Abstract
Although non-small-cell lung cancer patients with epidermal growth factor receptor (EGFR) mutations are responsive to EGFR-tyrosine kinase inhibitors, drug resistances are always inevitable. The secondary somatic EGFR threonine–methionine substitution at position 790 (T790M) mutation accounts for ∼50% of acquired resistance mechanisms. Small cell lung cancer (SCLC) transformation is a relatively rare mechanism, but has recently attracted considerable attention. The coexistence of both the mechanisms in one patient is much more scarce in clinic. In this case report, we described a 37-year-old woman who underwent refractory after second-line gefitinib therapy and was confirmed to have SCLC transformation without the T790M mutation in the left lobar nodule, but concomitant with the plasma-genotyped EGFR T790M mutation. Our case report uncovered the underling relationship between SCLC transformation and the T790M mutation, and the fluid biopsy approach may help overcome the problem of heterogeneity in acquired resistance to EGFR-tyrosine kinase inhibitors.
Keywords: acquired resistance, epidermal growth factor receptor-tyrosine kinase inhibitor, non-small-cell lung cancer, T790M, transformation
Introduction
Studies have shown that ∼50% of non-small-cell lung cancer (NSCLC) patients among Asians present with somatic mutations in exons of the EGFR gene 1 and 20% among Whites and African-Americans 2,3. Most of the NSCLC patients with EGFR mutations respond well to the treatment with epidermal growth factor receptor-tyrosine kinase inhibitors (EGFR-TKIs). However, most of the patients, even those with a good initial response, develop resistance to EGFR-TKIs with disease progression after an average of ∼12 months 4–6. Various mechanisms of resistance to EGFR-TKI have been identified; among these, about half are acquired with the threonine–methionine substitution at position 790 (T790M) in exon 20 as a secondary mutation of EGFR 7,8. In addition, transformation to small cell lung cancer (SCLC) was reported as one of the mechanisms. A series of repeat biopsy studies have shown that about 5–15% of NSCLC patients undergo transformation to SCLC histology upon acquisition of EGFR-TKI resistance 9–11.
However, cases with both of the above two mechanisms in one patient are rear, although individually reported, most of the mechanisms are detected in tumor tissue samples without the EGFR T790M mutation; the plasma-genotyped T790M mutation was positive 12–14. Here, we report a case of young woman, who had never smoked, who developed acquired resistance to EGFR-TKI therapy through the transformation to SCLC in a lung metastasis tissue sample without the EGFR T790M mutation; the plasmagenotyped T790M mutation was positive.
Case report
The patient was a 37-year-old nonsmoker woman presented with a 1-month history of progressive dyspnea. Computed tomography (CT) scan (November 2014) showed multiple occupying lesions in the left lobe (maximum diameter was 1.7 cm), with enlargement of the supraclavicular, axillary, and retroperitoneal lymph nodes. TNM staging was cT1bN3M1c. The laboratory data showed an elevation of tumor makers [carcinoembryonic antigen: 9.9 ng/ml, neuron-specific enolase (NSE): 11 ng/ml]. The minimal invasive axillary lymph node biopsy was adopted (5 November 2014) and showed that thyroid transcription factor-1 was positive, NapsinA was positive, CK7 was weakly positive, CK20 was negative, CDX2 was negative, villin was negative, ER was negative, PR was negative, Neu was positive, PAX-8 was negative, MAM was negative, GCDFP15 was negative, Calre was negative, D2-40 was negative, Berep4 was negative, and vimentin was weakly positive. CK5/6-negative adenocarcinoma cells harbored an exon 19 deletion of the EGFR gene without mutations in exons 18–21 of the EGFR gene using the amplification refractory mutation system (ARMS) method, in accordance with the result of plasma-genotype using the ARMS method. Thus, the patient was diagnosed with adenocarcinoma in the left lobe, staged cT1bN3M1c (IV).
One week after the diagnosis, she was initially treated with four cycles of first-line chemotherapy with cisplatin (75 mg/m2) and pemetrexed (500 mg/m2) every 3 weeks from 17 November 2014 to 21 January 2015. A partial response was found after the completion of two cycles of chemotherapy, but the patient was found to have pulmonary progression and liver metastasis at the fourth cycle on the basis of the Response Evaluation Criteria in Solid Tumors criteria, version 1.1. Gefitinib (250 mg/day) treatment was started in February 2015, which was 3 months after the original diagnosis. Regular CT examination was performed every 2 months, and progression was found on 1 July 2015 after a 5-month treatment with gefitinib.
