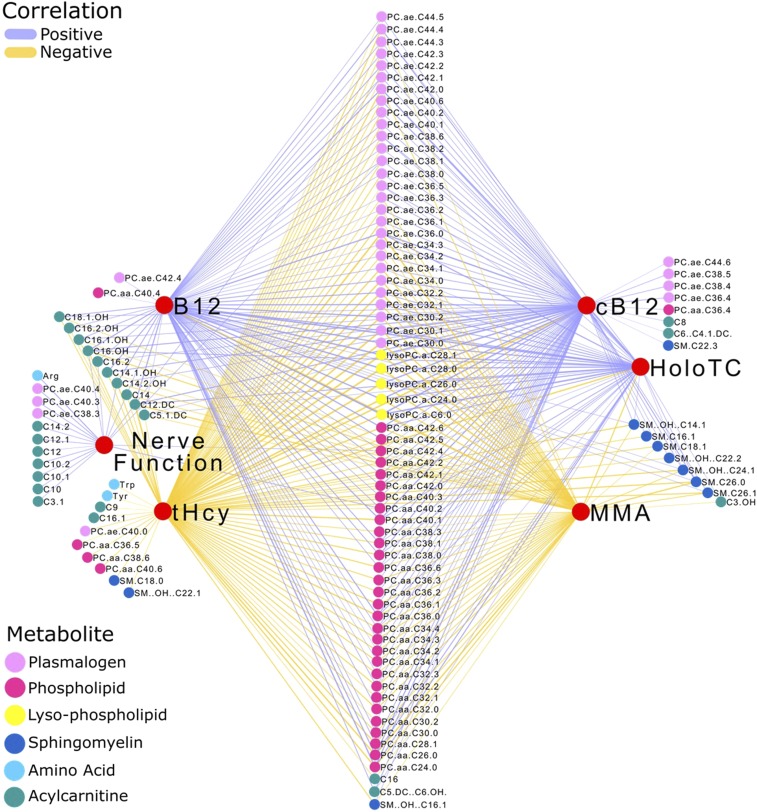
Relations between targeted metabolomics, vitamin B-12 status, and nerve function.
The addition of the group with adequate vitamin B-12 status allowed us to explore the relations with metabolomics over an expanded range of vitamin B-12 status (Figure 1). The correlation analyses were characterized by consistent significant (P < 0.05) direct correlations between acylcarnitines, plasmalogens, phospholipids, lyso-phospholipids, and sphingomyelins with vitamin B-12 status and nerve function (Figure 3). Serum vitamin B-12 and holotranscobalamin were directly correlated (P < 0.05) and tHcy and MMA were inversely correlated (P < 0.05) with targeted metabolomic variables. Correlations with cB-12 as a proxy of overall vitamin B-12 status confirmed these relations. A direct correlation (P < 0.05) was also observed between the nerve score and serum arginine, as was an inverse correlation (P < 0.05) between tHcy and tryptophan and tyrosine. Supplemental Table 3 shows the correlation coefficients and tests of the hypothesis for each correlation.
FIGURE 3.
Spearman rank correlations between targeted metabolomics, vitamin B-12 status, and nerve function in a data set of vitamin B-12–deficient elderly Chilean adults before (n = 14) and after (n = 13) treatment, and in those with adequate vitamin B-12 status (n = 18). The treatment was a single injection of 10 mg vitamin B-12 with 100 mg pyridoxine and 100 mg thiamin. B12, vitamin B-12; cB12, combined indicator of vitamin B-12 status; HoloTC, holotranscobalamin; MMA, methylmalonic acid; tHcy, total plasma homocysteine.
