Abstract
Infections on implanted medical devices are a challenging problem, especially when bacteria form difficult-to-treat biofilms. Antimicrobial peptides are considered as a solution due to their potency against antibiotic-resistant superbugs. Previously, we demonstrated the prevention of staphylococcal biofilm formation in an animal catheter model by injecting merecidin (formerly known as 17BIPHE2), a peptide engineered based on the only human cathelicidin. This study documents an alternative solution via covalent immobilization of FK-16, amino acid sequence FKRIVQRIKDFLRNLV-amide, which corresponds to the major antimicrobial region (residues 17–32) of LL-37. FK-16 is superior to the longer peptide LL-37 in terms of synthesis cost and the shorter peptide KR-12 in terms of activity spectrum. Indeed, the FK16-coated titanium surface showed a broad-spectrum activity against the ESKAPE pathogens, including Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter species. It also demonstrated anti-adhesion and biofilm inhibition capabilities against both S. aureus and E. coli.
Keywords: Biofilms, ESKAPE pathogens, FK-16, LL-37, Peptide immobilization, Titanium
Introduction
Biofilm-related infections on body implanted medical devices are a challenging issue (Darouiche 2004). Such infections can cause implant failures, increase treatment costs, and lead to high morbidity and mortality (Zhao et al. 2009; Xavier et al. 2016). Therefore, the best strategy is to prevent such infections (Costa et al. 2011; Onaizi and Leong 2011; Mishra et al. 2017). One promising method is to coat antimicrobials on the biomaterial surface. In the past years, both metals (e.g., silver, zinc, copper, and zirconium) and non-metals (e.g., selenium and antibiotics) have been used for coating (Gallo, Holinka, Moucha 2014). The effective use of metals such as silver, however, is complicated by leaching and cytotoxicity issues, whereas a prolonged use of antibiotics results in reduced efficacy due to the emergence of multi-drug resistance pathogens (Knetsch and Koole 2011; Zhao et al. 2009).
Antimicrobial peptides (AMPs), currently with over 2,800 entries in the antimicrobial peptide database at http://aps.unmc.edu/AP, are potent against resistant pathogens. They possess promising characteristics such as membrane disruption, rapid killing, immune modulation, and wound healing (Zasloff 2002; Wang 2010). The field is moving forward rapidly with approximately 100 new naturally occurring AMPs discovered annually from the six kingdoms (Wang et al. 2015; 2016). To date, over 100 AMPs have been documented from humans alone (Wang 2014). While multiple defensins have been identified, there is only one cathelicidin gene found in humans. LL-37, a 37-residue antimicrobial peptide starting with a pair of leucines, is one of the mature peptides released from the precursor of human cathelicidin. LL-37 is known to have broad-spectrum antimicrobial activity against bacteria, viruses, fungi, and parasites. In addition, it has wound healing, immune modulating, and anticancer effects (Durr, Sudheendra, Ramamoorthy 2006; Wang et al. 2014). Antimicrobial peptides are attractive as new coating agents to prevent microbial infection. The peptides can be surface coated non-covalently or covalently. While the non-covalent approach deals with surface adsorption of AMPs, the covalent immobilization method holds the molecule strongly onto the surface. Consequently, covalent immobilization is preferred because molecules coated in this manner are unlikely to leach into the surrounding environment (Santos et al. 2013). It is noticed that in covalent linking, the orientation of the peptide can be crucial. Site-specific immobilization of AMPs that keeps the peptide backbone intact is advantageous to random adsorption (Gabriel et al. 2006; Mishra et al. 2014). Indeed, some AMPs have been immobilized on polymeric and metal surfaces, including silicone, polyethylene terephthalate, silicon, titanium, and stainless steel, and were found to possess antibacterial and anti-biofilm characteristics (Costa et al. 2011). For instance, cecropin B (Xu et al. 2013) and hLf1-11, human lactoferrin-derived AMP (Godoy-Gallardo et al. 2014), are effective in reducing bacterial adhesion and biofilm formation of Bacillus subtilis, Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa, Streptococcus sanguinis and Lactobacillus salivarius. Tet213 (sequence KRWWKWWRRC) inhibits the growth of S. aureus and P. aeruginosa (Kazemzadeh-Narbat et al. 2013). Note that the treatment of polymicrobial biofilms could be more challenging due to the co-aggregation of microorganisms. Titanium is widely used in dental and orthopedic implants, because of its excellent mechanical strength, corrosion resistance, and biocompatibility (Bauer et al. 2013; Godoy-Gallardo et al. 2014). However, its use is limited due to ease of bacterial colonization, causing subsequent infection (Pye et al. 2009). In 2006, human cathelicidin LL-37 was successfully immobilized onto the titanium surface with retained antibacterial activity (Gabriel et al. 2006). However, human LL-37 is relatively long with 37 amino acids, which can be costly to synthesize chemically. Thus, there is a desire to shorten LL-37 by removing unwanted regions. Several fragments of LL-37 (e.g. P10, P60.4Ac, FK-16, IG-25, KR-12, KS-30, and KR-20) have since been evaluated with a goal of identifying better antimicrobial and anti-biofilm properties (Durr, Sudheendra, Ramamoorthy 2006; Feng et al. 2013; Wang et al. 2014b). Among them, FK-16 is the major antimicrobial fragment corresponding to residues 17–32 of human LL-37 (Li et al. 2006). GF-17, a glycine-appended FK-16 peptide (Wang et al. 2012), is more active than the full length LL-37 against both planktonic and biofilm formed S. aureus (Mishra et al. 2016). While we were preparing this manuscript on FK-16 for publication, Nie et al. (2016) and Song et al. (2016) reported the immobilization of KR-12, the minimal antimicrobial peptide of LL-37 we discovered (Wang, 2008). Different from FK-16, free KR-12 is only active against Gram-negative bacteria such as E. coli, but not Gram-positive S. aureus USA300. This study reports orientation-specific immobilization of FK-16, the most potent LL-37 peptide, on the titanium surface, which shows activity against a panel of the ESKAPE pathogens, including Enterococcus faecium, S. aureus, Klebsiella pneumoniae, Acinetobacter baumannii, P. aeruginosa and Enterobacter cloacae. The peptide coated surface also demonstrates anti-biofilm activity against methicillin-resistant S. aureus (MRSA) and E. coli. Significantly, we illustrate the initial inoculum-dependent biofilm inhibition property of the FK-16 peptide coated titanium surface for an extended time period.
Materials and Methods
Peptides, surface and chemicals
Peptides were chemically synthesized and purified to >95% when used in the free form (Genemed Synthesis, TX). Peptides were solubilized in autoclaved distilled water and their concentrations were determined by UV spectroscopy (Waddell 1956). Titanium foils (>99.6% pure) of 0.125 mm thickness were purchased from Goodfellow Corporation (PA, USA). All chemicals used in the immobilization were of analytical grade and purchased from Sigma (MO, USA) unless specified.
