Abstract
Introduction
We investigated safety, efficacy, and recurrence after postoperative hemithoracic intensity-modulated radiation therapy (IMRT) in patients with malignant pleural mesothelioma (MPM)treated with extrapleural pneumonectomy (EPP) during the past decade at a single institution.
Methods
In 2001–2011, 136 consecutive patients with MPM underwent EPP with planned adjuvant IMRT. Eighty-six patients (64%) underwent hemithoracic IMRT; the rest were not eligible because of postoperative complications, disease progression, or poor performance status. We assessed toxicity, survival, and patterns of failure in these 86 patients. Toxicity was scored with the Common Terminology Criteria for Adverse Events v4.0; survival outcomes were estimated with the Kaplan-Meier method; and local-regional patterns of failure were classified as in-field, marginal, or out-of-field. Risk factors related to survival were identified by univariate and multivariate Cox regression analysis.
Results
Median overall survival time for all 86 patients was 14.7 months. Rates of grade ≥ 3 toxicity were skin 17%, lung 12%, heart 2.3%, and gastrointestinal toxicity 16%. Five patients experienced grade 5 pulmonary toxicity. Rates of local-regional recurrence-free survival, distant metastasis-free survival (DMFS), and overall survival (OS) were 88%, 55%, and 55% at 1 year and 71%, 40%, and 32% at 2 years. On multivariate analysis, pretreatment forced expiratory volume in 1 second (FEV1), non-epithelioid histology, and nodal status were associated with DMFS and OS.
Conclusion
IMRT after EPP is associated with low rates of local-regional recurrence, though some patients experience life-threatening lung toxicity. Tumor histology and nodal status can be helpful for identifying patients for this aggressive treatment.
Keywords: malignant pleural mesothelioma, extrapleural pneumonectomy, intensity-modulated radiotherapy, IMRT
INTRODUCTION
Malignant pleural mesothelioma is a rare and deadly cancer that primarily occurs decades after exposure to asbestos. Outcomes have remained dismal despite aggressive treatment strategies. Extrapleural pneumonectomy (EPP) is an aggressive and increasingly controversial approach1 that can be used to surgically treat this malignancy, although patients remain at high risk for local-regional failure and nearly all die of disease even after EPP.2 Hemithoracic radiation therapy has been used to control microscopic residual disease and minimize the risk of local-regional recurrence. Although the initial reports of this approach involved conventional 3-dimensional conformal radiation therapy,3,4 subsequent studies suggest that postoperative radiation is feasible when delivered as intensity-modulated radiation therapy (IMRT), with promising rates of locoregional control.5 However, results of this technique from large databases6,7 other than the Surveillance, Epidemiology, and End Results (SEER) database are limited.8
The purpose of this study was to summarize our experience with this strategy at a high-volume tertiary cancer care center over the past decade, focusing on toxicity and survival outcomes. We also analyzed patterns of failure after this combined-modality technique to estimate the percentage of patients who experience recurrences at the radiation margin and to determine if the radiation fields currently being used for this purpose are appropriate.
METHODS
Eligibility
This retrospective study was approved by the institutional review board of The University of Texas MD Anderson Cancer Center. We identified 136 consecutive patients who had undergone EPP for malignant pleural mesothelioma at MD Anderson Cancer Center between January 2001 and April 2011. Eighty-six of these patients (64%) ultimately underwent hemithoracic IMRT, the characteristics of whom are listed in Table 1. We routinely recommend hemithoracic IMRT after EPP after patients recover from surgery, typically within 2–3 months if the patient can tolerate it. Of the 50 patients who did not undergo IMRT after EPP in this study, 22 were not treated because of poor performance status after surgery; 11 died before they could be considered for radiation (6 from pneumonia or acute respiratory distress syndrome, 1 from pulmonary embolism, 1 from bronchopleural fistula, 1 from intraoperative bleeding followed by cardiac arrest, and 2 from cardiac arrest of unknown causes); 12 were not considered candidates for radiation because of DM or significant local recurrence (LR) found at or before computed tomography (CT) treatment simulation; and 5 refused radiation or were lost to follow-up. Chemotherapy regimens included cisplatin/pemetrexed (n=25), cisplatin alone (n=4), cisplatin/gemcitabine (n=3), carboplatin/pemetrexed (n=2), and dasatinib+/− systemic therapy (n=8).
Table 1.
