Abstract
Background: Associations between childhood vitamin K consumption and cardiac structure and function have not been investigated.
Objective: We determined associations between phylloquinone (vitamin K-1) intake and left ventricular (LV) structure and function in adolescents.
Methods: We assessed diet with three to seven 24-h recalls and physical activity (PA) by accelerometry in 766 adolescents (aged 14–18 y, 50% female, 49% black). Fat-free soft tissue (FFST) mass and fat mass were measured by dual-energy X-ray absorptiometry. LV structure [LV mass (g)/height (m)2.7 (LV mass index) and relative wall thickness] and function [midwall fractional shortening (MFS) and ejection fraction] were assessed by echocardiography. Associations were evaluated by comparing the LV structure and function variables across tertiles of phylloquinone intake. Prevalence and OR of LV hypertrophy (LV mass index >95th percentile for age and sex) were also assessed by phylloquinone tertiles.
Results: The prevalence of LV hypertrophy progressively decreased across tertiles of phylloquinone intake (P-trend < 0.01). Multinomial logistic regression—adjusting for age, sex, race, Tanner stage, systolic blood pressure, FFST mass, fat mass, socioeconomic status, PA, and intakes of energy, fiber, calcium, vitamin C, vitamin D, and sodium—revealed that compared with the highest phylloquinone intake tertile (reference group), the adjusted OR for LV hypertrophy was 3.3 (95% CI: 1.2, 7.4) for those in the lowest phylloquinone intake tertile. When LV structure variables were compared across phylloquinone intake tertiles adjusting for the same covariates, there were significant linear downward trends for LV mass index (6.5% difference, tertile 1 compared with tertile 3) and relative wall thickness (9.2% difference, tertile 1 compared with tertile 3; both P-trend ≤ 0.02). Conversely, significant linear upward trends across phylloquinone intake tertiles were observed for MFS (3.4% difference, tertile 1 compared with tertile 3) and ejection fraction (2.6% difference, tertile 1 compared with tertile 3; both P-trend < 0.04).
Conclusion: Our adolescent data suggest that subclinical cardiac structure and function variables are most favorable at higher phylloquinone intakes.
Keywords: phylloquinone, children, cardiovascular disease, left ventricular mass, left ventricular hypertrophy, echocardiography
Introduction
Cardiac structural and functional abnormalities, including left ventricular (LV) hypertrophy, have been shown to be independent predictors of cardiovascular disease (CVD) events and mortality (1–4). Evidence suggests that cardiac structure and function abnormalities begin in childhood (5, 6), increasing the likelihood of CVD events in adulthood (7). Therefore, identifying key contributors to abnormalities in cardiac structure and function in childhood has wide-reaching implications for public health, particularly because CVD accounts for approximately one-third of all deaths in the United States (8). Dietary factors are important to consider because they can be modified, making them one of the main targets for interventions aimed at the prevention of CVD.
Recently, there has been growing interest in vitamin K, a fat-soluble vitamin, as a beneficial nutrient in cardiovascular health. In nature, vitamin K is present in the diet in the forms of phylloquinone (vitamin K-1) and menaquinones (vitamin K-2). Phylloquinone, the major dietary source of vitamin K, is found primarily in dark-green leafy vegetables and certain plant oils and is the vitamin K form best characterized in US food composition databases (9, 10). It is postulated from animal studies that increased phylloquinone intake may reduce the progression of CVD, possibly by means of counteracting vascular calcification in the arteries and by regulating lipoprotein metabolism (11, 12). However, the clinical evidence for the link between phylloquinone intake and CVD is scant and equivocal. For instance, some reports have found greater phylloquinone consumption linked to lower TGs (13), higher HDL-cholesterol (14), lower CVD risk (15, 16), and less deposition of calcified lesions in the aorta (17). In contrast, others have reported no association between phylloquinone intake and CVD risk or markers of CVD risk (18–22).
The discrepancies in the aforementioned dietary phylloquinone–CVD investigations can be partly attributed to differences in the populations studied and the study designs and instruments used. However, another notable limitation of these investigations was that all study participants were adults. Because cardiac structural and functional abnormalities have been shown to track during childhood and later life (23, 24) and may be affected by dietary intake (25, 26), it is of interest to examine relations between phylloquinone intake and cardiac structure and function in the pediatric population. Therefore, the objective of this study was to determine associations between phylloquinone intake and subclinical parameters of LV structure and function in an apparently healthy adolescent population living in the southeastern United States.
Methods
Participants.
The participants in this study were 766 adolescents who were recruited from local high schools in the Augusta, Georgia area. With approval from superintendents and school principals, flyers were distributed to all students in the high schools. Inclusion criteria for the study were white/Caucasian or black/African-American race and age 14–18 y. Adolescents were excluded if they were taking medications or had any medical conditions that could affect growth, maturation, physical activity, nutritional status, or metabolism. Informed consent and assent were obtained from all parents and adolescents, respectively. The study protocol was approved by the Augusta University Institutional Review Board. All measurements were performed at the Medical College of Georgia’s Georgia Prevention Institute at Augusta University between 2001 and 2005.
Anthropometry, blood pressure, pubertal stage, and socioeconomic status.
A trained laboratory technician collected height and weight measurements for calculating sex- and age-specific BMI percentiles (27). Seated blood pressure was measured 5 times at 1-min intervals after a 10-min rest using the Dinamap Pro 100 (Critikon Corporation), and the last 3 measures were averaged. Pubertal maturation stage (or Tanner stage) was measured with a 5-stage scale, ranging from I (prepubertal) to V (fully mature) as described by Tanner (28). Using this sex-specific questionnaire, participants reported their pubertal stage by comparing their own physical development to the 5 stages in standard sets of diagrams. A parent or research coordinator then reviewed the results with the children to make sure they understood the questionnaire. When an individual reported discordant stages of pubic hair and breast or genital development, the higher of the 2 stages was used. The socioeconomic status was assessed using the Hollingshead 4-factor index of social class (29), which combines the educational attainment and occupational prestige for the number of working parents in the child’s family. Scores ranged from 11 to 51, with higher scores indicating higher theoretical socioeconomic status.
Body composition.
Fat-free soft tissue (FFST) mass and fat mass were assessed by DXA (QDR-4500W; Hologic Inc.). For determination of measurement reproducibility, 1-factor random-effects model, single-measure intraclass correlation coefficients were calculated in participants aged 15–18 y (n = 219). Each participant was scanned twice within a 7-d period for FFST mass and fat mass (both r ≥ 0.97).
LV structure and function.
