Abstract
Low O2 pressures present in the microenvironment of epidermis control keratinocyte differentiation and epidermal barrier function through hypoxia inducible factors (HIFs) dependent gene expression. This study focuses on investigating relations of the retinoic acid receptor-related orphan receptor alpha (RORα) to HIF-1α in keratinocytes under hypoxic conditions. The expression level of RORα is significantly elevated under hypoxia in both human and murine keratinocytes. Gene silencing of RORA attenuates hypoxia-stimulated expression of genes related to late differentiation and epidermal barrier function, and leads to an enhanced apoptotic response. While the hypoxic induction of RORα is dependent on HIF-1α, RORα is in turn critical for nuclear accumulation of HIF-1α and activation of HIF transcriptional activity. These results collectively suggest that RORα functions as an important mediator of HIF-1α activities in regulating keratinocyte differentiation/survival and epidermal barrier function during the oxygen sensing stage.
Keywords: Hypoxia, RORα, HIF-1α, Keratinocytes, Differentiation, Apoptosis
INTRODUCTION
The epidermis of the skin is a stratified avascular tissue composed of a single basal layer of proliferative keratinocytes and multiple layers of progressively differentiating cells that constitute the cornified envelop, the outmost layer of epidermal permeability barrier (Blanpain and Fuchs, 2009; Fuchs, 2008). Characteristic structural proteins and signaling molecules are expressed in keratinocytes at different stages. In particular, during the initial proliferation-to-differentiation transition, keratinocytes switch from expressing keratin 5/14 (KRT5/14) in the basal layer to keratins 1/10 (KRT1/10) in the spinous layer. At later stages of differentiation in the granular layer, keratinocytes begin to express involucrin (IVL), loricrin (LOR), and filaggrin (FLG), which are structural proteins important to the cornification process, and to produce lipid granules containing cholesterol, free fatty acids, and ceramides (Feingold, 2007).
Life cycles of keratinocytes are intricately controlled through both intracellular transcriptional networks and environmental factors such as extracellular calcium gradient and the oxygen (O2) availability (Rezvani et al., 2011b; Tu et al., 2012; Wong et al., 2015). Due to the lack of vasculature, the skin epidermis acquires O2 mostly from the atmosphere with the O2 level at ~21% (Stucker et al., 2002). While the vascular dermis has an O2 level of 10%, the skin epidermis is characterized as moderately hypoxic with an O2 level ranging only between 0.2% and 8% (Evans et al., 2006; Rezvani et al., 2011a). In addition, reports have shown that areas of cutaneous wound are more hypoxic due to the disruption of injured blood vessels and high oxygen consumption caused by high cellular density and activities (Hunt et al., 1972; Tandara and Mustoe, 2004). Therefore, hypoxia represents a natural element in the epidermal environment and plays an intangible role in skin development and functions.
Hypoxia-inducible factors (HIFs) are master regulators that orchestrate a broad spectrum of adaptive responses to hypoxia through regulating the transcription of genes quintessential to angiogenesis, metabolism, proliferation, apoptosis, inflammation, and motility (Pouyssegur et al., 2006; Semenza, 2014). As the primary factor in these responses, HIF-1 is composed of an O2-sensitive HIF-1α subunit and a constitutively expressed subunit of aryl hydrocarbon nuclear translocator ARNT (also called HIF-1β). Under normoxic conditions, HIF-1α undergoes a sequence of post translational modifications mediated by prolyl hydroxylase domains (PHDs) and factor inhibiting HIF-1 (FIH-1), which allow the von Hippel-Lindau (VHL) E3 ligase to seize and poly-ubiquinate HIF-1α, leading to its ultimate proteasomal degradation (Brahimi-Horn and Pouyssegur, 2009; Jaakkola et al., 2001; Kamura et al., 2000; Lando et al., 2002). Low levels of O2 reduce activities of PHDs and FIH-1, thereby preventing hydroxylation and degradation of HIF-1α. The stabilized HIF-1α can translocate from the cytoplasm to the nucleus, where it forms a heterodimer with HIF-1β to activate transcriptions of target genes by recognizing hypoxia-response elements (HREs) and recruiting coactivators such as CBP/p300 (Kallio et al., 1998).
Given its natural hypoxic microenvironment, the skin epidermis constitutively expresses HIF-1α with mostly residing in the basal layer (Bedogni et al., 2005; Distler et al., 2004). Epidermal loss of HIF-1α accelerates skin aging and affects re-epithelialization due to a disturbance in the basement membrane involving laminin-332 and integrins (Rezvani et al., 2011b). In murine epidermis, simultaneous deletion of Hif1a and Hif2a genes results in dry flaky skin and defective epidermal permeability barrier that is associated with attenuated stratum granulosum and reduced expression of fillaggrin (FLG) (Wong et al., 2015). HIF-1α is also detected at the acute wound edge (Elson et al., 2000; Xing et al., 2011), and plays a positive role in wound healing by modulating skin angiogenesis, and proliferation and motility of keratinocytes and fibroblasts (Li et al., 2007; Rezvani et al., 2011a; Rezvani et al., 2011b; Tandara and Mustoe, 2004; Woodley et al., 2009).
The retinoic acid receptor-related orphan receptor alpha (RORα) belongs to the steroid nuclear hormone receptor superfamily and acts as a transcription factor through binding to the ROR responsive elements (ROREs) either as a monomer or homodimer in the regulatory region of target genes (Carlberg and Wiesenberg, 1995; Giguere et al., 1994; Jetten, 2009). Human RORA gene can generate four RORα isoforms (RORα 1–4) through alternative promoter usage or exon splicing. All four isoforms share the common DNA-binding and ligand-binding domains but differ in their N-terminal transactivation domains. RORα is widely expressed in a variety of tissues and has been implicated in an array of important biological processes such as metabolism, inflammation, and differentiation (Cook et al., 2015; Jetten, 2009). Intriguingly, Chauvet et al. have demonstrated that transcription of RORA gene could be up-regulated by hypoxia in a panel of cell lines derived from different tissues, albeit none from the skin (Chauvet et al., 2004; Chauvet et al., 2002). More importantly, additional studies have revealed that HIF1α/HIF1β heterodimers could transactivate RORα by binding to a hypoxia response element (HRE) within its promoter (Chauvet et al., 2004; Miki et al., 2004), while RORα stabilizes HIF-1α and amplifies its activity (Kim et al., 2008).
Despite these elegant precedents, precise roles of RORα in regulating hypoxic responses remain underexplored in different cell types especially in keratinocytes. Recently, we reported that RORα could be detected in all layers of human skin epidermis with RORα4 being the predominant isoform (Dai et al., 2013; Slominski et al., 2005). Furthermore, we found that RORα positively regulated the expression of genes related to keratinocyte differentiation and epidermal barrier formation, partially via the activation of FOXN1 in human keratinocytes (Dai et al., 2013). Given the aforementioned association of RORα with hypoxia, we investigated functions and underlying mechanisms of RORα in controlling gene expression in keratinocytes under hypoxic conditions. We wish to report herein our findings of hypoxic responses in relations to RORα in keratinocytes.