Patient Characteristics in EPP+IMRT group (n=86) and EPP alone (n=50)
| Characteristic | EPP+IMRT n (%) |
EPP alone n (%) |
p-value |
|---|---|---|---|
| Age at EPP, years | |||
| Median | 59.8 | 62.5 | |
| Mean | 60.6 | 61.9 | 0.132 |
| Range | 37.4–77.9 | 41.3–77.6 | |
| Sex | |||
| Male | 76 | 40 | |
| Female | 10 | 10 | 0.214 |
| Side | |||
| Right | 57 | 29 | |
| Left | 29 | 21 | 0.361 |
| ECOG PS Score at diagnosis | |||
| 0 | 35 | 14 | |
| 1 | 44 | 31 | 0.137 |
| 2 | 7 | 3 | |
| 3 | 0 | 2 | |
| Pretreatment FEV1 | |||
| % predicted value | |||
| Median | 70 | 69 | |
| Mean | 73 | 72 | 0.780 |
| Range | 40–109 | 50–110 | |
| Pretreatment DLCO | |||
| % predicted value | |||
| Median | 80 | 75 | 0.247 |
| Mean | 81 | 76 | |
| Range | 23–121 | 46–110 | |
| T-status | |||
| T2 | 9 | 7 | |
| T3 | 71 | 36 | 0.300 |
| T4 | 6 | 7 | |
| N-Stage | |||
| N0 | 44 | 24 | |
| N1 | 15 | 5 | 0.319 |
| N2 | 27 | 21 | |
| Tumor Histology | |||
| Epithelioid | 66 | 32 | 0.333 |
| Sarcomatoid/Biphasic | 23 | 18 | |
| Interval Between Surgery and Radiation, Months | |||
| Median | 2.4 | - | |
| Mean | 2.6 | N/A | |
| Range | 1.3–7.0 | ||
| Mean Lung Dose, Gy | |||
| Median | 6.61 | ||
| Mean | 6.74 | - | |
| Range | 4.28–8.7 | N/A | |
| Mean Esophageal Dose, Gy | |||
| Median | 35.3 | ||
| Mean | 35.4 | - | |
| Range | 26.0–44.9 | N/A | |
| Chemotherapy | |||
| None | 57 | 38 | |
| Induction | 20 | 12 | P=0.051 |
| Adjuvant | 12 | 0 | |
Abbreviations: EPP, extrapleural pneumonectomy; ECOG PS, Easter Cooperative Oncology Group performance status; FEV1, forced expiratory volume in 1 second; DLCO, carbon monoxide lung diffusion capacity; AJCC, American Joint Committee on Cancer.
It is evident from examining Table 1 that there are no significant differences in the majority of variables between those patients that received IMRT vs. those that did not, including performance status at the time of diagnosis, stage, and histology, further highlighting the “intention to treat” approach that is taken in all patients that receive an EPP. Indeed, no patient that underwent EPP alone received adjuvant chemotherapy, which supports the premise that this subgroup of patients was not eligible for any adjuvant therapy.
Surgery
All patients underwent staging studies including PET/CT, bronchoscopy, laparoscopy, and mediastinoscopy before surgery to identify transdiaphragmatic extension and contralateral hemithoracic disease; none had evidence of DM before EPP. Pulmonary and renal function was evaluated with spirometry, quantitative ventilation/perfusion scanning, and radioisotope renography. The surgical procedure involved an en bloc resection of the lung, parietal pleura, visceral pleura, ipsilateral pericardium, and diaphragm, with reconstruction of the pericardium and diaphragm with polytetrafluoroethylene, as described previously.9 Complications of surgical resection are depicted in Table 2.
Table 2.
Toxicity due to Extrapleural Pneumonectomy (n=136)
| Toxicity | N (%) |
|---|---|
| Pulmonary | |
| Pneumonia | 15 (11) |
| Acute Respiratory Distress Syndrome | 4 (2.9) |
| Cardiovascular | |
| Atrial arrhythmias | 59 (44) |
| Myocardial infarction | 3 (2.2) |
| Ventricular arrhythmias | 3 (2.2) |
| Deep vein thrombosis | 4 (2.9) |
| Pulmonary embolism | 3 (2.2) |
| Neurologic | |
| Cerebrovascular accident | 2 (1.5) |
| Technical | |
| Exsanguination (intraoperative) | 1 (0.7) |
| Bleeding requiring reoperation | 9 (6.6) |
| Pleural space infection | 2 (1.5) |
| Bronchopleural fistula | 4 (2.9) |
| Recurrent laryngeal nerve paralysis | 4 (2.9) |
| Chylothorax | 1 (0.7) |
| Patch dehiscence | 1 (0.7) |
| Other | |
| Empyema | 9 (6.6) |
| Sepsis | 11 (8.1) |
| Shock | 2 (1.5) |
Radiation Therapy
All patients underwent 4-dimensional CT-based treatment simulation before radiation therapy. Simulation took place while the patients were supine and immobilized in a Vac-Loc cradle, with both arms overhead grasping a T-bar. A bolus 0.5 cm thick and 3.5 cm in circumference was placed over incision and drainage sites. The ipsilateral hemithorax was contoured as the clinical target volume, which included the pleural space, scars, drain sites, and involved nodal stations. These contours were drawn in consultation with the operating surgeon to identify specific regions that required a boost. This volume was then expanded to include a margin for internal motion, and an additional 0.5- to 1.0-cm margin was added to account for patient movement (the planning target volume [PTV]). Details of our radiation treatment planning approach have been published elsewhere.10
The dose prescribed to the PTV was 45–50 Gy in 25 daily fractions. Thirteen patients received a radiation boost to 55–60 Gy in areas identified by the surgeon as being at high risk for residual disease or as positive margins. Standard dose constraints in the remaining lung included a mean lung dose <8.5 Gy, the percentage of lung receiving 20 Gy or more (V20) ≤ 7%; the esophagus V50 <50%, and the mean dose to the esophagus <34 Gy. Mean dose to the heart was kept <26 Gy and the heart V30 ≤45%. The maximum dose (Dmax) to the spinal cord was kept <45 Gy. Liver constraints were: mean dose to the liver was <30 Gy and the liver V30 ≤40%. Finally, the dose to at least two-thirds of the contralateral kidney was kept below 30 Gy. Interval histories and physical examinations were done weekly during treatment, after treatment events, and at each follow-up visit. CT imaging was obtained every 3 months for 2 years after treatment and yearly thereafter.
