Abstract
Background
Racial differences in endogenous pain facilitatory processes have been previously reported. Evidence suggests that psychological and behavioral factors, including depressive symptoms and sleep, can alter endogenous pain facilitatory processes. Whether depressive symptoms and sleep might help explain racial differences in endogenous pain facilitatory processes has yet to be determined.
Purpose
This observational, microlongitudinal study examined whether depressive symptoms and sleep were sequential mediators of racial differences in endogenous pain facilitatory processes.
Methods
A total of 50 (26 African American and 24 non-Hispanic White) community-dwelling adults without chronic pain (mean 49.04 years; range 21–77) completed the Center for Epidemiological Studies Depression Scale prior to seven consecutive nights of sleep monitoring with actigraphy in the home environment. Participants subsequently returned to the laboratory for assessment of endogenous pain facilitation using a mechanical temporal summation protocol.
Results
Findings revealed greater depressive symptoms, poorer sleep efficiency, and greater temporal summation of mechanical pain in African Americans compared to non-Hispanic Whites. In a sequential mediation model, greater depressive symptoms predicted poorer sleep efficiency (t = −2.55, p = .014), and poorer sleep efficiency predicted enhanced temporal summation of mechanical pain (t = −4.11, p < .001), particularly for African Americans.
Conclusions
This study underscores the importance of examining the contribution of psychological and behavioral factors when addressing racial differences in pain processing. Additionally, it lends support for the deleterious impact of depressive symptoms on sleep efficiency, suggesting that both sequentially mediate racial differences in endogenous pain facilitation.
Keywords: race, depressive symptoms, sleep efficiency, temporal summation of pain
INTRODUCTION
Temporal summation of pain refers to an increase in pain perception after application of a series of identical noxious stimuli delivered at sufficient frequency and intensity (1). Characterization of temporal summation responses provides information about endogenous pain facilitatory mechanisms underlying nociceptive processing in healthy individuals and those with chronic pain (2–4). Tests of experimental pain sensitivity like temporal summation responses are widely incorporated in psychophysical studies to invoke neural mechanisms related to central sensitization (5). Previous work conducted by our group has revealed significant racial differences in temporal summation of pain using heat and mechanical stimuli (6, 7), such that African Americans (AAs) demonstrate greater endogenous pain facilitation compared to non-Hispanic Whites (NHWs). Subsequently, we have begun to examine various factors that might help explain why AAs often demonstrate enhanced pain facilitatory processes (i.e., temporal summation) compared to their NHW counterparts.
Evidence suggests that psychological and behavioral factors, including depressive symptoms (8, 9) and sleep (10–12), can alter experimental tests of pain sensitivity including temporal summation of pain responses. Importantly, racial differences have also been previously documented in depressive symptoms and sleep quality. For example, significantly higher rates of depressive symptoms have been observed in community-dwelling AAs compared to NHWs (13). Additionally, higher levels of sleep disturbance have been observed on objective assessments in AAs versus NHWs, including greater odds of obstructive sleep apnea syndrome, shorter sleep duration, poorer sleep quality and sleep efficiency, and increased daytime sleepiness (14–16). AAs also subjectively report a high degree of sleep impairments in addition to objective parameters of poor sleep quality (17). Taken together, it may be that depressive symptoms and sleep quality are important factors to consider when evaluating temporal summation of pain responses in AA populations. When simultaneously considering depressive symptoms and sleep quality in relation to temporal summation of pain, it is also prudent to consider the directionality of these associations and the temporal precedence of variable measurement.
In a non-clinical sample, depressive symptoms were shown to be strongly linked to poor sleep quality (18). In clinical samples, difficulty initiating and/or maintaining sleep has been reported in about three-quarters of depressed patients (19–21). It has been argued that a bi-directional relationship likely exists between depressive symptoms and sleep quality; however, compelling evidence suggests that sleep problems often manifest secondary to the emergence of depressive symptoms (20, 22) and that depression is a risk factor for subsequent sleep difficulties (23, 24). These findings help support the assertion that the experience of depressive symptoms may subsequently beget poor sleep quality.
Ample evidence suggests that sleep quality is associated with pain, including experimental pain sensitivity (25, 26) as well as clinical pain outcomes (27, 28). A recent comprehensive review of the literature indicated that in (micro)longitudinal studies, poor sleep was generally a stronger, more reliable predictor of pain than pain was of poor sleep (17). Further, poor sleep also predicted experimental pain outcomes such as endogenous pain modulatory processes (29). This conclusion identifies a directional relationship between sleep and pain, rather than an equally bi-directional relationship as commonly thought.
To our knowledge, the majority of previous research examining predictors of experimental pain sensitivity has addressed race, depressive symptoms, and sleep quality independent of each another. Consideration of these factors together as part of a single microlongitudinal study may help identify a sequence of associations among the mechanisms underpinning racial differences in experimental pain sensitivity. Therefore, this study specifically addressed differences in depressive symptoms, sleep quality, and temporal summation of mechanical pain between AAs and NHWs. Temporal summation of pain is a pain sensitivity test that is widely incorporated in experimental studies to invoke neural mechanisms related to endogenous pain facilitation (30, 31). We hypothesized that AAs would demonstrate significantly greater depressive symptoms, poorer sleep quality, and greater temporal summation of mechanical pain compared to NHWs. It was further hypothesized that depressive symptoms and sleep quality would sequentially mediate the difference in temporal summation of mechanical pain between AAs and NHWs. Figure 1 illustrates our putative conceptual model.
Figure 1.
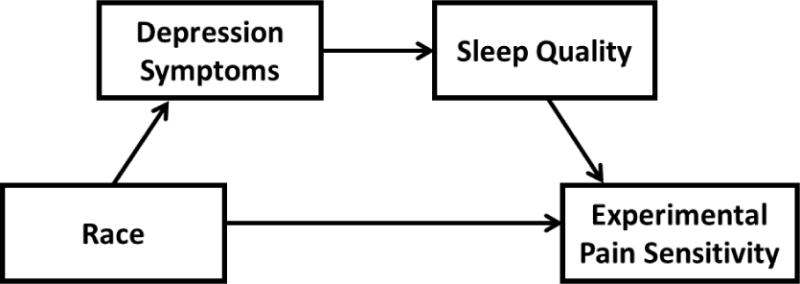
Putative conceptual model.
MATERIALS AND METHODS
Study Design & Participants
All participants underwent two study sessions in a laboratory setting that lasted approximately 1.5 hours each. All study protocols were approved by the local Institutional Review Board and carried out in accordance with guidelines for the ethical conduct of research. Written informed consent was obtained from each participant prior to the study and they were compensated for their involvement. Notably, a microlongitudinal study design (Figure 2) was utilized to provide a temporal precedence for arguing the directionality of associations hypothesized in our conceptual model.