LV structure and function variables were assessed by 2-dimensionally directed M-mode echocardiography (Hewlett-Packard Sonos 1500; Hewlett-Packard) by experienced sonographers using a standard institutional protocol in the Georgia Prevention Institute’s Echocardiography Laboratory (30). Measurements of LV structure included the following variables: LV mass index, relative wall thickness, interventricular septal wall thickness in diastole (SWTd), LV posterior wall thickness in diastole (PWTd), LV internal diameter in diastole (LVIDd), LV internal diameter in systole (LVIDs), end-diastolic volume (EDV), and end-systolic volume (ESV). SWTd, PWTd, LVIDd, and LVIDs were measured by American Society of Echocardiography guidelines (31). LV mass was calculated by using the following equation (32):
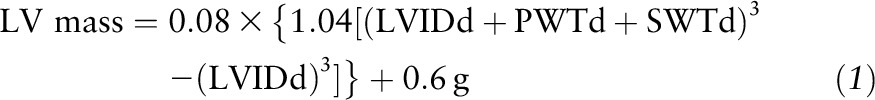 |
To minimize the effects of differences in body size of children and adolescents, LV mass needs to be adjusted relative to body size (33). Hence, LV mass was indexed to body height raised to the power of 2.7 (LV mass index), as recommended by de Simone et al. (34). LV hypertrophy was defined as LV mass index >95th percentile for age and sex (35). Relative wall thickness was calculated using the equation:
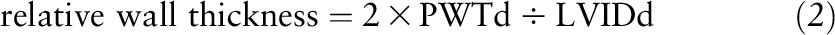 |
The LV volumes, EDV and ESV, were estimated from end-diastolic and end-systolic dimensions, respectively (36).
Measurements of LV systolic function included the following variables: endocardial fractional shortening (EFS), midwall fractional shortening (MFS), and ejection fraction. EFS, defined as [(LVIDd − LVIDs) ÷ LVIDd)] × 100, was calculated according to Lutas et al. (37). MFS was calculated using the following equation reported by de Simone et al. (38):
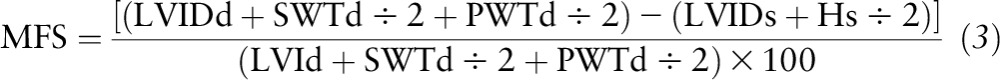 |
In Equation 3, Hs/2 is the LV inner shell myocardial thickness at end systole, taking into account the epicardial migration of the midwall during systole in a spherical model. Ejection fraction was calculated by using the following equation:
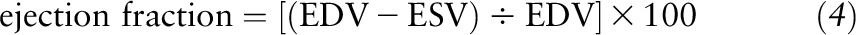 |
Physical activity.
The mean daily minutes spent in moderate and vigorous physical activity (PA) was assessed using MTI Actigraph monitors (model 7164; MTI Health Services). Subjects were instructed to wear the monitor for 7 d, remove it for sleep and any activity that may cause harm to the monitor or another person (e.g., during contact sports), and return the monitor 1 wk later. Data from day 1 and day 7 were discarded because a full day of information was not available for those days. Daily movement counts were converted to average minutes per day spent in moderate PA (3–6 metabolic equivalents) and vigorous PA (>6 metabolic equivalents) by the software accompanying the device.
Dietary intake.
To assess mean daily intakes of energy, protein, carbohydrate, fat, fiber, calcium, vitamin C, vitamin D, sodium, and vitamin K-1 (measured as phylloquinone), a trained registered dietitian conducted three to seven 24-h diet recalls (including 1 weekend day) using a multiple-pass, computer-assisted interview approach [Nutrition Data System for Research (NDS-R), Nutrition Coordinating Center, University of Minnesota, Minneapolis, Minnesota]. A total of 3 to 7 d of dietary information was collected in 3.3%, 8.7%, 17.2%, 18.4%, and 50.2% of the adolescents, respectively. Of the adolescents in our study, 98% (n = 750) and 95% (n = 725) completed ≥3- and 4-d dietary recalls, respectively. The first 2 recalls were performed in person at our institute with the use of food models, portion booklets, or serving containers to assist in estimating serving size, and the remaining interviews were conducted by telephone, with all 7 recalls completed within a 12-wk period. Participants were not interviewed on days when they had been ill or days that fell on a major holiday. To minimize the potential for undereating during the time frame for 24-h recalls, participants were blinded to the telephone recall schedule. A trained research assistant coded and analyzed dietary intake data using the NDS-R software version 2006.
Statistical analysis.
We examined the phylloquinone–cardiac structure and function association by comparing the LV structure and systolic function variables across tertile groups of phylloquinone intake. Phylloquinone intake values reported within each group are medians (range). Linear trends across tertile groups were tested by ANOVA with polynomial contrast for continuous variables (i.e., age, Tanner stage, blood pressure, BMI percentile, body composition, socioeconomic status, physical activity, and dietary intake variables) and by Mantel-Haenszel linear-by-linear association χ2 tests for categoric variables (i.e., sex, race, and LV hypertrophy). Descriptive statistics for the raw variables are presented as means ± SDs if not stated otherwise.
For comparison of the dependent variables (i.e., LV mass index, relative wall thickness, SWTd, PWTd, LVIDd, LVIDs, EDV, ESV, EFS, MFS, and ejection fraction), an F test was performed to test the assumption of homogeneity of regression slopes for the interactions between the independent variable (i.e., phylloquinone intake tertile groups) and the covariates (age, sex, race, Tanner stage, systolic blood pressure, FFST mass, fat mass, socioeconomic status, moderate and vigorous PA, and dietary intakes of total energy, fiber, calcium, vitamin C, vitamin D, and sodium). These covariates were chosen based on factors related to cardiovascular allometry (39, 40) and the established influence of lifestyle and dietary factors on cardiovascular health (41, 42). Because there were no interactions, ANCOVA with polynomial contrast was used to compare the dependent variables across tertile groups of phylloquinone intake after adjusting for the covariates. If the trend for difference in the dependent variable of interest across a tertile of phylloquinone intake was significant (P < 0.05), differences between individual tertiles, adjusted for multiple comparisons, were tested by using Tukey’s honestly significant difference adjustment. Multinomial logistic regression was used to estimate ORs and 95% CIs for the probability of LV hypertrophy according to tertiles of phylloquinone intake after adjusting for the same covariates. All statistical analyses were conducted with SAS version 9.4 software (SAS Institute), and statistical significance was set at P < 0.05.
