Abstract
Diabetic peripheral neuropathy (DPN) and diabetic kidney disease (DKD) are common diabetic complications with limited treatment options. Experimental studies show that targeting inflammation using chemokine receptor (CCR) antagonists ameliorates DKD, presumably by reducing macrophage accumulation or activation. As inflammation is implicated in DPN development, we assessed whether CCR2 and CCR5 antagonism could also benefit DPN. Five-week-old ob/ob mice were fed a diet containing MK-0812, a dual CCR2-CCR5 receptor antagonist, for 8 weeks and DPN, DKD, and metabolic phenotyping were then performed to determine the effect of CCR inhibition. Although MK-0812 reduced macrophage accumulation in adipose tissue, the treatment had largely no effect on metabolic parameters, nerve function, or kidney disease in ob/ob mice. These results conflict with published data demonstrating a benefit of CCR antagonists for DKD and hyperglycemia. We conclude that CCR signaling blockade is ineffective in ob/ob mice and suspect that this is due to the severe hyperglycemia found in this model. It remains to be determined whether MK-0812 treatment, alone or in combination with improved glycemic control, is useful in preventing diabetic complications in alternate animal models.
INTRODUCTION
Systemic inflammation, primarily driven by adipose tissue dysfunction, is recognized as an underlying contributor to the development of type 2 diabetes (T2D).1 Inflammation is also implicated in diabetic kidney disease (DKD) and diabetic peripheral neuropathy (DPN), two common microvascular complications of T2D.2,3 As T2D patients manifest with hyperglycemia, current prevention strategies focus on glycemic control; however, this approach does not guarantee protection from DKD or DPN.4,5 Thus, targeting inflammation may provide an adjuvant therapy for the prevention of diabetic complications.
A hallmark of DKD is leukocyte accumulation, including macrophages, within the kidney glomerulus where they secrete pro-inflammatory proteins such as tumor necrosis factor alpha (TNFα), promoting inflammation and subsequent kidney injury.3 Indeed, macrophage accumulation strongly correlates with renal dysfunction in patients with DKD.6 Though not as well characterized, studies support a similar inflammatory mechanism in DPN.7,8
During initial tissue injury, resident leukocytes become activated and secrete chemokines that bind to chemokine receptors (CCRs) found on circulating leukocytes. This chemokine-CCR signaling facilitates trafficking of circulating leukocytes to the site of injury where they propagate inflammation. Unlike inflammation triggered by external pathogens, metabolic inflammation is difficult to resolve, leading to low-grade, chronic activation of the immune response.1 Sustained inflammation promotes leukocyte accumulation in the damaged tissue, the continued production of pro-inflammatory mediators, and amplification of the inflammatory response via a positive feedback loop. Chemokine C-C ligand-2 (CCL2) is responsible for recruiting CCR2-expressing leukocytes, including macrophages, to sites of inflammation. Antagonizing CCL2 signaling attenuates features of T2D in mouse models by preventing leukocyte infiltration into adipose tissue.9 Importantly, these benefits appear to extend to diabetic complications, as CCR2 antagonism reduces renal tissue inflammation and ameliorates DKD in mouse models of T2D.10 Likewise, in patients with DKD, treatment with a CCR2 antagonist or an inhibitor of CCL2/MCP1 can reduce proteinuria.11 Though less well studied, CCL5-CCR5 mediated signaling has also been implicated in T2D patients and rodent models of DKD;12 however, unlike DKD, CCR2 and CCR5 activation in DPN has not yet been examined.
We recently completed microarray gene expression analyses and reported that biological pathways related to inflammation, including chemotaxis, immune response, and response to wounding, are dysregulated in the sciatic nerve of ob/ob and db/db mice.13 Moreover, the expression levels of chemokines Ccl2, Ccl4, and Ccl8, ligands for CCR2 and CCR5, were increased in sciatic nerve tissue of ob/ob mice. These data suggest that, similar to DKD, chemokine-CCR signaling is involved in DPN. Therefore, we tested whether blocking CCR2- and CCR5-mediated chemokine signaling would prevent DPN as well as DKD in ob/ob mice, a mouse model of both microvascular complications.13,14
RESEARCH DESIGN AND METHODS
Animals and Diets
Male ob/+ and ob/ob mice (BTBR.Cg-Lepob/WiscJ) were fed a standard diet (11.5% kcal fat) or a diet containing MK-0812 (10 mg/kg/mouse) ad libitum from 5–13 weeks of age. Fasting blood glucose (FBG), percent glycated hemoglobin (%GHb) and plasma insulin was measured at study conclusion. Additional details into experimental procedures are described in the Supplemental Information.