Although a partial response was achieved, acquired resistance developed 5 months later. On 1 July 2005, her chest-enhanced CT scan showed that two nodules in the left lobe had become larger and denser. Her chief complaint was not obvious. The laboratory data showed a slight increase in NSE from 11 to 22 ng/ml and the carcinoembryonic antigen level remained stable (9.6 ng/ml). According to the Response Evaluation Criteria in Solid Tumors (version 1.1), she was found to have progression disease and she agreed to participate in the clinical trial AURA 17 (NCT02442349).
Plasma circulating tumor DNA (ctDNA) was collected for EGFR mutation detection by ARMS and showed a positive result, but further biopsy of the left inferior lobe puncture showed that SCLC harbored exon 19 deletion of the EGFR gene without the T790M mutation by ARMS methods. Immunohistochemistry results [chromoganinA (a small amount, +); synaptophysin (+)] also showed SCLC transformation. Considering the possibility of false positivity of plasma-based detection of the T790M result, after 2 weeks, a second EGFR blood test was performed and the result was the same. The patient did not fulfill the requirements of AURA 17 trial and missed the treatment of AZD9291.
She was treated afterward with two cycles of chemotherapy of cisplatin (75 mg/m2) and etoposide (100 mg/m2, d1–d3) from August 2015, but the disease progressed after two cycles. Then, pemetrexed (500 mg/m2) was administered in October 2015, but her disease progressed again. The patient’s symptoms improved markedly in 2 months and a CT scan showed that the disease progressed obviously. She died on 15 December 2015 because of disease progression (Figs 1–5).
Fig. 1.
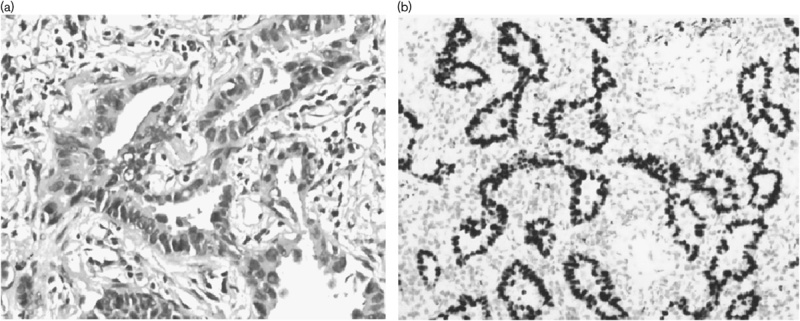
Pathological analysis of an axillary lymph node biopsy specimen at the time of diagnosis showing adenocarcinomatous cells (a: hematoxylin and eosin staining, ×20 original magnification) with thyroid transcription factor-1 staining positive (b: ×20 original magnification).
Fig. 5.
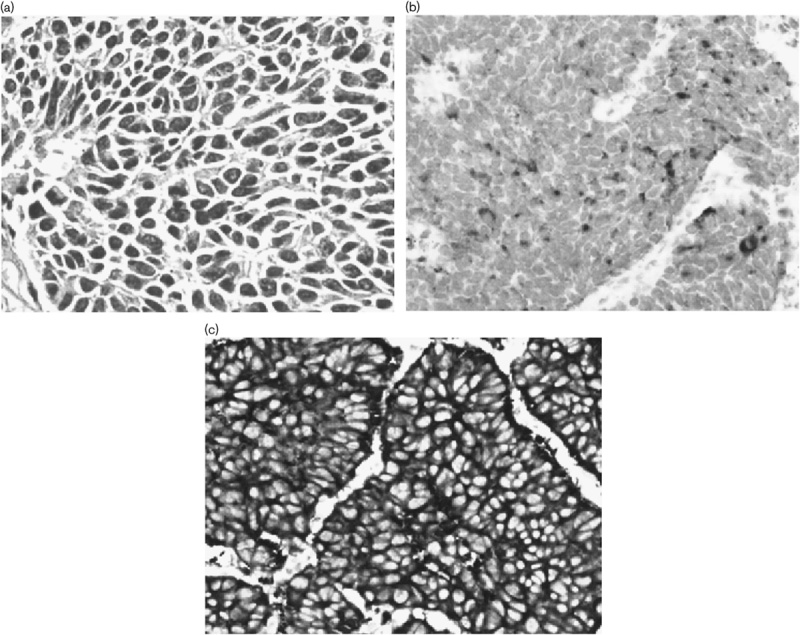
Small cell lung cancer transformation. (a) Pathological analysis of a left inferior lobe puncture biopsy specimen showing small cell lung cancer transformation (hematoxylin and eosin staining, ×20 original magnification). (b) Staining for chromoganinA showed partial positive (×20 original magnification). (c) Staining for synaptophysin was positive (×20 original magnification).