Results
The sample was composed of 766 white and black adolescents aged 14–18 y (50% female, 49% black). Most of the adolescents (91.7%) reported to be in pubertal stages IV and V; however, 55 were in pubertal stage III and 8 in stage II. Most of the females (96.5%) reported having started menstruation. In the total sample, mean dietary intake of phylloquinone was 64.5 μg/d (range: 8–386 μg/d), and 24.8% met the Adequate Intake (AI; 75 μg/d for males and females aged 14–18 y) for phylloquinone (10). In addition, the overall prevalence of LV hypertrophy was 9.6%.
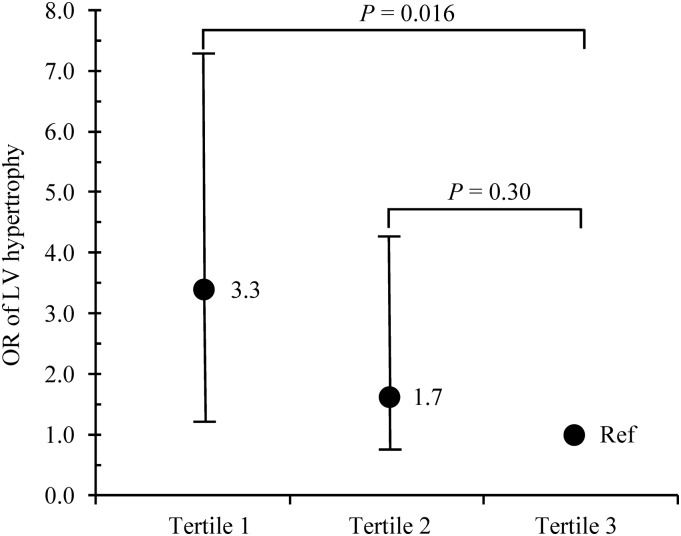
Participant characteristics by tertile categories of phylloquinone intake are described in Table 1. Age, race, Tanner stage, blood pressure, BMI percentile, socioeconomic status, moderate and vigorous PA, and intakes of macronutrients, calcium, vitamin C, vitamin D, and sodium did not differ between groups. However, significant linear upward trends in levels of FFST mass and intakes of energy and fiber were found across tertiles of phylloquinone intake (all P-trend ≤ 0.02). Significant linear downward trends across tertiles of phylloquinone intake were observed with sex distribution and levels of fat mass (both P-trend ≤ 0.02). In addition, the prevalence of LV hypertrophy progressively decreased across tertiles of phylloquinone intake (17% compared with 8% compared with 5%; P-trend < 0.01). After adjustment for age, sex, race, Tanner stage, systolic blood pressure, FFST mass, fat mass, socioeconomic status, moderate and vigorous PA, and dietary intakes of total energy, fiber, calcium, vitamin C, vitamin D, and sodium, multinomial logistic regression revealed that compared with the highest phylloquinone intake tertile (reference group), the adjusted OR for LV hypertrophy was 3.3 (95% CI: 1.2, 7.4) for those in the lowest phylloquinone intake tertile (Figure 1).
TABLE 1.
Characteristics by tertile categories of phylloquinone intake in 766 adolescents aged 14–18 y1
| Phylloquinone intake2 |
||||
| Tertile 1 | Tertile 2 | Tertile 3 | P-trend3 | |
| Age, y | 16.1 ± 1.2 | 16.1 ± 1.2 | 16.2 ± 1.3 | 0.28 |
| Females,4 % | 57 | 50 | 44 | 0.016 |
| Blacks,4 % | 55 | 44 | 49 | 0.07 |
| Tanner stage (I–V) | 4.7 ± 0.6 | 4.5 ± 0.7 | 4.6 ± 0.6 | 0.16 |
| Blood pressure, mm Hg | ||||
| Systolic | 112 ± 10 | 111 ± 11 | 111 ± 10 | 0.60 |
| Diastolic | 60 ± 7 | 60 ± 6 | 60 ± 6 | 0.53 |
| BMI percentile | 62.8 ± 28.9 | 60.0 ± 28.6 | 58.2 ± 27.9 | 0.10 |
| Fat-free soft tissue mass, kg | 45.7 ± 9.7b | 45.9 ± 9.7 | 47.6 ± 9.9a | 0.020 |
| Fat mass, kg | 17.7 ± 10.9a | 16.3 ± 10.5 | 15.0 ± 9.5b | 0.003 |
| Socioeconomic status | 32.8 ± 8.4 | 33.9 ± 9.0 | 34.4 ± 9.8 | 0.12 |
| Moderate and vigorous physical activity, min/d | 43.2 ± 29.8 | 42.5 ± 26.3 | 46.4 ± 30.7 | 0.25 |
| Dietary intake | ||||
| Energy, kcal/d | 1630 ± 463c | 2010 ± 473b | 2230 ± 617a | <0.001 |
| Protein, % of energy | 14 ± 3 | 14 ± 3 | 14 ± 3 | 0.49 |
| Carbohydrate, % of energy | 54 ± 7 | 53 ± 6 | 53 ± 6 | 0.70 |
| Fat, % of energy | 32 ± 6 | 33 ± 5 | 33 ± 4 | 0.50 |
| Fiber, g/d | 9.8 ± 3.0c | 10.8 ± 3.4b | 12.1 ± 5.1a | <0.001 |
| Calcium, mg/d | 739 ± 346 | 748 ± 310 | 747 ± 353 | 0.80 |
| Vitamin C, mg/d | 67.6 ± 38.6 | 68.4 ± 37.8 | 69.8 ± 24.4 | 0.54 |
| Vitamin D, μg/d | 4.0 ± 2.7 | 3.8 ± 2.6 | 4.0 ± 2.9 | 0.67 |
| Sodium, mg/d | 3210 ± 890 | 3320 ± 890 | 3310 ± 1020 | 0.07 |
| Left ventricular hypertrophy,4 % | 17 | 8 | 5 | <0.001 |
Values are means ± SDs unless othewise indicated. Values in a row without a common superscript letter differ, P < 0.05.
Median (range) intakes of phylloquinone: tertile 1 = 32 μg/d (8–42 μg/d), n = 255; tertile 2 = 54 μg/d (43–65 μg/d), n = 255; and tertile 3 = 90 μg/d (66–386 μg/d), n = 256.
P-trend based on ANOVA with polynomial contrast.
Based on Mantel-Haenszel linear-by-linear association χ2 test.
FIGURE 1.
Probability of LV hypertrophy across tertiles of daily phylloquinone intake in 766 adolescents aged 14–18 y. Median (range) intakes of phylloquinone were as follows: tertile 1 = 32 μg/d (8–42 μg/d), n = 255; tertile 2 = 54 μg/d (43–65 μg/d), n = 255; and tertile 3 = 90 μg/d (66–386 μg/d), n = 256. OR (95% CI) values were adjusted for age, sex, race, Tanner stage, systolic blood pressure, fat-free soft tissue mass, fat mass, socioeconomic status, moderate and vigorous physical activity, and dietary intakes of total energy, fiber, calcium, vitamin C, vitamin D, and sodium. LV, left ventricular; Ref, reference group.