MATERIALS AND METHODS
Cell lines and Cell culture
The immortal human keratinocyte cell line HaCat was obtained from COBIOER BIOSCIENCES CO. LTD (Nanjing, China). The mouse keratinocyte cell line PAM212 was generously provided by Dr. Stuart Yuspa (Bethesda, MD). Primary human keratinocytes (HKCs) were cultured in the CnT-07 media from CELLnTEC Advanced Cell Systems (Bern, Switzerland). HaCat and PAM212 keratinocytes were cultured in the DMEM medium (Corning) supplemented with 10% fetal bovine serum (Capricorn, Germany) and 1% penicillin/streptomycin (Solarbio, China). Cells were maintained at 37°C under normoxic conditions (21% O2, 5% CO2). Hypoxic condition was generated in a sealed Billups-Rothenburg chamber (Del Mar, CA) flushed with 1% O2, 5% CO2, and 94% N2.
SiRNA Transfection
Pre-designed human RORA siRNAs (s12103 and s12105) and negative control siRNA (#4613) were from Ambion-Invitrogen. SiGENOME human HIF1A siRNA SMARTpool (M-004018) and siGENOME mouse Rora siRNAs (D-040430-01 and D-040430-02) were from GE Healthcare Dharmacon Inc. (Pittsburgh, PA). HaCat, HKCs, or PAM212 keratinocytes were reversely transfected with 20 nM of siRNA duplexes using lipidoid (Love et al., 2010). At 48 h post transfection, cells were cultured in the hypoxic chamber for an additional 8 h, 24 h, or 48 h.
Real time RT-PCR
Total RNA was isolated from cells using Eastep® Super Total RNA Extraction kit (Promega, Madison, WI), and was reverse-transcribed into cDNA using the HiFiScript cDNA Synthesis Kit (Cwbiotech, China). Real time RT-PCR with UltraSYBR Mixture (Cwbiotech, China) was performed on the ABI QuantStudio™ 6 Flex Real-Time PCR System (Foster City, CA) according to manufacturer’s instructions. The mRNA levels of target genes were normalized to the expression of the housekeeping 36B4 gene. The list of gene-specific primers for RT-PCR is provided in Supplementary Table S1. RT-PCR primers and conditions used for the amplification of human RORA1-4 genes in Supplementary Fig. S1 were described in (Pozo et al., 2004).
Western blot analysis
Whole cell lysates for immunoblotting were prepared with the SDS-sample buffer. Proteins were separated by SDS-PAGE and transferred onto the PVDF membrane (Millipore, Darmstadt, Germany). The following antibodies were used for immunoblotting: RORα (Santa Cruz Biotechnology Inc., Cat# sc-28612, RRID: AB-218011), HIF-1α (Novus, Cat#, NB100-105, RRAD: AB-10001154), Filaggrin (Santa Cruz Biotechnology Inc., Cat# sc-66192, RRID: AB-1122916), Involucrin (Sigma, Cat# I9018, RRID: AB-477129), cleaved PARP (Cell Signaling, Cat# 9541, RRID: AB-331426), α-tubulin (Sigma, Cat# T9026, RRID: AB-477593), AQP3 (BA1559; Boster, China), Keratin 1 or Keratin 10 (Biolegend, Cat# Poly19056, Poly19054). Chemiluminescence images were acquired with Amersham Imager 600 from GE Healthcare Life Sciences (Pittsburgh, PA). The level of target proteins was quantified by densitometry scanning with the Image J software, and normalized to the amount of α-tubulin.
Plasmids and viruses
The retroviral pinco-Flag-RORA4 plasmids were generated as described before through cloning the Flag-tagged full-length cDNAs of RORA4 into the pinco-GFP vector (Dai et al., 2013). The pGL2-HRE-luciferase, containing three HREs (24-mers) from the Pgk-1 gene, was a gift from Navdeep Chandel (# 26731; Addgene, Cambridge, MA). The lentiviral pLKO-RORA shRNA #1 against all human RORA isoforms was designed based on the sequence of RORA siRNA (s12103, Ambion), with the EcoR1 restriction site at the 5′ end, and the Agel site at the 3′ end. The sequences of the DNA oligos were listed in Supplementary Table S1. The annealing product of the DNA fragment was cloned into the EcoR1 and Agel sites of pLKO.1 lenti-viral vector. The lenti-viral MISSION RORA shRNA #2 (TRCN0000022154) was obtained from Sigma. Conditions employed for retro- and lenti-virus production and infection were as previously reported (Nguyen et al., 2006). After HaCat cells were infected with virus made from pLKO.1 or pLKO.1-RORA shRNAs, stable cell lines were generated by selection with 2 μg/ml of puromycin.
Fluorescence microscopy
HaCat cells were cultured on coverslips. After 8 h of culturing under either normoxia or hypoxia, cells were fixed with 4% paraformaldehyde/PBS at room temperature for 10 min. After 5 min of permeabilization with 0.5% Triton X-100/PBS, coverslips were first incubated with the antibody against HIF-1α (NB100-105, Novus) at 4° C overnight, and then with Alexa488-conjugated secondary antibody (Invitrogen, Grand Island, NY, USA) along with propidium iodide (PI) for DNA staining. Fluorescence images were acquired with UltraVIEW VoX Spinning Disk Confocal Microscope (PerkinElmer). The fluorescence intensity of HIF-1α signal in each cell was quantified by the Image J software, and normalized to the intensity of PI staining. For statistic analysis, 50 cells from 10 independent fields were quantified for each treatment.
Transient transfection and luciferase assay
HaCat cells were seeded into 96-well (3000/well). After 24 h, cells were co-transfected with the pGL2-HRE-luciferase and renilla luciferase vectors at a ratio of 20:1 by using the Polyethylenimine (PEI) transfection regent (#23966; Polysciences, Inc, Warrington, PA). At 24 h post transfection, cells were incubated under normoxic or hypoxic conditions for additional 24 h, and lysed with 1X Passive Lysis Buffer (Promega). The activities of HRE-driven firefly luciferase and renilla luciferase were sequentially measured with the Dual-Luciferase Reporter Assay System (Promega). The renilla luciferase activity was used as an internal control.
Caspase 3/7 activity-based apoptosis assay
HaCat cells stably expressing control or RORA shRNAs were seeded in a 96-well plate (6000/well), and incubated under normal conditions for 24 h to allow attachment. After 24 h of culturing under either normoxic or hypoxic conditions, the caspase 3/7 activity was measured with the Caspase-Glo 3/7 Assay System (Promega), according to manufacturer’s instruction. The caspase 3/7 activity was normalized to the number of viable cells measured with the CellTiter Glo Luminescent Cell Viability Assay (Promega).
