Abstract
Background
Peripheral artery disease (PAD) in heart failure with preserved ejection fraction (HFpEF) is associated with an increased mortality risk, but the risk of individual outcomes associated with PAD in this patient group is less clear.
Hypothesis
PAD is associated with adverse outcomes in HFpEF, including hospitalization and specific cardiovascular outcomes.
Methods
We examined the association between PAD and adverse outcomes in 3385 patients with HFpEF (mean age, 69 ± 9.6 years; 49% male; 89% white) from the Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist Trial (TOPCAT). Baseline PAD was identified by self‐reported history and medical‐record review. The following outcomes were adjudicated by a clinical endpoint committee: hospitalization, hospitalization for heart failure (HF), myocardial infarction, stroke, death, and cardiovascular death.
Results
Over a median follow‐up of 3.4 years (interquartile range, 2.0–4.9 years), an increased risk for hospitalization (hazard ratio [HR]: 1.36, 95% confidence interval [CI]: 1.16‐1.60), myocardial infarction (HR: 1.69, 95% CI: 1.07‐2.67), death (HR: 1.56, 95% CI: 1.22‐1.99), and cardiovascular death (HR: 1.53, 95% CI: 1.12‐2.10) was observed for those with PAD compared with those without PAD. PAD was not associated with incident stroke. The association between PAD and hospitalization for HF was limited to participants with prior history of HF hospitalization (n = 2449; HR: 1.51, 95% CI: 1.09‐2.13).
Conclusions
PAD increases the risk for adverse outcomes in HFpEF and is associated with HF rehospitalization. Practitioners should be aware of the inherent risk associated with PAD in HFpEF.
Keywords: Peripheral Artery Disease, Heart Failure, Preserved Ejection Fraction
1. INTRODUCTION
Heart failure with preserved ejection fraction (HFpEF) is an emerging public health problem, representing nearly half of all heart failure (HF) cases.1 Peripheral artery disease (PAD) and HFpEF share similar cardiovascular risk factors (eg, hypertension, diabetes mellitus [DM]),2, 3 and this condition is present in ~15% of patients with HFpEF.3 PAD also is associated with an increased risk of mortality in patients who have HFpEF.3, 4 However, the actual risk of death associated with PAD possibly is greater, as 50% of PAD cases are asymptomatic and PAD symptoms often are masked by coexisting HF.5
Due to the reported mortality risk associated with PAD in HFpEF, PAD likely represents a comorbid condition that is associated with significant morbidity regarding healthcare utilization and the development of specific cardiovascular outcomes (eg, myocardial infarction [MI]). However, prior studies have not specifically examined the influence of PAD on outcomes in HFpEF. Such a finding would alert practitioners to a group of HFpEF patients in whom intense risk‐factor modification strategies and the optimization of HF therapies are warranted. Therefore, we examined the impact of PAD on outcomes in patients with HFpEF in the Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist Trial (TOPCAT).6
2. METHODS
2.1. Study design and patients
TOPCAT was a multicenter, international, randomized, double‐blind, placebo‐controlled study to examine the efficacy of spironolactone in patients with HFpEF. The design, inclusion criteria, and baseline characteristics of the trial have been published previously.7, 8 Briefly, 3445 patients with symptomatic HFpEF from 270 sites in 6 countries were enrolled between August 2006 and January 2012. The primary goal of the trial was to determine if spironolactone was associated with a reduction in the composite outcome of cardiovascular mortality, aborted cardiac arrest, or HF hospitalization in patients with HFpEF (eg, documented ejection fraction ≥45%).
The aim of this analysis was to examine the influence of PAD on hospitalization, hospitalization for HF, MI, stroke, death, and cardiovascular death in patients with HFpEF. We included 3385 patients from TOPCAT (mean age, 69 ± 9.6 years; 49% male; 89% white) who had complete baseline information and follow‐up data.