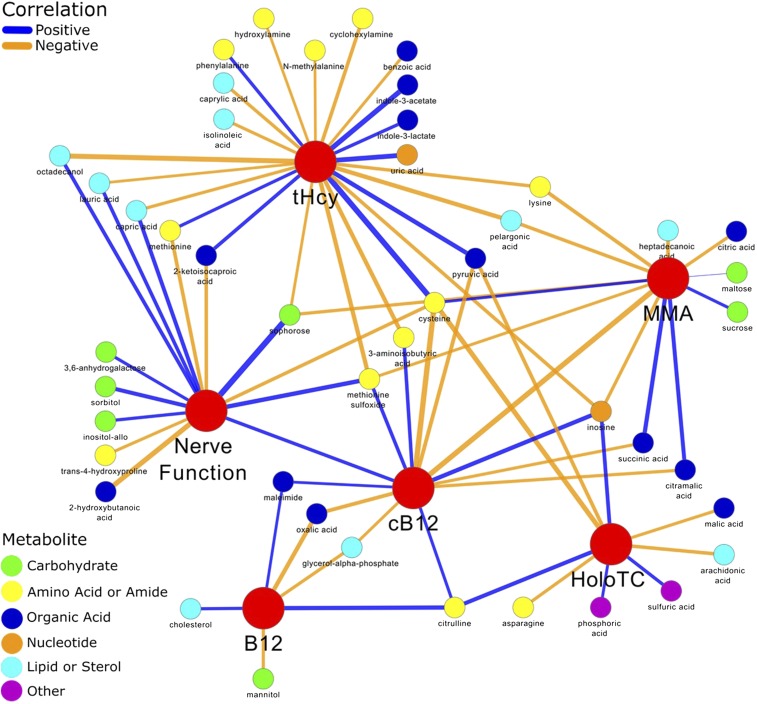
Relations between untargeted metabolomic variables, vitamin B-12 status, and nerve function.
Direct correlations (P < 0.05) between vitamin B-12 status, nerve function, or both were identified with the following metabolites: sophorose, sulfuric acid, sorbitol, phosphoric acid, pelargonic acid, octadecanol, N-methylalanine, methionine sulfoxide, maleimide, lysine, lauric acid, isolinoleic acid, inositol-allo, inosine, hydroxylamine, heptadecanoic acid, cyclohexylamine, citrulline, citric acid, cholesterol, caprylic acid, capric acid, benzoic acid, 3,6-anhydrogalactose, and 3-aminoisobutyric acid. By contrast, inverse correlations (P < 0.05) were found between vitamin B-12 status and peripheral nerve function with uric acid, trans-4-hydroxyproline, sucrose, succinic acid, pyruvic acid, phenylalanine, oxalic acid, methionine, mannitol, maltose, malic acid, indole-3-lactate, indole-3-acetate, glycerol-α-phosphate, cysteine, citramalic acid, asparagine, arachidonic acid, 2-ketoisocaproic acid, and 2-hydroxybutanoic acid (Figure 4). Supplemental Table 4 shows the correlation coefficients and tests of hypothesis for each correlation.
FIGURE 4.
Spearman rank correlations between untargeted metabolomics, vitamin B-12 status, and nerve function in a data set from vitamin B-12–deficient elderly Chilean adults before (n = 14) and after treatment (n = 13). The treatment was a single injection of 10 mg vitamin B-12 with 100 mg pyridoxine and 100 mg thiamin. B12, vitamin B-12; cB12, combined indicator of vitamin B-12 status; HoloTC, holotranscobalamin; MMA, methylmalonic acid; tHcy, total plasma homocysteine.
Discussion
This study characterizes the human serum metabolome in subclinical vitamin B-12 deficiency and repletion. Although much is known about the biochemical role of vitamin B-12 in the 2 reactions that require this vitamin as a cofactor, beyond the observed accumulation of homocysteine and methylmalonic acid in vitamin B-12 deficiency, the biochemical perturbations that occur in vitamin B-12 deficiency, and especially in subclinical vitamin B-12 deficiency, have not been well characterized (1). We determined changes in vitamin B-12 status over 4 mo after a single treatment (injection of 10 mg vitamin B-12). Our analyses were complemented by a correlation analysis that explored connections with metabolomics in an expanded range of vitamin B-12 status. To identify metabolic pathways influenced by vitamin B-12 status, metabolomics profiling was coupled to measures of changing peripheral nerve conductivity. The ability to assess multiple metabolites simultaneously provided an opportunity to generate hypotheses regarding the metabolic consequences of subclinical vitamin B-12 deficiency from observations of changes and connections in the metabolomics profile of individuals who were replete with vitamin B-12 after administration of a single large dose of vitamin B-12. We demonstrated the biochemical influence of vitamin B-12 in asymptomatic, elderly Chileans who showed improvements in conductivity in myelinated peripheral nerves after treatment (6). Notably, for example, increases in acylcarnitines might reflect an improvement in mitochondrial function or increases in metabolic markers of myelin integrity and membrane constituents, such as plasmalogens and other phospholipids. Metabolomics also revealed an influence of vitamin B-12 on primary intermediates of one-carbon metabolites and the Krebs cycle, as well as on a number of carbohydrates, amino acids, amides, organic acids, nucleotides, lipids, and sterols.