IMRT Toxicity Assessment
Treatment-related toxicity was assessed weekly during radiation therapy, at 4–6 weeks after treatment, and then every 3 months thereafter. Toxicity was scored according to the Common Terminology Toxicity Criteria for Adverse Events version 4.0 and focused on esophageal, pulmonary, and cardiac toxicity.
Patterns of Failure
Patterns of failure were characterized in all patients who experienced disease recurrence. Treatment failure within the ipsilateral hemithorax was considered local-regional recurrence (LR), and all other sites of failure were considered DM. For patients who experienced LR, archived radiation treatment plans were obtained and fused with the image that revealed the recurrence to establish whether the failure was in-field, marginal, or out-of-field. Marginal failures were those in which the recurrence lay partially within and partially outside the isodose line representing the prescribed dose.
Statistical Considerations
Data were analyzed with Stata/SE 11.1 (StataCorp LP, College Station, Texas). Actuarial rates of local-regional recurrence-free survival (LRFS), distant metastasis-free survival (DMFS), and overall survival (OS) were estimated by using the Kaplan-Meier method and compared with log-rank tests. Cox proportional hazards models were developed to identify associations between patient- and disease-related characteristics and the survival endpoints. Significance was determined using α = 0.05, and variables found to be significant in univariate analysis were then tested in multivariate analysis. Survival was calculated from the date of surgery.
RESULTS
The median follow-up time for all 86 patients receiving EPP+IMRT was 10.2 months (range <1–99.3 months), and the median follow-up time for all patients alive at the time of analysis was 13.9 months (range 2.7–99.3 months). The median interval between surgery and IMRT was 2.4 months (range 1.3–7.0 months).
Toxicity
Toxic effects secondary to IMRT are shown in Table 2. Almost all patients experienced grade ≥2 gastrointestinal symptoms, primarily nausea, esophagitis, or both. Rates of grade ≥3 or higher toxicity were as follows: skin, 17.4% (n=15); gastrointestinal (e.g. esophagitis/nausea), 16.3% (n=14); heart, 2.3% (n=2); and lung, 11.6% (n=10). Five patients experienced grade 5 toxicity, all pulmonary (3 radiation pneumonitis and 2 bronchopleural fistulae). Of the two patients who experienced grade ≥3 cardiac toxicity, one had a pericardial effusion (grade 3), and the other developed severe cardiomyopathy with markedly reduced ejection fraction (grade 4). Other grade 3 toxicities included grade 3 dyspnea 2–6 months after completing IMRT (n=2) and a seroma, possibly caused by radiation-related chest wall disruption, requiring surgery. Patients who received induction chemotherapy did not experience higher rates of severe toxicity (p>0.05), though the small number of such patients (n=20) precluded full statistical analysis.
Survival and Patterns of Failure
The median OS rate for patients undergoing combined modality therapy of EPP+IMRT was At 1 year, OS rates were 54%, LRFS 88%, and DMFS 71%; corresponding rates at 2 years were OS 32%, LRFS 55%, and DMFS 40%, and at 3 years were OS 22%, LRFS 54%, and DMFS 21% (Fig. 1). Fourteen patients (16%) experienced LR and 51 patients (59%) had DM. Only 2 patients experienced LR alone; the other 12 patients had both LR and DM. Sites of distant metastasis were as follows: contralateral hemithorax, 41% (n=35); abdomen/pelvis, including liver, 28% (n=24); and bones, n=7% (n=6). Some patients experienced recurrence at more than one distant site. Of the 14 patients who experienced LR, 8 had multiple recurrences within the high-dose region, 5 patients had recurrences on the margin of a hemithoracic field (in a subcarinal lymph node, an ipsilateral subpectoral lymph node, a retrosternal mass, within a solitary pleural mass in the ipsilateral hemithorax, and in multiple mediastinal lymph nodes near the margin of the radiation field), and 1 patient had a recurrence in the ipsilateral supraclavicular region. Recurrence patterns for two patients who experienced LR, one in a cold spot within the radiation field and the other in both a high-dose region and near the margin of the field, are shown in Figure 2.