Fig. 2.
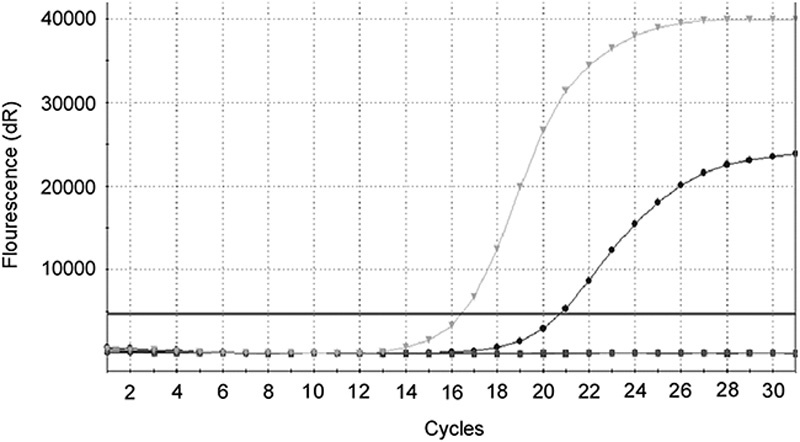
The epidermal growth factor receptor mutation was identified using the amplification refractory mutation system method in tumor tissue after progression on gefitinib therapy: epidermal growth factor receptor 19 deletion detected without the T790M point mutation.
Fig. 3.
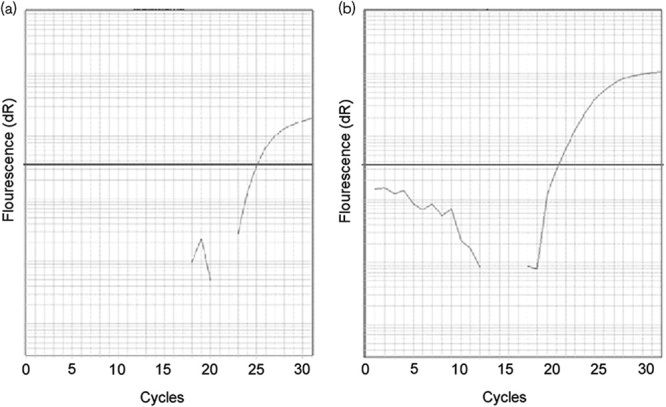
Epidermal growth factor receptor mutations were identified using the Scorpions-amplification refractory mutation system method in peripheral blood after progression on gefitinib therapy: (a) epidermal growth factor receptor 19 deletion detected. (b) T790M point mutation detected.
Fig. 4.
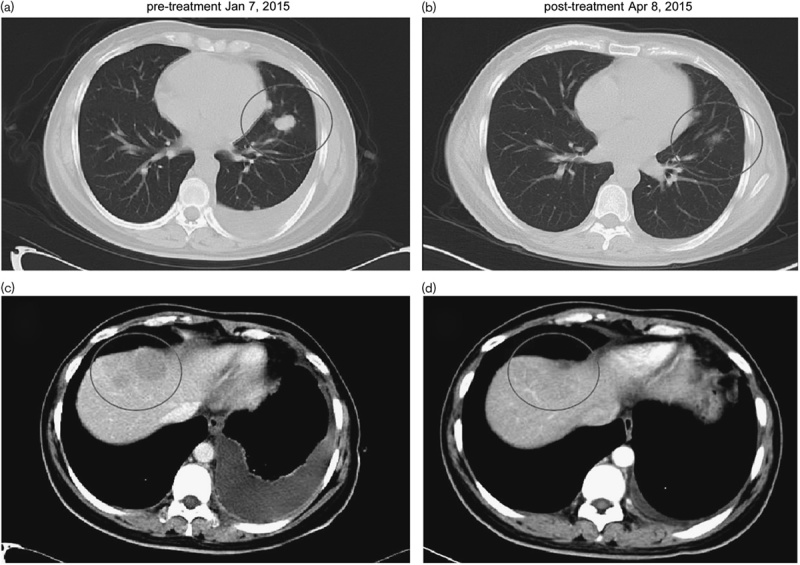
A chest computed tomographic scan after 2 months of gefitnib treatment showing that the tumor in the left lobe and the liver had shrunk (b, d) compared with before gefitinib treatment (a, c). The circles represent the location of the tumor.