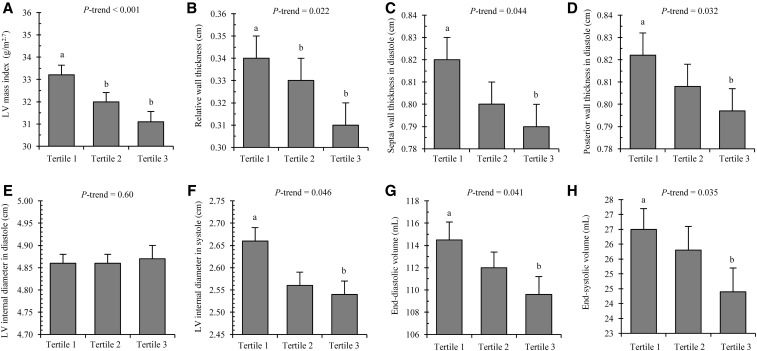
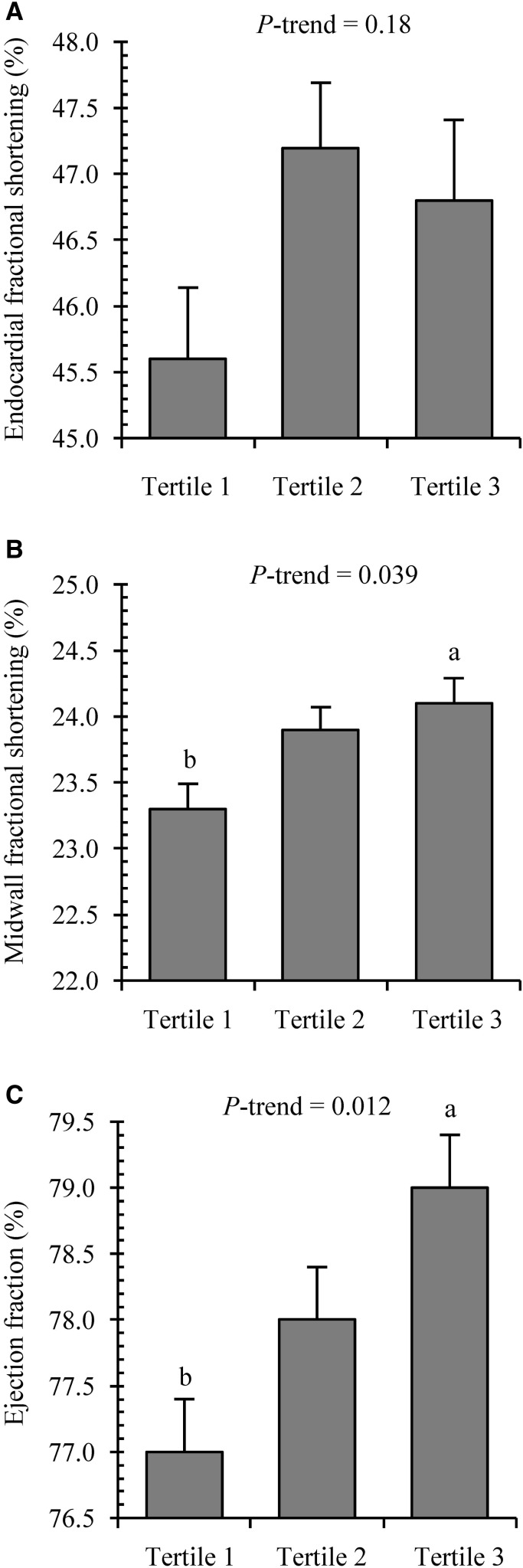
Figures 2 and display measures of LV structure and LV systolic function, respectively, across tertiles of phylloquinone intake when we adjusted for age, sex, race, Tanner stage, systolic blood pressure, FFST mass, fat mass, socioeconomic status, moderate and vigorous PA, and dietary intakes of total energy, fiber, calcium, vitamin C, vitamin D, and sodium. There were significant linear downward trends for LV mass index (6.5% difference, tertile 1 compared with tertile 3), relative wall thickness (9.2% difference, tertile 1 compared with tertile 3), SWTd (3.7% difference, tertile 1 compared with tertile 3), PWTd (3.7% difference, tertile 1 compared with tertile 3), LVIDs (4.6% difference, tertile 1 compared with tertile 3), EDV (4.3% difference, tertile 1 compared with tertile 3), and ESV (8.3% difference, tertile 1 compared with tertile 3; all P-trend ≤ 0.05; Supplemental Table 1, Figure 2). Conversely, significant linear upward trends across tertiles of phylloquinone intake were observed for MFS (3.4% difference, tertile 1 compared with tertile 3) and ejection fraction (2.6% difference, tertile 1 compared with tertile 3; both P-trend < 0.04; Supplemental Table 2, Figure 3). There were no differences in LVIDd or EFS across tertiles of phylloquinone intake (both P-trend > 0.05)
FIGURE 2.
Cardiac structure variables, including LV mass index (A), relative wall thickness (B), septal wall thickness in diastole (C), posterior wall thickness in diastole (D), LV internal diameter in diastole (E), LV internal diameter in systole (F), end-diastolic volume (G), and end-systolic volume (H), across tertiles of daily phylloquinone intake in 766 adolescents aged 14–18 y. Median (range) intakes of phylloquinone were as follows: tertile 1 = 32 μg/d (8–42 μg/d), n = 255; tertile 2 = 54 μg/d (43–65 μg/d), n = 255; and tertile 3 = 90 μg/d (66–386 μg/d), n = 256. Values are adjusted means ± SEMs. Means were adjusted for age, sex, race, Tanner stage, systolic blood pressure, fat-free soft tissue mass, fat mass, socioeconomic status, moderate and vigorous physical activity, and dietary intakes of total energy, fiber, calcium, vitamin C, vitamin D, and sodium. Labeled means without a common lowercase letter differ, P < 0.05. LV, left ventricular.
FIGURE 3.