Figure 2.
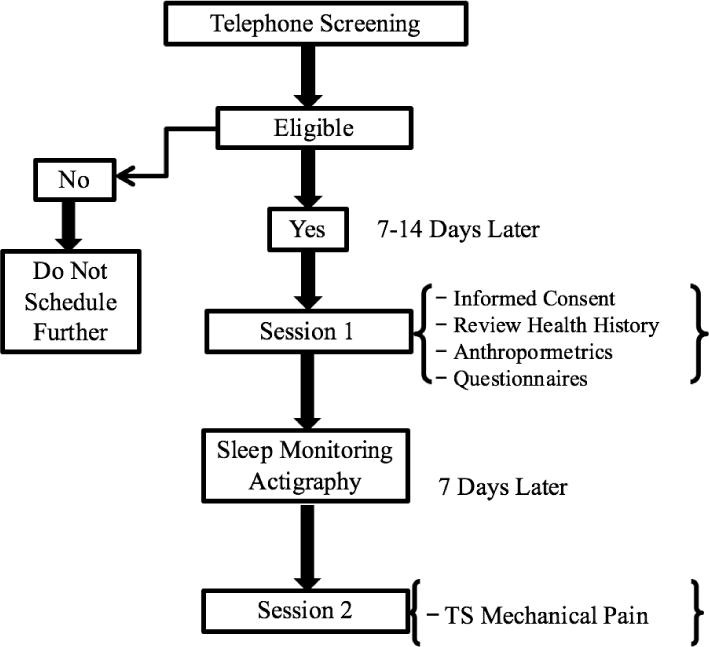
Microlongitudinal study design.
The study’s final sample was composed of 50 healthy adults recruited from the Birmingham, Alabama community who reported their ethnicity as non-Hispanic and their race as either white (24 participants) or African American (26 participants). The participants were divided by sex and race in nearly equal proportions (56% female, 52% AA) and ranged in age from 21 to 77 years old. Individuals interested in being part of this study were assessed for eligibility during an initial telephone screening. Healthy participants were chosen for this study in large part to eliminate extraneous factors that may influence pain perception, depressive symptoms, or sleep quality, such as pre-existing pain conditions and chronic systemic medical disorders. To this end, interested participants were only included in the study if they denied any ongoing chronic pain problems and also denied any episodes of acute pain within the 2 weeks prior to study participation. Additionally, all study participants fulfilled the following criteria for inclusion: not currently pregnant, no evidence of uncontrolled hypertension (i.e., resting blood pressure > 150/95 mmHg) assessed via sphygmomanometer, no circulatory disorders, and no history of cardiac events, stroke, seizure, metabolic disease, neuropathy, and cancer. Eligible participants then presented for an initial study session, during which time they provided demographic and anthropometric information. They also completed measures of obstructive sleep apnea risk and depressive symptoms prior to seven consecutive nights of sleep monitoring with wrist actigraphy in the home environment. Upon completion of sleep monitoring, participants returned to the laboratory for a quantitative sensory testing session to assess endogenous pain facilitation using a mechanical temporal summation protocol. All study participants underwent the same set of procedures.
Measures
CES-D
The Center for Epidemiological Studies-Depression Scale (CES-D) is a 20-item measure of depressive symptoms including negative mood, guilt/worthlessness, helplessness/hopelessness, psychomotor retardation, loss of appetite, and sleep disturbance (32). Individuals report on their experiences of depressive symptoms over the past week. This measure has been shown to be reliable and valid in general populations, including when used in racial minority groups (33). The CES-D is generally accepted as a useful tool for screening depressive symptomatology (34), and was therefore used to characterize depressive symptoms in the current study. The total score ranges from 0 to 60, and scores above 16 are suggestive of possible clinical levels of depressive symptomatology (35).
STOP-BANG
The STOP-BANG Sleep Apnea Questionnaire is used to screen patients for obstructive sleep apnea (OSA). The STOP-BANG consists of eight yes/no questions, scored 0 = no/1 = yes (range 0–8). The STOP-BANG assesses for major risk factors of OSA including snoring, daytime fatigue, and high blood pressure, external observation of the participant not breathing while sleeping, a body mass index (BMI) >35kg/m2, age over 50 years, neck circumference > 16 inches, and male gender (36). Risk for OSA is considered high if the participant answers “yes” to 5 or more of the questions. Intermediate risk is indicated by a score of 3–4, while a score of <3 suggests low risk (37). High internal validity and sensitivity has been demonstrated in patients without history of sleep disorders. As such, the STOP-BANG questionnaire has been recommended for use due to its high methodological quality and ease of administration (38). None of the participants were excluded due to sleep apnea risk, rather the intent was to quantify risk and include it as a covariate in data analysis. This was done to control for the variance in sleep quality and depressive symptoms that was attributable to obstructive sleep apnea.
Sleep Assessment
Actigraphy
Objective sleep data was acquired using the Actiwatch2 (Respironics, Bend, OR), a wrist-worn, watch-like actigraph. The Actiwatch2 is a solid-state accelerometer, or movement detector, designed to measure ambulatory activity. It was used to measure daily sleep-wake patterns and record body movement. The Actiwatch2 has good reliability and criterion validity (39, 40). Actigraphy has been shown to be comparable to polysomnography (41, 42), and actigraphic measurement produces valid data in persons with and without chronic pain (43, 44). Study participants were instructed to depress a button (event marker) on the Actiwatch2 at bedtime and upon waking in the morning. These events were also compared to the corresponding sleep diaries participants completed daily. With the aid of these diaries and event markers, researchers used a protocol for actigraphy scoring and set sleep periods. Sleep-wake patterns were extracted from the actigraphy data using the Actiware Sleep version 6.0.8, which bases its algorithm on the amplitude and frequency of detected movements, which were scored in 30-second epochs.
The following parameters were derived from the actigraphy data: total sleep time, sleep onset latency, wake after sleep onset time, and sleep efficiency. Each parameter was averaged across seven nights of actigraphy for overall measures of sleep quality a week prior to the quantitative sensory testing session. Total sleep time was scored as sleep in minutes from sleep onset to sleep offset. Sleep onset latency represents the length of time in minutes it took to transition from fully awake to asleep. Wake after sleep onset was calculated by adding the number of minutes in which participants were awake from sleep onset to final awakening. Sleep efficiency is the ratio of estimated total sleep time divided by total time spent in bed as a percentage times 100, with values closer to 100 meaning the most efficient sleep.