Discussion
Repeat biopsy of growing tumors at clinical progression has become increasingly important as the results may better predict prognosis and guide therapy 15,16. Understanding the acquired resistance mechanisms is essential as new specific resistance mechanism-based therapies are becoming more common and are effective. For instance, relapsed tumors with the EGFR T790M secondary mutation or SCLC transformation can be treated by T790M-specific EGFR-TKI in clinical trial settings 17 or cytotoxic chemotherapy and radiation for SCLC 9.
In previously reported cases that experienced acquired resistance to EGFR-TKI by SCLC transformation 9–13, all the SCLC components harbored the same activating EGFR mutation as the adenocarcinoma component. In our case, the SCLC in metastatic lesions also harbored the original exon 19 deletion of the EGFR gene. Majority experts tended to believe that the subsequently occurring small cell cancers developed directly from the initial cancer, rather than being a distinct, second primary cancer. We believed that it was a transformation also because the NSE level increased immediately after gefitinib treatment and rebiopsy pathology showed SCLC with EGFR exon 19 deletion as well. Our case also showed an increase in the serum NSE level accompanied by tumor progression, which may indicate that the SCLC transformation will happen. Thus, detection of persistent elevated NSE levels might need attention during EGFR-TKIs treatment.
In our clinical practice, we found a case with SCLC transformation through rebiopsy in the progression lesion after EGFR-TKI therapy and with the result of plasma T790M positivity. We analyzed previous literatures and found several cases with both SCLC transformation and the T790M mutation 12–14 by histological detection. Suda et al. 13 reported an autopsy case with nine EGFR-TKI-refractory tumor lesions that consisted of six SCLCs, two adenocarcinomas with T790M, and one retroperitoneum lymph node that included each histology independently. The author concluded on a complementary and reciprocal relationship between SCLC transformation and the EGFR T790M secondary mutation. However, this phenomenon is rarely encountered in clinical cases because of the restriction of sampling and intratumoral genetic heterogeneity (IGH).
In this case, we found the SCLC transformation in tissue sample without the T790M mutation; otherwise, the T790M mutation was positive in circulating free DNA from plasma samples. The plasma-based detection of T790M has become more reliable and feasible with technological development 18–21, but because of the low sensitivity of ARMS in ctDNA detection, we verified the result repeatedly. The genetic heterogeneity and the represent activeness of the histological sample might be the reason that T790M mutation only detected in the plasmid sample but not in the tissue sample in this case. Other dormant metastasis lesions harbored the T790M mutation as a result of the positive plasma T790M genotyping. According to Oxnard et al.’s 22 research, the detection of the T790M mutation may appear 16 weeks before radiographic progression. We believed that T790M was not always the dominant growth driver as observed in Sequist et al.’s 23 study, in which serial plasma data showed that T790M mutation burden was decreased in most patients including even nonresponders after they received rociletinib therapy.
Histological pathology is always the gold standard for the detection of genotyping; however, this position has been challenged recently. One primary reason is that IGH may exist in spatially separated subclones of the same tumor 24,25 and the tissue genotype may yield a false-negative result. In the research of rociletinib (CO-1686) in NSCLC patients 23, among 113 patients with plasma genotyping, there were 17 patients with T790M positivity in plasma, but nine results were negative and eight failed tissue genotyping. The rate of clinical response to rociletinib was almost identical in patients with a positive T790M mutation in plasma and tissue.
Another study of EGFR mutation detection in ctDNA from NSCLC patients showed high specificity (97%) for EGFR-sensitizing mutations with the cobas Mutation Test. The tissue sample was used as a nonreference standard. However, for the T790M mutation, the specificity was 67%, Thress et al. 18 concluded that genomic heterogeneity of T790M-mediated resistance may explain the reduced specificity that was observed with the detection of T790M mutations in plasma ctDNA.