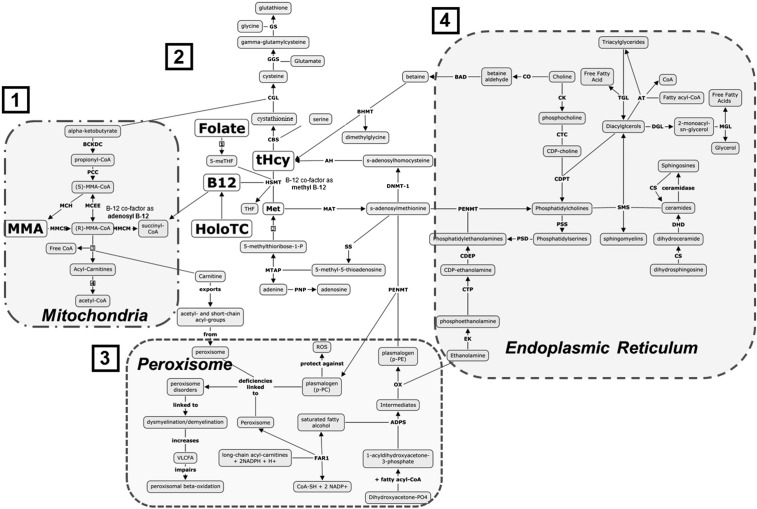
Figure 5 illustrates the direct and indirect relations between vitamin B-12 and one-carbon metabolism, mitochondrial function, FA oxidation, plasmalogen synthesis, and glycerophospholipid and triacylglyceride production and breakdown, in the context of our findings. Several long-chain hydroxylated and nonhydroxylated acylcarnitines (C10–C-18) increased after vitamin B-12 treatment. The connection between acylcarnitine, vitamin B-12 status, and nerve function was confirmed in the correlation analyses. This suggests some changes in the β-oxidation of long-chain FAs and in the tricarboxylic acid cycle. Acylcarnitines facilitate the entry of long-chain FAs into the mitochondria via the carnitine shuttle. Long-chain FAs act as an important source of fuel for many tissues (e.g., skeletal and cardiac muscle), where acyl-CoAs are converted to acylcarnitines (17). Further, the synthesis of carnitine requires the methylation of lysine by SAM to form trimethyllysine (18). Therefore, the impairment of methionine (and SAM) formation in vitamin B-12 deficiency might interfere with carnitine biosynthesis. Vitamin B-12 deficiency is associated with the redistribution of carnitine and a consequent higher loss due to the formation of propionyl and other carnitine derivatives (19). Studies in animals have shown the consequences of methyl donor–deficient diets impairing mitochondrial FA oxidation and consequently decreasing free and total circulating carnitines (20). Data also indicate new, multifactorial roles for acylcarnitines in neuroprotection through mechanisms that include modifying synthesizing lipids, stabilizing membrane composition, modulating genes and proteins, increasing antioxidant activity, and enhancing cholinergic neurotransmission (21). A redistribution of serum carnitines was reported to distinguish between patients with Alzheimer disease, those with mild cognitive impairment, and healthy controls (22).
FIGURE 5.