Annexin V staining – based apoptosis analysis
HaCat cells were plated in the 60-mm dish (8×105/dish), and incubated for 24 h to allow attachment. After 48 h of culturing under normoxic or hypoxic conditions, cells were trypsinized and stained with Annexin V-FITC and propidium iodide, using the Annexin V-FITC Apoptosis Detection Kit (#A211-02; Vazyme, China). The percentage of live and apoptotic cells were analyzed by flow cytometry on a FACSCalibur flow cytometer (Becton Dickinson, San Diego, CA).
Statistics
All statistical evaluations were carried out using the GraphPad Prism 7.0 software. Real time RT-PCR analysis and the luciferase assay were performed in duplicates, and repeated at least three times. Data were analyzed by Student’s t-test for comparison between two groups or two-way ANNOVA for comparison between multiple groups. Combined data were presented as mean-fold over control ± S.E.M. P-values < 0.05 were considered significant.
RESULTS
1. Hypoxia induces RORα expression and late differentiation in keratinocytes
To study the hypoxic functions of RORα, we chose to employ the human HaCat and murine PAM212 immortal cell lines of keratinocytes. Real time RT-PCR and western blot analysis showed that the RORα expression was significantly increased at both mRNA and protein levels in HaCat cells cultured under 1% O2 (Fig. 1A). It is noteworthy that only RORA4 gene transcript was detected in these cells, with an increased level during hypoxia (Supplementary Fig. S1). In addition to RORα, low O2 tension also triggered the expression of several other genes that are related to late differentiation and epidermal barrier function. These genes are filaggrin (FLG), involucrin (IVL), loricrin (LOR), aquaporin 3 (AQP3), and adipose-differentiation related protein (ADRP) (Fig. 1B). Significant inductions of FLG and AQP3 (but not IVL) were confirmed at the protein level by western blot analysis (Fig. 1B). It has been shown in mouse primary keratinocytes that the mRNA levels of the early differentiation markers KRT1 and KRT10 were slightly decreased during hypoxia, albeit the protein level of KRT10 was not affected (Wong et al., 2015). In our study, we detected a similar expression pattern of KRT10 in hypoxic HaCat cells (Supplementary Fig. S2A). Although the mRNA level of KRT1 was significantly increased during hypoxia, its protein level was unchanged (Supplementary Fig. S2B). Therefore, instead of early differentiation genes, transcriptional activations of late differentiation genes could represent a functionally more important response to hypoxia in keratinocytes. In addition, expression levels of RORα and the few late differentiation genes were also elevated in PAM212 cells during hypoxia (Fig. 1C and 1D). This outcome is consistent with results from HaCat cells and suggests a conservation of hypoxic responses between human and murine keratinocytes.
Figure 1. Hypoxia induces the expression of RORα and genes related to late differentiation and epidermal barrier function in keratinocytes.
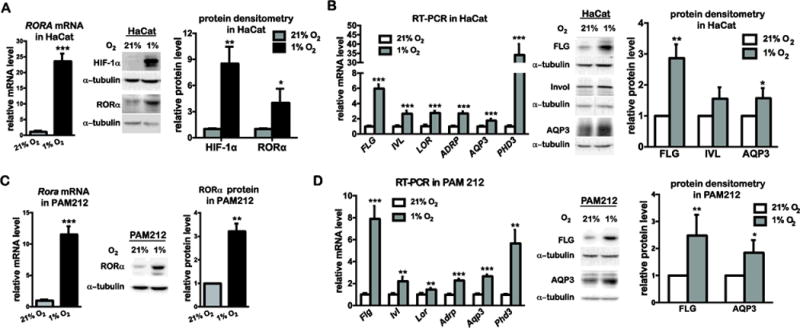
Real time RT-PCR analysis and western blot analysis of the expression of indicated genes and proteins. (A, B) Human HaCat keratinocytes were cultured under normoxic (21% O2) and hypoxic (1% O2) conditions for 24 h, and harvested for RT-PCR and western blot analysis of RORα (A) or indicated genes (B). (C, D) Mouse PAM21 keratinocytes were cultured under normoxic (21% O2) or hypoxic (1% O2) conditions for 24 h, and harvested for RT-PCR and western blot analysis of RORα (C) or the indicated genes (D). The mRNA level of each gene was normalized to 36B4. The protein level of indicated genes was quantified by densitometry scanning, and normalized to α-tubulin. Values are presented as mean-fold over control ± S.E.M. *, p < 0.05, **, p < 0.01 ***, p < 0.001, N=3 independent experiments.
2. RORα and HIF-1α are essential for hypoxia-induced late differentiation in human keratinocytes
As hypoxia-induced genes (Fig. 1) partially overlap with those positively regulated by RORα (Dai et al., 2013), we evaluated the ability of RORα to modulate gene expression in keratinocytes under hypoxic conditions. Towards that goal, we adopted the loss-of-function approach. Two lenti-viral shRNAs targeting all RORα isoforms were able to knock down hypoxia-induced RORα expression at both mRNA and protein levels in HaCat cells (Fig. 2A). Except for PHD3, hypoxia-induced expression of FLG, AQP3, IVL, and ADRP was greatly attenuated by these two RORA shRNAs (Fig. 2B–2D). Similar effects were observed with two Rora siRNAs in PAM212 mouse keratinocytes (Supplementary Fig. S3), thereby indicating the conservation of RORα functions in hypoxic keratinocytes of different species.
Figure 2. Silencing of RORA attenuates hypoxia-induced expression of genes involved in late differentiation and epidermal barrier function in human keratinocytes.