2.2. Baseline characteristics
Patients who participated in TOPCAT underwent a detailed baseline visit to obtain medical histories, and a physical examination was performed.8 Baseline PAD cases were identified by self‐reported history and medical‐record review during the initial study visit. Age, sex, and race were obtained by self‐reported history. Smoking was defined as the current use of cigarettes and ascertained by self‐report. Medical history for the following diagnoses was obtained by self‐report and medical‐record review: DM, coronary heart disease (CHD), stroke, New York Heart Association Class, prior HF hospitalization, and atrial fibrillation. Systolic blood pressure and body mass index were obtained by trained staff, and laboratory data included serum creatinine. Medication data also were obtained during the initial study visit and the following were included in this analysis: aspirin, β‐blockers, angiotensin‐converting enzyme inhibitors/angiotensin II receptor blockers, and statins.
2.3. Outcomes
Outcomes in TOPCAT were adjudicated by a clinical endpoint committee, and the details of this process and definitions for each outcome examined have been described previously.6, 7 The outcomes examined in this analysis included hospitalization, hospitalization for HF, MI, stroke, death, and cardiovascular death. Briefly, hospitalization for HF was defined as the unexpected presentation to an acute‐care facility requiring overnight stay with symptoms and physical examination findings consistent with HF, and treatment with intravenous vasodilators, inotropes, mechanical fluid removal, or hemodynamic support. MI was defined as the presence of positive cardiac markers with either electrocardiogram changes or clinically apparent ischemic symptoms (eg, chest pain, dyspnea, or pressure). Stroke was defined as a focal neurological deficit of sudden onset that was not reversible within 24 hours of onset or a focal neurological deficit of sudden onset with brain imaging consistent with infarction or hemorrhage. Cardiovascular death was defined as death due to one of the following: MI, worsening HF, sudden death, stroke, pulmonary embolism, death occurring during a cardiovascular‐related procedure, or other cardiovascular death. Death included the composite of cardiovascular and noncardiovascular death.
2.4. Statistical analysis
Baseline characteristics were compared by the presence of baseline PAD. Categorical variables were reported as frequency and percentage, and continuous variables were recorded as mean ± SD. Statistical significance for categorical variables was tested using the χ2 method, and for continuous variables the Student's t test was used. Kaplan‐Meier estimates were used to examine the unadjusted cumulative incidence estimates of each outcome associated with baseline PAD. Cox regression was used to examine the risk of each outcome associated with PAD.
Multivariable models were constructed as follows: Model 1 adjusted for age, sex, and race; Model 2 adjusted for Model 1 covariates with the addition of smoking, systolic blood pressure, DM, body mass index, angiotensin‐converting enzyme inhibitors/angiotensin II receptor blockers, β‐blockers, statins, randomization group, New York Heart Association class, atrial fibrillation, CHD, and stroke. A secondary analysis was performed in patients with prior HF hospitalization to determine if the magnitude of the association between PAD and each outcome was dependent on prior admission for decompensated HF, and interaction P values were computed. Additionally, due to differences in the baseline characteristics and event rates observed between patients recruited in Russia and Georgia vs the Americas,9 we examined if our findings varied by region (Russia/Georgia vs the Americas). The parallel hazards assumption was not violated in our analyses. Statistical significance was defined as P < 0.05. SAS software version 9.4 (SAS Institute Inc., Cary, NC) was used for all analyses.
3. RESULTS
A total of 309 patients (9.1%) had PAD. The baseline characteristics stratified by the presence of PAD are shown in Table 1. Patients with PAD were more likely to be male, report current smoking, and to have DM and prior history of CHD and stroke than those without PAD. PAD patients also were more likely to report the use of aspirin and statins, and to have higher serum creatinine values than patients without PAD.
Table 1.