Quantitative Sensory Testing
Temporal Summation of Mechanical Pain
Participants underwent a mechanical procedure designed to assess temporal summation of mechanical pain using a nylon monofilament (Touchtest Sensory Evaluator 6.65). This filament is calibrated to bend at 300g of pressure. We have successfully used this procedure in our previous work (7, 45). Testing sites included the back of the non-dominant hand and the ipsilateral superior trapezius, in randomized order. These testing sites were selected due to ease of accessibility and because they allowed for a more comprehensive assessment of temporal summation given that the back of the hand (C7) and superior trapezius (C4) are innervated by separate spinal nerves with distinct dermatomal distributions. To assess temporal summation of mechanical pain at each site, participants were subjected to a single contact of the monofilament and then asked to rate the intensity of the pain resulting from this one contact using a 0–100 numeric rating scale where 0 = “no pain” and 100 = “the most intense pain imaginable.” Participants were then subjected to a series of 10 additional contacts at a rate of one contact per second, after which they were again asked to provide one additional 0–100 rating representing the greatest pain intensity experienced during the series of 10 contacts. This procedure was repeated twice at each anatomical location. Pain ratings for the single and multiple contacts performed at each anatomical location were averaged across the two trials. The magnitude of temporal summation (i.e., Δ change scores for temporal summation at the hand and trapezius) was calculated by subtracting pain intensity ratings following the 1st contact from pain intensity ratings following the 10th contact.
Data Analysis
All data were analyzed using SPSS, version 22 (IBM, Chicago, IL, USA). All participants provided complete demographic and quantitative sensory testing data. Descriptive statistics were computed for the overall sample as well as separately by racial group; data are presented as percentages or as means and standard deviations. Differences in categorical data were examined using Chi-square. Two separate paired samples t-tests comparing the pain rating following a single contact to the maximal pain rating following 10 contacts were used to evaluate whether significant temporal summation occurred at each site. Univariate ANCOVAs were then utilized to examine racial differences in depressive symptoms, objective sleep data, and temporal summation of mechanical pain. Finally, a sequential mediation model was used to evaluate predictive relationships among race, depressive symptoms, objective sleep parameters, and temporal summation of mechanical pain (46). Although all of the objective sleep parameters were examined, it was anticipated that sleep efficiency would be the most relevant given that its calculation is derived from the other parameters (e.g., total sleep time = time in bed — sleep onset latency — wake after sleep onset; sleep efficiency = (total sleep time/time in bed) * 100) (47).
Covariates
Even in healthy participants there are likely still confounding factors that relate to depressive symptoms, sleep quality, and temporal summation of mechanical pain. Demographic factors including age, sex, and indicators of socioeconomic status (e.g., education level) have all been shown to contribute to the experience of pain (7, 48, 49). Further, obstructive sleep apnea is a risk factor for poor depressive symptoms (50) and poor sleep quality (51). Accordingly, age, sex (coded 0 = women, 1 = men), education level (coded 0 = high school or less, 1 = some college or more), and risk for obstructive sleep apnea (coded 0 = low/intermediate risk, 1 = high risk) were included in all analyses as covariates.
RESULTS
Participant characteristics
Characteristics of the 50 healthy adults who participated in the study are presented in Table 1. The mean age of the sample was 49.04 (SD = 12.07, range 21–77), with slightly greater proportion of women participating (56% women). NHW and AA races were approximately equally represented (52% AA). A significantly greater proportion of NHWs (87.5%) than AAs (42.3%) reported completing some college or greater (χ2 = 11.06, p = .001). On the STOP-BANG questionnaire, which measures risk for obstructive sleep apnea, approximately 22% of the overall sample met criteria for “high risk.” The remaining 78% were found to have “low to intermediate risk” of obstructive sleep apnea. Obstructive sleep apnea risk did not significantly differ between AAs and NHWs. Neither acute pain nor a history of chronic pain was endorsed by any of the participants, and none reported taking any prescribed or over-the-counter pain medications prior to the first or second study sessions.
Table 1.
Participant Characteristics
| Overall (N = 50) |
African-American (N = 26) |
Non-Hispanic White (N = 24) |
|
|---|---|---|---|
| Variable | |||
| Age (y ± SD) | 49.0 ± 12.1 | 49.0 ± 9.5 | 49.0 ± 14.6 |
| Gender | |||
| Male (%) | 44.0 | 46.2 | 41.7 |
| Female (%) | 56.0 | 53.8 | 58.3 |
| Education | |||
| High school or less (%) | 36.0 | 57.7 | 12.5 |
| Some college or greater (%) | 64.0 | 42.3 | 87.5 |
| STOP-Bang | |||
| High Risk (%) | 22.0 | 30.8 | 12.5 |
| Intermediate/Low Risk (%) | 78.0 | 69.2 | 87.5 |
| BMI (kg/m2 ± SD) | 30.1 ± 9.1 | 31.6 ± 9.6 | 28.4 ± 8.4 |
Note. SD, standard deviation; BMI, Body Mass Index is calculated by dividing an individual’s weight (kg) by height (m2); STOP-Bang, the STOP-Bang Sleep Apnea Questionnaire is a risk assessment for Obstructive Sleep Apnea (OSA). Individuals that score 5 or higher are considered High Risk for OSA. Individuals that score less than 5 are considered Intermediate to Low Risk for OSA
Descriptive data representing depressive symptoms and actigraphic sleep parameters both overall and by race differences are shown in Table 2. Scores on the CES-D, an index of depressive symptoms, ranged from 2–43 with a mean of 13.45 (SD = 10.1). Overall total sleep time was 380.69 minutes (SD = 65.41), or 6.34 hours, per night with an average efficiency of 79.66% (SD = 9.58). Sleep onset latency was approximately 25.61 (SD = 44.79) minutes, with an average of 53.38 (SD = 26.82) minutes of wake time after the onset of sleep.
Table 2.
Descriptive data representing depressive symptoms and actigraphic sleep parameters for African-American and non-Hispanic white community-dwelling adults
| Total M (SD) |
AA M (SD) |
NHW M (SD) |
p | |
|---|---|---|---|---|
| CES-D | 13.45 (10.11) | 17.72 (11.32) | 9.00 (6.26) | 0.043 |
| Sleep efficiency | 79.66 (9.58) | 75.60 (9.11) | 83.89 (8.25) | 0.046 |
| Sleep onset latency | 25.61 (44.79) | 35.51 (58.31) | 15.29 (20.55) | 0.444 |
| Total sleep time | 380.69 (65.41) | 354.48 (60.52) | 408.00 (59.85) | 0.035 |
| Wake after sleep onset | 53.38 (26.82) | 59.51 (27.74) | 46.99 (24.79) | 0.518 |
Note. CES-D, Center for Epidemiological Studies Depression Scale.