FIGURE 1.
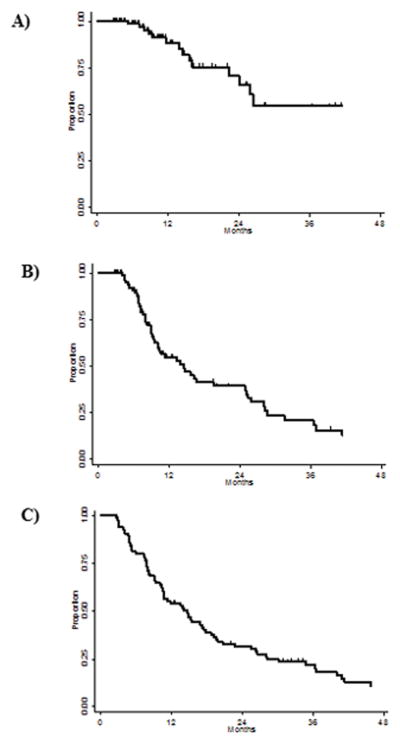
Kaplan-Meier survival curves for patients treated with extrapleural pneumonectomy followed by intensity-modulated radiation therapy for malignant pleural mesothelioma. (A) Local-regional–progression free survival; (B) distant metastasis–free survival; (C) overall survival.
FIGURE 2.
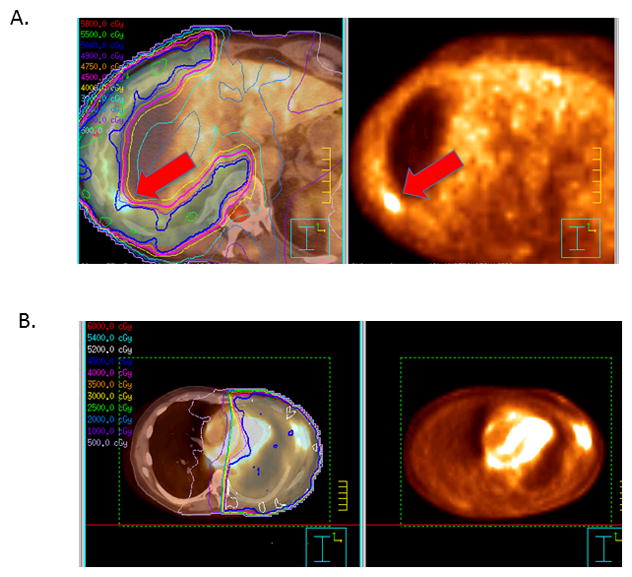
Fused PET/CT scans and radiation isodose curves (left) and diagnostic PET scans (right) of treatment failures in two different patients after extrapleural pneumonectomy followed by intensity-modulated radiation therapy for malignant pleural mesothelioma. (A) Arrows indicate failure within a “cold spot” where the isodose lines curved inward along the chest wall and thus compromised target coverage in this portion of the radiation field. (B) Recurrence in high-dose region and within subcutaneous tissues adjacent to the surgical scar.
In Figure 3, we have depicted a comparison of those patients that completed IMRT after EPP (median OS 12.9 months) vs. those patients that received EPP alone (median OS 4.5 months), both with respect to OS and disease-free survival. It is evident that survival outcomes are substantially improved with combined modality therapy, primarily because inherent selection criteria for IMRT after EPP are factors that would affect these survival endpoints, such as poor performance status, early treatment failure, and death (with only 5 patients foregoing IMRT due strictly to patient refusal).
FIGURE 3.
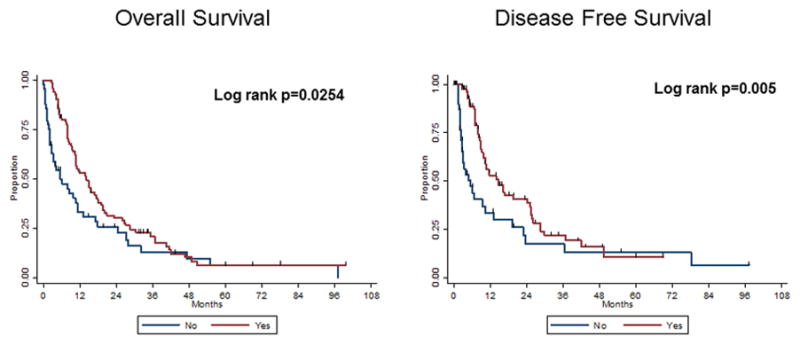
Comparison of EPP alone with EPP + IMRT with regards to, a) overall survival, and b) disease-free survival.