Metabolic pathways. 1: Role of vitamin B-12 in the mitochondria (acyl-CoA utilization). Acyl-CoAs are released into the cytoplasm. 2: Participation of vitamin B-12 in folate metabolism and methionine salvaging. 3: Acyl-CoA enters the peroxisome, where biosynthesis of plasmalogens occurs. 4: Phospholipid synthesis occurs in the endoplasmic reticulum. Vitamin B-12 is required for the mitochondrial-mediated conversion of methylmalonyl-CoA to succinyl-CoA. This conversion apparently results in the liberation of free CoA, which is consequently used to generate acylcarnitines (1). Vitamin B-12 and 5-methyltetrahydrofolate are cofactors required to methylate homocysteine into methionine, the primary precursor of the principal methyl donor, SAM (2). Inadequate vitamin B-12 availability results in a reduced remethylation potential of homocysteine, thereby limiting methionine pools and redirection of homocysteine into the transsulfuration pathway, a source of free cysteine for glutathione synthesis (1). Reduced methionine availability limits SAM biosynthesis. SAM participates in the biosynthesis of PCs and plasmalogen-PCs via the addition of 3 SAM-derived methyl groups to the phosphoethanolamine headgroup of PEs and plasmalogen-PEs (3 and 4). Perturbed generation of plasmalogens has been linked to demyelination and dysmyelination. Sphingomyelin, an important component of the myelin sheath, is generated through the transfer of the phosphocholine headgroup from PCs to ceramides (4). ADPS, 1-alkyldihydroxyacetone-3-phosphate synthetase; AH, denosylhomocysteinase; AT, acyltransferase; BAD, betaine aldehyde dehydrogenase; BCKDC, branched chain alpha-keto acid dehydrogenase complex; BHMT, betaine-homocysteine s-methyltransferase; B12, vitamin B-12; CAT, carnitine-acylcarnitine translocase; CBS, cystathionine beta synthase; CDEP, CDP-ethanolamine:1,2-diacylglycerol ethanolaminephosphotransferase; CDPT, CDP-choline-diacylglycerol phosphocholine transferase; CGL, cystathionine gamma lyase; CK, choline kinase; CO, choline oxidase; CS, ceramide synthase; CTC, CTP-choline cytidylyltransferase; CTP, phosphoethanolamine cytidylytransferase; DGL, diacylglyceride lipase; DHD, dihydroceramide desaturase; DNMT, DNA (cytosine-5)-methyltransferase 1; EK, ethanolamine kinase; FAR1, fatty acyl-reductase; GGS, gamma-glutamylcysteine synthase; GS, glutathione synthase; HMST, homocysteine s-methyltransferase; HoloTC, holotranscobalamin; MAT, s-adenosylmethionine synthetase; MCEE, methylmalonyl-CoA/ethylmalonyl-CoA epimerase; MCH, (S)-methylmalonyl-CoA hydrolase; Met, methionine; MGL, monoacylglyceride lipase; MMA, methylmalonic acid; MMCM, methylmalonyl-CoA mutase; MMCS, malonyl-CoA/methylmalonyl-CoA synthetase; MTAP, MTA phosphorylase; OX, oxidase; PC, phosphatidylcholine; PCC, propionyl-CoA carboxylase; PE, phosphatidylethanolamine; PENMT, phosphatidylethanolamine N-methyltransferase; PNP, purine-nucleoside phosphorylase; PSD, phosphatidylserine decarboxylase; PSS, phosphatidylserine synthase; ROS, reactive oxygen species; SAM, S-adenosylmethionine; SMS, sphingomyelin synthase; SS, spermidine synthetase; TGL, triacylglyceride lipase; tHcy, total plasma homocysteine; VLCFA, very-long-chain FA.
Vitamin B-12 treatment increased metabolic markers of myelin integrity and modulators of membrane dynamics, such as plasmalogens (a specific type of ether phospholipids) and other long-chain phospholipids (C38–40). Changes were also observed in lyso-phospholipids, which are considered to be potent biologically active lipid mediators that exert cellular effects through the specific G protein–coupled receptors (23). The correlation analyses confirmed direct relations between plasmalogens, phospholipids, lyso-phospholipids, and sphingomyelins, and vitamin B-12 status and nerve function. Plasmalogens are biosynthesized in peroxisomes; this involves the transesterification of dihydroxyacetone phosphate with a long-chain acyl-CoA (24, 25). Acyl groups are required in this process and might be delivered by newly produced carnitines as a consequence of treatment with vitamin B-12. Plasmalogens are particularly important because of their multiple biological roles, as described in the literature (24, 25). Plasmalogens may have an antioxidant function: they act as scavengers, protecting other phospholipids, lipids, and lipoproteins from reactive oxygen species (ROS) (24, 25). They are considered to be mediators of membrane dynamics and signaling, and can also serve as storage sites of PUFAs (24–26). The importance of plasmalogens in human health is highlighted by their potential role in Alzheimer disease and other neurologic disorders, such as Down syndrome and Parkinson disease (24, 25). Decreasing concentrations of serum plasmalogens correlate with functional decline in patients with Alzheimer disease (27). Plasmalogens represent ≤20% of the total phospholipid mass in humans and are widely distributed in tissues (28). These compounds constitute a large proportion of ethanolamine glycerophospholipids—≥50% in the brain, heart, neutrophils, and eosinophils, and up to ∼90% of ethanolamine derivatives in some regions of the brain (28). They are concentrated in specialized membranes, such as muscle sarcolemma and axonal myelin (28). The total amount of brain plasmalogens increases dramatically during the developmental phase of myelination (28). Plasmalogens are crucial for the Schwann cell development and differentiation required for myelination of peripheral nerves, and plasmalogen defects impair myelin structure (29). The consistent increase in plasmalogens and other phospholipids in response to vitamin B-12 treatment suggests novel mechanisms linking vitamin B-12 to neuroprotection (4, 30, 31).