Cardiac function variables, including endocardial fractional shortening (A), midwall fractional shortening (B), and ejection fraction (C), across tertiles of daily phylloquinone intake in 766 adolescents aged 14–18 y. Median (range) intakes of phylloquinone were as follows: tertile 1 = 32 μg/d (8–42 μg/d), n = 255; tertile 2 = 54 μg/d (43–65 μg/d), n = 255; and tertile 3 = 90 μg/d (66–386 μg/d), n = 256. Values are adjusted means ± SEMs. Means were adjusted for age, sex, race, Tanner stage, systolic blood pressure, fat-free soft tissue mass, fat mass, socioeconomic status, moderate and vigorous physical activity, and dietary intakes of total energy, fiber, calcium, vitamin C, vitamin D, and sodium. Labeled means without a common lowercase letter differ, P < 0.05.
Discussion
To the best of our knowledge, this is the first study to investigate associations of vitamin K intake with cardiac structure and function in a pediatric population. We found that greater phylloquinone consumption in adolescents was associated with multiple indicators of LV structure and function. These associations were independent of potentially confounding factors such as age, sex, race, pubertal stage, blood pressure, body composition, physical activity, and other factors of dietary intake. Our study findings in adolescents suggest that greater phylloquinone consumption may favorably influence subclinical parameters of cardiac structure and function implicated in CVD risk.
To date, there are no published data in children or adolescents on relations between vitamin K intake and LV structure and function or any other CVD-related outcomes. Findings from adult studies do suggest the importance of vitamin K intake on cardiovascular health; however, results have been inconsistent (13–22). In the Framingham Offspring Cohort Study (13), researchers found that higher phylloquinone intake was associated with higher HDL-cholesterol and lower TGs reflecting a blood lipid profile indicative of lower CVD risk. Data from NHANES 1999–2004 also revealed a direct relation between phylloquinone intake and HDL-cholesterol (14). Investigators in another study suggested that vitamin K intake might be linked to the development of atherosclerosis, because they found that phylloquinone intakes were significantly lower in postmenopausal women with aortic calcifications than in those without aortic calcifications (17). In the Nurses’ Health Study, researchers reported that a higher phylloquinone intake was associated with a lower risk of coronary artery disease (15). However, other population-based studies found no cardioprotective effect of phylloquinone intake. In 4807 Dutch men and women (mean age: 67 y; 38% men) from the Rotterdam Study (20) and 807 US Army personnel (mean age: 42 y; 82% men) from the Prospective Army Coronary Calcium study (18), there were no associations of phylloquinone intake with risk of coronary artery disease or the presence of coronary artery calcification, respectively. Likewise, in 1689 Dutch postmenopausal women (mean age: 57 y) participating in the Predictors of Response to Cardiac Resynchronization Therapy study (19), the prevalence of breast artery calcifications did not differ across quartiles of phylloquinone intake.
Although the disparate findings from the adult investigations may result from differences in study designs and populations studied, they may also be attributed to the differential effects of vitamin K subtypes (vitamin K-1 compared with vitamin K-2) on cardiovascular health. For instance, menaquinone-4 and menaquinone-7 are 2 subtypes of vitamin K-2 that have been postulated to be more effective in improving cardiovascular health than phylloquinone (43). Two population-based studies that compared vitamin K-1 and vitamin K-2 found that higher intakes of vitamin K-2 but not K-1 were associated with lower coronary artery disease risk (20, 22), with Gast et al. (22) showing that menaquinone-7 accounted in large part for the protective effect of vitamin K-2. Furthermore, in a study of 564 postmenopausal women, Beulens et al. (21) reported that higher intake of vitamin K-2, attributed mostly to menaquinone-4, was associated with lower coronary calcification, whereas vitamin K-1 was not. Our study was limited to assessment of phylloquinone. Dietary intakes of menaquinone-4 and menaquonine-7, which have a bacterial origin found primarily in animal meats and fermented foods, were not measured in our study. Thus, total vitamin K intake may have been underestimated, even though phylloquinone is the predominant vitamin K form in the US diet (9) and can be endogenously converted to menaquinone-4 (44).
In adult investigations, LV structure and systolic function measurements, including LV mass index, relative wall thickness, MFS, and ejection fraction, have been associated with subsequent CVD (1–4). Because cardiac structural and functional abnormalities have been shown to track during childhood and later life (23, 24) and may be affected by dietary intake (25, 26), it is of clinical relevance to study dietary determinants of cardiac structures and function. In our study, LV mass index and relative wall thickness were significantly greater (6.5% and 9.2%, respectively) and MFS and ejection fraction were significantly lower (3.4% and 2.6%, respectively) in the lowest than in the highest tertile of phylloquinone intake. The magnitude of difference in these subclinical risk markers of CVD between tertiles 1 and 3 was statistically significant but was relatively small and may not reflect clinically relevant differences. Moreover, the effect of small cardiovascular developmental differences on health in later life is largely unknown. Prospective studies with follow-up in adulthood will be needed to address these questions. Nevertheless, the present study expands our knowledge of adolescent phylloquinone consumption and CVD.
Without an estimated average requirement value, our study could not estimate the prevalence of inadequacy of vitamin K intake. However, only 25% of the adolescents met AI values for phylloquinone intake and thus had a high probability of having AI, whereas the proportion with inadequate intakes is less certain. Another important finding in our study is that adolescents who consumed ≤42 μg phylloquinone/d were 3.3 times more likely to have LV hypertrophy than those who consumed ≥90 μg phylloquinone/d. The biological basis by which a low or inadequate intake of phylloquinone may adversely influence cardiac structure and function or cardiovascular health in general remains to be determined. The vitamin K–cardiovascular health relation is linked through vascular calcification, a key factor in the development of LV hypertrophy that has been shown to alter the pulsatile dynamics and thereby contribute to an increase in LV load (45–47). Several vitamin K–dependent proteins (VKDPs) have been implicated in the vascular calcification process. For instance, it has been postulated that matrix Gla protein activated via vitamin K–dependent γ-carboxylation counteracts vascular calcification in arteries (48–51), whereas inactive matrix Gla protein, seen in cases of vitamin K insufficiency, has been associated with intimal and medial calcification (11, 52). Dhore et al. (53) reported that osteocalcin, another VKDP, was expressed in arteries at all stages of atherosclerosis development, acting as a calcification inhibitor. Gla-rich protein, a recently discovered VKDP (54), and growth arrest-specific gene 6 protein have also been shown to have roles in vascular calcification (55–57). Collectively, the biological function of vitamin K appears to extend to VKDPs involved in vascular disease. Whether increasing intake of vitamin K, via diet or supplementation, might be able to counteract or attenuate vascular disease progression remains to be determined.
The present study has several strengths. First, the collection of 3–7 independent 24-h recalls over a 12-wk period provided a more accurate dietary assessment of phylloquinone intake compared with fewer recalls used in other epidemiologic studies (14, 58), which reduces bias from measurement error and random error due to within-person variability over time. Second, we had a relatively large, apparently healthy adolescent population with nearly equal distributions of males and females and whites and blacks. Another strength is the consideration of potential confounding variables in our analyses with phylloquinone intake.