DPN and DKD Phenotyping
DPN and DKD phenotyping was performed on the same animals in accordance with Diabetic Complications Consortium guidelines (www.diacomp.org) and are described in the Supplemental Information. Briefly, DPN phenotyping consisted of assessing hindpaw withdrawal from a thermal stimulus, sensory and motor nerve conduction velocities (NCV), and intraepidermal nerve fiber (IENF) density; DKD phenotyping consisted of measuring urinary albumin excretion (UAE), the albumin creatinine ratio (ACR), and the mesangial index quantified by calculating the percentage of the total glomerular tuft area that was PAS-positive.
Statistical Analysis
Statistical analysis consisted of one-way ANOVA with Tukey’s post-test for multiple comparisons or Kruskal-Wallis test with Dunn’s post-test for multiple comparisons, as appropriate, using GraphPad Prism Software, Version 6 (GraphPad Software, La Jolla, California). Error bars represent SEM (n = 6 mice/group). A p-value of < 0.05 was considered statistically significant.
RESULTS
MK-0812 Treatment Does Not Affect FBG, %GHb, Insulin, or Body Weight
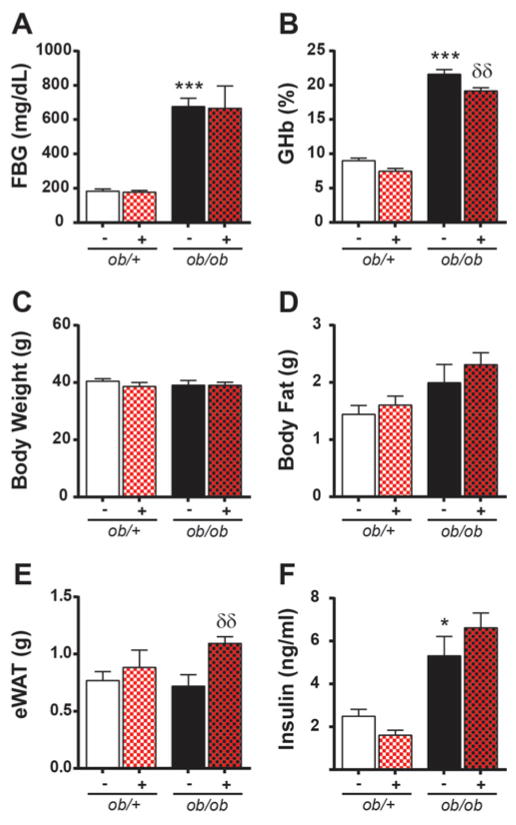
At 13 weeks, ob/ob mice had increased FBG, %GHb, and insulin when compared to non-diabetic controls (Fig. 1A,B,F). Body weight was similar in ob/+ and ob/ob mice (Fig. 1C); however, the increase in total adipose tissue mass in ob/ob mice (Fig. 1D) suggests that ob/+ mice have greater lean muscle mass. MK-0812 had no effect on FBG, weight, total adipose mass, or insulin levels in ob/+ or ob/ob mice (Fig. 1A,C,D,F) and only a modest effect on %GHb (Fig. 1B). Moreover, MK-0812 treatment increased eWAT mass in ob/ob mice (Fig. 1E).
Figure 1. Effects of MK-0812 on metabolic parameters.
Terminal FBG (A), GHb%, body weights (C), collective weight of adipose tissue depots (D), eWAT weight (E), and insulin (F). ***P < 0.0001 ob/ob mice versus ob/+ mice. δδP < 0.001 MK-0812-treated ob/+ and ob/ob mice versus respective non-treated controls.