One ongoing clinical trial [blood detection of EGFR mutation for Iressa treatment (NCT02282267)] was proposed to validate the efficacy of gefitinib in advanced lung adenocarcinoma with the EGFR mutation determined by plasma circulating free DNA. In addition to the homogeneity, the blood specimen also has the advantages of being noninvasive, the fact that it can be carried out in real time, repeatability, and accessibility. On 13 February 2015, the China Food and Drug Administration and on 26 September 2014, the European Directorate for Quality Medicines approved the revision of labels for gefitinib, allowing the use of ctDNA for the assessment of EGFR mutation status in patients whose tumor sample could not be accessed.
Unfortunately, the patient was not enrolled in the AURA 17 trial and did not receive AZD9291 treatment; thus, we could not evaluate the efficacy of third-generation EGFR-TKI targeting EGFR T790M mutation in this patient.
Conclusion
Here, we have reported a case of acquired resistance to EGFR-TKI therapy through transformation to SCLC without the T790M mutation concomitant with plasma-genotyped T790M positivity. Combining with a literature study, we advocate and look forward to more researches to clarify the complementary relationship between SCLC transformation and the EGFR T790M secondary mutation. Also, the inconsistency of the plasma and tissue genotyping results may indicate the existence of IGH. The noninvasive fluid biopsy approaches may help overcome the problem of heterogeneity and its value in acquired resistance to tyrosine kinase inhibitor therapy should be researched further for confirmation. In summary, we conclude that SCLC transformation is the resistance mechanism to EGFR-TKI for our case and emphasized the importance of rebiopsy. Both histopathology and molecular test with tissue samples or plasmid samples are useful, and may provide more evidence to enable a patient’s specific treatment.
Acknowledgements
This work was supported by the Natural Science Foundation of China (81402429) and the Natural Science Foundation of Zhejiang Province, China (LQ14H160003). This study was carried out in accordance with the Declaration of Helsinki and has been approved by the ethics committee of Zhejiang Cancer Hospital. We had obtained informed consent for the publication from the patient and her husband.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Shi Y, Au JS, Thongprasert S, Srinivasan S, Tsai CM, Khoa MT, et al. A prospective, molecular epidemiology study of EGFR mutations in Asian patients with advanced non-small-cell lung cancer of adenocarcinoma histology (PIONEER). J Thorac Oncol 2014; 9:154–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pham D, Kris MG, Riely GJ, Sarkaria IS, McDonough T, Chuai S, et al. Use of cigarette-smoking history to estimate the likelihood of mutations in epidermal growth factor receptor gene exons 19 and 21 in lung adenocarcinomas. J Clin Oncol 2006; 24:1700–1704. [DOI] [PubMed] [Google Scholar]
- 3.Suda K, Tomizawa K, Mitsudomi T. Biological and clinical significance of KRAS mutations in lung cancer: an oncogenic driver that contrasts with EGFR mutation. Cancer Metastasis Rev 2010; 29:49–60. [DOI] [PubMed] [Google Scholar]
- 4.Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, et al. Gefitinib or carboplatin–paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009; 361:947–957. [DOI] [PubMed] [Google Scholar]
- 5.Rosell R, Moran T, Queralt C, Porta R, Cardenal F, Camps C, et al. Spanish Lung Cancer Group. Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med 2009; 361:958–967. [DOI] [PubMed] [Google Scholar]
- 6.Ohashi K, MaruvkaYE, Michor F, Pao W. Epidermal growth factor receptor tyrosine kinase inhibitor-resistant disease. J Clin Oncol 2013; 31:1070–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kobayashi S, Boggon TJ, Dayaram T, Jänne PA, Kocher O, Meyerson M, et al. EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. N Engl J Med 2005; 352:786–792. [DOI] [PubMed] [Google Scholar]