However, we acknowledge several study limitations. Given that our study used cross-sectional data, we cannot be certain that phylloquinone intake has a direct effect on cardiac structure and function. Also, the absence of circulating vitamin K biomarkers precludes us from assessing whether a better vitamin K status reflected by a higher intake of phylloquinone would have a beneficial effect on cardiac structure and function. Last, our study findings are limited to adolescents living in the southeastern United States; thus, differences in socioeconomic status, geographic location, social environment, lifestyle, or food habits of the study population may preclude generalizability of the study findings. However, given that the mean phylloquinone consumption (65 μg/d) in our sample is comparable to the most recent national average in 12- to 19-y-olds (74 μg/d) (58), our findings are likely generalizable to many other settings.
In conclusion, our data suggest that greater phylloquinone consumption may favorably influence subclinical markers of cardiac structure and function in a population of US adolescents. Additional pediatric investigations are needed to clarify the importance of phylloquinone intake to cardiovascular development and to understand the role of VKDP as the biological basis for the phylloquinone–cardiovascular health relation. This could eventually lead to phylloquinone interventions in childhood aimed to improve cardiovascular development and to reduce the subsequent risk of CVD.
Acknowledgments
The authors’ responsibilities were as follows—NKP and BG: conceived of and designed the study and were the grant holders; MKD, MEF, and NKP: wrote the paper; MKD, MEF, BG, and NKP: critically revised the manuscript for important intellectual content; NKP: performed the statistical analysis and had primary responsibility for the final content; and all authors: acquired, analyzed, or interpreted the data and read and approved the final manuscript.
Footnotes
Abbreviations used: AI, Adequate Intake; CVD, cardiovascular disease; EDV, end-diastolic volume; EFS, endocardial fractional shortening; ESV, end-systolic volume; FFST, fat-free soft tissue mass; LV, left ventricular; LVIDd, left ventricular internal diameter in diastole; LVIDs, left ventricular internal diameter in systole; MFS, midwall fractional shortening; PA, physical activity; PWTd, posterior wall thickness in diastole; SWTd, septal wall thickness in diastole; VKDP, vitamin K-dependent protein.
References
- 1.Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Left ventricular mass and incidence of coronary heart disease in an elderly cohort. The Framingham Heart Study. Ann Intern Med 1989;110:101–7. [DOI] [PubMed] [Google Scholar]
- 2.Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med 1990;322:1561–6. [DOI] [PubMed] [Google Scholar]
- 3.de Simone G, Devereux RB, Mureddu GF, Roman MJ, Ganau A, Alderman MH, Contaldo F, Laragh JH. Influence of obesity on left ventricular midwall mechanics in arterial hypertension. Hypertension 1996;28:276–83. [DOI] [PubMed] [Google Scholar]
- 4.Shah AM, Claggett B, Sweitzer NK, Shah SJ, Anand IS, O’Meara E, Desai AS, Heitner JF, Li G, Fang J, et al. Cardiac structure and function and prognosis in heart failure with preserved ejection fraction: findings from the echocardiographic study of the Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist (TOPCAT) Trial. Circ Heart Fail 2014;7:740–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davis CL, Kapuku G, Snieder H, Kumar M, Treiber FA. Insulin resistance syndrome and left ventricular mass in healthy young people. Am J Med Sci 2002;324:72–5. [DOI] [PubMed] [Google Scholar]
- 6.Movahed MR, Martinez A, Greaves J, Greaves S, Morrell H, Hashemzadeh M. Left ventricular hypertrophy is associated with obesity, male gender, and symptoms in healthy adolescents. Obesity (Silver Spring) 2009;17:606–10. [DOI] [PubMed] [Google Scholar]
- 7.Bao W, Srinivasan SR, Wattigney WA, Berenson GS. Persistence of multiple cardiovascular risk clustering related to syndrome X from childhood to young adulthood. The Bogalusa Heart Study. Arch Intern Med 1994;154:1842–7. [PubMed] [Google Scholar]
- 8.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Després JP, Fullerton HJ, Howard VJ, et al. ; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics–2015 update: a report from the American Heart Association. Circulation 2015;131:e29–322. [DOI] [PubMed] [Google Scholar]
- 9.Booth SL, Pennington JA, Sadowski JA. Food sources and dietary intakes of vitamin K-1 (phylloquinone) in the American diet: data from the FDA Total Diet Study. J Am Diet Assoc 1996;96:149–54. [DOI] [PubMed] [Google Scholar]
- 10.Food and Nutrition Board, Institute of Medicine. Vitamin K. Dietary reference intakes for vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. Washington (DC): National Academy Press; 2001. [PubMed] [Google Scholar]
- 11.Schurgers LJ, Spronk HM, Soute BA, Schiffers PM, DeMey JG, Vermeer C. Regression of warfarin-induced medial elastocalcinosis by high intake of vitamin K in rats. Blood 2007;109:2823–31. [DOI] [PubMed] [Google Scholar]
- 12.Sogabe N, Maruyama R, Baba O, Hosoi T, Goseki-Sone M. Effects of long-term vitamin K(1) (phylloquinone) or vitamin K(2) (menaquinone-4) supplementation on body composition and serum parameters in rats. Bone 2011;48:1036–42. [DOI] [PubMed] [Google Scholar]
- 13.Braam L, McKeown N, Jacques P, Lichtenstein A, Vermeer C, Wilson P, Booth S. Dietary phylloquinone intake as a potential marker for a heart-healthy dietary pattern in the Framingham Offspring cohort. J Am Diet Assoc 2004;104:1410–4. [DOI] [PubMed] [Google Scholar]
- 14.Pan Y, Jackson RT. Dietary phylloquinone intakes and metabolic syndrome in US young adults. J Am Coll Nutr 2009;28:369–79. [DOI] [PubMed] [Google Scholar]