Temporal summation of mechanical pain
Across all participants, paired samples t-tests revealed that the mean pain intensity rating following the 10th contact with the mechanical stimulus was significantly greater than the mean pain intensity rating following the 1st contact when assessed at the hand (t49 = 5.78, p < .001) and the trapezius (t49 = 5.11, p < .001). These increases in pain intensity ratings represent statistically significant temporal summation of mechanical pain for both anatomical testing sites.
Racial differences in temporal summation of mechanical pain, depressive symptoms, and sleep quality
In an adjusted model controlling for all covariates, AAs demonstrated significantly greater temporal summation of mechanical pain compared to NHWs at both the hand (F(1, 44) = 4.15, p = .048) and the trapezius (F(1, 44) = 5.67, p = .022) (Table 3). AAs also reported significantly greater severity of depressive symptoms than their NHW counterparts (F(1, 44) = 4.34, p = .043). AAs experienced poorer nightly actigraphic sleep efficiency than NHWs (F(1, 44) = 4.21, p = 0.046). Additionally, AAs spent less total time asleep each night on average (i.e., total sleep time) compared to their NHW counterparts (F(1, 44) = 4.75, p = 0.035). In contrast, no significant racial differences were observed for either the sleep onset latency or wake after sleep onset parameters (both p’s > 0.05).
Table 3.
Assessment of temporal summation of mechanical pain at the hand, trapezius, and overall between African American (AA) and non-Hispanic white (NHW) community-dwelling adults.
| Variables | Racial Group | Mean (SD) | p |
|---|---|---|---|
| Hand | |||
| Pain rating – 1st contact | NHW AA |
3.15 (4.12) 5.38 (8.25) |
0.227 |
| Pain rating – 10 contacts | NHW AA |
6.54 (6.62) 13.96 (13.10) |
0.015 |
| Temporal summation – Hand | NHW AA |
3.39 (4.20) 8.58 (8.89) |
0.048 |
| Trapezius | |||
| Pain rating – 1st contact | NHW AA |
3.33 (3.60) 4.58 (6.48) |
0.402 |
| Pain rating – 10 contacts | NHW AA |
7.21 (4.72) 15.94 (16.39) |
0.014 |
| Temporal summation – Trapezius | NHW AA |
3.88 (3.43) 11.37 (13.69) |
0.022 |
| Overall Temporal Summation* | NHW AA |
3.64 (3.37) 8.93 (8.83) |
0.012 |
Note: All pain intensity ratings were provided using a 0–100 numeric rating scale with 0 = “no pain” and 100 = “the most intense pain imaginable”. The magnitude of temporal summation represents a difference score (10th contact–1st contact) at each site.
Overall temporal summation is the average of site specific temporal summation at the hand and trapezius.
Zero-order correlations
Temporal summation of mechanical pain was found to be significantly correlated between the two anatomical locations — hand and trapezius (r = .54, p < .001). Therefore, temporal summation of mechanical pain was averaged across the two anatomical locations to create overall temporal summation of mechanical pain that was used in subsequent analyses. Additional Pearson correlations are presented in Table 4. Heightened overall temporal summation of mechanical pain was significantly correlated with all four actigraphic sleep quality parameters including, less sleep efficiency and total sleep time as well as greater sleep onset latency and wake after sleep onset. Greater depressive symptoms were significantly correlated with both lower sleep efficiency and less total sleep time. However, depressive symptoms were not significantly correlated with overall temporal summation of mechanical pain.
Table 4.
Correlation Matrix
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
|---|---|---|---|---|---|---|---|---|---|
| 1. Sex | — | ||||||||
| 2. Age | .11 | — | |||||||
| 3. Education | −.18 | −.05 | — | ||||||
| 4. Stop-Bang | .31* | .38** | −.01 | — | |||||
| 5. CES-D | −.08 | .11 | −.40** | −.02 | — | ||||
| 6. Sleep | −.19 | .03 | .32* | −.13 | −0.46** | — | |||
| 7. Sleep onset | .21 | −.07 | −.27 | −.01 | 0.19 | −0.62** | — | ||
| 8. Total sleep | −.34* | .03 | .22 | −.19 | −0.30* | 0.71** | −0.60** | — | |
| 9. Wake after | .11 | .03 | −.25 | .08 | 0.23 | −0.55** | 0.03 | −0.16 | — |
| 10. Overall | .12 | .20 | −.11 | .12 | 0.11 | −0.53** | 0.44** | −0.29* | 0.36* |
Note p < 0.05,
p < 0.01.
Sex coded 0 = women, 1 = men), education level coded 0 = high school or less, 1 = some college or more, and risk for obstructive sleep apnea (Stop-Bang) coded 0 = low/intermediate risk, 1 = high risk; CES-D = Center for Epidemiological Studies Depression Scale.
Sequential mediation
A sequential mediation analysis controlling for covariates was conducted via bootstrapping methods (41). This analysis provides estimates of path coefficients and significance tests for specified mediational models, as well as estimates of indirect effects. All paths for the full model are illustrated in Figure 3. In this analysis sleep efficiency was chosen as the primary indicator of sleep quality given that it was most strongly related to race, depressive symptoms, and overall temporal summation of mechanical pain. The indirect effect of AA versus NHW race on overall temporal summation of mechanical pain through both depressive symptoms and sleep efficiency was significant with a point estimate of −0.99 and a 95% confidence interval of −2.325 to −0.086. These results support the hypothesized sequential mediation model. AA race predicted greater severity of depressive symptoms (t = −2.08, p = .043). In turn, greater severity of depressive symptoms predicted poorer sleep efficiency (t = −2.55, p = .014). While poorer sleep efficiency subsequently predicted greater temporal summation of mechanical pain for AAs (t = −4.11, p = .0002). This model was then tested again with the mediators reversed, such that sleep efficiency predicted depressive symptoms, and the sequential mediation results were non-significant in this instance. Lastly, total sleep time was examined as an alternative indicator of sleep quality in a subsequent analysis; however, the results showed that sequential mediation in the model including total sleep time was also non-significant.
Figure 3.