Bacteria and growth media
Bacterial strains used in this study included Enterococcus faecium ATCC51559, Staphylococcus aureus USA300 LAC, Klebsiella pneumoniae ATCC13883, Acinetobacter baumannii B2367-12, Pseudomonas aeruginosa PAO1, Enterobacter cloacae B2366-12 and Escherichia coli ATCC 25922, the first six constituting the ESKAPE pathogens. Tryptic soy broth (TSB) for bacterial growth was obtained from BD Bioscience (MD, USA).
Antimicrobial and hemolytic activities of free peptides
Antimicrobial assays
The antibacterial activity of peptides was evaluated using a standard broth microdilution protocol as described (Wang et al. 2012). In brief, 5 μL of each bacterium (stored at −80°C) was inoculated into TSB medium (2 mL) and grown overnight. A second inoculation was made in the morning by delivering 10 μL of the overnight culture to a fresh TSB medium. After 2–3 h growth at 37°C, 225 rpm, logarithmic phase bacterial cultures (i.e., OD600 ≈ 0.5) were diluted and partitioned into a 96-well polystyrene microplate with ~105 colony forming units (CFU) per well (90 μL aliquots). After treatment with 10 μL of peptide solutions at various concentrations, microplates were incubated at 37°C overnight and read on a ChroMate 4300 Microplate Reader at 630 nm (GMI, Ramsey, MN). The medium was used as a blank where no bacteria should grow. The minimal inhibitory concentration (MIC) was defined as the lowest peptide concentration that completely inhibited bacterial growth.
Measurement of peptide hemolysis
Blood was obtained from the Blood Bank of the University of Nebraska Medical Center (UNMC) and washed three times (800 g, 10 min) with normal saline to remove plasma. A final solution containing 2% human red blood cells (hRBC) was then prepared in normal saline and used for the assay. 90 μL of this solution was added to 10 μL of serially diluted peptide solutions and was incubated at 37°C for 1 h. It was then centrifuged at 15,700 g for 5 min on an Eppendorf bench-top centrifuge 5415D. Aliquots of the supernatant (~80 μL) was carefully transferred to a fresh 96 well microplate (Costar, Corning, NY) and absorbance was read at 545 nm to detect the amount of hemoglobin released. Percent lysis was calculated based on the extent of hemoglobin released, where 100% release is assumed in the presence of 1% Triton X-100 and 0% release is assumed in saline.
Peptide immobilization and characterization
Preparation of peptides
In order to couple the peptide to a titanium surface, a cysteine residue was appended to either the N or C-terminus of FK-16. These peptides were characterized by mass spectrometry and HPLC with purity at least 90% (Genemed Synthesis, TX).
Modification of the titanium surface
Prior to any chemical reactions, titanium (Ti) foils (1 cm × 1 cm) were first cleaned ultrasonically with ethanol, distilled water and acetone, each for 15 min at 50 Hz. Initial surface activation was accomplished by alkaline etching with 5 M NaOH for 24 h at 60°C (Godoy-Gallardo et al. 2014). Samples were then thoroughly cleaned by immersion in distilled water for 30 min twice, washed with acetone and dried. These hydroxylated surfaces were silanized with (3-Aminopropyl)triethoxysilane (APTES, 0.5% vol) in anhydrous toluene for 1 h at 70°C to obtain free amine groups. Excess and adsorbed reactants were removed by cleaning the foils ultrasonically with distilled water for 10 min. Surfaces were again profusely washed with ethanol, isopropanol, distilled water and acetone, and dried.
Orientation-specific immobilization of FK-16Cys to the titanium surface
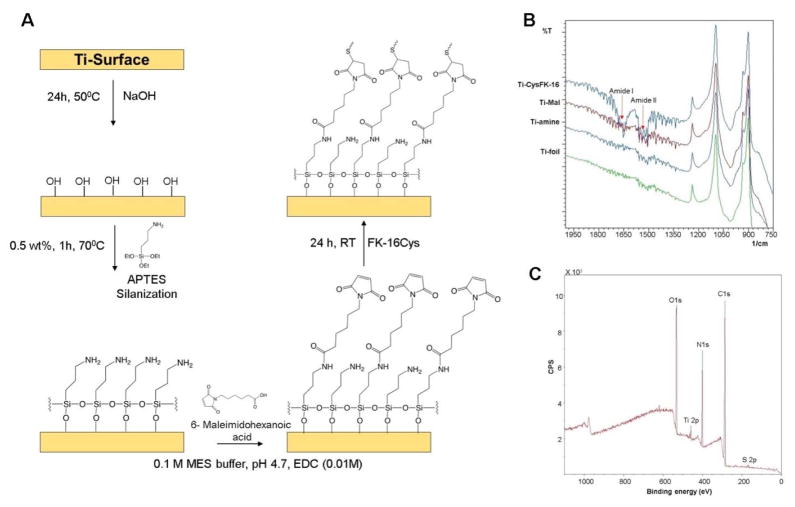
The amino silanized Ti surfaces were reacted with a short bifunctional cross linker 6-maleimidoheaxanoic acid. The reaction of the free amines and cross linker (2 mg/mL) were carried out in 0.1 M 2-(N-morpholino)ethanesulfonic acid (MES) buffer at pH 4.7 in the presence of 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC) (0.01% w/V) for 24 h at room temperature. The amide reaction generated free maleimide groups that enabled the coupling with the C-terminal cysteine of FK-16Cys, leading to a similar orientation on the Ti-surface. Reaction was conducted overnight at room temperature in phosphate buffer (PBS) at a peptide concentration of 2.5 mg/mL. Finally, the surfaces were washed with ethanol, isopropanol, distilled water and acetone to remove any unbound peptide. The FK-16 peptide coated titanium surfaces (Ti-FK-16Cys) were dried and kept at −20°C till other assays were performed. The detailed scheme of the reaction is illustrated in Figure 1A.
Figure 1.
(A) Scheme for peptide coating showing stepwise immobilization of FK-16Cys onto the titanium surface. (B) FT-IR analysis of different reaction steps toward the immobilization of FK-16. (C) XPS analysis showing the wide range spectra of different elements on a FK-16 Coated surface.
Physical and chemical analyses of the Ti-FK-16Cys coated titanium surface
Fourier-transformed infrared (FT-IR) spectroscopy
The presence of signature bands in the IR spectrum confirmed the success of the stepwise coupling leading to the final product. The spectrum was recorded 20 scans from 600 cm−1 to 4000 cm−1 with an IR Prestige-21 instrument (Shimadzu) using Happ-Genzel apodization function at Creighton University (Omaha, USA).