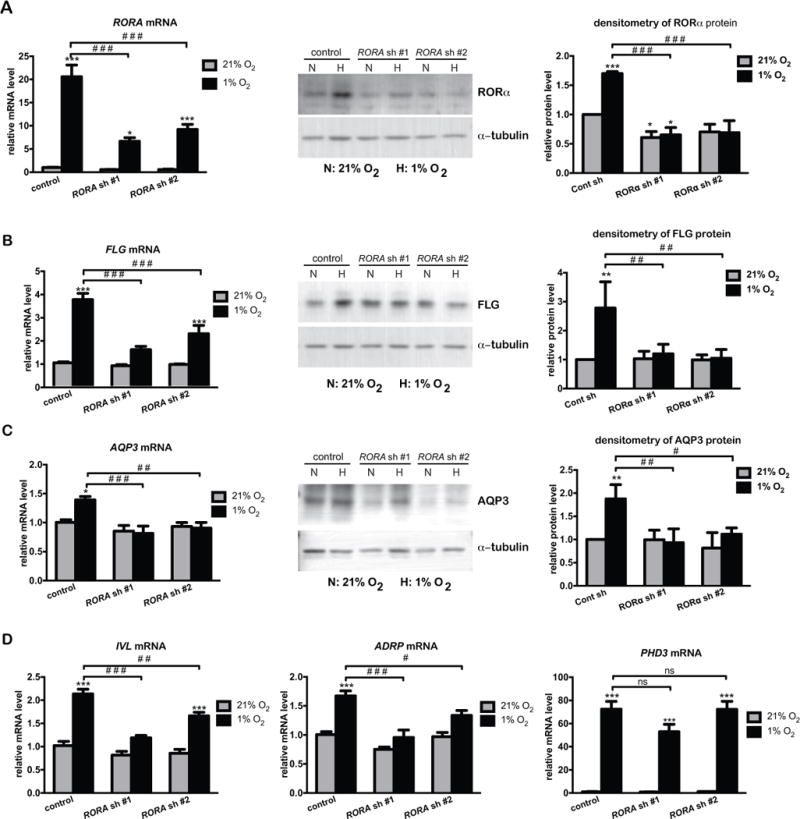
Real time RT-PCR analysis and western blot analysis of the expression of indicated genes. HaCat cells were stably transduced with lentivirus prepared from pLKO.1 vector (control) or two pLKO.1-RORA shRNAs. Cells were cultured under normoxic (21% O2) or hypoxic (1% O2) conditions for 24 h, and harvested for real time RT-PCR or western blot analysis of RORα (A), FLG (B), AQP3 (C), or other indicated genes (D). The mRNA level of each gene is normalized to 36B4. The protein level of indicated genes was quantified by densitometry scanning, and normalized to α-tubulin. Values are shown as mean-fold over control ± S.E.M. *, p < 0.05, **, p < 0.01 ***, p < 0.001, N=3 independent experiments.
Given the significance of HIF-1 as master regulator of hypoxic responses, we again employed loss-of-function approach to directly compare functions of RORα and HIF-1α in controlling gene expression during hypoxia. Hypoxic inductions of RORα mRNA/protein and HIF-1α protein were efficiently knocked down by their respective siRNAs in HaCat cells (Fig. 3A). As shown in Fig. 3B and 3C, RORA siRNAs and HIF1A siRNAs elicited comparable inhibitory effects on hypoxia-induced expression of FLG, IVL, and ADRP genes. Importantly, HIF1A gene silencing also abrogated the hypoxic expression of RORα at both mRNA and protein levels (Fig. 3A). These outcomes suggest that HIF-1α likely functions upstream of RORα in the pathway that leads to transactivation of genes associated with late differentiation and epidermal barrier function. It is noteworthy that while not affected by RORA siRNAs, the induction of PHD3 gene was significantly attenuated under conditions of HIF1A gene silencing (Fig. 3C). This distinct contrast indicates that target genes of RORα and HIF-1α only partially overlap in hypoxic keratinocytes.
Figure 3. RORA siRNAs and HIF1A siRNAs elicit similar blocking effects on hypoxia-induced expression of genes related to late differentiation in human keratinocytes.
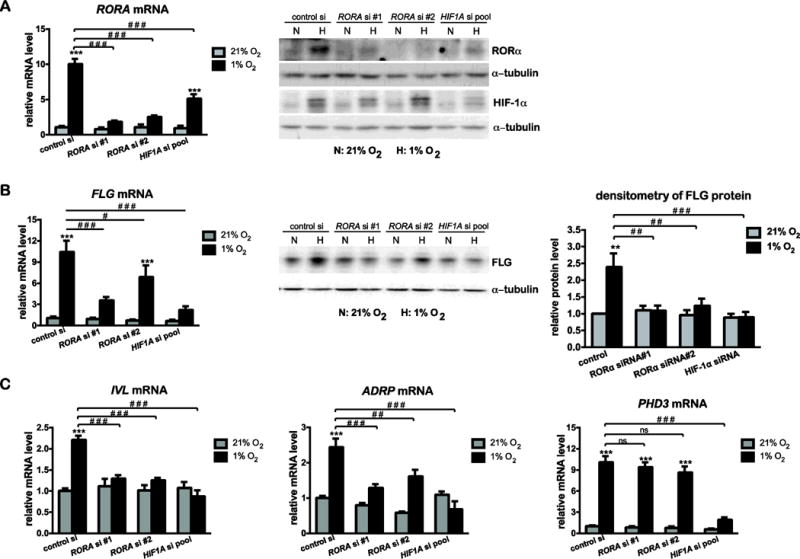
HaCat cells were transfected with control siRNA, two separate siRNAs against all RORA isoforms, or SMARTpool siRNAs against HIF1A. At 48 h post transfection, cells were cultured under normoxic (21% O2) or hypoxic (1% O2) conditions for additional 24 h, and harvested for real time RT-PCR or western blot analysis of RORα (A), FLG (B), or other indicated genes (C). The mRNA level of each gene is normalized to 36B4. The protein level was quantified by densitometry scanning, and normalized to α-tubulin. Values are presented as mean-fold over control ± S.E.M. *, p < 0.05, **, p < 0.01 ***, p < 0.001, ns, not significant, N=3 independent experiments.
More importantly, hypoxia-induced expression of RORA, FLG, IVL, ADRP, and PHD3 genes was also observed in primary human keratinocytes (HKCs) [Supplementary Fig. S4]. As in Hacat cells, RORA siRNAs and HIF1A siRNAs showed a similar blocking effect on hypoxic induction of genes related to late differentiation and epidermal barrier formation in HKCs (Supplementary Fig. S4). These results further confirmed that RORα and HIF-1α play critical roles in controlling hypoxia responses in keratinocytes especially for those in late differentiation.
3. RORα is essential for HIF-1α accumulation in the nucleus and HIF transcriptional activity in human keratinocytes
As shown in Fig. 3A, hypoxia-induced RORα expression was dependent upon HIF-1α in HaCat cells. We examined possible roles of RORα in regulating HIF-1α protein expression and transcriptional activity. Western blot analysis indicated that the robust induction of HIF-1α protein during hypoxia was moderately reduced by RORA gene silencing mediated by its shRNAs (Fig. 4A). While confocal microscopy revealed a strong accumulation of HIF-1α protein in the nucleus for the control cells under hypoxia, the intensity of HIF-1α nuclear signals was significantly weakened in cells with RORA knockdown (Fig. 4B). To evaluate the effect of RORα on HIF transcription activity, HaCat cells were transfected with a luciferase reporter driven by three copies of HRE (24-mers) from the Pgk-1 gene promoter. Hypoxia stimulated HRE-luciferase activity was markedly suppressed by RORA shRNAs (Fig. 4C). In contrast, overexpression of RORα4 in HaCat cells showed an opposite effect by enhancing HIF transcriptional activity under both normoxic and hypoxic conditions (Fig. 4D), thereby revealing a positive regulatory effect of RORα on HIF activity in human keratinocytes.
Figure 4. RORα is required for HIF-1α nuclear accumulation and transcriptional activity in human keratinocytes.