MK-0812 Treatment Does Not Prevent Development of Diabetic Microvascular Complications
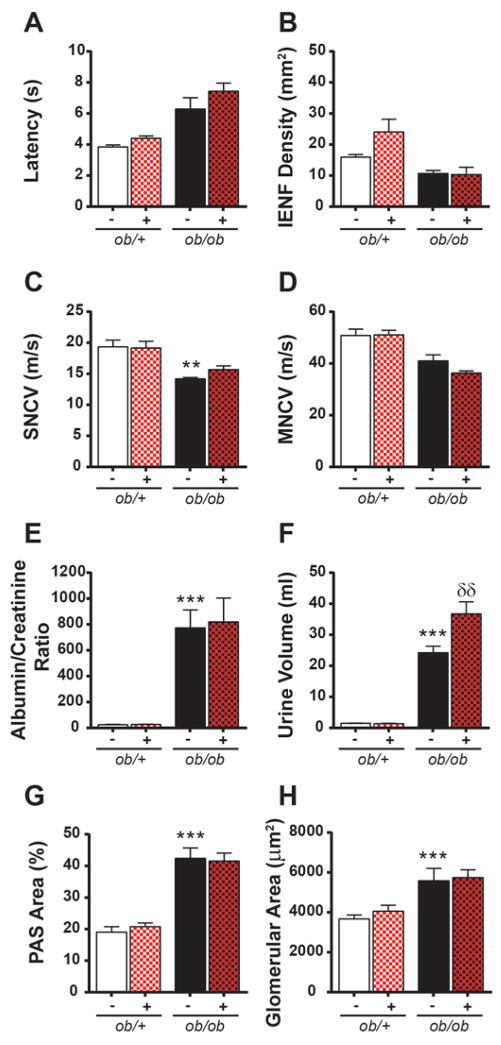
DPN and DKD measures were examined in ob/ob mice to determine the effects of MK-0812 on diabetic complications. ob/ob mice developed DPN, with increased hindpaw withdrawal latency (Fig. 2A), reduced IENF (Fig. 2B), and reduced sensory (Fig. 2C) and motor (Fig. 2D) NCV compared to ob/+ mice. ob/ob mice also exhibited features of DKD, with increased ACR (Fig. 2E), polyuria (Fig. 2F), mesangial matrix expansion (Fig. 2G), and glomerular thickening (Fig. 2H). MK-0812-treated ob/ob mice showed no improvement of diabetic nerve (Fig. 2A–D) or kidney changes (Fig. 2E–H) relative to untreated controls, indicating that MK-0812 treatment did not prevent DPN or DKD.
Figure 2. Effects of MK-0812 on DPN and DKD.
Neuropathy phenotyping consisted of measuring latency of hindpaw withdrawal from a thermal stimulus (A), quantification of intraepidermal nerve fiber (IENF) density (B), and assessment of sural sensory (C; SNCV) and sciatic motor (D; MNCV) nerve conduction velocity. Nephropathy was assessed by urinary ACR (E), urine volume (F), mesangial index, expressed as PAS-positive glomerular area divided by total glomerular area (G), and total glomerular area (H). **P < 0.001; ***P < 0.0001 ob/ob mice versus ob/+ mice. δδP < 0.001 MK-0812-treated ob/+ and ob/ob mice versus respective non-treated controls.
Effects of MK-0812 Treatment on Systemic, Neuronal, Renal, and Adipose Inflammation
To determine the effects of MK-0812 treatment on systemic and local inflammation, a cytokine/chemokine multiplex array was performed on plasma and nerve (SCN/DRG) and kidney (glomerulus) tissue lysates. Interestingly, little to no difference in inflammatory protein levels was observed between ob/ob mice and ob/+ mice in plasma or tissue (Supplementary Table 1). The multiplex data were validated by more sensitive ELISAs for plasma TNF-α, IL-6, and CCL2 (Supplementary Fig. 1). As adipose tissue inflammation drives insulin resistance and is implicated in T2D development,1 changes in markers of macrophage inflammation were also assessed in eWAT. ob/ob mice had increased mRNA expression of inflammation-related mediators, including Cd68, F4/80, Pai-1, and Tnfα (Supplementary Fig. 2A–D), while MK-0812 treatment decreased expression of Cd68, F4/80, and Tnfα. These data suggest that MK-0812 effectively suppressed macrophage infiltration in ob/ob adipose tissue.
DISCUSSION
The recruitment of leukocytes to kidney tissue during T2D is an early event in the pathogenesis of DKD,10 and evidence suggests a similar mechanism in DPN.2 Previously, we reported that ob/ob mice develop an early and robust DPN, with transcriptomic bioinformatics analysis implicating ‘chemotaxis’ and ‘inflammatory response’ in DPN pathogenesis.13 As these animals manifest with early DKD,14 the current study sought to investigate whether targeting a common inflammatory pathway would have restorative effects by attenuating microvascular complications.