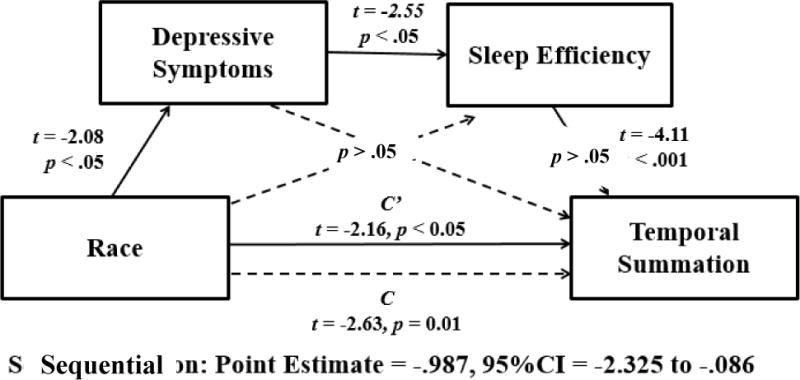
Sequential Mediation Model.
DISCUSSION
Previous studies that have addressed racial differences in clinical pain consistently revealed that AAs reported more severe pain compared to NHWs (52). One explanation for greater clinical pain severity in AA populations has been that AAs often demonstrate enhanced endogenous pain facilitation when assessed using experimental protocols of heat and/or mechanical temporal summation (6, 7). It has been suggested by Yarnitsky and colleagues that an individual (or population of individuals such as AAs) expressing enhanced pain facilitatory processes could be characterized as pro-nociceptive (53). As a result, this individual (or population of individuals) would express a more pain sensitive phenotype that increases the risk of developing (or perpetuating) a chronic pain condition. Understanding how different racial groups experience pain is crucial to enhancing pain assessment and treatment by allowing providers to personalize pain medicine based on the sources of this variation. Specifically studying differences in how pain is experienced between AAs and NHWs seems worthwhile because each represents major population groups that show significant differences in pain-related phenotypes (45, 54, 55). Also, the influence of race on pain responses is driven by complex interactions among multiple pain-relevant biological, psychological, behavioral, and sociocultural factors (52, 56). This study included two important psychological and behavioral factors: depressive symptoms and sleep quality, respectively. The ultimate goal of this investigation was to characterize the relationships amongst race, depressive symptoms, sleep quality, and endogenous pain facilitatory processes in community-dwelling adults without chronic pain.
A noteworthy shortcoming of the previous research that specifically investigated racial differences in pain has been the lack of consideration for clinically-relevant mechanisms that may help explain how these racial differences manifest. The goal of this study was to specifically address this shortcoming in the published literature. Along this line, AAs reported significantly greater depressive symptoms, poorer sleep efficiency, and attenuated total sleep time in comparison to their NHW counterparts. Our results indicate AA participants on average reported clinically relevant levels of depressive symptoms (mean CES-D > 16) (35) and poor sleep efficiency suggestive of insomnia (mean sleep efficiency < 85%) (57). This is consistent with prior research in community-dwelling samples indicating that AAs often report greater mood disturbances (13, 58) and lower quality sleep (14–16) than their NHW counterparts. Lastly, results indicated that AAs demonstrated greater temporal summation of mechanical pain than NHWs at the hand and the trapezius, as well as the overall index of endogenous pain facilitation.
Given the above findings, a sequential mediation model was tested to determine whether the impact of depressive symptoms on sleep quality might help explain the observed racial difference in mechanical temporal summation between AAs and NHWs. Findings demonstrated that AA race predicted greater severity of depressive symptoms, which in turn predicted poorer sleep efficiency, which subsequently predicted increased endogenous pain facilitation (i.e., mechanical temporal summation). This model was not replicated when using total sleep time in place of sleep efficiency. In addition, the microlongitudinal nature of the study design allows for the careful interpretation that greater depressive symptoms predicted poorer sleep quality (i.e., efficiency) in this context, rather than the reverse. Overall, the study’s hypothesis that depressive symptoms and sleep quality would sequentially mediate the racial difference in temporal summation of mechanical pain was confirmed. To our knowledge, we are the first to evaluate all of these factors simultaneously in one model to ascertain directional relationships.
Interestingly, this investigation suggests that not all parameters of actigraphic sleep quality mediate racial differences in endogenous pain facilitation. In fact, only two of the four sleep quality parameters were found to have significant racial differences (sleep efficiency and total sleep time), and only one of those parameters was significant in the tested sequential mediation model. This suggests that sleep efficiency may be of particular interest when characterizing poor sleep in AAs across the adult lifespan. As noted previously, one explanation may be that sleep efficiency is the best indicator of overall sleep quality, given that its calculation takes total sleep time, sleep onset latency, and wake after sleep onset into account (47). Thus, it is the most relevant for examining in relation to depressive symptoms and experimental pain sensitivity. Another explanation may be related to differences in relationships amongst depressive symptoms, total sleep time, and sleep efficiency. Depressive symptoms may confer a propensity towards insomnia symptoms (59) or hypersomnolence (60), both of which can increase total sleep time. However, such an increase in total sleep time is not necessarily suggestive of better or more efficient sleep. Indeed, depressive symptoms such as fatigue, anhedonia, and physical inactivity may lead to more time spent sleeping throughout the night and day (61), but less time obtaining efficient, restful, and uninterrupted sleep (18).
Overall, these results indicate that even in relatively healthy, community-dwelling adults, clinically relevant levels of depression, insomnia, and pain sensitivity may still be present. Further clinical implications of our findings, particularly for AAs, are underscored by previous research that has related depression, poor sleep, and experimental pain responses to clinical pain outcomes. Depression and sleep quality are well documented predictors of chronic pain development and severity, as well as related disability (17, 62). Further, enhanced pain facilitatory processes have been shown to be predictive of post-operative chronic pain development and severity (7, 63). It has even been suggested that responses to experimental pain testing may be better related to the clinical pain experiences of AAs compared to NHWs (52). Importantly, AAs often experience a greater prevalence and severity of chronic pain across a variety of clinical conditions relative to NHWs (64), and it may be that depressive symptoms, poor sleep, and endogenous pain facilitation play a contributory role. Additional research on this topic is needed to confirm or refute such a hypothesis.
Results of this investigation suggest that efforts to better assess mood and sleep in the clinical setting may prove crucial for identifying modifiable treatment targets. Also, our findings lend further support to the incorporation of treatments intended to reduce depressive symptoms and improve sleep quality in those at risk for (or who already have) chronic pain, particularly AA populations. Use of evidence-based treatments (e.g., cognitive behavioral therapy) designed to mitigate depressive symptoms in AAs may also exert beneficial downstream effects on sleep quality and pain sensitivity. Given the bi-directional overlap between depression and sleep reported in other studies (65, 66), it may be that behavioral sleep medicine interventions designed to improve sleep quality also concomitantly exert positive impacts on depressive symptoms and pain sensitivity, particularly for AAs. Ideally, depressive symptoms and sleep quality should be equally addressed as part of a comprehensive pain treatment plan.