- 15.Erkkilä AT, Booth SL, Hu FB, Jacques PF, Manson JE, Rexrode KM, Stampfer MJ, Lichtenstein AH. Phylloquinone intake as a marker for coronary heart disease risk but not stroke in women. Eur J Clin Nutr 2005;59:196–204. [DOI] [PubMed] [Google Scholar]
- 16.Erkkilä AT, Booth SL, Hu FB, Jacques PF, Lichtenstein AH. Phylloquinone intake and risk of cardiovascular diseases in men. Nutr Metab Cardiovasc Dis 2007;17:58–62. [DOI] [PubMed] [Google Scholar]
- 17.Jie KS, Bots ML, Vermeer C, Witteman JC, Grobbee DE. Vitamin K intake and osteocalcin levels in women with and without aortic atherosclerosis: a population-based study. Atherosclerosis 1995;116:117–23. [DOI] [PubMed] [Google Scholar]
- 18.Villines TC, Hatzigeorgiou C, Feuerstein IM, O’Malley PG, Taylor AJ. Vitamin K1 intake and coronary calcification. Coron Artery Dis 2005;16:199–203. [DOI] [PubMed] [Google Scholar]
- 19.Maas AH, van der Schouw YT, Beijerinck D, Deurenberg JJ, Mali WP, Grobbee DE, van der Graaf Y. Vitamin K intake and calcifications in breast arteries. Maturitas 2007;56:273–9. [DOI] [PubMed] [Google Scholar]
- 20.Geleijnse JM, Vermeer C, Grobbee DE, Schurgers LJ, Knapen MH, van der Meer IM, Hofman A, Witteman JC. Dietary intake of menaquinone is associated with a reduced risk of coronary heart disease: the Rotterdam Study. J Nutr 2004;134:3100–5. [DOI] [PubMed] [Google Scholar]
- 21.Beulens JW, Bots ML, Atsma F, Bartelink ML, Prokop M, Geleijnse JM, Witteman JC, Grobbee DE, van der Schouw YT. High dietary menaquinone intake is associated with reduced coronary calcification. Atherosclerosis 2009;203:489–93. [DOI] [PubMed] [Google Scholar]
- 22.Gast GC, de Roos NM, Sluijs I, Bots ML, Beulens JW, Geleijnse JM, Witteman JC, Grobbee DE, Peeters PH, van der Schouw YT. A high menaquinone intake reduces the incidence of coronary heart disease. Nutr Metab Cardiovasc Dis 2009;19:504–10. [DOI] [PubMed] [Google Scholar]
- 23.Urbina EM, Gidding SS, Bao W, Pickoff AS, Berdusis K, Berenson GS. Effect of body size, ponderosity, and blood pressure on left ventricular growth in children and young adults in the Bogalusa Heart Study. Circulation 1995;91:2400–6. [DOI] [PubMed] [Google Scholar]
- 24.Gruppen MP, Groothoff JW, Prins M, van der Wouw P, Offringa M, Bos WJ, Davin JC, Heymans HS. Cardiac disease in young adult patients with end-stage renal disease since childhood: a Dutch cohort study. Kidney Int 2003;63:1058–65. [DOI] [PubMed] [Google Scholar]
- 25.Haufe S, Utz W, Engeli S, Kast P, Böhnke J, Pofahl M, Traber J, Haas V, Hermsdorf M, Mähler A, et al. Left ventricular mass and function with reduced-fat or reduced-carbohydrate hypocaloric diets in overweight and obese subjects. Hypertension 2012;59:70–5. [DOI] [PubMed] [Google Scholar]
- 26.van den Hooven EH, de Jonge LL, Kiefte-de Jong JC, Raat H, Villamor E, Hofman A, Felix JF, Jaddoe VW, Moll HA, Franco OH. Infant macronutrient composition is associated with differences in cardiovascular structures and function in childhood. J Nutr 2013;143:1989–98. [DOI] [PubMed] [Google Scholar]
- 27.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, Flegal KM, Guo SS, Wei R, Mei Z, Curtin LR, Roche AF, Johnson CL. CDC growth charts: United States. Adv Data 2000;314:1–27. [PubMed] [Google Scholar]
- 28.Tanner J. Growth and adolescence. 2nd ed. Oxford (United Kingdom): Blackwell Scientific Publications; 1962. [Google Scholar]
- 29.Cirino PT, Chin CE, Sevcik RA, Wolf M, Lovett M, Morris RD. Measuring socioeconomic status: reliability and preliminary validity for different approaches. Assessment 2002;9:145–55. [DOI] [PubMed] [Google Scholar]
- 30.Kapuku GK, Treiber FA, Davis HC, Harshfield GA, Cook BB, Mensah GA. Hemodynamic function at rest, during acute stress, and in the field: predictors of cardiac structure and function 2 years later in youth. Hypertension 1999;34:1026–31. [DOI] [PubMed] [Google Scholar]
- 31.Gottdiener JS, Bednarz J, Devereux R, Gardin J, Klein A, Manning WJ, Morehead A, Kitzman D, Oh J, Quinones M, et al. ; American Society of Echocardiography. American Society of Echocardiography recommendations for use of echocardiography in clinical trials. J Am Soc Echocardiogr 2004;17:1086–119. [DOI] [PubMed] [Google Scholar]
- 32.Devereux RB, Alonso DR, Lutas EM, Gottlieb GJ, Campo E, Sachs I, Reichek N. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol 1986;57:450–8. [DOI] [PubMed] [Google Scholar]
- 33.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise J, et al. ; American Society of Echocardiography’s Nomenclature and Standards Committee; Task Force on Chamber Quantification; American College of Cardiology Echocardiography Committee; American Heart Association; European Association of Echocardiography, European Society of Cardiology. Recommendations for chamber quantification. Eur J Echocardiogr 2006;7:79–108. [DOI] [PubMed] [Google Scholar]
- 34.de Simone G, Daniels SR, Devereux RB, Meyer RA, Roman MJ, de Divitiis O, Alderman MH. Left ventricular mass and body size in normotensive children and adults: assessment of allometric relations and impact of overweight. J Am Coll Cardiol 1992;20:1251–60. [DOI] [PubMed] [Google Scholar]
- 35.Khoury PR, Mitsnefes M, Daniels SR, Kimball TR. Age-specific reference intervals for indexed left ventricular mass in children. J Am Soc Echocardiogr 2009;22:709–14. [DOI] [PubMed] [Google Scholar]
- 36.Teichholz LE, Kreulen T, Herman MV, Gorlin R. Problems in echocardiographic volume determinations: echocardiographic-angiographic correlations in the presence of absence of asynergy. Am J Cardiol 1976;37:7–11. [DOI] [PubMed] [Google Scholar]
- 37.Lutas EM, Devereux RB, Reis G, Alderman MH, Pickering TG, Borer JS, Laragh JH. Increased cardiac performance in mild essential hypertension. Left ventricular mechanics. Hypertension 1985;7:979–88. [DOI] [PubMed] [Google Scholar]