Metabolite intermediaries of one-carbon metabolism, including methionine and cysteine, were reduced after treatment with vitamin B-12, in keeping with the higher metabolic utilization of these compounds via vitamin B-12–dependent pathways. Cysteine is an important substrate for glutathione biosynthesis (a recognized primary antioxidant). We speculate that cystine, an oxidized dimer of cysteine, could serve as an additional source of cysteine that can be taken up via cystine-glutamate antiporters and reduced to free cysteine, thereby maintaining redox balance and glutathione content (32). The reduction in cysteine and cystine coupled with elevations in methionine sulfoxide, an oxidation product of methionine, implicates altered redox status, which may be linked to the observed changes in circulating acyl carnitines. Fat broken down through FA oxidation is a prominent source of mitochondrial ROS in kidney cortical tubules in early diabetes (33). The reduction in cysteine and cystine may therefore reflect a compensatory mechanism of increased glutathione synthesis to counteract toxic ROS buildup. In addition, an inverse relation was found between vitamin B-12 biomarkers and tryptophan, tyrosine, pyruvic acid, and succinic acid, among other intermediaries of the citric acid cycle (34). Consequently, citric acid was also inversely related to vitamin B-12 status. We observed a direct correlation between the nerve score and arginine; this is consistent with the role of arginine in nerve regeneration (22, 35). We also observed a decrease in proline. This was unexpected but may reflect a role for vitamin B-12 status in the integrity of bone. Vitamin B-12 deficiency is associated with osteopenia (36), and proline and hydroxyproline concentrations increase in conditions associated with defective synthesis of collagen in the bone matrix (36). Changes in or connections between other metabolites (e.g., maleimide, oxalic acid, 2-hydroxyvaleric acid, N-acetylglycine, methionine sulfoxide, sulfuric acid, and pelargonic acid) and vitamin B-12 status cannot be explained at this time.
We selected for metabolomics profiling those participants who initially had low serum vitamin B-12 concentrations (<120 pmol/L) and whose poor vitamin B-12 status was confirmed by ≥3 vitamin B-12 biomarkers, thereby reducing the sample size from 51 to 27. Our smaller sample size may have resulted in some possibly meaningful before-and-after responses to supplementation becoming statistically nonsignificant. Although we complemented these data sets with a group of participants with adequate vitamin B-12 status for analyses against targeted metabolomics, only 9 of those 18 participants had available peripheral nerve conduction assessment, reducing our capacity to detect correlations with nerve function. The before-and-after data set created for the correlation analyses was constructed by selecting 14 (of 27) individuals before treatment; the data from the remaining 13 participants were used only after treatment to ensure independence between groups, although this also reduced our power to detect significant correlations. A total of 34 unknown moieties were significantly correlated with vitamin B-12 status, and their identification may be important for future research on vitamin B-12 deficiency in humans. Because of the limited statistical power provided by this small sample size, folate–vitamin B-12 interaction was not analyzed in this study. We previously reported a weaker vitamin B-12 biochemical response to vitamin B-12 treatment in participants from this study who had moderately high and high folate at baseline (6). Another limitation of this study is the treatment with a vitamin mixture (the single intramuscular dose of 10 mg cyanocobalamin contained 100 mg thiamin and 100 mg pyridoxine). Although it is possible that the thiamin or pyridoxine contributed to the metabolomic changes, a pyridoxine effect is unlikely because we measured pyridoxal-5-phosphate by HPLC with fluorescence detection in a subsample and did not observe significant changes before and after injection [median: 26.0 nM (IQR: 12.3 nM) compared with 14.8 nM (IQR: 13.0 nM)] (6). Pyridoxine supplementation may have affected the catalytic conversion of homocysteine to cystathione by pyridoxine-dependent cystathione B synthase and ultimately to cysteine and glutathione. However, the high dose of vitamin B-12 might have also limited the availability of homocysteine for cystathione B synthase by stimulating catalytic conversion of homocysteine to methionine by methionine synthase. We cannot rule out a confounding effect of thiamin and did not measure change in status. Some of the changes in mitochondrial metabolites could be attributed to thiamin, given that this vitamin is a cofactor for 1) α-ketoglutarate dehydrogenase in the Krebs cycle, which catalyses the formation of succinyl-CoA; and 2) the branched-chain α-ketoacid dehydrogenase complex, which catalyzes the formation of branched-chain acyl-CoA. Given the fast turnover rate and minimal storage of thiamin, however, the effect of the single supplemental dose would not likely be seen at follow-up. A more conclusive result could have been obtained if the intervention involved only an injection of vitamin B-12. This study lacked a control group. Our findings need to be replicated in a larger sample with an appropriate control group in order to clarify and further understand the metabolic role of vitamin B-12 and the implications of these findings for public health. We acknowledge the uncertainty of whether these serum changes actually reflect intracellular changes or changes in interorgan transport. Finally, we advise confirming the plasmalogen response to vitamin B-12 treatment using newer, more specific, and more accurate methods (37, 38).