X-ray photoelectron spectroscopy (XPS)
Samples were analyzed using a Surface Science Instrument SSX-100 with an operating pressure of ~2×10−9 torr at Cornell University (New York, USA). Monochromatic Al K-alpha X rays (1486.6 eV) were used with a beam diameter of 1 mm. Photoelectrons were collected at a 55 degree emission angle. A hemispherical analyzer determined electron kinetic energy, using a pass energy of 150 V for wide/survey scans, and 50 V for high resolution scans. A flood gun was used for charge neutralization of non-conductive samples. Elements were identified from the survey spectra. High resolution spectra of carbon, nitrogen, oxygen and sulfur were recorded individually for comparison of the element ratio.
Quantification of surface attached FK-16Cys using sulfosuccinimidyl-4-o-(4,4-dimethoxytrityl) butyrate (Sulfo-SDTB)
The amount of the covalently attached FK-16Cys on the Ti surface was measured using a spectrophotometric assay. This method utilizes the high extinction coefficient value of a complex ion formed by the reaction of the surface amino groups and Sulfo-SDTB. The immobilized peptide concentration was determined using an established protocol (Gaur and Gupta 1989). In brief, 1 mL of the Sulfo-SDTB (3.0 mg/mL) solution was prepared in dimethylformamide (DMF) and then diluted to 50.0 mL with a 50 mM sodium bicarbonate solution (pH 8.5). 1.0 mL of this solution was added to the peptide coated Ti samples followed by 1 h incubation at room temperature. The samples were then washed twice with 5.0 mL of distilled water to remove any unused reactant, and immersed in 2.0 mL of perchloric acid for another 30 min. Absorbance of the solution was measured at 498 nm to detect the 4,4′-dimethyloxytrityp (DMTr) cation. The number of amine groups on the surface of each sample was quantified using the Beer–Lambert law with an extinction coefficient of 70,000 M−1 cm−1 and finally the amount of immobilized FK-16Cys was calculated based on the number of amines present in the peptide.
Microbiological assays of immobilized FK-16Cys
Antibacterial activity of the Ti-FK-16Cys surfaces was evaluated according to the previously published International Organization for Standardization (ISO) 22196 protocol with minor modifications (Kowalczuk, Ginalska, Golus 2010). Briefly, a mid-exponential phase bacterial culture was washed with sterile PBS and adjusted to 105 CFU/mL. 20 μL of the cell suspension was added on the top of each Ti surface placed in each well of a 24-well culture plate. Further, the plates were incubated at 37°C for 2 h. After incubation, 980 μL of fresh PBS was added and mixed to allow all the adhered cells to come into the PBS solution. 100 μL of the solution was then spread on LB agar plates for CFU determination after 18 h incubation at 37°C. The Ti surface coated up to maleimide but without the peptide was used as a control.
In addition, the effects of serum and salt on the antimicrobial activity of the peptide immobilized surfaces were also analyzed. The samples, containing 10% of human serum (Human Serum, Pooled, MP Biomedicals, CA, USA) or 150 mM NaCl, were tested using S. aureus USA300. The samples were processed in the same manner as detailed above.
Inhibition of bacterial adhesion onto the FK-16Cys coated Ti surface
A quantitative estimation of bacterial adhesion inhibition by the FK-16Cys coated Ti surface was made using the 2,3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide (XTT) assay (Mishra et al. 2016). In short, S. aureus USA300 was grown in TSB overnight. 1 mL of the culture was then added to the wells of 24 wells culture plate with surfaces coated with the linker and the FK-16 peptide, or the linker but no peptide. The plates were then incubated at 37°C for 1 h to allow for bacterial attachment. After removal of media, surfaces were washed three times with fresh PBS to remove unattached cells. Finally, fresh TSB media containing 10% XTT solution with 1% PMS (N-methylphenazonium methyl sulfate) were further incubated for 2 h at 37°C. Plates were read at 450 nm for calorimetric estimation of the bacterial attachment. Comparison was made between the surfaces with and without the coated peptide to measure the anti-adhesion activity. The anti-adhesion activity was also assayed for E. coli in the same manner.
Inhibition of biofilm formation by FK-16Cys coated titanium
Inhibition of the biofilm formation by the Ti-FK-16Cys surface was also evaluated using S. aureus and E. coli. In short, bacteria at the exponential phase were adjusted to 105 or 103 CFU/mL in a fresh TSB medium. One mL of the culture was then added to the wells of 24-well culture plate containing the surface that was coated up to maleimide or peptide. The plates were then incubated at 37°C for 24 h to allow for biofilm formation. Media were then removed and surfaces were washed with fresh PBS three times. Quantification of live bacteria in the biofilm was performed using the XTT assay as described above, except for the use of 20% XTT.
Cytotoxicity assays of Ti-FK-16Cys to human keratinocytes
In vitro spontaneously transformed keratinocytes from histologically normal human skin (HaCaT cells) from Fisher (Cat # T0020001) were maintained in DMEM High Glucose media with 4 mM L-Glutamine (NyClone) and 100 U/mL penicillin, 100 μg/mL streptomycin (pen/strep, p/s) (Life Technologies), and 10% (v/v) inactivated fetal bovine serum (FBS, NyClone), DMEM with 10% FBS, p/s. Cells were grown in 5% CO2 at 37°C and were detached from culturing dish at 100% confluency by treating with 0.025% trypsin-EDTA (NyClone), seeded (100,000 cells/well) in 24-well plates (Corning Life Science) and grown overnight in 200 μL DMEM media to reach a 70% confluency. The peptide-coated surfaces (1.0 × 1.0 cm) were placed in a 24-well plate DMEM with or without 10% FBS, p/s for 1 hour. Samples were taken out and 60 μL of CellTiter96® MTS was added. Plates were further incubated for another 2 h at 37°C. Subsequently, 100 μL of the solution was placed in a clean 96-well plate to measure the absorbance at 492 nm on a ChroMate reader (GMI, Ramsey, MN). For comparison, we also measured the toxicity of the soluble peptides and treated them in the same way as described above. The culture medium and a 0.2% SDS solution were used as negative and positive controls, respectively.
Hemolysis of the FK-16Cys coated Ti surface
Blood cells were prepared as described above for soluble peptides. 450 μL of this solution was added to the peptide coated surfaces (1.0 × 1.0 cm) placed in a 24-well plate. The rest 50 μL contained PBS, Triton-X100, or soluble peptides. The plate was incubated for 1 h at 37°C. Then 100 μL of the cell suspension was centrifuged at 15,700 g for 5 min on an Eppendorf bench-top centrifuge 5415D. Supernatants were transferred to a clean 96-well plate (Costar, Corning, NY) and absorbance was read at 545 nm to detect the amount of hemoglobin released. 1% Triton X-100 was used as a positive control (100% lysis), while PBS served as a negative control (0% lysis).