Baseline characteristics of patients (N = 3385)
| Characteristic | PAD, n = 309 | No PAD, n = 3076 | P Valuea |
|---|---|---|---|
| Age, y | 69 ± 9.3 | 68 ± 9.6 | 0.11 |
| Male sex | 187 (61) | 1456 (47) | <0.001 |
| White race | 280 (91) | 2729 (89) | 0.31 |
| Current smoker | 45 (15) | 314 (10) | 0.018 |
| DM | 144 (47) | 947 (31) | <0.001 |
| CHD | 197 (64) | 1016 (33) | <0.001 |
| Stroke | 47 (15) | 214 (6.9) | <0.001 |
| SBP, mm Hg | 128 ± 15 | 129 ± 14 | 0.18 |
| BMI, kg/m2 | 32 ± 7.1 | 32 ± 7.1 | 0.27 |
| sCr, mg/dL | 1.16 ± 0.33 | 1.08 ± 0.29 | <0.001 |
| NYHA class III–IV | 112 (36) | 1006 (33) | 0.21 |
| Prior HF hospitalization | 211 (68) | 2238 (73) | 0.094 |
| AF | 96 (31) | 1095 (36) | 0.11 |
| Medications | |||
| ASA use | 228 (74) | 1990 (65) | 0.0013 |
| β‐Blocker | 242 (78) | 2394 (78) | 0.84 |
| ACEI/ARB | 259 (84) | 2593 (84) | 0.83 |
| Statin | 233 (75) | 1535 (50) | <0.001 |
| Spironolactone | 166 (54) | 1531 (50) | 0.19 |
Abbreviations: ACEI, angiotensin‐converting enzyme inhibitor; AF, atrial fibrillation; ARB, angiotensin II receptor blocker; ASA, acetylsalicylic acid (aspirin); BMI, body mass index; CHD, coronary heart disease; DM, diabetes mellitus; HF, heart failure; NYHA, New York Heart Association; PAD, peripheral artery disease; SBP, systolic blood pressure; sCr, serum creatinine; SD, standard deviation.
Data are presented as n (%) or mean ± SD.
Statistical significance for continuous data was tested using the Student's t test and categorical data were tested using the χ2 test.
Over a median follow‐up of 3.4 years (interquartile range, 2.0–4.9 years), a total of 1524 (45%) hospitalizations, 437 (13%) hospitalizations for HF, 125 (3.7%) MIs, 115 (3.4%) strokes, 516 (15%) deaths, and 330 (10%) cardiovascular deaths occurred. The unadjusted cumulative incidence estimates for hospitalization, hospitalization for HF, MI, stroke, death, and cardiovascular death are depicted in Figures 1, 2, and 3. The unadjusted cumulative incidence estimates for all outcomes examined were higher among patients with PAD than those without PAD.
Figure 1.
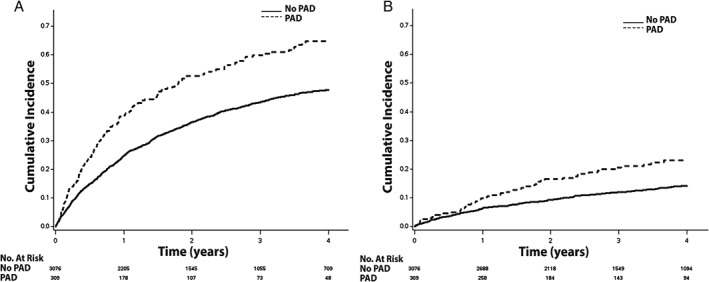
The cumulative incidence curves for (A) hospitalization and (B) hospitalization for HF. The cumulative incidence curves are statistically different for both hospitalization (log‐rank P < 0.001) and hospitalization for HF (log‐rank P < 0.001). Abbreviations: HF, heart failure; PAD, peripheral artery disease.
Figure 2.
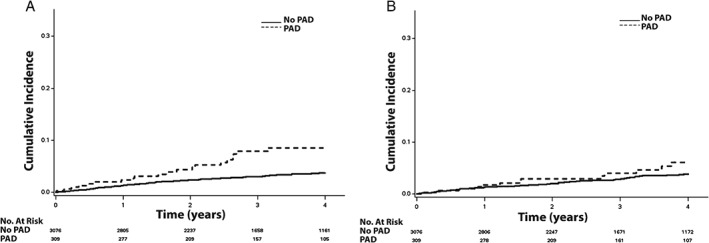
The cumulative incidence curves for (A) MI and (B) stroke. The cumulative incidence curves are statistically different for both MI (log‐rank P < 0.001) and stroke (log‐rank P = 0.046). Abbreviations: MI, myocardial infarction; PAD, peripheral artery disease.