- 38.de Simone G, Devereux RB, Roman MJ, Ganau A, Saba PS, Alderman MH, Laragh JH. Assessment of left ventricular function by the midwall fractional shortening/end-systolic stress relation in human hypertension. J Am Coll Cardiol 1994;23:1444–51. [DOI] [PubMed] [Google Scholar]
- 39.Lopez L, Colan SD, Frommelt PC, Ensing GJ, Kendall K, Younoszai AK, Lai WW, Geva T. Recommendations for quantification methods during the performance of a pediatric echocardiogram: a report from the Pediatric Measurements Writing Group of the American Society of Echocardiography Pediatric and Congenital Heart Disease Council. J Am Soc Echocardiogr 2010;23:465–95, quiz 576–7. [DOI] [PubMed] [Google Scholar]
- 40.Daniels SR, Kimball TR, Morrison JA, Khoury P, Witt S, Meyer RA. Effect of lean body mass, fat mass, blood pressure, and sexual maturation on left ventricular mass in children and adolescents. Statistical, biological, and clinical significance. Circulation 1995;92:3249–54. [DOI] [PubMed] [Google Scholar]
- 41.Writing Group Members, Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Després JP, Fullerton HJ, et al. Heart disease and stroke statistics-2016 update: a report from the American Heart Association. Circulation 2016;133:e38–360. [DOI] [PubMed] [Google Scholar]
- 42.Poslusna K, Ruprich J, de Vries JH, Jakubikova M, van’t Veer P. Misreporting of energy and micronutrient intake estimated by food records and 24 hour recalls, control and adjustment methods in practice. Br J Nutr 2009;101 Suppl 2:S73–85. [DOI] [PubMed] [Google Scholar]
- 43.Beulens JW, Booth SL, van den Heuvel EG, Stoecklin E, Baka A, Vermeer C. The role of menaquinones (vitamin K(2)) in human health. Br J Nutr 2013;110:1357–68. [DOI] [PubMed] [Google Scholar]
- 44.Nakagawa K, Hirota Y, Sawada N, Yuge N, Watanabe M, Uchino Y, Okuda N, Shimomura Y, Suhara Y, Okano T. Identification of UBIAD1 as a novel human menaquinone-4 biosynthetic enzyme. Nature 2010;468:117–21. [DOI] [PubMed] [Google Scholar]
- 45.Girerd X, Laurent S, Pannier B, Asmar R, Safar M. Arterial distensibility and left ventricular hypertrophy in patients with sustained essential hypertension. Am Heart J 1991;122:1210–4. [DOI] [PubMed] [Google Scholar]
- 46.Nitta K, Akiba T, Uchida K, Otsubo S, Otsubo Y, Takei T, Ogawa T, Yumura W, Kabaya T, Nihei H. Left ventricular hypertrophy is associated with arterial stiffness and vascular calcification in hemodialysis patients. Hypertens Res 2004;27:47–52. [DOI] [PubMed] [Google Scholar]
- 47.Cho IJ, Chang HJ, Park HB, Heo R, Shin S, Shim CY, Hong GR, Chung N. Aortic calcification is associated with arterial stiffening, left ventricular hypertrophy, and diastolic dysfunction in elderly male patients with hypertension. J Hypertens 2015;33:1633–41. [DOI] [PubMed] [Google Scholar]
- 48.Schurgers LJ, Cranenburg EC, Vermeer C. Matrix Gla-protein: the calcification inhibitor in need of vitamin K. Thromb Haemost 2008;100:593–603. [PubMed] [Google Scholar]
- 49.Cranenburg EC, Koos R, Schurgers LJ, Magdeleyns EJ, Schoonbrood TH, Landewe RB, Brandenburg VM, Bekers O, Vermeer C. Characterisation and potential diagnostic value of circulating matrix Gla protein (MGP) species. Thromb Haemost 2010;104:811–22. [DOI] [PubMed] [Google Scholar]
- 50.Wallin R, Cain D, Hutson SM, Sane DC, Loeser R. Modulation of the binding of matrix Gla protein (MGP) to bone morphogenetic protein-2 (BMP-2). Thromb Haemost 2000;84:1039–44. [PubMed] [Google Scholar]
- 51.Wallin R, Schurgers L, Wajih N. Effects of the blood coagulation vitamin K as an inhibitor of arterial calcification. Thromb Res 2008;122:411–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schurgers LJ, Teunissen KJ, Knapen MH, Kwaijtaal M, van Diest R, Appels A, Reutelingsperger CP, Cleutjens JP, Vermeer C. Novel conformation-specific antibodies against matrix gamma-carboxyglutamic acid (Gla) protein: undercarboxylated matrix Gla protein as marker for vascular calcification. Arterioscler Thromb Vasc Biol 2005;25:1629–33. [DOI] [PubMed] [Google Scholar]
- 53.Dhore CR, Cleutjens JP, Lutgens E, Cleutjens KB, Geusens PP, Kitslaar PJ, Tordoir JH, Spronk HM, Vermeer C, Daemen MJ. Differential expression of bone matrix regulatory proteins in human atherosclerotic plaques. Arterioscler Thromb Vasc Biol 2001;21:1998–2003. [DOI] [PubMed] [Google Scholar]
- 54.Viegas CS, Simes DC, Laize V, Williamson MK, Price PA, Cancela ML. Gla-rich protein (GRP), a new vitamin K-dependent protein identified from sturgeon cartilage and highly conserved in vertebrates. J Biol Chem 2008;283:36655–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Viegas CS, Rafael MS, Enriquez JL, Teixeira A, Vitorino R, Luís IM, Costa RM, Santos S, Cavaco S, Neves J, et al. Gla-rich protein acts as a calcification inhibitor in the human cardiovascular system. Arterioscler Thromb Vasc Biol 2015;35:399–408. [DOI] [PubMed] [Google Scholar]
- 56.Son BK, Kozaki K, Iijima K, Eto M, Kojima T, Ota H, Senda Y, Maemura K, Nakano T, Akishita M, et al. Statins protect human aortic smooth muscle cells from inorganic phosphate-induced calcification by restoring Gas6-Axl survival pathway. Circ Res 2006;98:1024–31. [DOI] [PubMed] [Google Scholar]
- 57.Clauser S, Meilhac O, Bieche I, Raynal P, Bruneval P, Michel JB, Borgel D. Increased secretion of Gas6 by smooth muscle cells in human atherosclerotic carotid plaques. Thromb Haemost 2012;107:140–9. [DOI] [PubMed] [Google Scholar]
- 58.US Department of Agriculture, Agricultural Research Service. Total nutrient intakes: percent reporting and mean amounts of selected vitamins and minerals from food and beverages and dietary supplements, by gender and age. What We Eat In America, NHANES 2011–2012. c2014. [cited 2017 Feb 5]. Available from: www.ars.usda.gov/nea/bhnrc/fsrg. [Google Scholar]