Results
Impact of terminal cysteine appendage on antimicrobial and cytotoxic activity of free FK-16
To preserve peptide orientation on the surface, a specific maleimide-thiol based coupling method was selected. In this method, cysteine from the peptide reacts with maleimide from the linker (Hermanson 2013). This requires the addition of a cysteine amino acid to FK-16. The FK-16 variant with a cysteine added to the N-terminus is named as CysFK-16, while the variant with a cysteine at the C-terminus is referred to as FK-16Cys. The impact of the addition of the cysteine on peptide activity was evaluated. Antimicrobial activities of FK-16 and its two variants are summarized in Table 1. Similar to the engineered peptide merecidin (Wang et al. 2014a), FK-16 was found to be active against almost all the tested ESKAPE pathogens with a minimal inhibitory concentration (MIC) in the range of 3.1–12.5 μM, although it was less active against P. aeruginosa PAO1 (MIC = 50 μM). CysFK-16 lost activity against K. pneumonia, P. aeruginosa, E. cloacae and E. coli (MIC > 50 μM); it only retained activity against E. faecium at 6.25 μM, S. aureus at 18 μM, and A. baumannii at 25 μM, respectively. Interestingly, the FK-16Cys peptide showed broader activity (Table 1). It is active against most of the strains in the range of 12.5–50 μM. Therefore, the addition of a cysteine at the C-terminus of FK-16 retained more peptide activity. It is likely that the cationic N-terminus of FK-16, including K18 and R19 (as numbered in LL-37), can rapidly recognize anionic pathogens via electrostatic interactions followed by the anchoring into bacterial membranes through the hydrophobic surface containing the aromatic ring of F17 (Wang 2008; Wang et al. 2012). Thus, FK-16Cys was chosen as a candidate for surface immobilization.
Table 1.
Antibacterial activities of FK-16 and cysteine-attached variants against the ESKAPE pathogens.1
| Peptide | MIC (μM) | ||||||
|---|---|---|---|---|---|---|---|
| EF | SA | KP | AB | PA | ECl | EC | |
| FK-16 | 3.1 ± 0 | 4.67 ± 1.8 | 4.67 ± 1.5 | 4.67 ± 2.2 | 50 ± 0 | 12.5 ± 0 | 4.67 ± 2.2 |
| CysFK-16 | 6.25 ± 0 | 18 ± 7.2 | ≥50 | 25 ± 0 | > 50 | > 50 | >50 |
| FK-16Cys | 12.5 ± 0 | 18 ± 7.2 | 12.5 ± 0 | 12.5 ± 0 | > 50 | 50 ± 0 | 37.5 ± 17.6 |
| Merecidin | ≤3.1 | 3.1 ± 0 | ≤3.1 | 3.1 ± 0 | 6.25 ± 0 | 6.25 ± 0 | 3.1 ± 0 |
Abbreviations used: EF, E. faecium ATCC51559; SA, S. aureus USA300 LAC; KP, K. pneumonia ATCC 13883; AB, A. baumannii B2367-12; PA, P. aeruginosa PAO1; ECl, E. cloacae B2366-12 and EC, E. coli ATCC 25922. Merecidin, known to have activity against the ESKAPE pathogens, is used as a positive control. This peptide is engineered to achieve protease stability based on the major antimicrobial region of human cathelicidin LL-37 (Wang et al. 2014a).
We also evaluated cytotoxicity of FK-16Cys before immobilization. Free FK-16Cys showed a 50% lethal concentration (LC50) to HaCaT cells over 100 μM and 50% hemolytic concentration (HL50) of ~35 μM. We previously found a HL50 of ~100 μM for GF-17, which contains one additional glycine at the N-terminus compared to FK-16 (Mishra et al. 2016). Therefore, the addition of a cysteine at the C-terminus of FK-16 made the peptide more hemolytic. However, this toxicity could be masked after surface coating of this peptide (see below).
Orientation-specific immobilization of FK-16Cys
Next, we generated hydroxyls on the titanium surface by alkaline treatment (5 M NaOH) overnight at 50°C. The resultant hydroxyl groups were covalently silanized with (3-aminopropyl)triethoxysilane (APTES) to generate surface amines. Subsequently, a bifunctional short spacer, 6-Maleimidohexanoic acid, was coupled to the APTES amine via a 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC) coupling reaction, leading to a free maleimide. The free maleimide group can react very specifically with thiol (peptide-cysteine) in the pH range of 6.5–7.5 to form a stable thioether linkage (Hermanson 2013). The scheme of the series of reactions for peptide coating is depicted in Figure 1A.
Surface characterization of Ti-FK-16Cys surfaces
The stepwise proceeding of the reaction was monitored using FT-IR spectroscopy (Figure 1B). The signature stretching vibration band of amide I at 1650 cm−1 and amide II at 1560 cm−1 confirmed the immobilization of the peptide onto the Ti surface. Although the same bands also appeared on the Ti-Mal coated surface because of the amide reaction between the surface amine and carboxyl group of the spacer, sharp increases in their intensities yielded clear evidence for peptide grafting. We also employed XPS to verify the peptide immobilization. The wide range spectra for carbon 1s, nitrogen 1s, and oxygen 1s at corresponding binding energy confirmed the presence of respective elements as anticipated during the proceeding of the reaction. However, the signal of the sulfur 2p peak at 164 eV provided strong evidence for successful immobilization of FK-16Cys because it only existed in the peptide (Figure 1C). This is also evident in the zoomed region in Figure S1. In addition, the increments of the nitrogen and carbon elemental composition, and the decrease in the oxygen content from 48.04% to 17.53% of samples coated with maleimide and peptide, also support immobilization (Supplementary Table S1).
Immobilized peptide quantification
Having established the chemical platform, we also quantitated the amount of FK-16Cys on the surface using a spectroscopic method (Gaur and Gupta 1989). The free amines of the peptide were titrated with sulfo-SDTB. The resultant product by acid lysis led to the chromophore, 4,4′-dimethoxytrityl cation (DMTr), which has a high extinction coefficient of 70,000 M−1 cm−1 at 498 nm. Beer’s Lambert law was employed to calculate the amount of cation released, which was correlated with the amount of free amines of the immobilized peptide, which include two lysines and the N-terminus (Gabriel et al. 2006). Based on this, we obtained a surface peptide density at 6 × 10−10 mol/cm2. This FK-16Cys coated peptide density is 4-fold the amount of LL-37 (1.47 × 10−10mol/cm2) coated to the same titanium surface (Gabriel et al. 2006).