Figure 3.
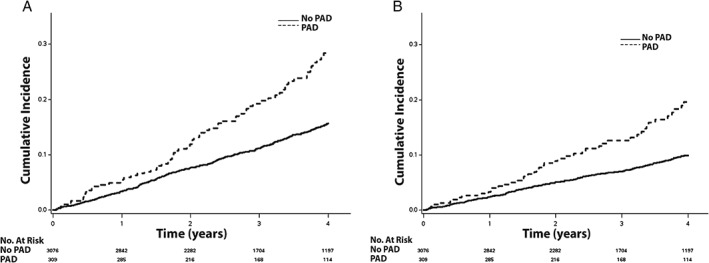
The cumulative incidence curves for (A) death and (B) cardiovascular death. The cumulative incidence curves are statistically different for both death (log‐rank P < 0.001) and cardiovascular death (log‐rank P < 0.001). Abbreviations: PAD, peripheral artery disease.
An increased risk for hospitalization (hazard ratio [HR]: 1.36, 95% confidence interval [CI]: 1.16‐1.60), MI (HR: 1.69, 95% CI: 1.07‐2.67), death (HR: 1.56, 95% CI: 1.22‐1.99), and cardiovascular death (HR: 1.53, 95% CI: 1.12‐2.10) was observed for those with PAD compared with those without PAD (Table 2). PAD was not associated with incident stroke or hospitalization for HF. When the analysis was limited to participants who reported prior hospitalization for HF (n = 2449), the magnitudes of the association for hospitalization (HR: 1.39, 95% CI: 1.15‐1.69; P for interaction: 0.42), MI (HR: 1.69, 95% CI: 0.95‐3.01; P for interaction: 0.89), death (HR: 1.60, 95% CI: 1.18‐2.17; P for interaction: 0.78), and cardiovascular death (HR: 1.56, 95% CI: 1.07‐2.27; P for interaction: 0.89) were not substantively different from the main analysis. However, PAD (HR: 1.51, 95% CI: 1.09‐2.13; P for interaction: 0.14) was significantly associated with hospitalization for HF among patients who reported prior hospitalization for HF. When we examined the association between PAD and each outcome by country of origin (Russia/Georgia vs the Americas), the association between PAD and each outcome was similar between groups (see Supporting Information, Table 1, in the online version of this article).
Table 2.
Risk of hospitalization, MI, stroke, and death with PAD (N = 3385)
| Outcome | Events | Model 1a, HR (95% CI) | P Value | Model 2b, HR (95% CI) | P Value |
|---|---|---|---|---|---|
| Hospitalization | 1524 | 1.64 (1.40‐1.91) | <0.001 | 1.36 (1.16‐1.60) | <0.001 |
| Hospitalization for HF | 437 | 1.61 (1.22‐2.12) | <0.001 | 1.29 (0.97‐1.71) | 0.081 |
| MI | 125 | 2.52 (1.62‐3.91) | <0.001 | 1.69 (1.07‐2.67) | 0.025 |
| Stroke | 115 | 1.68 (0.99‐2.86) | 0.055 | 1.40 (0.81‐2.42) | 0.23 |
| Death | 516 | 1.74 (1.37‐2.21) | <0.001 | 1.56 (1.22‐1.99) | <0.001 |
| CV death | 330 | 1.68 (1.24‐2.28) | <0.001 | 1.53 (1.12‐2.10) | <0.001 |
Abbreviations: ACEI, angiotensin‐converting enzyme inhibitor; AF, atrial fibrillation; ARB, angiotensin II receptor blocker; ASA, acetylsalicylic acid (aspirin); BMI, body mass index; CHD, coronary heart disease; CI, confidence interval; CV, cardiovascular; DM, diabetes mellitus; HF, heart failure; HR, hazard ratio; MI, myocardial infarction; NYHA, New York Heart Association; PAD, peripheral artery disease; SBP, systolic blood pressure.
Adjusted for age, sex, and race.
Adjusted for Model 1 covariates plus smoking, SBP, DM, BMI, ASA, ACEIs/ARBs, β‐blockers, statins, randomization group, NYHA class, AF, CHD, and stroke.