Factors Predicting Survival
Multivariate analysis indicated that the following factors were predictive of worse DMFS and OS in those patients receiving EPP+IMRT: non-epithelioid histology, higher nodal status, and lower pretreatment FEV1 tested as a continuous variable (Table 3). Higher hazard ratios in this table indicate an increased risk of an event (e.g. distant metastasis or death, respectively). No variables were found to be associated with LRFS. Grouping patients according to nodal status and histology also produced subgroups with distinct prognoses in terms of DM (Fig. 3A) and OS (Fig. 3B). Chemotherapy was not associated with any survival outcomes (P>0.05).
Table 3.
Toxicity in Patients Treated with Extrapleural Pneumonectomy plus Hemithoracic Intensity-Modulated Radiation Therapy (n=86)
| Toxicity Type and Grade | No. of Patients |
|---|---|
| Skin | |
| 0 | 2 |
| 1 | 59 |
| 2 | 10 |
| 3 | 15 |
| Lung | |
| 0 or 1 | 74 |
| 2 | 3 |
| 3 | 4 |
| 4 | 1 |
| 5 | 5 |
| Heart | |
| 0 | 80 |
| 1 | 0 |
| 2 | 4 |
| 3 | 1 |
| 4 | 1 |
| Gastrointestinal | |
| 0 | 6 |
| 1 | 0 |
| 2 | 66 |
| 3 | 14 |
DISCUSSION
Outside of SEER analyses, the current study represents the largest known series with complete clinical information for evaluating survival outcomes after EPP followed by IMRT for malignant pleural mesothelioma. Our pertinent findings can be summarized as follows: First, a significant number of patients (49, or 36%) who underwent EPP with planned adjuvant radiation did not receive IMRT because of poor performance status, postoperative complications, or the early development of DM. In addition, some patients did not receive postoperative radiation therapy for several months after treatment, either due to the administration of chemotherapy or recovery from surgery, thereby questioning the “adjuvant” nature of this approach. Second, the predominant pattern of failure after this combined-modality approach was DM, and among those patients who experienced LR, most had treatment failures at multiple sites within the high-dose radiation region, suggesting that radiation doses currently being used for this purpose do not control disease in at least some of these patients. Third, although this aggressive approach could produce significant toxicity (primarily gastrointestinal and dermatologic effects that required rigorous monitoring and care), the incidence of grade ≥3 cardiopulmonary toxicity was much lower (14%). Nevertheless, patients considering this treatment should be informed that even when stringent dosimetric constraints are used, this approach carries a low, although non-negligible, risk (about 6%) of potentially fatal complications. Finally, histologic type and nodal status were the two primary disease factors that influenced survival outcomes—further evidence that this aggressive approach should be reserved mainly for patients with epithelioid tumors and negative mediastinal lymph nodes.
We previously reported outcomes and patterns of failure among 100 patients treated with EPP with or without adjuvant IMRT.5 In that study, for the 61 patients who underwent IMRT from 1999–2005, median OS was 14.2 months, 3-year OS was 20%, and 8 patients (13%) had LR. The current analysis, with a greatly expanded number of patients and with the increased use of PET scan to evaluate recurrence and patterns of failure in the recent cohort, corroborated our previous finding of high rates of local control while more closely evaluating the benefit of IMRT. Indeed, results from the current study compare favorably with those in other published studies of radiation after EPP. In one such study, among 13 patients treated with IMRT at Duke University, 6 (46%) had local failure.11 In another study at Memorial Sloan Kettering Cancer Center, a matched photon/electron (MPE) technique was used to deliver 54 Gy of hemithoracic radiation after EPP to a series of 61 patients.4 Although the median OS time in that study was 17 months, the median OS for the subset of patients most analogous to those in the current study, those with stage III or IV disease, was just 10 months.3
Use of IMRT to deliver hemithoracic RT after EPP has been linked with high rates of fatal pneumonitis.12 Another risk factor for the development of severe pulmonary events has been the use of intraoperative cisplatin13. However, since these studies were published, several others have demonstrated that IMRT can provide both dosimetric superiority and good clinical outcomes when appropriate dose constraints are used.14 For example, in one analysis of 78 patients treated with MPE, 67% of isolated local failures were in areas of dose inhomogeneity that would have had better coverage with IMRT.15 Other studies from MD Anderson5 and other institutions6,7 have shown low rates of high-grade pneumonitis (5% in this study) and median survival times of 16 months or more with the use of IMRT. With the increasing adoption of IMRT and the growing acceptance that the mean dose to the ipsilateral lung should not exceed 8.5 Gy, the rates of adverse clinical consequences of the low-dose bath created by IMRT have been sharply reduced. Use of newer technologies such as volumetric-modulated arc therapy may also reduce patient treatment times while offering target volume coverage similar to IMRT with reduced contralateral lung doses.16
Our finding that IMRT after EPP led to a low rate of LR (16%), defined as recurrence in the ipsilateral hemithorax, is encouraging. Indeed, we found that OS thus correlated much more directly with time to DM, which led to a median OS time of 14.7 months. These findings are consistent with prior phase II studies examining the efficacy of trimodality therapy, in which OS times in excess of 20 months have been consistently achieved.17 In one such analysis, patients in a phase II trial of neoadjuvant cisplatin and pemetrexed followed by EPP and IMRT/MPE had a median OS time of 29.1 months.7 In another series, patients with N0 disease who successfully completed trimodality therapy at Princess Margaret Hospital had median OS times as long as 59 months.6 Achieving OS for longer than 5 years has proven to be much more difficult, and will likely necessitate the development of improved systemic regimens. Over the past decade, the addition of antifolates to platinum regimens has been shown to improve OS rates in several trials.18 With the recognition that malignant pleural mesotheliomas often express activated Src kinase, prospective trials of dasatinib are also being undertaken in potentially resectable cases.19 Further results from studies such as these are eagerly anticipated.