Antibacterial activity of immobilized FK-16Cys against the ESKAPE pathogens
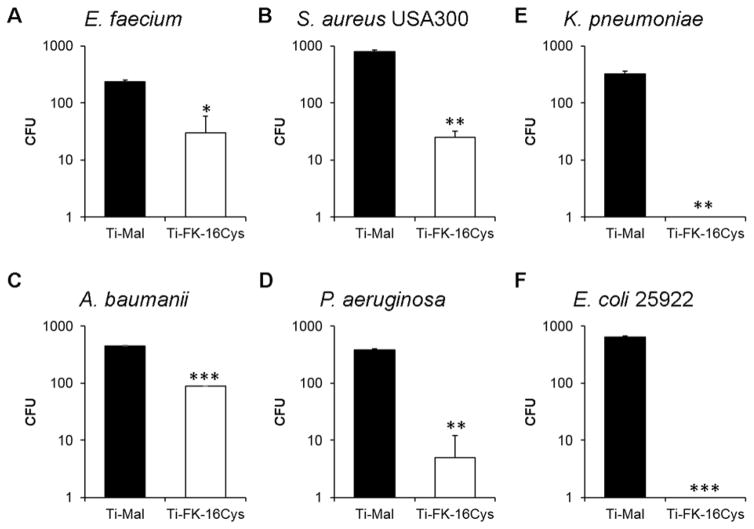
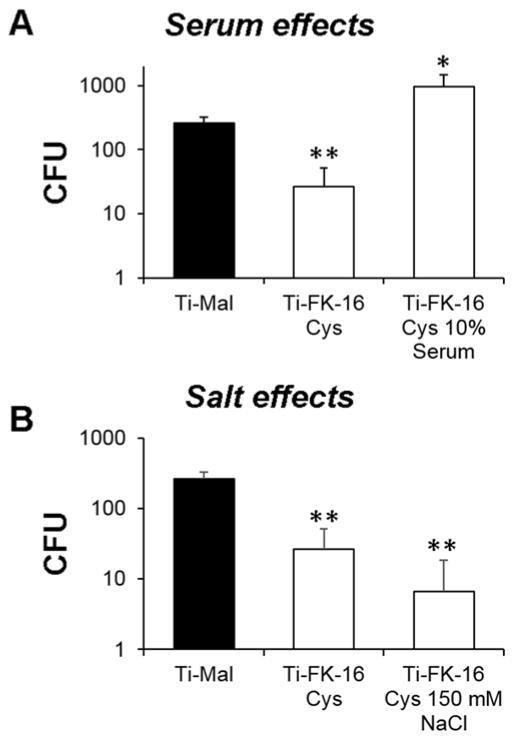
We evaluated antimicrobial activity of the FK16-immobilized surface against the ESKAPE pathogens, which account for over 90% of the nosocomial infections. Using the ISO 22196 protocol, a method for evaluation of the antibacterial activity on non-porous surfaces (Kowalczuk, Ginalska, Golus 2010), we found the surface very active (Figure 2). It reduced the growth of E. faceium by ~ 1 log (~80% in Figure 2A) and S. aureus by ~1.5 log (over ~95% in Figure 2B). Further, it completely inhibited the growth of K. pneumoniae and E. coli (Figure 2, C and F), although it only reduced the CFU of A. baumannii by 0.5 log (Figure 2D). A 2 log CFU reduction was observed for P. aeruginosa (~98% inhibition in Figure 2E). Only E. cloacae was not inhibited by the FK16-coated surface. Thus, the new surface immobilized with FK-16 possesses broad antimicrobial activity against planktonic bacterial cells, which are in concordance with the activity of free FK-16 (Table 1). In addition, we also determined the activity of the peptide coated surfaces in the presence of 10% human serum (Figure 3A) and 150 mM NaCl (Figure 3B) against S. aureus USA300. The FK-16 immobilized surface was ineffective in the presence of serum, but remained active in the presence of the salt.
Figure 2.
Antimicrobial activity of the Ti-FK-16Cys surfaces against different ESKAPE pathogens (2×103 CFU): (A) E. faecium, (B) S. aureus USA300, (C) K. pneumoniae, (D) A. baumannii, (E) P. aeruginosa, and (F) E. coli ATCC 25922. Killing efficiency is expressed as the CFU changes of live bacteria compared to the surface coated up to maleimide but no peptide coupling (Ti-Mal). Experiments were done in duplicates and average results were reported. For confirmation, the entire experiment was also repeated on a different date. The error bars represent the standard deviation of the values from the mean. The level of significance was determined by performing Student t-Test with parameters of one tailed distribution with samples of equal variance (*: p < 0.05; **: p < 0.005 and ***: p < 0.0005).
Figure 3.
Antimicrobial activity of the Ti-FK-16Cys surfaces against S. aureus USA300 (2×103 CFU) in the presence of 10% human serum (A) or 150 mM NaCl (B). Killing efficiency is expressed as the CFU changes of live bacteria compared to the surface coated up to maleimide without the peptide (Ti-Mal). Experiments were performed, data processed, and statistically analyzed as described in the legend of Figure 2.
Anti-biofilm activity of FK-16Cys coated Ti surface
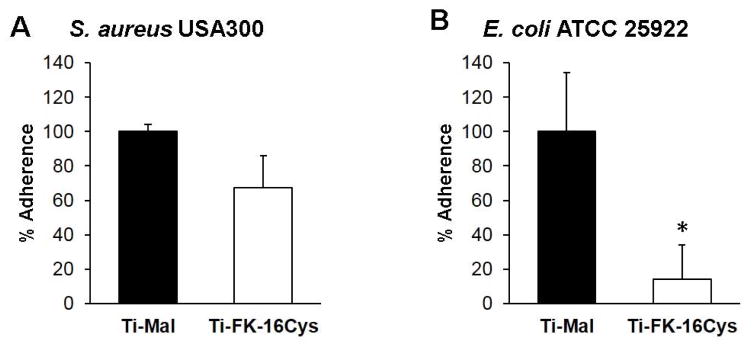
Because it is difficult to remove biofilms from implanted medical devices, we also tested the anti-biofilm property of the FK-16Cys coated titanium surface using both Gram-positive S. aureus USA300 and Gram-negative E. coli ATCC 25922. Bacterial surface attachment is regarded as the first stage of biofilm formation. To test this peptide property, we investigated the anti-attachment potential of this surface against a high density overnight bacterial culture (~5.0 × 109 CFU/mL for both bacteria). It reduced the attachment of S. aureus by ~30% (Figure 4A). However, the new surface was even more active in the case of E. coli; a ~85% reduction was observed (Figure 4B). Following bacterial attachment to the substratum, biofilms could further develop into a complicated structure covered with extra cellular materials such as carbohydrates, eDNA, and proteins.
Figure 4.
Anti-adherent property of the FK-16Cys immobilized titanium surfaces. An overnight bacterial culture of S. aureus USA300 (A) and E. coli ATCC 25922 (B) was allowed to attach to the titanium surface with (open bar) and without (filled bar) the peptide. Experiments were performed, data processed, and statistically analyzed as described in the legend of Figure 2.
We also tested the biofilm inhibition capability of our peptide coated surfaces. At an initial bacterial inoculum of 105 CFU, they reduced the biofilms of S. aureus and E. coli by ~75% and ~80%, respectively (Figure 5, A and B). We also asked how long the FK-16Cys coated surface could retain anti-biofilm property. Unfortunately, the surface showed no biofilm inhibitory activity at 105 CFU when the biofilm forming time was extended to 48 or 72 h (Figure 5, C–F). However, this FK-16 coated titanium surface remained effective at 48 or 72 h when a reduced initial inoculum of 103 CFU was used (Figure 5, G–L).