Contrary to our hypothesis, MK-0812 treatment evoked no improvement in DKD or DPN in ob/ob mice and was also insufficient in mitigating hyperglycemia or hyperinsulinemia. Despite the absence of predicted effects, however, MK-0812 decreased adipose inflammation in ob/ob mice by reducing concentrations of Cd68, F4/80, and Tnfα.13 MK-0812-treated ob/ob mice also displayed a modest decrease in %GHb relative to untreated controls that could be attributed to the decrease in adipose tissue inflammation, but the improvement was insufficient to surmount the metabolic derangements characteristic of T2D.10 These results conflict with independent studies in which CCR2 inhibition prevented DKD in mice10 and humans.11 Kang et al. observed improvements in renal function of C57BKS db/db mice treated for 12 weeks with the CCR2 antagonist RS504393,10 while CCR2 inhibition reduced proteinuria in humans with T2D and DKD.11
We anticipate that the primary reason why MK-0812 had largely no effect on metabolic parameters or diabetic complications is related to features of the ob/ob mouse model itself. Previous studies demonstrating a positive effect of CCR2 antagonists on DKD have almost exclusively used db/db mice10 that do not develop the aggressive hyperglycemia or DKD phenotype seen in ob/ob mice.14 This suggests that the likelihood of an intervention successfully attenuating disease in db/db mice will be higher than in a more aggressive T2D model. In addition, though db/db mice lack the long isoforms of hypothalamic leptin receptor, short leptin receptor isoforms are expressed on the kidney and vasculature, and leptin-leptin receptor binding may confer a slight protection against DKD development that is absent in leptin-deficient ob/ob mice.15 Another caveat of this model stems from the fact that we observed no difference in markers of inflammation in plasma, kidney, or nerve between control and ob/ob mice. Inflammation is heavily implicated in DKD development in mouse models of T2D that have functional leptin.3 As leptin has numerous important immunomodulatory actions a deficiency can compromise normal immune homeostasis. Hence, the atypical inflammatory signaling present in ob/ob mice may not be suitable to explore therapies targeting inflammation. Observations from this study further suggest that DKD and DPN pathogenesis are independent of increases in kidney or nerve pro-inflammatory mediators in this model. Finally, we ensured that the lack of a positive effect on DKD/DPN was not due to an ineffective dose of MK-0812 by looking directly at adipose tissue inflammation, a known driver of metabolic dysfunction.1 Similar to Kang et al. who demonstrated that CCR2 antagonism decreases adipose tissue inflammation in C57BKS db/db mice,10 we observed a decrease in inflammatory markers following MK-0812 treatment in ob/ob mice, thus confirming that the lack of MK-0812 therapeutic efficacy on microvascular complications in ob/ob mice was not simply due to inadequate drug activity.
In summary, MK-0812 had no effect on the metabolic parameters or the severity of DPN and DKD in ob/ob mice. These observations are unexpected given previous independent reports of its efficacy in improving both glycemic control and DKD in db/db mice.10 We speculate that MK-0812 treatment in alternate models, such as db/db or diet-induced obese mice, should be pursued based on the existing literature supporting a role for inflammation in the onset and development of microvascular complications in humans as well as these other models.
Supplementary Material
Acknowledgments
The authors acknowledge the technical expertise of Ms. Jacqueline Dauch, Ms. Chelsea Lindblad, Ms. Jharna Saha, and Dr. Sang Su Oh in conducting animal experiments. The authors thank the Hormone Core at Vanderbilt University and the Lipid Laboratory at the Mouse Metabolic Phenotyping Center at the University of Washington for mouse plasma measurements, respectively, and the Chemistry Core of the MDRTC (930DK020572) at the U-M for %GHb measurements. The authors thank Dr. Charlie Alpers at the University of Washington for expert advice on the ob/ob mouse strain.
FUNDING
Funding was provided by the National Institutes of Health (1DP3DK094292, 1R24082841 to E.L.F and F.C.B.); Novo Nordisk Foundation (NNF14SA0006 to E.L.F.), Juvenile Diabetes Research Foundation (Post-doctoral Fellowship to L.M.H.), Milstein, Nathan and Rose Research Fund; Sinai Medical Staff Foundation Neuroscience Scholar Fund 2; Robert C Graham Fund; Walbridge Aldinger Graduate Fellowship Fund (Post-doctoral Fellowship to P.D.O); American Diabetes Association; Program for Neurology Research & Discovery; A. Alfred Taubman Medical Research Institute; and the University of Michigan Center for Organogenesis Non-Traditional Post-Doctoral Fellowship (to S.D.P).
Footnotes
DUALITY OF INTEREST
L.M. is employed as an Associate Principal Scientist at Merck Pharmaceuticals, Kenilworth, New Jersey, USA. No other potential conflicts of interest relevant to this article were reported.