Several limitations are worthy of mention when considering the findings of this study. First, this study included only community-dwelling adults without any chronic pain conditions. This limits the ability to generalize our findings to clinical pain populations who may very well demonstrate greater severity of depressive symptoms, sleep impairments, and altered pain sensitivity. Second, we attempted to recruit only community-dwelling individuals who were healthy; however, as mentioned above, AAs demonstrated significantly greater levels of depressive symptoms that were clinically relevant (mean CES-D > 16). Elevated depressive symptoms are known to be associated with other factors that can influence pain sensitivity such as stress (67) and inflammation (68), which were not examined as part of this study. Therefore, we cannot rule out the possibility that other depression-related mediators besides sleep might account for racial differences in temporal summation of mechanical pain. Third, although the microlongitudinal design of this study helps to establish a temporal precedence among key variables, the ability to make reliable causal inferences is limited by the lack of a true longitudinal study design with repeat measurements. Therefore, the directionality of reported effects in this study should be considered tentative and interpreted with caution. Future research extending the timeline of assessment and examining the development of depression and sleep impairments in AAs with and without chronic pain will be necessary to further validate the clinical relevance of the current study’s findings. Finally, the sample size included in the current study was rather small. Although the significant findings preclude concerns about statistical power, additional studies with larger sample sizes are necessary to replicate our findings and further establish their reliability.
Despite these limitations, this study provides evidence of the detrimental impact of depressive symptoms on sleep efficiency, suggesting that both sequentially mediate the difference in endogenous pain facilitation between AAs and NHWs when assessed by temporal summation of mechanical pain. Further, incorporation of interventions intended to reduce depressive symptoms and improve sleep efficiency in those at risk for chronic pain may be warranted. Although these results suggest a significant impact of race, depressive symptoms, and sleep efficiency on the experimental pain sensitivity of community-dwelling AAs, it will be important to examine and validate these relationships in clinical populations (e.g., chronic pain patients). If additional research indicates that the model in this study is relevant for clinical pain outcomes, then treatments that improve the mood and sleep of AAs may help reduce the high burden of chronic pain currently experienced by this racial group. Continuing research in this area could result in more effective, individually tailored pain treatments for those at risk for chronic pain.
Acknowledgments
Support for this study was provided by an NIH/NIA Health Disparities Research Pilot Award through the Deep South Resource Center for Minority Aging Research (RCMAR); 5P30AG031054.
Footnotes
None of the authors have any conflict of interest to report.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
References
- 1.Staud R. The important role of CNS facilitation and inhibition for chronic pain. Int J Clin Rheumatol. 2013;8(6):639–646. doi: 10.2217/ijr.13.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Magerl W, Wilk SH, Treede RD. Secondary hyperalgesia and perceptual wind-up following intradermal injection of capsaicin in humans. Pain. 1998;74(2–3):257–268. doi: 10.1016/s0304-3959(97)00177-2. [DOI] [PubMed] [Google Scholar]
- 3.Price DD, Hu JW, Dubner R, Gracely RH. Peripheral suppression of first pain and central summation of second pain evoked by noxious heat pulses. Pain. 1977;3(1):57–68. doi: 10.1016/0304-3959(77)90035-5. [DOI] [PubMed] [Google Scholar]
- 4.Staud R, Weyl EE, Riley JL, 3rd, Fillingim RB. Slow temporal summation of pain for assessment of central pain sensitivity and clinical pain of fibromyalgia patients. PloS one. 2014;9(2):e89086. doi: 10.1371/journal.pone.0089086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain. 2011;152(3):S2–15. doi: 10.1016/j.pain.2010.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bulls HW, Goodin BR, McNew M, Gossett EW, Bradley LA. Minority Aging and Endogenous Pain Facilitatory Processes. Pain Med. 2016;17(6):1037–48. doi: 10.1093/pm/pnv014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goodin BR, Bulls HW, Herbert MS, et al. Temporal summation of pain as a prospective predictor of clinical pain severity in adults aged 45 years and older with knee osteoarthritis: ethnic differences. Psychosom Med. 2014;76(4):302–310. doi: 10.1097/PSY.0000000000000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bar KJ, Brehm S, Boettger MK, Boettger S, Wagner G, Sauer H. Pain perception in major depression depends on pain modality. Pain. 2005;117(1–2):97–103. doi: 10.1016/j.pain.2005.05.016. [DOI] [PubMed] [Google Scholar]
- 9.de Souza JB, Potvin S, Goffaux P, Charest J, Marchand S. The deficit of pain inhibition in fibromyalgia is more pronounced in patients with comorbid depressive symptoms. Clin J Pain. 2009;25(2):123–7. doi: 10.1097/AJP.0b013e318183cfa4. [DOI] [PubMed] [Google Scholar]
- 10.Edwards RR, Grace E, Peterson S, Klick B, Haythornthwaite JA, Smith MT. Sleep continuity and architecture: associations with pain-inhibitory processes in patients with temporomandibular joint disorder. Eur J Pain. 2009;13(10):1043–1047. doi: 10.1016/j.ejpain.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee YC, Lu B, Edwards RR, et al. The role of sleep problems in central pain processing in rheumatoid arthritis. Arthritis Rheum. 2013;65(1):59–68. doi: 10.1002/art.37733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith MT, Edwards RR, McCann UD, Haythornthwaite JA. The effects of sleep deprivation on pain inhibition and spontaneous pain in women. Sleep. 2007;30(4):494–505. doi: 10.1093/sleep/30.4.494. [DOI] [PubMed] [Google Scholar]