The metabolomics profiling study was conducted in the context of low vitamin B-12 status before and after correction, and was greatly enhanced by the available measurements of the change in neurological function. We did not limit our analyses to before-and-after treatment comparisons, and we expanded the spectrum of vitamin B-12 status. We used a refined nerve score as a proxy of nerve function and the 4 biochemical markers to determine vitamin B-12 status; we also used cB-12, which confirmed strong metabolic connections with overall vitamin B-12 status. Laboratory analyses were performed with the most advanced instruments available for metabolomic profiling. Our results highlight the application of metabolomics as a useful and powerful approach to identify metabolic pathways associated with subclinical functional impairment, to assess responses to interventions, and to identify new biomarkers of vitamin B-12 status. The metabolites connected to vitamin B-12 status in this study (i.e., acylcarnitines and plasmalogens) have been implicated in neuroprotection. Our findings have the potential to help clarify the role of vitamin B-12 in neurological function and the metabolites involved in the etiology or prevention of diseases such as Alzheimer or Parkinson.
In conclusion, this is to our knowledge the first characterization of the human serum metabolome in subclinical vitamin B-12 deficiency. Metabolomics revealed connections between vitamin B-12 status and serum metabolic markers of mitochondrial function, myelin integrity, oxidative stress, and peripheral nerve function, that is, acylcarnitines and plasmalogens.
Acknowledgments
The authors’ responsibilities were as follows—AB: conceived and designed the study, performed the statistical analyses, interpreted the data, and wrote the manuscript; DG and JF: supported the targeted and untargeted metabolomics data analyses; JF: led the postulated pathways; D Harvey: assisted with the statistical analyses; JWM and RG: interpreted the data; SNF: developed cB-12, interpreted the data, and conceptualized the development of the nerve score; SS-F: conducted the biochemical analyses; D Hampel and TLP: supported the targeted metabolomics analyses; JWN: oversaw integration of the targeted metabolomic analyses; OF: led the untargeted metabolomics analyses; RU: acted as the principal investigator of the supplementation trial and conceived the study; LHA: conceptualized the main study and had final responsibility for the manuscript; and all authors: provided input into and read and approved the final version of the manuscript.
Footnotes
Abbreviations used: cB-12, combined indicator of vitamin B-12 status; EAR, estimated average requirement; FDR, false discovery rate; MMA, methylmalonic acid; PACAM, Chile's national nutritional supplementation program for the elderly; ROS, reactive oxygen species; SAM, S-adenosylmethionine; tHcy, total plasma homocysteine.