Finally, consistent with prior reports from our institution and others5,20,21, we found that patients with sarcomatoid or biphasic histology and mediastinal nodal involvement had substantially reduced survival outcomes with this aggressive technique. It is important to emphasize that largely based on findings over the past decade that have shown, even with modern surgical and radiation techniques, prognosis is poor in patients that have these disease characteristics, the practice patterns at our institution have changed over time. The standard practice at our institution for high-risk patients is currently as follows. For patients with sarcomatoid disease, no surgery is recommended. Patients with biphasic histology or N2 stage are offered either lung sparing surgery or no surgery, but an EPP is not recommended.
In addition to the weaknesses inherent in any retrospective study, the current analysis had several limitations. First, our patterns-of-failure analysis was limited by the lack of histologic confirmation in some cases, as would be available in a controlled prospective analysis. In these instances, the assessment of failure was made based on the clinical and imaging data available in the medical record. Second, small patient numbers precluded our fully analyzing the effect of chemotherapy (e.g., the specific regimen, number of cycles, or timing) with regard to patterns of failure and survival outcomes. Third, as alluded to above, it is important to stress that recent trials of multimodality therapy involving lung-sparing procedures have been published that demonstrate similar to improved outcomes with these techniques when compared to EPP.1,22–24 Therefore, there remains no consensus as to the optimal approach in this setting, and lung-sparing techniques such as pleurectomy/decortication should be strongly considered, particularly in patients that are borderline candidates for EPP. Finally, while we have included a comparison of OS and DFS outcomes in patients that received EPP + IMRT vs. EPP alone, as noted above this retrospective comparison issubject to substantial bias inherent in the use of a comparison group that received EPP alone because of underlying factors that portend a worse prognosis. Indeed, we found in the current study that the vast majority of patients who did not undergo combined-modality treatment could not because of recurrence, toxicity, or poor performance status that rendered postoperative radiation unfeasible. Or, put another way, our approach in all patients that undergo EPP is an “intention to treat” with IMRT as well, with unanticipated early recurrences, postoperative complications, or declines in performance status precluding treatment with postoperative radiation.
CONCLUSIONS
While lung-sparing surgery has emerged as a viable approach to patients with malignant pleural mesothelioma, our results demonstrate that definitive EPP followed by IMRT for MPM produces high rates of local-regional control. Although the rates of dermatitis and gastrointestinal symptoms were significant, the incidence of high-grade cardiopulmonary toxicity was low (albeit non-negligible) when stringent dosimetric constraints were used. Technical advances may eventually permit better local control with less toxicity. However, even patients in whom local-regional control is achieved are at risk of developing DM, such that similar survival outcomes have been shown in trials utilizing chemotherapy alone25. Thus, further trials of systemic agents, including targeted therapies, are indicated.
FIGURE 4.
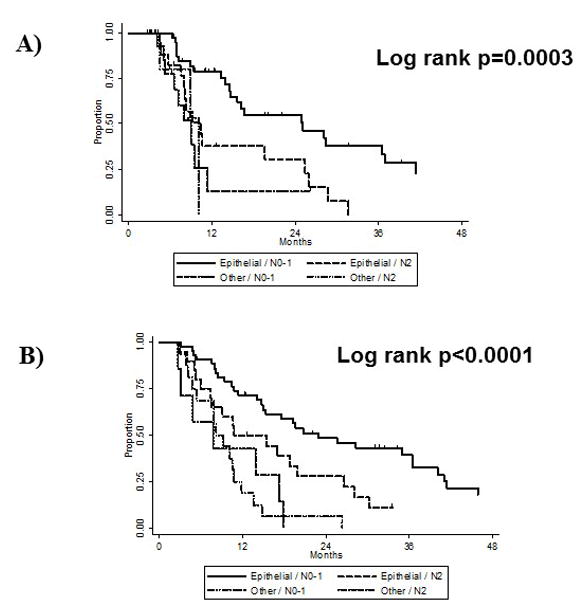
Kaplan-Meier curves illustrating (A) distant metastasis and (B) overall survival according to nodal status and histologic subtype in patients treated with extrapleural pneumonectomy plus intensity modulated radiation therapy.
Table 4.