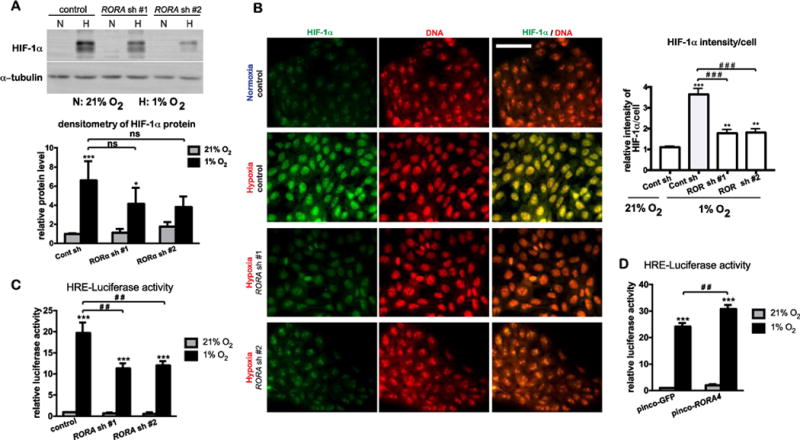
(A–C) HaCat cells stably transduced with lentivirus prepared from pLKO.1 vector (control) or two different pLKO.1-RORA shRNAs were cultured under normoxic (21% O2) or hypoxic (1% O2) conditions. (A) After 8-h culture under hypoxia, cells were harvested for western blot analysis of HIF-1α. (B) After 8-h culture under hypoxia, cells were fixed and subjected to immununostaining with an antibody against HIF-1α (green). DNA was counter stained with propidium iodide (PI). Bar = 70 μm. Fluorescence intensity of HIF-1α signal/cell was normalized to the intensity of DNA staining. Fifty cells from 10 independent fields were measured for each condition. Data are presented as mean-fold over control ± S.E.M. **, p < 0.01 ***, p < 0.001, N=3. (C) HaCat stable cells were transiently transfected with the pGL2-HRE-luciferase and renilla constructs. At 24 h post transfection, cells were cultured under normoxia or hypoxia for 24 h, and lysed for measurement of luciferase activities. (D) HaCat cells stably transduced with the retrovirus of pinco-GFP or pinco-RORα4 were transiently transfected with the pGL2-HRE-luciferase and renilla constructs, followed by the steps as described in (C). The HRE-luciferase activity was normalized to renilla activity, and presented as mean-fold over control ± S.E.M. **, p < 0.01 ***, p < 0.001, N=3.
4. RORα has a pro-survival function in the hypoxic response of keratinocytes
It has been shown that down regulation of HIF-1α triggers cell cycle arrest and apoptosis in human keratinocytes (Rezvani et al., 2011b). We found that hypoxia triggered a slight increase in caspase 3/7 activity in control HaCat cells (Fig. 5A). The caspase 3/7 activity was significantly enhanced in cells with RORA gene silencing (Fig. 5A), which suggests a protective function of RORα during hypoxia. The greater apoptotic responses caused by RORα depletion was confirmed by two other parameters: An increased level of caspase 3-cleaved form of PARP protein measured by western blot analysis (Fig. 5B); and a markedly increased number of early and late apoptotic cells measured by Annexin V staining – FACS analysis (Fig. 5C). On the contrary, the caspase 3/7 activity was markedly reduced in HaCat cells stably expressing RORα4 (Fig. 5D), thereby further confirming the protective action of RORα in hypoxic human keratinocytes. It is noteworthy that the level of cleaved PARP and percentage of apoptotic cells were also higher in cells expressing RORA shRNAs under normoxic conditions (Fig 5B and 5C). This suggests that the pro-survival function of RORα in keratinocytes may be not restricted to hypoxic conditions.
Figure 5. RORα protects keratinocytes during hypoxia.
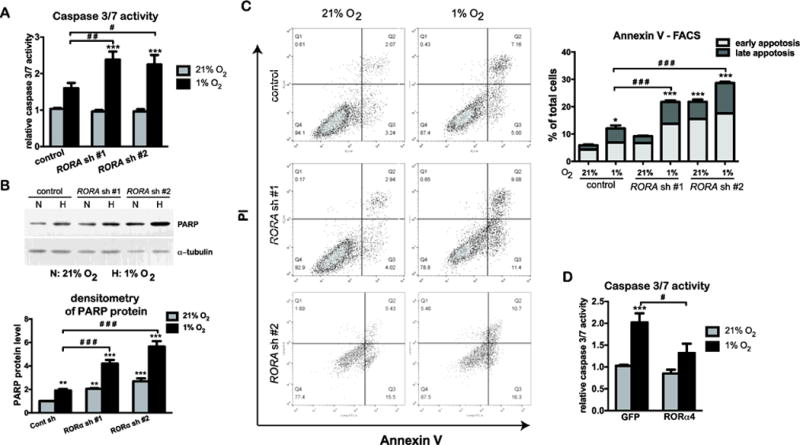
(A–C) HaCat cells stably transduced with lentivirus prepared from pLKO.1 vector (control) or two different pLKO.1-RORA shRNAs were cultured under normoxic (21% O2) or hypoxic (1% O2) conditions. (A) After 24 h of normoxic or hypoxic culture, HaCat cells were analyzed for the caspase 3/7 activity, which was normalized to the number of viable cells measure by the Celltiter Glo assay. The value is presented as mean-fold over control ± S.E.M. N=3. (B) After 24 h of normoxic or hypoxic culture, cells were harvested for western blot analysis using an antibody against the cleaved form of PARP. The protein level of cleaved PARP was quantified as described in Fig. 1. (C) After 48 h of culture under normoxia or hypoxia, HaCat cells were double stained with Annexin V-FITC and propidium iodide (PI), and analyzed with flow cytometry for apoptotic cell death. Cells that stain positive for Annexin V-FITC and negative for PI are categorized as in the early apoptotic stage. Cells that stain positive for both Annexin V-FITC and PI are categorized as in the late apoptosis. Values show mean-percentage of total cells ± S.E.M., N=3 independent experiments. (D) HaCat cells stably transduced with the retrovirus expressing GFP or RORα4 were culture under normoxia or hypoxia for 24 h, and analyzed for the caspase 3/7 activity, as described in (A).
DISCUSSION
Because of its avascular structure, the skin epidermis is located in a mildly hypoxic microenvironment (Distler et al., 2004; Evans et al., 2006) that favors the constitutive expression of HIF-1α mainly in the basal layer (Bedogni et al., 2005; Distler et al., 2004). As the principle coordinator of cellular responses to hypoxia, HIF-1α transactivates numerous target genes that are pivotal in skin homeostasis (Rezvani et al., 2011a). In this study, we have identified RORα as a critical regulator of hypoxic responses and HIF-1α activities in keratinocytes. RORα expression is highly induced by hypoxia in both human and murine keratinocytes. Gene silencing of RORA attenuates HIF-1α dependent transcriptional activation of genes associated with late differentiation and epidermal barrier function, and enhances apoptotic responses of keratinocytes under hypoxic stress. Moreover, while the hypoxic induction of RORα relies on HIF-1α, nuclear accumulation of HIF-1α as well as stimulation of HIF transcriptional activity would require RORα. These collective results strongly suggest that RORα acts as an indispensible cofactor of HIF-1α in promoting differentiation and survival of keratinocytes under low O2 tension.