AUTHOR CONTRIBUTIONS
P.D.O. and L.M.H. directed the study, interpreted the data, and wrote the manuscript. S.D.P. provided consultation, performed experimental work, contributed to the discussion of the manuscript. J.M.H, C.B., and H.Z. conducted animal experiments. L.M. was involved in study design, provided consultation and interpreted the pharmacokinetic data. S.A.S reviewed and edited the manuscript. E.L.F. and F.C.B. designed and directed the study, contributed to discussion, and edited the manuscript. All authors critically revised the manuscript. E.L.F. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and accuracy of the data analysis.
References
- 1.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444(7121):860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 2.Wilson NM, Wright DE. Inflammatory Mediators in Diabetic Neuropathy. J Diabetes Metab. 2011;S5:004. [Google Scholar]
- 3.Navarro-Gonzalez JF, Mora-Fernandez C, Muros de Fuentes M, Garcia-Perez J. Inflammatory molecules and pathways in the pathogenesis of diabetic nephropathy. Nature reviews Nephrology. 2011;7(6):327–340. doi: 10.1038/nrneph.2011.51. [DOI] [PubMed] [Google Scholar]
- 4.Callaghan BC, Little AA, Feldman EL, Hughes RA. Enhanced glucose control for preventing and treating diabetic neuropathy. The Cochrane database of systematic reviews. 2012;6:CD007543. doi: 10.1002/14651858.CD007543.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nadkarni GN, Yacoub R, Coca SG. Update on glycemic control for the treatment of diabetic kidney disease. Current diabetes reports. 2015;15(7):42. doi: 10.1007/s11892-015-0612-7. [DOI] [PubMed] [Google Scholar]
- 6.Nguyen D, Ping F, Mu W, Hill P, Atkins RC, Chadban SJ. Macrophage accumulation in human progressive diabetic nephropathy. Nephrology (Carlton) 2006;11(3):226–231. doi: 10.1111/j.1440-1797.2006.00576.x. [DOI] [PubMed] [Google Scholar]
- 7.Herder C, Bongaerts BW, Rathmann W, et al. Differential association between biomarkers of subclinical inflammation and painful polyneuropathy: results from the KORA F4 study. Diabetes Care. 2015;38(1):91–96. doi: 10.2337/dc14-1403. [DOI] [PubMed] [Google Scholar]
- 8.Doupis J, Lyons TE, Wu S, Gnardellis C, Dinh T, Veves A. Microvascular reactivity and inflammatory cytokines in painful and painless peripheral diabetic neuropathy. The Journal of clinical endocrinology and metabolism. 2009;94(6):2157–2163. doi: 10.1210/jc.2008-2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sullivan TJ, Miao Z, Zhao BN, et al. Experimental evidence for the use of CCR2 antagonists in the treatment of type 2 diabetes. Metabolism: clinical and experimental. 2013;62(11):1623–1632. doi: 10.1016/j.metabol.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 10.Kang YS, Lee MH, Song HK, et al. CCR2 antagonism improves insulin resistance, lipid metabolism, and diabetic nephropathy in type 2 diabetic mice. Kidney international. 2010;78(9):883–894. doi: 10.1038/ki.2010.263. [DOI] [PubMed] [Google Scholar]
- 11.de Zeeuw D, Bekker P, Henkel E, et al. The effect of CCR2 inhibitor CCX140-B on residual albuminuria in patients with type 2 diabetes and nephropathy: a randomised trial. Lancet Diabetes Endocrinol. 2015;3(9):687–696. doi: 10.1016/S2213-8587(15)00261-2. [DOI] [PubMed] [Google Scholar]
- 12.Ruster C, Wolf G. The role of chemokines and chemokine receptors in diabetic nephropathy. Front Biosci. 2008;13:944–955. doi: 10.2741/2734. [DOI] [PubMed] [Google Scholar]
- 13.O’Brien PD, Hur J, Hayes JM, Backus C, Sakowski SA, Feldman EL. BTBR ob/ob mice as a novel diabetic neuropathy model: Neurological characterization and gene expression analyses. Neurobiology of disease. 2014;73C:348–355. doi: 10.1016/j.nbd.2014.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hudkins KL, Pichaiwong W, Wietecha T, et al. BTBR Ob/Ob mutant mice model progressive diabetic nephropathy. Journal of the American Society of Nephrology : JASN. 2010;21(9):1533–1542. doi: 10.1681/ASN.2009121290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang W, Poole B, Mitra A, et al. Role of leptin deficiency in early acute renal failure during endotoxemia in ob/ob mice. Journal of the American Society of Nephrology : JASN. 2004;15(3):645–649. doi: 10.1097/01.asn.0000113551.14276.0b. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.