Cox Proportional Hazard Models for Overall, Local-Regional Recurrence-Free, and Distant Metastasis-Free Survival. Hazard ratios represent the risk of death, locoregional recurrence, and distant metastasis, respectively.
| Overall Survival | Locoregional Recurrence-Free Survival | Distant Metastasis-Free Survival | ||||
|---|---|---|---|---|---|---|
| HR [95% CI] | P Value | HR [95% CI] | P Value | HR [95% CI] | P Value | |
| Non-epithelioid histology | 3.64 [1.47–5.70] | <0.001 | -- | -- | 3.46 [1.70–7.04] | 0.001 |
| N Status (N0-N1 vs N2) | 2.05 [1.14–3.69] | 0.016 | -- | -- | 1.95 [1.08–3.54] | 0.028 |
| Higher pretreatment FEV1 | 0.11 [0.01–0.95] | 0.045 | -- | -- | 0.10 [0.01–0.88] | 0.038 |
Abbreviations: HR, hazard ratio; CI, confidence interval; FEV1, forced expiratory volume in 1 second.
Acknowledgments
The authors thank Christine Wogan, M.S., of The University of Texas MD Anderson Cancer Center for her editorial contributions to this report.
Footnotes
Disclaimers: The authors declare no conflicts of interest regarding this study.
References
- 1.Treasure T, Lang-Lazdunski L, Waller D, et al. Extra-pleural pneumonectomy versus no extra-pleural pneumonectomy for patients with malignant pleural mesothelioma: clinical outcomes of the Mesothelioma and Radical Surgery (MARS) randomised feasibility study. Lancet Oncol. 2011;12:763–72. doi: 10.1016/S1470-2045(11)70149-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wolf AS, Daniel J, Sugarbaker DJ. Surgical techniques for multimodality treatment of malignant pleural mesothelioma: extrapleural pneumonectomy and pleurectomy/decortication. Semin Thorac Cardiovasc Surg. 2009;21:132–48. doi: 10.1053/j.semtcvs.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 3.Rusch VW, Rosenzweig K, Venkatraman E, et al. A phase II trial of surgical resection and adjuvant high-dose hemithoracic radiation for malignant pleural mesothelioma. Journal of Thoracic and Cardiovascular Surgery. 2001;122:788–795. doi: 10.1067/mtc.2001.116560. [DOI] [PubMed] [Google Scholar]
- 4.Yajnik S, Rosenzweig KE, Mychalczak B, et al. Hemithoracic radiation after extrapleural pneumonectomy for malignant pleural mesothelioma. International Journal of Radiation Oncology Biology Physics. 2003;56:1319–1326. doi: 10.1016/s0360-3016(03)00287-6. [DOI] [PubMed] [Google Scholar]
- 5.Rice DC, Stevens CW, Correa AM, et al. Outcomes after extrapleural pneumonectomy and intensity-modulated radiation therapy for malignant pleural mesothelioma. Ann Thorac Surg. 2007;84:1685–92. doi: 10.1016/j.athoracsur.2007.04.076. discussion 1692–3. [DOI] [PubMed] [Google Scholar]
- 6.de Perrot M, Feld R, Cho BC, et al. Trimodality therapy with induction chemotherapy followed by extrapleural pneumonectomy and adjuvant high-dose hemithoracic radiation for malignant pleural mesothelioma. J Clin Oncol. 2009;27:1413–8. doi: 10.1200/JCO.2008.17.5604. [DOI] [PubMed] [Google Scholar]
- 7.Krug LM, Pass HI, Rusch VW, et al. Multicenter phase II trial of neoadjuvant pemetrexed plus cisplatin followed by extrapleural pneumonectomy and radiation for malignant pleural mesothelioma. J Clin Oncol. 2009;27:3007–13. doi: 10.1200/JCO.2008.20.3943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Milano MT, Zhang H. Malignant pleural mesothelioma: a population-based study of survival. Journal of Thoracic Oncology. 2010;5:1841. doi: 10.1097/JTO.0b013e3181f1cf2b. [DOI] [PubMed] [Google Scholar]
- 9.Cao CQ, Yan TD, Bannon PG, et al. A systematic review of extrapleural pneumonectomy for malignant pleural mesothelioma. J Thorac Oncol. 2010;5:1692–703. doi: 10.1097/JTO.0b013e3181ed0489. [DOI] [PubMed] [Google Scholar]
- 10.Ahamad A, Stevens CW, Smythe WR, et al. Intensity-modulated radiation therapy: a novel approach to the management of malignant pleural mesothelioma. Int J Radiat Oncol Biol Phys. 2003;55:768–75. doi: 10.1016/s0360-3016(02)04151-2. [DOI] [PubMed] [Google Scholar]