Figure 5.
Inhibition of bacterial biofilm formation on the FK-16Cys coated surfaces at bacterial CFU 105 (panels A–F, left) or 103 (panels G–L, right) against S. aureus USA300 and E. coli ATCC 25922, respectively. In each experiment, comparison was made between the FK-16Cys peptide coated Ti surfaces (Ti-FK16Cys) and the maleimide coated surfaces devoid of the FK-16 peptide (Ti-Mal). Experiments were performed, data processed, and statistically analyzed as described in the legend of Figure 2.
Biocompatibility of Ti-FK-16Cys against human keratinocytes and red blood cells
An important prerequisite of implanted medical devices is biocompatibility. We also tested cytotoxicity of the FK-16Cys coated titanium surface using human epidermal keratinocytes (HaCaT cells) and human erythrocytes hRBCs. While no significant reduction in HaCaT cell growth was observed with or without FBS (Figure 6A), the surface was devoid of hemoglobin release after incubation with hRBCs (Figure 6B). These results indicate that the cell toxicity of the FK-16Cys immobilized surface was little to none.
Figure 6.
Cytotoxicity evaluation of the FK-16Cys coated surface to human epidermal keratinocytes HaCaT cells in the absence and presence of FBS (A) and human red blood cells (hRBCs) (B). In each experiment, comparison was made between the peptide coated Ti surface (Ti-FK-16Cys) and the maleimide coated surface devoid of the FK-16 peptide (Ti-Mal). Additional controls (filled columns) were used for the case of HaCaT cells without any treatment (100% live in panel A) and for hRBCs treated with 1% Triton X-100 (100% lysis in panel B). Experiments were performed, data processed, and statistically analyzed as described in the legend of Figure 2.
Discussion
Biofilms usually consist of complex bacterial communities covered with biopolymers such as polysaccharides and eDNA. Such a structure is resistant to host defense or antibiotic treatment. Therefore, a preferred strategy would be to prevent the formation of biofilms on implanted medical devices. Although traditional antibiotics have been coated to surfaces, they may not provide sufficient protection as a consequence of the growing antibiotic resistance problem. Antimicrobial peptides are regarded as useful candidates for surface coatings (Costa et al. 2011; Onaizi and Leong 2011; Mishra et al., 2017). Several laboratories have tested surface coating of human cathelicidin LL-37. Gabriel et al. (2006) found that the PEG linker between the peptide and surface, as well as orientational coupling via the maleimide chemistry, is essential for E. coli killing by the peptide-immobilized titanium surface. Likewise, Han et al. found that both peptide structure and activity could be influenced by the length of the linker as well as the orientation of the peptide coated to the surface (Han et al., 2014). Furthermore, the LL-37 molecule, after being immobilized onto the poly-hydroxyethyl methacrylate (pHEMA) surface, is active against P. aeruginosa, but not S. aureus (Dutta, Kumar, Willcox 2016). However, LL-37 (37 amino acids) is relatively long and costly to manufacture. Consequently, there is a great desire to use its active fragments. IG-25 is a C-terminal fragment of LL-37 corresponding to residues 13–37, which possesses both antibacterial and anticancer activities (Li et al. 2006). This 25mer peptide, when attached to a fluorous surface via the specific click chemistry (Santos et al. 2013), kills P. aeruginosa by permeating bacterial membranes. Further, the lens modified with IG-25 reduces the colonization of P. aeruginosa PAO1 by 98%. Some non-peptide agents also have this property. For instances, Xavier et al. (2016) found that lactam can inhibit Streptococcus mutans growth on titanium. In addition, KR-12, the smallest antibacterial peptide (i.e., residues 18–29) of human LL-37, retains activity against E. coli K12, but not S. aureus USA300 (Wang 2008). KR-12 was connected to the surface via a reaction between amine of the silanization agent and the carboxylic acid of the peptide for 8 h at room temperature. The KR-12 coated surface reduces the adherence of Staphylococcus epidermidis (Nie et al. 2016). It is evident that only one to two bacterial strains were tested for each of the above LL-37 peptide coated surfaces. Therefore, our study represents the first case where the surfaces immobilized with FK-16 are potent against a wide range of bacteria, including nearly all the ESKAPE pathogens (Figure 2). Note that the presence of serum makes the surface ineffective, probably due to the serum binding property of the LL-37 cathelicidin peptides documented in the literature (Durr, Sudheendra, Ramamoorthy 2006). However, 150 mM NaCl did not reduce the antimicrobial activity of the surface (Figure 3B) against S. aureus USA300.
While both the IG-25 and KR-12 surfaces were subjected to anti-adhesion tests, no biofilm inhibition was tested using the LL-37 peptide coated surfaces prior to the current study. Therefore, our demonstration of the biofilm inhibition ability of the FK-16 coated titanium surface fills our knowledge gap in this aspect (Figures 4 and 5). Significantly, we also illustrate the potential of this new surface in inhibiting biofilm formation at an extended time period of 48 h and 72 h when an initial 103 CFU of bacteria is inoculated. Note that it is a common practice to minimize the potential pathogen contamination when medical devices are implanted via surgery. Such a practice will lead to low initial bacterial CFU if there is any. Therefore, our use of a low starting CFU at 103 in our assays may better mimic the starting conditions after surgery. Under such a “clean” condition, we anticipate the coated surfaces will work well. In other words, a high starting CFU such as 105 or more may not be realistic unless there is already infection, which needs pre-treatment before inserting any medical device. Interestingly, the same 103 inoculum is reported to be critical to evaluate the immune response to various S. aureus strains in a mouse orthopedic-implant biofilm infection model. This is because all the differences disappeared at a higher CFU (Vidlak and Kielian 2016). Thus, our use of 103 CFU could be medically relevant.
There are reports that commensal bacteria can prevent colonization of invading pathogens (Hwang et al. 2016). While maintaining an elegant balance of commensal microbiota can be a helpful strategy to fight antibiotic resistance, it may not be a general practice for implanted medical devices. Depending on the location of the device in human bodies, however, the colonization of commensal bacteria may not be desired, especially those opportunistic pathogens. The broad antimicrobial activity of the FK-16 coated surface may not allow the colonization of such bacteria. On the other side of the coin, rapid attachment of human cells to implanted devices could be preferred so that the device becomes an integral part of the body. Whether the FK-16 coated surface is able to do so remains tested. However, the KR-12 coated titanium surface can improve the attachment and proliferation of human bone marrow mesenchymal stem cells (Nie et al. 2016). It is also important that the coated surfaces have negligible cytotoxicity to the surrounding tissues. Under the conditions tested, the FK-16 coated surface showed no hemolysis and little toxicity to human skin cells. Therefore, our results underscore biocompatibility of the FK-16 coated anti-biofilm surfaces.