References
- 1.Green R, Miller JW. Vitamin B12. In: Zempleni J, Suttie JW, Gregory JF III, Stover PJ, editors. Handbook of vitamins. 5th ed. Boca Raton (FL): CRC Press; 2014. p. 447–89. [Google Scholar]
- 2.Selhub J, Miller JW. The pathogenesis of homocysteine: interruption of the coordinate regulation by S-adenosylmethionine of the remethylation and transsulfuration of homocysteine. Am J Clin Nutr 1992;55:131–8. [DOI] [PubMed] [Google Scholar]
- 3.Hemmer B, Clocker FX, Schumacher M, Deuschl G, Lucking CH. Subacute combined degeneration: clinical, electrophysiological, and magnetic resonance imaging findings. J Neurol Neurosurg Psychiatry 1998;65:822–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosenberg IH. Effects of folate and vitamin B12 on cognitive function in adults and the elderly. Food Nutr Bull 2008;29(2 Suppl):S132–42. [DOI] [PubMed] [Google Scholar]
- 5.Allen LH. Causes of vitamin B12 and folate deficiency. Food Nutr Bull 2008;29(2 Suppl):S20–34; discussion S35–7. [DOI] [PubMed] [Google Scholar]
- 6.Brito A, Verdugo R, Hertrampf E, Miller JW, Green R, Fedosov SN, Shahab-Ferdows S, Sanchez H, Albala C, Castillo JL, et al. . Vitamin B-12 treatment of asymptomatic deficient elderly Chileans improves conductivity in myelinated peripheral nerves, but high serum folate impairs B-12 status response assessed by the combined indicator cB-12. Am J Clin Nutr 2016;103:250–7. [DOI] [PubMed] [Google Scholar]
- 7.Fedosov SN, Brito A, Miller JW, Green R, Allen LH. Combined indicator of vitamin B-12 status: modification for missing biomarkers and folate status, and recommendations for revised cut-points. Clin Chem Lab Med 2015;53:1215–25. [DOI] [PubMed] [Google Scholar]
- 8.Sánchez H, Albala C, Lera L, Castillo JL, Verdugo R, Lavados M, Hertrampf E, Brito A, Allen L, Uauy R. Comparison of two modes of vitamin B-12 supplementation on neuroconduction and cognitive function among older people living in Santiago, Chile: a cluster randomized controlled trial. a study protocol [ISRCTN 02694183]. Nutr J 2011;10:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Institute of Medicine. Dietary reference intakes: thiamin, riboflavin, niacin, vitamin B6, folate, vitamin B-12, pantothenic acid, biotin, and choline. Washington (DC): National Academies Press; 2000. [PubMed] [Google Scholar]
- 10.Pedersen TL, Keyes WR, Shahab-Ferdows S, Allen LH, Newman JW. Methylmalonic acid quantification in low serum volumes by UPLC-MS/MS. J Chromatogr B Analyt Technol Biomed Life Sci 2011;879:1502–6. [DOI] [PubMed] [Google Scholar]
- 11.Grapov D, Fahrmann J, Hwang J, Poudel A, Jo J, Periwal V, Fiehn O, Hara M. Diabetes associated metabolomic perturbations in NOD mice. Metabolomics 2015;11:425–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brito A, Fedosov S, Miller J, Green R, Uauy R, Allen LH. Reply to LR Solomon. Am J Clin Nutr 2016;103:1379. [DOI] [PubMed] [Google Scholar]
- 13.Yoav B, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol 1995;57:289–300. [Google Scholar]
- 14.R Core Team. R: a language and environment for statistical computing [Internet]. Vienna (Austria): R Foundation for Statistical Computing [cited 2015 Dec 14]. Available from: http://www.R-project.org/.
- 15.Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, Ideker T. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res 2003;13:2498–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cañas AJ, Carff R, Hill G, Carvalho M, Arguedas M, Eskridge TC, Lott J, Carvajal R. Concept maps: integrating knowledge and information visualization In: Tergan S-O, Keller T, editors. Knowledge and information visualization: searching for synergies. Heidelberg (Germany): Springer Lecture Notes in Computer Science; 2005. p. 205–19. [Google Scholar]