- 11.Miles EF, Larrier NA, Kelsey CR, et al. Intensity-modulated radiotherapy for resected mesothelioma: the Duke experience. Int J Radiat Oncol Biol Phys. 2008;71:1143–50. doi: 10.1016/j.ijrobp.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 12.Allen AM, Czerminska M, Janne PA, et al. Fatal pneumonitis associated with intensity-modulated radiation therapy for mesothelioma. Int J Radiat Oncol Biol Phys. 2006;65:640–5. doi: 10.1016/j.ijrobp.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 13.Komaki R, Liao Z, Liu H, et al. Fatal pneumonitis associated with intensity-modulated radiation therapy for mesothelioma: in regard to Allen et al. (Int J Radiat Oncol Biol Phys 2006;65:640–645) Int J Radiat Oncol Biol Phys. 2006;66:1595–6. doi: 10.1016/j.ijrobp.2006.09.005. author reply 1596. [DOI] [PubMed] [Google Scholar]
- 14.Krayenbuehl J, Oertel S, Davis JB, et al. Combined photon and electron three-dimensional conformal versus intensity-modulated radiotherapy with integrated boost for adjuvant treatment of malignant pleural mesothelioma after pleuropneumonectomy. Int J Radiat Oncol Biol Phys. 2007;69:1593–9. doi: 10.1016/j.ijrobp.2007.07.2370. [DOI] [PubMed] [Google Scholar]
- 15.Gupta V, Krug LM, Laser B, et al. Patterns of local and nodal failure in malignant pleural mesothelioma after extrapleural pneumonectomy and photon-electron radiotherapy. J Thorac Oncol. 2009;4:746–50. doi: 10.1097/JTO.0b013e3181a5292c. [DOI] [PubMed] [Google Scholar]
- 16.Scorsetti M, Bignardi M, Clivio A, et al. Volumetric Modulation Arc Radiotherapy Compared with Static Gantry Intensity-Modulated Radiotherapy for Malignant Pleural Mesothelioma Tumor: A Feasibility Study. International Journal of Radiation Oncology Biology Physics. 2010;77:942–949. doi: 10.1016/j.ijrobp.2009.09.053. [DOI] [PubMed] [Google Scholar]
- 17.Chi A, Liao Z, Nguyen NP, et al. Intensity-modulated radiotherapy after extrapleural pneumonectomy in the combined-modality treatment of malignant pleural mesothelioma. J Thorac Oncol. 2011;6:1132–41. doi: 10.1097/JTO.0b013e3182199819. [DOI] [PubMed] [Google Scholar]
- 18.Tsao AS, Wistuba I, Roth JA, et al. Malignant pleural mesothelioma. J Clin Oncol. 2009;27:2081–90. doi: 10.1200/JCO.2008.19.8523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsao ASH, DD, Saigal B, Liu S, Lee J, Bakkannagari S, Ordonez N, Hong W, Wistuba I, Johnson F. Activated Src kinase is expressed in malignant pleural mesothelioma tumors; dasatinib inhibition leads to cytotoxicity, cell cycle inhibition, and prevention of invasion and migration. J Clin Oncol. 2007;25:7713. [Google Scholar]
- 20.Rusch VW, Venkatraman ES. Important prognostic factors in patients with malignant pleural mesothelioma, managed surgically. Ann Thorac Surg. 1999;68:1799–804. doi: 10.1016/s0003-4975(99)01038-3. [DOI] [PubMed] [Google Scholar]
- 21.Sugarbaker DJ, Flores RM, Jaklitsch MT, et al. Resection margins, extrapleural nodal status, and cell type determine postoperative long-term survival in trimodality therapy of malignant pleural mesothelioma: results in 183 patients. J Thorac Cardiovasc Surg. 1999;117:54–63. doi: 10.1016/s0022-5223(99)70469-1. discussion 63–5. [DOI] [PubMed] [Google Scholar]
- 22.Bolukbas S, Manegold C, Eberlein M, et al. Survival after trimodality therapy for malignant pleural mesothelioma: Radical Pleurectomy, chemotherapy with Cisplatin/Pemetrexed and radiotherapy. Lung Cancer. 2011;71:75–81. doi: 10.1016/j.lungcan.2009.08.019. [DOI] [PubMed] [Google Scholar]
- 23.Friedberg JS, Culligan MJ, Mick R, et al. Radical pleurectomy and intraoperative photodynamic therapy for malignant pleural mesothelioma. Ann Thorac Surg. 2012;93:1658–65. doi: 10.1016/j.athoracsur.2012.02.009. discussion 1665–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lang-Lazdunski L, Bille A, Lal R, et al. Pleurectomy/decortication is superior to extrapleural pneumonectomy in the multimodality management of patients with malignant pleural mesothelioma. J Thorac Oncol. 2012;7:737–43. doi: 10.1097/JTO.0b013e31824ab6c5. [DOI] [PubMed] [Google Scholar]
- 25.Vogelzang NJ, Rusthoven JJ, Symanowski J, et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol. 2003;21:2636–44. doi: 10.1200/JCO.2003.11.136. [DOI] [PubMed] [Google Scholar]