It is clear that the FK-16 coated titanium surface retained antimicrobial and antibiofilm effects of the major antimicrobial region of LL-37. It also retained the serum binding property known to the parent peptide LL-37 (Durr, Sudheendra, Ramamoorthy 2006). These results indicate that the chemistry used here is proper. While the antimicrobial and antibiofilm capabilities are desired here, serum binding of FK-16 is a disadvantage because it can prevent the peptide from bacterial killing. However, our finding is helpful in developing potential uses of human cathelicidin peptide coated surfaces reported herein and perhaps those in the literature. First, the current FK-16 coated surface is best suited for serum-free cases (e.g., medical tools and surfaces for operation). Second, it can also be applied to situations where serum is limited and, when generated, is only limited to the initial stage. Under such circumstances, the areas may be rinsed with saline to remove serum to mitigate the problem. Third, when serum cannot be avoided, this template can be re-designed to minimize its binding to serum in future studies. There are also alternative strategies. Recently, we have demonstrated that injection of merecidin, a peptide designed based also on the major antimicrobial region of human LL-37, can prevent staphylococcal biofilm formation in an animal catheter model and boost immune responses (Wang et al. 2014a). Taken together, the major antimicrobial region of human cathelicidin LL-37 can be developed into different medical treatment options to control bacterial biofilms involving the ESKAPE pathogens.
Conclusions
In summary, we have developed a titanium-based antimicrobial and anti-biofilm platform where FK-16, a peptide corresponding to the major antimicrobial region of the human cathelicidin LL-37, is directionally coupled to the surface via a short six-carbon linker. Except for E. cloacae, the FK-16 coated titanium surfaces are able to inhibit not only superbugs such as the ESKAPE pathogens but also bacterial surface adhesion of both S. aureus USA300 and E. coli ATCC 25922. Importantly, we also demonstrate the inhibition of biofilm formation by the FK-16 immobilized surface for an extended time frame from 24 to 72 h. Also, these antimicrobial effects could be achieved at a concentration not toxic to human cells. Thus, the titanium surfaces covalently immobilized with the major antimicrobial peptide of human LL-37 may be harnessed to prevent biofilm-related infection on implanted medical devices.
Supplementary Material
Acknowledgments
We thank Tamara Lushnikova for cultivating HaCaT cells and Dr. Alekha K. Dash (Creighton University, Omaha) for allowing us to access to the FT-IR instrument. The XPS analysis was conducted on a fee basis by the Cornell Center for Materials Research Shared Facilities supported through the NSF MRSEC program (DMR-1120296).
Funding
This study was supported by the NIAID/NIH grant R01AI105147 to GW.
Footnotes
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Bauer J, Siala W, Tulkens PM, Van Bambeke F. A combined pharmacodynamic quantitative and qualitative model reveals the potent activity of daptomycin and delafloxacin against staphylococcus aureus biofilms. Antimicrob Agents Chemother. 2013;57(6):2726–37. doi: 10.1128/AAC.00181-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa F, Carvalho IF, Montelaro RC, Gomes P, Martins MC. Covalent immobilization of antimicrobial peptides (AMPs) onto biomaterial surfaces. Acta Biomater. 2011;7(4):1431–40. doi: 10.1016/j.actbio.2010.11.005. [DOI] [PubMed] [Google Scholar]
- Darouiche RO. Treatment of infections associated with surgical implants. N Engl J Med. 2004;350(14):1422–9. doi: 10.1056/NEJMra035415. [DOI] [PubMed] [Google Scholar]
- Durr UH, Sudheendra US, Ramamoorthy A. LL-37, the only human member of the cathelicidin family of antimicrobial peptides. Biochim Biophys Acta. 2006;1758(9):1408–25. doi: 10.1016/j.bbamem.2006.03.030. [DOI] [PubMed] [Google Scholar]
- Dutta D, Kumar N, Willcox MDP. Antimicrobial activity of four cationic peptides immobilised to poly-hydroxyethylmethacrylate. Biofouling. 2016;32(4):429–38. doi: 10.1080/08927014.2015.1129533. [DOI] [PubMed] [Google Scholar]
- Epand RF, Wang G, Berno B, Epand RM. Lipid segregation explains selective toxicity of a series of fragments derived from the human cathelicidin LL-37. Antimicrob Agents Chemother. 2009;53(9):3705–3714. doi: 10.1128/AAC.00321-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng X, Sambanthamoorthy K, Palys T, Paranavitana C. The human antimicrobial peptide LL-37 and its fragments possess both antimicrobial and antibiofilm activities against multidrug-resistant acinetobacter baumannii. Peptides. 2013;49:131–7. doi: 10.1016/j.peptides.2013.09.007. [DOI] [PubMed] [Google Scholar]
- Gabriel M, Nazmi K, Veerman EC, Nieuw Amerongen AV, Zentner A. Preparation of LL-37-grafted titanium surfaces with bactericidal activity. Bioconjug Chem. 2006;17(2):548–50. doi: 10.1021/bc050091v. [DOI] [PubMed] [Google Scholar]
- Gallo J, Holinka M, Moucha CS. Antibacterial surface treatment for orthopaedic implants. Int J Mol Sci. 2014;15(8):13849–80. doi: 10.3390/ijms150813849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaur RK, Gupta KC. A spectrophotometric method for the estimation of amino groups on polymer supports. Anal Biochem. 1989;180(2):253–8. doi: 10.1016/0003-2697(89)90426-0. [DOI] [PubMed] [Google Scholar]
- Godoy-Gallardo M, Mas-Moruno C, Fernandez-Calderon MC, Perez-Giraldo C, Manero JM, Albericio F, Gil FJ, Rodriguez D. Covalent immobilization of hLf1-11 peptide on a titanium surface reduces bacterial adhesion and biofilm formation. Acta Biomater. 2014;10(8):3522–34. doi: 10.1016/j.actbio.2014.03.026. [DOI] [PubMed] [Google Scholar]
- Han X, Liu Y, Wu FG, Jansensky J, Kim T, Wang Z, Brooks CL, 3rd, Wu J, Xi C, Mello CM, Chen Z. Different interfacial behaviors of peptides chemically immobilized on surfaces with different linker lengths and via different termini. J Phys Chem B. 2014;118(11):2904–12. doi: 10.1021/jp4122003. [DOI] [PubMed] [Google Scholar]
- Hermanson GT. Chapter 8 - dendrimers and dendrons. In: Hermanson GT, editor. Bioconjugate techniques. 3. Boston: Academic Press; 2013. p. 351. [Google Scholar]