4. DISCUSSION
In this analysis from TOPCAT, PAD was associated with an increased risk for hospitalization, MI, death, and cardiovascular death in patients with HFpEF. Additionally, our data suggest that the risk of hospitalization for HF is limited to HFpEF patients with history of prior HF hospitalization. Overall, the findings of this analysis suggest that PAD portends a poor prognosis in patients with HFpEF and identifies several outcomes associated with this common comorbid condition among HFpEF patients.
The influence of PAD on adverse outcomes in HFpEF has not been widely examined. To our knowledge, only 1 report has studied the association of PAD with adverse outcomes in HFpEF. An examination of 880 HFpEF patients admitted to Canadian hospitals reported that PAD was associated with an increased mortality risk (HR: 2.00, 95% CI: 1.31‐3.08).3 This analysis supports the hypothesis that PAD increases mortality in HFpEF and extends this previous finding to include cardiovascular death. Additionally, we were able to examine specific outcomes and demonstrated that PAD increases the risk for MI and rehospitalization for HF.
The underlying mechanism that links PAD with adverse events in HFpEF is unknown. Patients with PAD have a higher prevalence of cardiovascular comorbid conditions,2 likely increasing the risk for adverse events and utilization of healthcare resources (eg, hospitalization). Additionally, PAD has a more pronounced impact on physical impairment in HFpEF due to limited exercise capacity.10 Thus, the coexistence of PAD and HFpEF would further reduce cardiovascular fitness and negatively affect patient prognosis.11 Furthermore, patients with PAD have widespread atherosclerotic burden and greater disease progression that likely contributes to adverse cardiovascular outcomes, such as MI.12
PAD is not currently considered in the management of HF,13 despite the worse prognosis and increased utilization of healthcare resources. As evidenced by the findings in this analysis, a concurrent diagnosis of PAD is indicative of a high‐risk group in which intense risk‐factor modification strategies and the optimization of HF therapies are warranted. Due to the expected increase in the prevalence of HFpEF,14 and associated cost,15 further research is needed to reduce the future burden that HFpEF will place on the healthcare system. Additionally, there is a need for increased focus on PAD as an important comorbid condition in HFpEF, as well as the development of appropriate management strategies in this population.
4.1. Study limitations
The current study should be interpreted in the context of several limitations. This analysis was a secondary examination of clinical trial data that did not have a primary aim of determining the risk of outcomes associated with PAD in HFpEF. However, the effect estimates for outcomes associated with PAD were statistically significant, suggesting that our analysis was appropriately powered. Our analysis also was subjected to recall bias, as several baseline characteristics were self‐reported. Similarly, some cases of PAD were ascertained at baseline by self‐report, and there were no vascular function studies to quantitate the presence, location, or severity of PAD. Additionally, it is possible that the effect of PAD on outcomes in HFpEF is larger than reported in this analysis, as the majority of patients with PAD are asymptomatic.5 Furthermore, it is possible that certain outcomes were missed despite rigorous methodology to ascertain all events. Finally, we acknowledge the possibility of residual confounding in our multivariable models.
5. CONCLUSION
PAD increases the risk for several adverse outcomes in patients with HFpEF and is associated with HF rehospitalization. Practitioners should be aware of the inherent risk of adverse outcomes associated with PAD in HFpEF, and further research is needed to develop preventive strategies to reduce the burden in this high‐risk group.
Conflicts of interest
The authors declare no conflicts of interest.
Supporting information
Table S1. Risk of Hospitalization, Myocardial Infarction, Stroke, and Death with PAD by Region of Enrollment (N = 3,385)
Sandesara PB, Hammadah M, Samman‐Tahhan A, Kelli HM and O'Neal WT. Peripheral artery disease and risk of adverse outcomes in heart failure with preserved ejection fraction. Clinical Cardiology. 2017;40:692‐696. 10.1002/clc.22716
Funding Information Research reported in this publication was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under award no. F32‐HL134290. This manuscript was prepared using Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist Trial (TOPCAT) research materials obtained from the National Heart, Lung, and Blood Institute Biologic Specimen and Data Repository Information Coordinating Center. The content is solely the responsibility of the authors and does not necessarily reflect the opinions or official views of the TOPCAT Study; the National Heart, Lung, and Blood Institute; or the National Institutes of Health.