Our data revealed that a group of genes associated with epidermal barrier formation could be up regulated by hypoxia (Fig. 1). One of these genes is filaggrin (FLG) that is responsible for cross-linking of keratin intermediate filament in the cornified envelop and crucial for the development of integrate skin barrier (Irvine et al., 2011). This finding is consistent with the literature report by (Wong et al., 2015). In addition, other genes induced by hypoxia and relevant to cornification are: (a) Involucrin (IVL) and loricrin (LOR), which are the structural proteins of cornification; (b) ADRP, a membrane-associated fatty acid binding protein that is involved in lipid accumulation at the terminal stage of epidermal differentiations (Schmuth et al., 2004); and (c) AQP3, a membrane transporter of water and glycerol that is involved in maintenance of water content and elasticity of stratum corneum (Hara-Chikuma and Verkman, 2008). It is noteworthy that the hypoxic induction of these genes occurs in both human and murine keratinocytes (Fig. 1). This suggests that initiation of the late differentiation program may represent an evolutionarily conserved response of keratinocytes to low O2 tension from human to murine.
Although increased expression of RORα during hypoxia has been described in a number of cell types (Chauvet et al., 2004), the hypoxic functions of RORα have not been well investigated. Our loss-of-function studies show that RORα is essential for hypoxia-induced expression of FLG, IVL, ADRP, and AQP3, but is dispensable for the induction of PHD3 (Fig. 2, Fig. 3, supplementary Fig. S3 and S4). PHD3 is a HIF prolyl-4-hydroxylase involved in hydroxylations of HIF-α, a key modification prior to its complexation with VHL E3 ligase in preparation of the proteasomal degradation under normoxic conditions (Bruick and McKnight, 2001; Place and Domann, 2013). These results suggest that hypoxic induction of RORα is critical for the expression of a select group of genes with specific epidermal functions in human keratinocytes.
As a master regulator of hypoxic responses, HIF-1α is also responsible for hypoxic expression of RORα (Fig. 3A). This is consistent with the finding in hepatoma HepG2 cells, where HIF-1α up-regulates the expression of RORA gene by directly binding to the putative HREs in its promoter regions (Chauvet et al., 2004; Miki et al., 2004). On the other hand, gene silencing of RORA significantly reduced hypoxia stimulated HIF transcriptional activity in keratinocytes (Fig. 4C). This reduction is accompanied by a decrease in HIF-1α protein level and its nuclear accumulation (Fig. 4A and 4B). Therefore, RORα may facilitate the transcriptional activity of HIF-1α, at least in part, by stabilizing HIF-1α protein in the nucleus of hypoxic keratinocytes. These findings are again in consistence with those reported for human hepatoma HepG2 cells in which RORα enhanced HIF-1α protein stability through interacting with HIF-1α via its DNA binding domain (Kim et al., 2008). HIF-1α is constitutively expressed in the human skin, especially the basal layer of epidermis, where RORα is also present (Dai et al., 2013). Future in vivo studies should offer greater insight into the role of HIF-1α and RORα interaction in controlling epidermal development and pathophysiological skin conditions such as aging and wound healing.
In addition to its role in promoting terminal differentiation, we have also uncovered an important function of RORα in protecting keratinocytes against apoptosis during hypoxia (Fig. 5). It has been shown that keratinocyte depletion of HIF-1α leads to decreased cell growth and increased apoptosis (Rezvani et al., 2011b). Our data suggest that pro-survival functions of HIF-1α may also be facilitated by RORα in hypoxic keratinocytes. Both HIF-1α and RORα have shown opposing effects on apoptosis depending upon their specific cellular context (Kim et al., 2011; Sendoel and Hengartner, 2014). In UVB-irradiated keratinocytes, HIF-1α promotes apoptosis via up-regulation of pro-apoptotic genes, such as Noxa, BCL2/adenovirus E1B 19-kDa-interacting protein (BNIP3), and tumor necrosis factor (ligand) (TRAIL) (Nys et al., 2010; Turchi et al., 2008). Pro-apoptotic functions of RORα have been reported for colon cancer cells and mouse embryonic fibroblasts in response to DNA damage (Kim et al., 2011). It remains to be clarified whether and how relations between RORα and HIF-1α regulate survival among different types of cells and in response to different kinds of environmental stresses.
In conclusion, we have identified RORα as a regulator imperative to HIF-1α activities in promoting terminal differentiation, epidermal barrier function, and survival of keratinocytes under low O2 tension. As a member of the nuclear receptor superfamily, RORα is ligand regulated, and thus, its conformation and activities can be modulated using small molecules. A number of endogenous and synthetic ligands of RORα have recently been identified (Solt and Burris, 2012). Given highly diversified functions of HIF targets in maintaining skin homeostasis, manipulations of HIF activities through RORα ligands can represent a novel strategy for therapeutic treatment of pathophysiological conditions such as cutaneous diseases related to defective epidermal barrier functions and wound healing.
Supplementary Material
Acknowledgments
Grant information
Grant sponsor: The National Institutes of Health
Grant number: K01AR062132
Authors are grateful for generous funding from School of Pharmaceutical Science and Technologies at Tianjin University and The National Institutes of Health (K01AR062132 to JD). Authors would like to thank Professor David E. Fisher and Professor G. Paolo Dotto of Cutaneous Biology Research Center at Massachusetts General Hospital for invaluable discussions. Authors also thank Professor Richard P. Hsung of School of Pharmacy at University of Wisconsin–Madison for the preparation of this manuscript, and Mr. Gentao Li for his technical support on making the pLKO.1-RORA shRNA construct.
Footnotes
CONFLICT OF INTEREST
The authors state no conflict of interest.