- 17.Schooneman MG, Vaz FM, Houten SM, Soeters MR. Acylcarnitines. Reflecting or inflicting insulin resistance. Diabetes 2013;62:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cox RA, Hoppel CL. Biosynthesis of carnitine and 4-N-trimethylaminobutyrate from 6-N-trimethyl-lysine. Biochem J 1973;136:1083–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brass EP, Stabler SP. Carnitine metabolism in the vitamin B-12-deficient rat. Biochem J 1988;255:153–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pooya S, Blaise S, Garcia MM, Giudicelli J, Alberto JM, Guéant-Rodriguez RM, Jeannesson E, Gueguen N, Bressenot A, Nicolas B, et al. . Methyl donor deficiency impairs fatty acid oxidation through PGC-1α hypomethylation and decreased ER-α, ERR-α, and HNF-4α in the rat liver. J Hepatol 2012;57:344–51. [DOI] [PubMed] [Google Scholar]
- 21.Jones LL, McDonald DA, Borum PR. Acylcarnitines: role in brain. Prog Lipid Res 2010;49:61–75. [DOI] [PubMed] [Google Scholar]
- 22.González‐Domínguez R, García A, García‐Barrera T, Barbas C, Gómez‐Ariza JL. Metabolomic profiling of serum in the progression of Alzheimer’s disease by capillary electrophoresis–mass spectrometry. Electrophoresis 2014;35:3321–30. [DOI] [PubMed] [Google Scholar]
- 23.Rivera R, Chun J. Biological effects of lysophospholipids. Rev Physiol Biochem Pharmacol 2008;160:25–46. [DOI] [PubMed] [Google Scholar]
- 24.Brites P, Waterham HR, Wanders RJ. Functions and biosynthesis of plasmalogens in health and disease. Biochim Biophys Acta 2004;1636:219–31. [DOI] [PubMed] [Google Scholar]
- 25.Nagan N, Zoeller R. Plasmalogens: biosynthesis and functions. Prog Lipid Res 2001;40:199–229. [DOI] [PubMed] [Google Scholar]
- 26.Wallner S, Schmitz G. Plasmalogens the neglected regulatory and scavenging lipid species. Chem Phys Lipids 2011;164:573–89. [DOI] [PubMed] [Google Scholar]
- 27.Wood PL, Mankidy R, Ritchie S, Heath D, Wood JA, Flax J, Goodenowe DB. Circulating plasmalogen levels and Alzheimer disease assessment scale-cognitive scores in Alzheimer patients. J Psychiatry Neurosci 2010;35:59–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Braverman NE, Moser AB. Functions of plasmalogen lipids in health and disease. Biochim Biophys Acta 2012;1822:1422–52. [DOI] [PubMed] [Google Scholar]
- 29.da Silva TF, Eira J, Lopes AT, Malheiro AR, Sousa V, Luoma A, Avila RL, Wanders RJ, Just WW, Kirschner DA, et al. . Peripheral nervous system plasmalogens regulate Schwann cell differentiation and myelination. J Clin Invest 2014;124:2560–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reynolds E. Vitamin B12, folic acid, and the nervous system. Lancet Neurol 2006;5:949–60. [DOI] [PubMed] [Google Scholar]
- 31.Savage DG, Lindenbaum J. Neurological complications of acquired cobalamin deficiency: clinical aspects. Baillieres Clin Haematol 1995;8:657–78. [DOI] [PubMed] [Google Scholar]
- 32.Ishii T, Mann GE. Redox status in mammalian cells and stem cells during culture in vitro: critical roles of Nrf2 and cystine transporter activity in the maintenance of redox balance. Redox Biol 2014;2:786–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rosca MG, Vazquez EJ, Chen Q, Kerner J, Kern TS, Hoppel CL. Oxidation of fatty acids is the source of increased mitochondrial reactive oxygen species production in kidney cortical tubules in early diabetes. Diabetes 2012;61:2074–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Berg JM, Tymoczko JL, Stryer L. Amino acids are made from intermediates of the citric acid cycle and other major pathways. In: Biochemistry. 5th ed. New York: W H Freeman; 2002. [Google Scholar]
- 35.Cestaro B. Effects of arginine, S‐adenosylmethionine and polyamines on nerve regeneration. Acta Neurol Scand Suppl 1994;154:32–41. [DOI] [PubMed] [Google Scholar]
- 36.Pohlídal A, Husek P, Palicka V, Slabík D, Hill M, Matucha P. Novel and traditional biomarkers of bone turnover in postmenopausal women. Clin Chem Lab Med 2003;41:74–8. [DOI] [PubMed] [Google Scholar]
- 37.Otoki Y, Nakagawa K, Kato S, Miyazawa T. MS/MS and LC-MS/MS analysis of choline/ethanolamine plasmalogens via promotion of alkali metal adduct formation. J. Chromatogr B Analyt Technol Biomed Life Sci 2015;1004:85–92. [DOI] [PubMed] [Google Scholar]
- 38.Otoki Y, Kato S, Kimura F, Furukawa K, Yamashita S, Arai H, Miyazawa T, Nakagawa K. Accurate quantitation of choline and ethanolamine plasmalogen molecular species in human plasma by liquid chromatography–tandem mass spectrometry. J Pharm Biomed Anal 2017;134:77–85. [DOI] [PubMed] [Google Scholar]