- 13.Dunlop DD, Song J, Lyons JS, Manheim LM, Chang RW. Racial/ethnic differences in rates of depression among preretirement adults. Am J Public Health. 2003;93(11):1945–1952. doi: 10.2105/ajph.93.11.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen X, Wang R, Zee P, et al. Racial/Ethnic Differences in Sleep Disturbances: The Multi-Ethnic Study of Atherosclerosis (MESA) Sleep. 2015;38(6):877–888. doi: 10.5665/sleep.4732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ruiter ME, DeCoster J, Jacobs L, Lichstein KL. Sleep disorders in African Americans and Caucasian Americans: a meta-analysis. Behav Sleep Med. 2010;8(4):246–259. doi: 10.1080/15402002.2010.509251. [DOI] [PubMed] [Google Scholar]
- 16.Ruiter ME, Decoster J, Jacobs L, Lichstein KL. Normal sleep in African-Americans and Caucasian-Americans: A meta-analysis. Sleep Med. 2011;12(3):209–214. doi: 10.1016/j.sleep.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 17.Finan PH, Goodin BR, Smith MT. The association of sleep and pain: an update and a path forward. J Pain. 2013;14(12):1539–1552. doi: 10.1016/j.jpain.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lacruz ME, Schmidt-Pokrzywniak A, Dragano N, et al. Depressive symptoms, life satisfaction and prevalence of sleep disturbances in the general population of Germany: results from the Heinz Nixdorf Recall study. BMJ open. 2016;6(1):e007919. doi: 10.1136/bmjopen-2015-007919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hamilton M. Frequency of symptoms in melancholia (depressive illness) Br J Psychiatry. 1989;154:201–206. doi: 10.1192/bjp.154.2.201. [DOI] [PubMed] [Google Scholar]
- 20.Nutt D, Wilson S, Paterson L. Sleep disorders as core symptoms of depression. Dialogues Clin Neurosci. 2008;10(3):329–336. doi: 10.31887/DCNS.2008.10.3/dnutt. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yates WR, Mitchell J, John Rush A, et al. Clinical features of depression in outpatients with and without co-occurring general medical conditions in STAR*D: confirmatory analysis. Prim Care Companion J Clin Psychiatry. 2007;9(1):7–15. doi: 10.4088/pcc.v09n0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buysse DJ, Angst J, Gamma A, Ajdacic V, Eich D, Rossler W. Prevalence, course, and comorbidity of insomnia and depression in young adults. Sleep. 2008;31(4):473–480. doi: 10.1093/sleep/31.4.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fok M, Stewart R, Besset A, Ritchie K, Prince M. Incidence and persistence of sleep complaints in a community older population. Int J Geriatr Psychiatry. 2010;25(1):37–45. doi: 10.1002/gps.2295. [DOI] [PubMed] [Google Scholar]
- 24.Jaussent I, Dauvilliers Y, Ancelin ML, et al. Insomnia symptoms in older adults: associated factors and gender differences. Am J Geriatr Psychiatry. 2011;19(1):88–97. doi: 10.1097/JGP.0b013e3181e049b6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haack M, Scott-Sutherland J, Santangelo G, Simpson NS, Sethna N, Mullington JM. Pain sensitivity and modulation in primary insomnia. Eur J Pain. 2012;16(4):522–533. doi: 10.1016/j.ejpain.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sivertsen B, Lallukka T, Petrie KJ, Steingrimsdottir OA, Stubhaug A, Nielsen CS. Sleep and pain sensitivity in adults. Pain. 2015;156(8):1433–1439. doi: 10.1097/j.pain.0000000000000131. [DOI] [PubMed] [Google Scholar]
- 27.Anderson RJ, McCrae CS, Staud R, Berry RB, Robinson ME. Predictors of clinical pain in fibromyalgia: examining the role of sleep. J Pain. 2012;13(4):350–358. doi: 10.1016/j.jpain.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buenaver LF, Quartana PJ, Grace EG, et al. Evidence for indirect effects of pain catastrophizing on clinical pain among myofascial temporomandibular disorder participants: the mediating role of sleep disturbance. Pain. 2012;153(6):1159–1166. doi: 10.1016/j.pain.2012.01.023. [DOI] [PubMed] [Google Scholar]
- 29.Petrov ME, Goodin BR, Cruz-Almeida Y, et al. Disrupted sleep is associated with altered pain processing by sex and ethnicity in knee osteoarthritis. J Pain. 2015;16(5):478–490. doi: 10.1016/j.jpain.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Staud R, Cannon RC, Mauderli AP, Robinson ME, Price DD, Vierck CJ., Jr Temporal summation of pain from mechanical stimulation of muscle tissue in normal controls and subjects with fibromyalgia syndrome. Pain. 2003;102(1–2):87–95. doi: 10.1016/s0304-3959(02)00344-5. [DOI] [PubMed] [Google Scholar]
- 31.Staud R, Craggs JG, Robinson ME, Perlstein WM, Price DD. Brain activity related to temporal summation of C-fiber evoked pain. Pain. 2007;129(1–2):130–142. doi: 10.1016/j.pain.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 33.Turner AD, Capuano AW, Wilson RS, Barnes LL. Depressive symptoms and cognitive decline in older african americans: two scales and their factors. Am J Geriatr Psychiatry. 2015;23(6):568–578. doi: 10.1016/j.jagp.2014.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Knight RG, Williams S, McGee R, Olaman S. Psychometric properties of the Centre for Epidemiologic Studies Depression Scale (CES-D) in a sample of women in middle life. Behav Res Therapy. 1997;35(4):373–80. doi: 10.1016/s0005-7967(96)00107-6. [DOI] [PubMed] [Google Scholar]
- 35.Vilagut G, Forero CG, Barbaglia G, Alonso J. Screening for Depression in the General Population with the Center for Epidemiologic Studies Depression (CES-D): A Systematic Review with Meta-Analysis. PloS one. 2016;11(5):e0155431. doi: 10.1371/journal.pone.0155431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chung F, Abdullah HR, Liao P. STOP-Bang Questionnaire: A Practical Approach to Screen for Obstructive Sleep Apnea. Chest. 2016;149(3):631–638. doi: 10.1378/chest.15-0903. [DOI] [PubMed] [Google Scholar]
- 37.Chung F, Subramanyam R, Liao P, Sasaki E, Shapiro C, Sun Y. High STOP-Bang score indicates a high probability of obstructive sleep apnoea. Br J Anaesth. 2012;108(5):768–75. doi: 10.1093/bja/aes022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abrishami A, Khajehdehi A, Chung F. A systematic review of screening questionnaires for obstructive sleep apnea. Can J Anaesth. 2010;57(5):423–438. doi: 10.1007/s12630-010-9280-x. [DOI] [PubMed] [Google Scholar]
- 39.Gironda RJ, Lloyd J, Clark ME, Walker RL. Preliminary evaluation of reliability and criterion validity of Actiwatch-Score. J Rehabil Res Dev. 2007;44(2):223–230. doi: 10.1682/jrrd.2006.06.0058. [DOI] [PubMed] [Google Scholar]