References
- Bedogni B, Welford SM, Cassarino DS, Nickoloff BJ, Giaccia AJ, Powell MB. The hypoxic microenvironment of the skin contributes to Akt-mediated melanocyte transformation. Cancer Cell. 2005;8(6):443–454. doi: 10.1016/j.ccr.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Blanpain C, Fuchs E. Epidermal homeostasis: a balancing act of stem cells in the skin. Nature reviews Molecular cell biology. 2009;10(3):207–217. doi: 10.1038/nrm2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahimi-Horn MC, Pouyssegur J. HIF at a glance. J Cell Sci. 2009;122(Pt 8):1055–1057. doi: 10.1242/jcs.035022. [DOI] [PubMed] [Google Scholar]
- Bruick RK, McKnight SL. A conserved family of prolyl-4-hydroxylases that modify HIF. Science. 2001;294(5545):1337–1340. doi: 10.1126/science.1066373. [DOI] [PubMed] [Google Scholar]
- Carlberg C, Wiesenberg I. The orphan receptor family RZR/ROR, melatonin and 5-lipoxygenase: an unexpected relationship. J Pineal Res. 1995;18(4):171–178. doi: 10.1111/j.1600-079x.1995.tb00157.x. [DOI] [PubMed] [Google Scholar]
- Chauvet C, Bois-Joyeux B, Berra E, Pouyssegur J, Danan JL. The gene encoding human retinoic acid-receptor-related orphan receptor alpha is a target for hypoxia-inducible factor 1. Biochem J. 2004;384(Pt 1):79–85. doi: 10.1042/BJ20040709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauvet C, Bois-Joyeux B, Danan JL. Retinoic acid receptor-related orphan receptor (ROR) alpha4 is the predominant isoform of the nuclear receptor RORalpha in the liver and is up-regulated by hypoxia in HepG2 human hepatoma cells. Biochem J. 2002;364(Pt 2):449–456. doi: 10.1042/BJ20011558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook DN, Kang HS, Jetten AM. Retinoic Acid-Related Orphan Receptors (RORs): Regulatory Functions in Immunity, Development, Circadian Rhythm, and Metabolism. Nucl Receptor Res. 2015;2 doi: 10.11131/2015/101185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai J, Brooks Y, Lefort K, Getsios S, Dotto GP. The retinoid-related orphan receptor RORalpha promotes keratinocyte differentiation via FOXN1. PLoS One. 2013;8(7):e70392. doi: 10.1371/journal.pone.0070392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Distler O, Distler JH, Scheid A, Acker T, Hirth A, Rethage J, Michel BA, Gay RE, Muller-Ladner U, Matucci-Cerinic M, Plate KH, Gassmann M, Gay S. Uncontrolled expression of vascular endothelial growth factor and its receptors leads to insufficient skin angiogenesis in patients with systemic sclerosis. Circ Res. 2004;95(1):109–116. doi: 10.1161/01.RES.0000134644.89917.96. [DOI] [PubMed] [Google Scholar]
- Elson DA, Ryan HE, Snow JW, Johnson R, Arbeit JM. Coordinate up-regulation of hypoxia inducible factor (HIF)-1alpha and HIF-1 target genes during multi-stage epidermal carcinogenesis and wound healing. Cancer Res. 2000;60(21):6189–6195. [PubMed] [Google Scholar]
- Evans SM, Schrlau AE, Chalian AA, Zhang P, Koch CJ. Oxygen levels in normal and previously irradiated human skin as assessed by EF5 binding. J Invest Dermatol. 2006;126(12):2596–2606. doi: 10.1038/sj.jid.5700451. [DOI] [PubMed] [Google Scholar]
- Feingold KR. Thematic review series: skin lipids. The role of epidermal lipids in cutaneous permeability barrier homeostasis. J Lipid Res. 2007;48(12):2531–2546. doi: 10.1194/jlr.R700013-JLR200. [DOI] [PubMed] [Google Scholar]
- Fuchs E. Skin stem cells: rising to the surface. J Cell Biol. 2008;180(2):273–284. doi: 10.1083/jcb.200708185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giguere V, Tini M, Flock G, Ong E, Evans RM, Otulakowski G. Isoform-specific amino-terminal domains dictate DNA-binding properties of ROR alpha, a novel family of orphan hormone nuclear receptors. Genes Dev. 1994;8(5):538–553. doi: 10.1101/gad.8.5.538. [DOI] [PubMed] [Google Scholar]
- Hara-Chikuma M, Verkman AS. Roles of aquaporin-3 in the epidermis. J Invest Dermatol. 2008;128(9):2145–2151. doi: 10.1038/jid.2008.70. [DOI] [PubMed] [Google Scholar]
- Hunt TK, Niinikoski J, Zederfeldt B. Role of oxygen in repair processes. Acta Chir Scand. 1972;138(2):109–110. [PubMed] [Google Scholar]
- Irvine AD, McLean WH, Leung DY. Filaggrin mutations associated with skin and allergic diseases. N Engl J Med. 2011;365(14):1315–1327. doi: 10.1056/NEJMra1011040. [DOI] [PubMed] [Google Scholar]
- Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, Gaskell SJ, von Kriegsheim A, Hebestreit HF, Mukherji M, Schofield CJ, Maxwell PH, Pugh CW, Ratcliffe PJ. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;292(5516):468–472. doi: 10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- Jetten AM. Retinoid-related orphan receptors (RORs): critical roles in development, immunity, circadian rhythm, and cellular metabolism. Nucl Recept Signal. 2009;7:e003. doi: 10.1621/nrs.07003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallio PJ, Okamoto K, O’Brien S, Carrero P, Makino Y, Tanaka H, Poellinger L. Signal transduction in hypoxic cells: inducible nuclear translocation and recruitment of the CBP/p300 coactivator by the hypoxia-inducible factor-1alpha. EMBO J. 1998;17(22):6573–6586. doi: 10.1093/emboj/17.22.6573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamura T, Sato S, Iwai K, Czyzyk-Krzeska M, Conaway RC, Conaway JW. Activation of HIF1alpha ubiquitination by a reconstituted von Hippel-Lindau (VHL) tumor suppressor complex. Proc Natl Acad Sci U S A. 2000;97(19):10430–10435. doi: 10.1073/pnas.190332597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim EJ, Yoo YG, Yang WK, Lim YS, Na TY, Lee IK, Lee MO. Transcriptional activation of HIF-1 by RORalpha and its role in hypoxia signaling. Arterioscler Thromb Vasc Biol. 2008;28(10):1796–1802. doi: 10.1161/ATVBAHA.108.171546. [DOI] [PubMed] [Google Scholar]
- Kim H, Lee JM, Lee G, Bhin J, Oh SK, Kim K, Pyo KE, Lee JS, Yim HY, Kim KI, Hwang D, Chung J, Baek SH. DNA damage-induced RORalpha is crucial for p53 stabilization and increased apoptosis. Molecular cell. 2011;44(5):797–810. doi: 10.1016/j.molcel.2011.09.023. [DOI] [PubMed] [Google Scholar]
- Lando D, Peet DJ, Whelan DA, Gorman JJ, Whitelaw ML. Asparagine hydroxylation of the HIF transactivation domain a hypoxic switch. Science. 2002;295(5556):858–861. doi: 10.1126/science.1068592. [DOI] [PubMed] [Google Scholar]