- Hwang IY, Koh E, Kim HR, Yew WS, Chang MW. Reprogrammable microbial cell-based therapeutics against antibiotic-resistant bacteria. Drug Resist Updat. 2016;27:59–71. doi: 10.1016/j.drup.2016.06.002. [DOI] [PubMed] [Google Scholar]
- Kazemzadeh-Narbat M, Lai BF, Ding C, Kizhakkedathu JN, Hancock RE, Wang R. Multilayered coating on titanium for controlled release of antimicrobial peptides for the prevention of implant-associated infections. Biomaterials. 2013;34(24):5969–77. doi: 10.1016/j.biomaterials.2013.04.036. [DOI] [PubMed] [Google Scholar]
- Knetsch MLW, Koole LH. New strategies in the development of antimicrobial coatings: The example of increasing usage of silver and silver nanoparticles. Polymers. 2011;3(1) [Google Scholar]
- Kowalczuk D, Ginalska G, Golus J. Characterization of the developed antimicrobial urological catheters. Int J Pharm. 2010;402(1–2):175–83. doi: 10.1016/j.ijpharm.2010.10.014. [DOI] [PubMed] [Google Scholar]
- Li X, Li Y, Han H, Miller DW, Wang G. Solution structures of human LL-37 fragments and NMR-based identification of a minimal membrane-targeting antimicrobial and anticancer region. J Am Chem Soc. 2006;128(17):5776–85. doi: 10.1021/ja0584875. [DOI] [PubMed] [Google Scholar]
- Mishra B, Basu A, Chua RRY, Saravanan R, Tambyah PA, Ho B, Chang MW, Leong SSJ. Site specific immobilization of a potent antimicrobial peptide onto silicone catheters: Evaluation against urinary tract infection pathogens. Journal of Materials Chemistry B. 2014;2(12):1706–16. doi: 10.1039/c3tb21300e. [DOI] [PubMed] [Google Scholar]
- Mishra B, Basu A, Saravanan R, Xiang L, Yang LK, Leong SSJ. Lasioglossin-III: Antimicrobial characterization and feasibility study for immobilization applications. RSC Adv. 2013;3(24):9534–43. [Google Scholar]
- Mishra B, Golla RM, Lau K, Lushnikova T, Wang G. Anti-staphylococcal biofilm effects of human cathelicidin peptides. ACS Med Chem Lett. 2016;7(1):117–21. doi: 10.1021/acsmedchemlett.5b00433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra B, Lushnikova T, Golla RM, Wang X, Wang G. Design and surface immobilization of short anti-biofilm peptides. Acta Biomater. 2017;49:316–328. doi: 10.1016/j.actbio.2016.11.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie B, Ao H, Chen C, Xie K, Zhou J, Long T, Tang T, Yue B. Covalent immobilization of KR-12 peptide onto a titanium surface for decreasing infection and promoting osteogenic differentiation. RSC Adv. 2016;6(52):46733–43. [Google Scholar]
- Onaizi SA, Leong SS. Tethering antimicrobial peptides: Current status and potential challenges. Biotechnol Adv. 2011;29(1):67–74. doi: 10.1016/j.biotechadv.2010.08.012. [DOI] [PubMed] [Google Scholar]
- Pye AD, Lockhart DE, Dawson MP, Murray CA, Smith AJ. A review of dental implants and infection. J Hosp Infect. 2009;72(2):104–10. doi: 10.1016/j.jhin.2009.02.010. [DOI] [PubMed] [Google Scholar]
- Santos CM, Kumar A, Kolar SS, Contreras-Caceres R, McDermott A, Cai C. Immobilization of antimicrobial peptide IG-25 onto fluoropolymers via fluorous interactions and click chemistry. ACS Appl Mater Interfaces. 2013;5(24):12789–93. doi: 10.1021/am404591n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidlak D, Kielian T. Infectious dose dictates the host response during Staphylococcus aureus orthopedic-implant biofilm infection. Infect Immun. 2016;84(7):1957–65. doi: 10.1128/IAI.00117-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddell WJ. A simple ultraviolet spectrophotometric method for the determination of protein. J Lab Clin Med. 1956;48:311–314. [PubMed] [Google Scholar]
- Wang G. Structures of human host defense cathelicidin LL-37 and its smallest antimicrobial peptide KR-12 in lipid micelles. J Biol Chem. 2008;283(47):32637–43. doi: 10.1074/jbc.M805533200. [DOI] [PubMed] [Google Scholar]
- Wang G. Antimicrobial peptides: Discovery, design and novel therapeutic strategies. Oxfordshire, UK: CABI; 2010. [Google Scholar]
- Wang G. Human antimicrobial peptides and proteins. Pharmaceuticals. 2014;7(5):545–94. doi: 10.3390/ph7050545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Epand RF, Mishra B, Lushnikova T, Thomas VC, Bayles KW, Epand RM. Decoding the functional roles of cationic side chains of the major antimicrobial region of human cathelicidin LL-37. Antimicrob Agents Chemother. 2012;56(2):845–56. doi: 10.1128/AAC.05637-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Hanke ML, Mishra B, Lushnikova T, Heim CE, Chittezham Thomas V, Bayles KW, Kielian T. Transformation of human cathelicidin LL-37 into selective, stable, and potent antimicrobial compounds. ACS Chem Biol. 2014a;9(9):1997–2002. doi: 10.1021/cb500475y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Li X, Wang Z. APD3: the antimicrobial peptide database as a tool for research and education. Nucleic Acids Res. 2016;44(D1):D1087–93. doi: 10.1093/nar/gkv1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Mishra B, Epand RF, Epand RM. High-quality 3D structures shine light on antibacterial, anti-biofilm and antiviral activities of human cathelicidin LL-37 and its fragments. Biochim Biophys Acta. 2014b;1838(9):2160–72. doi: 10.1016/j.bbamem.2014.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Mishra B, Lau K, Lushnikova T, Golla R, Wang X. Antimicrobial peptides in 2014. Pharmaceuticals. 2015;8(1):123–150. doi: 10.3390/ph8010123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xavier JG, Geremias TC, Montero JF, Vahey BR, Benfatti CA, Souza JC, Magini RS, Pimenta AL. Lactam inhibiting Streptococcus mutans growth on titanium. Mater Sci Eng C Mater Biol Appl. 2016;68:837–841. doi: 10.1016/j.msec.2016.07.013. [DOI] [PubMed] [Google Scholar]
- Xu D, Yang W, Hu Y, Luo Z, Li J, Hou Y, Liu Y, Cai K. Surface functionalization of titanium substrates with cecropin B to improve their cytocompatibility and reduce inflammation responses. Colloids Surf B Biointerfaces. 2013;110:225–35. doi: 10.1016/j.colsurfb.2013.04.050. [DOI] [PubMed] [Google Scholar]
- Zasloff M. Antimicrobial peptides of multicellular organisms. Nature. 2002;415(6870):389–95. doi: 10.1038/415389a. [DOI] [PubMed] [Google Scholar]
- Zhao L, Chu PK, Zhang Y, Wu Z. Antibacterial coatings on titanium implants. J Biomed Mater Res B Appl Biomater. 2009;91(1):470–80. doi: 10.1002/jbm.b.31463. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.