- 40.Wood AC, Kuntsi J, Asherson P, Saudino KJ. Actigraph data are reliable, with functional reliability increasing with aggregation. Behav Res Methods. 2008;40(3):873–878. doi: 10.3758/brm.40.3.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Blackwell T, Redline S, Ancoli-Israel S, et al. Comparison of sleep parameters from actigraphy and polysomnography in older women: the SOF study. Sleep. 2008;31(2):283–291. doi: 10.1093/sleep/31.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kushida CA, Chang A, Gadkary C, Guilleminault C, Carrillo O, Dement WC. Comparison of actigraphic, polysomnographic, and subjective assessment of sleep parameters in sleep-disordered patients. Sleep Med. 2001;2(5):389–396. doi: 10.1016/s1389-9457(00)00098-8. [DOI] [PubMed] [Google Scholar]
- 43.O’Donoghue GM, Fox N, Heneghan C, Hurley DA. Objective and subjective assessment of sleep in chronic low back pain patients compared with healthy age and gender matched controls: a pilot study. BMC Musculoskel Disord. 2009;10:122. doi: 10.1186/1471-2474-10-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sadeh A. The role and validity of actigraphy in sleep medicine: an update. Sleep Med Rev. 2011;15(4):259–267. doi: 10.1016/j.smrv.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 45.Cruz-Almeida Y, Sibille KT, Goodin BR, et al. Racial and ethnic differences in older adults with knee osteoarthritis. Arthritis Rheum. 2014;66(7):1800–1810. doi: 10.1002/art.38620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hayes AF. Introduction to mediation, moderation, and conditional process analysis: a regression-based approach. 1st. New York, NY: The Guilford Press; 2013. [Google Scholar]
- 47.Ancoli-Israel S, Cole R, Alessi C, Chambers M, Moorcroft W, Pollak CP. The role of actigraphy in the study of sleep and circadian rhythms. Sleep. 2003;26(3):342–392. doi: 10.1093/sleep/26.3.342. [DOI] [PubMed] [Google Scholar]
- 48.Bulls HW, Freeman EL, Anderson AJ, Robbins MT, Ness TJ, Goodin BR. Sex differences in experimental measures of pain sensitivity and endogenous pain inhibition. J Pain Res. 2015;8:311–320. doi: 10.2147/JPR.S84607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Riley JL, 3rd, Cruz-Almeida Y, Glover TL, et al. Age and race effects on pain sensitivity and modulation among middle-aged and older adults. J Pain. 2014;15(3):272–282. doi: 10.1016/j.jpain.2013.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Harris M, Glozier N, Ratnavadivel R, Grunstein RR. Obstructive sleep apnea and depression. Sleep Med Rev. 2009;13(6):437–444. doi: 10.1016/j.smrv.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 51.Roure N, Gomez S, Mediano O, et al. Daytime sleepiness and polysomnography in obstructive sleep apnea patients. Sleep Med. 2008;9(7):727–731. doi: 10.1016/j.sleep.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 52.Campbell CM, Edwards RR. Ethnic differences in pain and pain management. Pain Manage. 2012;2(3):219–230. doi: 10.2217/pmt.12.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yarnitsky D, Granot M, Granovsky Y. Pain modulation profile and pain therapy: between pro- and antinociception. Pain. 2014;155(4):663–665. doi: 10.1016/j.pain.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 54.Cruz-Almeida Y, Riley JL, 3rd, Fillingim RB. Experimental pain phenotype profiles in a racially and ethnically diverse sample of healthy adults. Pain Med. 2013;14(11):1708–1718. doi: 10.1111/pme.12203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rahim-Williams B, Riley JL, 3rd, Williams AK, Fillingim RB. A quantitative review of ethnic group differences in experimental pain response: do biology, psychology, and culture matter? Pain Med. 2012;13(4):522–540. doi: 10.1111/j.1526-4637.2012.01336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mossey JM. Defining racial and ethnic disparities in pain management. Clin Orthop Relat Res. 2011;469(7):1859–1870. doi: 10.1007/s11999-011-1770-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schutte-Rodin S, Broch L, Buysse D, Dorsey C, Sateia M. Clinical guideline for the evaluation and management of chronic insomnia in adults. J Clin Sleep Med. 2008;4(5):487–504. [PMC free article] [PubMed] [Google Scholar]
- 58.Plant EA, Sachs-Ericsson N. Racial and ethnic differences in depression: the roles of social support and meeting basic needs. J Consult Clin Psychol. 2004;72(1):41–52. doi: 10.1037/0022-006X.72.1.41. [DOI] [PubMed] [Google Scholar]
- 59.Lustberg L, Reynolds CF. Depression and insomnia: questions of cause and effect. Sleep Med Rev. 2000;4(3):253–262. doi: 10.1053/smrv.1999.0075. [DOI] [PubMed] [Google Scholar]
- 60.Liu X, Buysse DJ, Gentzler AL, et al. Insomnia and hypersomnia associated with depressive phenomenology and comorbidity in childhood depression. Sleep. 2007;30(1):83–90. doi: 10.1093/sleep/30.1.83. [DOI] [PubMed] [Google Scholar]
- 61.Patel SR, Malhotra A, Gottlieb DJ, White DP, Hu FB. Correlates of long sleep duration. Sleep. 2006;29(7):881–889. doi: 10.1093/sleep/29.7.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ericsson M, Poston WS, Linder J, Taylor JE, Haddock CK, Foreyt JP. Depression predicts disability in long-term chronic pain patients. Disabil Rehabil. 2002;24(6):334–340. doi: 10.1080/09638280110096241. [DOI] [PubMed] [Google Scholar]
- 63.Weissman-Fogel I, Granovsky Y, Crispel Y, et al. Enhanced presurgical pain temporal summation response predicts post-thoracotomy pain intensity during the acute postoperative phase. J Pain. 2009;10(6):628–636. doi: 10.1016/j.jpain.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 64.Green CR, Anderson KO, Baker TA, et al. The unequal burden of pain: confronting racial and ethnic disparities in pain. Pain Med. 2003;4(3):277–294. doi: 10.1046/j.1526-4637.2003.03034.x. [DOI] [PubMed] [Google Scholar]
- 65.Gregory AM, Rijsdijk FV, Lau JY, Dahl RE, Eley TC. The direction of longitudinal associations between sleep problems and depression symptoms: a study of twins aged 8 and 10 years. Sleep. 2009;32(2):189–199. doi: 10.1093/sleep/32.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sivertsen B, Salo P, Mykletun A, et al. The bidirectional association between depression and insomnia: the HUNT study. Psychosom Med. 2012;74(7):758–65. doi: 10.1097/PSY.0b013e3182648619. [DOI] [PubMed] [Google Scholar]
- 67.Blackburn-Munro G, Blackburn-Munro RE. Chronic pain, chronic stress and depression: coincidence or consequence? J Neuroendocrinol. 2001;13(12):1009–1023. doi: 10.1046/j.0007-1331.2001.00727.x. [DOI] [PubMed] [Google Scholar]
- 68.Kojima M, Kojima T, Suzuki S, et al. Depression, inflammation, and pain in patients with rheumatoid arthritis. Arthritis Rheum. 2009;61(8):1018–1024. doi: 10.1002/art.24647. [DOI] [PubMed] [Google Scholar]