- 8.Pao W, Miller VA, Politi KA, Riely GJ, Somwar R, Zakowski MF, et al. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS Med 2005; 2:e73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sequist LV, Waltman BA, Dias-Santagata D, Digumarthy S, Turke AB, Fidias P, et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. SciTransl Med 2011; 375ra26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu HA, Arcila ME, Rekhtman N, Sima CS, Zakowski MF, Pao W, et al. Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers. Clin Cancer Res 2013; 19:2240–2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Niederst MJ, Sequist LV, Poirier JT, Mermel CH, Lockerman EL, Garcia AR, et al. RB loss in resistant EGFR mutant lung adenocarcinomas that transform to small-cell lung cancer. Nat Commun 2015; 6:6377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Furugen M, Uechi K, Hirai J, Aoyama H, Saio M, Yoshimi N, et al. An autopsy case of two distinct, acquired drug resistance mechanisms in epidermal growth factor receptor-mutant lung adenocarcinoma: small cell carcinoma transformation and epidermal growth factor receptor T790M mutation. Intern Med 2015; 54:2491–2496. [DOI] [PubMed] [Google Scholar]
- 13.Suda K, Murakami I, Sakai K, Mizuuchi H, Shimizu S, Sato K, et al. Small cell lung cancer transformation and T790M mutation: complimentary roles in acquired resistance to kinase inhibitors in lung cancer. Sci Rep 2015; 5:14447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fallet V, Ruppert AM, Poulot V, Lacave R, Belmont L, Antoine M, et al. Secondary resistance to erlotinib: acquired T790M mutation and small-cell lung cancer transformation in the same patient. J Thorac Oncol 2012; 7:1061–1063. [DOI] [PubMed] [Google Scholar]
- 15.Oxnard GR, Arcila ME, Sima C, Riely GJ, Chmielecki J, Kris MG, et al. Acquired resistance to EGFR tyrosine kinase inhibitors in EGFR mutant lung cancer: distinct natural history of patients with tumors harboring the T790M mutation. Clin Cancer Res 2011; 17:1616–1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hata A, Katakami N, Yoshioka H, Takeshita J, Tanaka K, Nanjo S, et al. Rebiopsy of non-small cell lung cancer patients with acquired resistance to EGFR-TKI: comparison between T790M mutation-positive and mutation-negative populations. Cancer 2013; 119:4325–4332. [DOI] [PubMed] [Google Scholar]
- 17.Sequist LV, Soria JC, Goldman JW, Wakelee HA, Gadgeel SM, Varga A, et al. Rociletinib in EGFR-mutated non-small-cell lung cancer. N Engl J Med 2015; 372:1700–1709. [DOI] [PubMed] [Google Scholar]
- 18.Thress KS, Brant R, Carr TH, Dearden S, Jenkins S, Brown H, et al. EGFR mutation detection in ctDNA from NSCLC patient plasma: a cross-platform comparison of leading technologies to support the clinical development of AZD9291. Lung Cancer 2015; 90:509–515. [DOI] [PubMed] [Google Scholar]
- 19.Ishii H, Azuma K, Sakai K, Kawahara A, Yamada K, Tokito T, et al. Digital PCR analysis of plasma cell-free DNA for non-invasive detection of drug resistance mechanisms in EGFR mutant NSCLC: correlation with paired tumor samples. Oncotarget 2015; 6:30850–30858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuang Y, Rogers A, Yeap BY, Wang L, Makrigiorgos M, Vetrand K, et al. Noninvasive detection of EGFR T790M in gefitinib or erlotinib resistant non-small cell lung cancer. Clin Cancer Res 2009; 15:2630–2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sorensen BS, Wu L, Wei W, Tsai J, Weber B, Nexo E, Meldgaard P. Meldgaard P.Monitoring of epidermal growth factor receptor tyrosine kinase inhibitor-sensitizing and resistance mutations in the plasma DNA of patients with advanced non-small cell lung cancer during treatment with erlotinib. Cancer 2014; 120:3896–3901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oxnard GR, Paweletz CP, Kuang Y, Mach SL, O’Connel A, Messineo MM, et al. Noninvasive detection of response and resistance in EGFR-mutant lung cancer using quantitative next-generation genotyping of cell-free plasma DNA. Clin Cancer Res 2014; 20:1698–1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sequist LV, Goldman JW, Wakelee HA, Camidge DR, Yu HA, Varga A, et al. Efficacy of rociletinib (CO-1686) in plasma-genotyped T790M-positive non-small cell lung cancer (NSCLC) patients (pts). J Clin Oncol 2015; 33 (Suppl): 8001. [Google Scholar]
- 24.Cai W, Lin D, Wu C, Li X, Zhao C, Zheng L, et al. Intratumoral heterogeneity of ALK-rearranged and ALK/EGFR coaltered lung adenocarcinoma. J Clin Oncol 2015; 33:3701–3709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Birkbak NJ, Hiley CT, Swanton C. Evolutionary precision medicine:a role for repeat epidermal growth factor receptor analysis in ALK-rearranged lung adenocarcinoma? J Clin Oncol 2015; 33:3681–3683. [DOI] [PubMed] [Google Scholar]