- Li W, Li Y, Guan S, Fan J, Cheng CF, Bright AM, Chinn C, Chen M, Woodley DT. Extracellular heat shock protein-90alpha: linking hypoxia to skin cell motility and wound healing. EMBO J. 2007;26(5):1221–1233. doi: 10.1038/sj.emboj.7601579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love KT, Mahon KP, Levins CG, Whitehead KA, Querbes W, Dorkin JR, Qin J, Cantley W, Qin LL, Racie T, Frank-Kamenetsky M, Yip KN, Alvarez R, Sah DW, de Fougerolles A, Fitzgerald K, Koteliansky V, Akinc A, Langer R, Anderson DG. Lipid-like materials for low-dose, in vivo gene silencing. Proc Natl Acad Sci U S A. 2010;107(5):1864–1869. doi: 10.1073/pnas.0910603106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki N, Ikuta M, Matsui T. Hypoxia-induced activation of the retinoic acid receptor-related orphan receptor alpha4 gene by an interaction between hypoxia-inducible factor-1 and Sp1. J Biol Chem. 2004;279(15):15025–15031. doi: 10.1074/jbc.M313186200. [DOI] [PubMed] [Google Scholar]
- Nguyen BC, Lefort K, Mandinova A, Antonini D, Devgan V, Della Gatta G, Koster MI, Zhang Z, Wang J, di Vignano AT, Kitajewski J, Chiorino G, Roop DR, Missero C, Dotto GP. Cross-regulation between Notch and p63 in keratinocyte commitment to differentiation. Genes Dev. 2006;20(8):1028–1042. doi: 10.1101/gad.1406006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nys K, Van Laethem A, Michiels C, Rubio N, Piette JG, Garmyn M, Agostinis P. A p38(MAPK)/HIF-1 pathway initiated by UVB irradiation is required to induce Noxa and apoptosis of human keratinocytes. J Invest Dermatol. 2010;130(9):2269–2276. doi: 10.1038/jid.2010.93. [DOI] [PubMed] [Google Scholar]
- Place TL, Domann FE. Prolyl-hydroxylase 3: Evolving Roles for an Ancient Signaling Protein. Hypoxia (Auckl) 2013;2013(1):13–17. doi: 10.2147/HP.S50091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouyssegur J, Dayan F, Mazure NM. Hypoxia signalling in cancer and approaches to enforce tumour regression. Nature. 2006;441(7092):437–443. doi: 10.1038/nature04871. [DOI] [PubMed] [Google Scholar]
- Pozo D, Garcia-Maurino S, Guerrero JM, Calvo JR. mRNA expression of nuclear receptor RZR/RORalpha, melatonin membrane receptor MT, and hydroxindole-O-methyltransferase in different populations of human immune cells. J Pineal Res. 2004;37(1):48–54. doi: 10.1111/j.1600-079X.2004.00135.x. [DOI] [PubMed] [Google Scholar]
- Rezvani HR, Ali N, Nissen LJ, Harfouche G, de Verneuil H, Taieb A, Mazurier F. HIF-1alpha in epidermis: oxygen sensing, cutaneous angiogenesis, cancer, and non-cancer disorders. J Invest Dermatol. 2011a;131(9):1793–1805. doi: 10.1038/jid.2011.141. [DOI] [PubMed] [Google Scholar]
- Rezvani HR, Ali N, Serrano-Sanchez M, Dubus P, Varon C, Ged C, Pain C, Cario-Andre M, Seneschal J, Taieb A, de Verneuil H, Mazurier F. Loss of epidermal hypoxia-inducible factor-1alpha accelerates epidermal aging and affects re-epithelialization in human and mouse. J Cell Sci. 2011b;124(Pt 24):4172–4183. doi: 10.1242/jcs.082370. [DOI] [PubMed] [Google Scholar]
- Schmuth M, Haqq CM, Cairns WJ, Holder JC, Dorsam S, Chang S, Lau P, Fowler AJ, Chuang G, Moser AH, Brown BE, Mao-Qiang M, Uchida Y, Schoonjans K, Auwerx J, Chambon P, Willson TM, Elias PM, Feingold KR. Peroxisome proliferator-activated receptor (PPAR)-beta/delta stimulates differentiation and lipid accumulation in keratinocytes. J Invest Dermatol. 2004;122(4):971–983. doi: 10.1111/j.0022-202X.2004.22412.x. [DOI] [PubMed] [Google Scholar]
- Semenza GL. Oxygen sensing, hypoxia-inducible factors, and disease pathophysiology. Annu Rev Pathol. 2014;9:47–71. doi: 10.1146/annurev-pathol-012513-104720. [DOI] [PubMed] [Google Scholar]
- Sendoel A, Hengartner MO. Apoptotic cell death under hypoxia. Physiology (Bethesda) 2014;29(3):168–176. doi: 10.1152/physiol.00016.2013. [DOI] [PubMed] [Google Scholar]
- Slominski A, Fischer TW, Zmijewski MA, Wortsman J, Semak I, Zbytek B, Slominski RM, Tobin DJ. On the role of melatonin in skin physiology and pathology. Endocrine. 2005;27(2):137–148. doi: 10.1385/ENDO:27:2:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solt LA, Burris TP. Action of RORs and their ligands in (patho)physiology. Trends Endocrinol Metab. 2012;23(12):619–627. doi: 10.1016/j.tem.2012.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stucker M, Struk A, Altmeyer P, Herde M, Baumgartl H, Lubbers DW. The cutaneous uptake of atmospheric oxygen contributes significantly to the oxygen supply of human dermis and epidermis. J Physiol. 2002;538(Pt 3):985–994. doi: 10.1113/jphysiol.2001.013067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tandara AA, Mustoe TA. Oxygen in wound healing–more than a nutrient. World J Surg. 2004;28(3):294–300. doi: 10.1007/s00268-003-7400-2. [DOI] [PubMed] [Google Scholar]
- Tu CL, Crumrine DA, Man MQ, Chang W, Elalieh H, You M, Elias PM, Bikle DD. Ablation of the calcium-sensing receptor in keratinocytes impairs epidermal differentiation and barrier function. J Invest Dermatol. 2012;132(10):2350–2359. doi: 10.1038/jid.2012.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turchi L, Aberdam E, Mazure N, Pouyssegur J, Deckert M, Kitajima S, Aberdam D, Virolle T. Hif-2alpha mediates UV-induced apoptosis through a novel ATF3-dependent death pathway. Cell Death Differ. 2008;15(9):1472–1480. doi: 10.1038/cdd.2008.74. [DOI] [PubMed] [Google Scholar]
- Wong WJ, Richardson T, Seykora JT, Cotsarelis G, Simon MC. Hypoxia-inducible factors regulate filaggrin expression and epidermal barrier function. J Invest Dermatol. 2015;135(2):454–461. doi: 10.1038/jid.2014.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodley DT, Fan J, Cheng CF, Li Y, Chen M, Bu G, Li W. Participation of the lipoprotein receptor LRP1 in hypoxia-HSP90alpha autocrine signaling to promote keratinocyte migration. J Cell Sci. 2009;122(Pt 10):1495–1498. doi: 10.1242/jcs.047894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing D, Liu L, Marti GP, Zhang X, Reinblatt M, Milner SM, Harmon JW. Hypoxia and hypoxia-inducible factor in the burn wound. Wound Repair Regen. 2011;19(2):205–213. doi: 10.1111/j.1524-475X.2010.00656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