REFERENCES
- 1. Yancy CW, Lopatin M, Stevenson LW, et al; ADHERE Scientific Advisory Committee and Investigators. Clinical presentation, management, and in‐hospital outcomes of patients admitted with acute decompensated heart failure with preserved systolic function: a report from the Acute Decompensated Heart Failure National Registry (ADHERE) Database [published correction appears in J Am Coll Cardiol. 2006;47:1502]. J Am Coll Cardiol. 2006;47:76–84. [DOI] [PubMed] [Google Scholar]
- 2. Selvin E, Erlinger TP. Prevalence of and risk factors for peripheral arterial disease in the United States: results from the National Health and Nutrition Examination Survey, 1999–2000. Circulation. 2004;110:738–743. [DOI] [PubMed] [Google Scholar]
- 3. Bhatia RS, Tu JV, Lee DS, et al. Outcome of heart failure with preserved ejection fraction in a population‐based study. N Engl J Med. 2006;355:260–269. [DOI] [PubMed] [Google Scholar]
- 4. Wei B, Qian C, Fang Q, et al. The prognostic value of peripheral artery disease in heart failure: insights from a meta‐analysis. Heart Lung Circ. 2016;25:1195–1202. [DOI] [PubMed] [Google Scholar]
- 5. Inglis SC, Hermis A, Shehab S, et al. Peripheral arterial disease and chronic heart failure: a dangerous mix. Heart Fail Rev. 2013;18:457–464. [DOI] [PubMed] [Google Scholar]
- 6. Pitt B, Pfeffer MA, Assmann SF, et al; TOPCAT Investigators. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med. 2014;370:1383–1392. [DOI] [PubMed] [Google Scholar]
- 7. Desai AS, Lewis EF, Li R, et al. Rationale and design of the treatment of preserved cardiac function heart failure with an aldosterone antagonist trial: a randomized, controlled study of spironolactone in patients with symptomatic heart failure and preserved ejection fraction. Am Heart J. 2011;162:966.e10–972.e10. [DOI] [PubMed] [Google Scholar]
- 8. Shah SJ, Heitner JF, Sweitzer NK, et al. Baseline characteristics of patients in the treatment of preserved cardiac function heart failure with an aldosterone antagonist trial. Circ Heart Fail. 2013;6:184–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pfeffer MA, Claggett B, Assmann SF, et al. Regional variation in patients and outcomes in the Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist (TOPCAT) trial. Circulation. 2015;131:34–42. [DOI] [PubMed] [Google Scholar]
- 10. Edelmann F, Stahrenberg R, Gelbrich G, et al. Contribution of comorbidities to functional impairment is higher in heart failure with preserved than with reduced ejection fraction. Clin Res Cardiol. 2011;100:755–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fletcher GF, Ades PA, Kligfield P, et al. Exercise standards for testing and training: a scientific statement from the American Heart Association. Circulation. 2013;128:873–934. [DOI] [PubMed] [Google Scholar]
- 12. Hussein AA, Uno K, Wolski K, et al. Peripheral arterial disease and progression of coronary atherosclerosis. J Am Coll Cardiol. 2011;57:1220–1225. [DOI] [PubMed] [Google Scholar]
- 13. Yancy CW, Jessup M, Bozkurt B, et al. 2016 ACC/AHA/HFSA Focused Update on New Pharmacological Therapy for Heart Failure: an update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol. 2016;68:1476–1488. [DOI] [PubMed] [Google Scholar]
- 14. Oktay AA, Rich JD, Shah SJ. The emerging epidemic of heart failure with preserved ejection fraction. Curr Heart Fail Rep. 2013;10:401–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Heidenreich PA, Albert NM, Allen LA, et al. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail. 2013;6:606–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Risk of Hospitalization, Myocardial Infarction, Stroke, and Death with PAD by Region of Enrollment (N = 3,385)


