Abstract
Whether APOL1 polymorphisms contribute to the excess risk of hypertension among blacks is unknown. To assess this we evaluated whether self-reported race and, in blacks, APOL1 risk variants (high-risk [2 risk alleles] versus low-risk [0–1 risk allele]) were associated with longitudinal blood pressure. Blood pressure trajectories were determined using linear mixed-effects (slope) and latent class models (5 distinct groups) during 25 years of follow-up in the Coronary Artery Risk Development in Young Adults Study. Associations of race and APOL1 genotypes with blood pressure change, separately, using linear mixed-effects and multinomial logistic regression models, adjusting for demographic, socioeconomic, and traditional hypertension risk factors, anti-hypertensive medication use, and kidney function were evaluated. Among 1700 whites and 1330 blacks (13% APOL1 high-risk, mean age 25 years; 46% male) mean mid-, ([systolic + diastolic blood pressure]/2), systolic and diastolic blood pressures were 89, 110, 69 mmHg, respectively. One percent of participants used anti-hypertensive medications at baseline. Compared to whites, blacks, regardless of APOL1 genotype, had significantly greater increases in mid-blood pressure and were more likely to experience significantly increasing mid-blood pressure trajectories with adjusted relative risk ratios of 5.21 and 7.27 for moderate-increasing and elevated-increasing versus low-stable blood pressure, respectively. Among blacks, longitudinal mid-blood pressure changes and mid-blood pressure trajectory classification were similar by APOL1 risk status. Modeling systolic and diastolic blood pressure as outcomes yielded similar findings. From young adulthood to mid-life, blacks have greater blood pressure increases versus whites that are not fully explained by traditional risk factors. Thus APOL1 variants are not associated with longitudinal blood pressure in blacks.
Keywords: APOL1, apolipoprotein L1, hypertension, blood pressure, CARDIA
INTRODUCTION
Hypertension is a well-established risk factor for both cardiovascular and chronic kidney disease (CKD) progression. Studies to date have demonstrated that racial disparities exist in the incidence, prevalence, and control of hypertension in the United States (U.S.).1–4 A report from the Multi-Ethnic Study of Atherosclerosis (MESA) showed that black participants had 2-fold higher odds of having hypertension compared to white participants, even after adjusting for relevant demographic, clinical, and socioeconomic risk factors. Moreover, a greater percentage of black individuals had treated but uncontrolled hypertension (35% vs. 24%, compared to white individuals).3 Prior reports in the Coronary Artery Risk Development in Young Adults (CARDIA) study have also suggested higher blood pressure levels in young blacks compared to whites longitudinally.5,6 In clinical trials, black participants typically require more medications to achieve equivalent blood pressure control as white participants.7,8 While environmental factors, such as access to care and/or medications, account for some of these observed differences in blood pressure by race, genetic factors may also play a role.
Numerous genetic variants conferring increased susceptibility for hypertension have been identified; however, none appear to fully explain the excess burden of hypertension among U.S. blacks.9–11 In recent years, two variants in the APOL1 gene encoding apolipoprotein L1 have emerged as a risk factor for various forms of kidney disease, including focal segmental glomerulosclerosis, HIV-associated nephropathy, and CKD attributed to hypertension.12–17 Approximately 12–14% of black Americans carry APOL1 high-risk genotypes (2 risk alleles, G1 or G2).12,14,16 While the mechanisms by which APOL1 risk variants lead to kidney disease are unclear, several lines of evidence suggest that they may also directly contribute to hypertension. First, APOL1 is expressed in not only podocytes but also vascular endothelial and smooth muscle cells within the kidney.18,19 Second, pre-eclampsia, of which hypertension is a hallmark manifestation, and eclampsia have been described in transgenic mice bearing the APOL1 G2 variant.20 Finally, in humans, APOL1 was one of nineteen serum peptide biomarkers that helped differentiate women with pre-eclampsia from pregnant controls.21 Alternatively, the APOL1 risk variants may lead to hypertension via their effects on the kidney. Early renal impairment was associated with an increased risk for incident hypertension in middle-aged persons.22
We designed the present study to evaluate whether APOL1 high-risk genotypes account for some of the excess burden of hypertension observed among black Americans. To date, the association between APOL1 variants and blood pressure levels over time among individuals transitioning from young adulthood to middle age is not established. This is potentially important due to the strong association between blood pressure, kidney disease, and cardiovascular disease.23,24 We hypothesize that APOL1 risk variants will partially explain the more aggressive blood pressure trajectories observed in black compared to white individuals.
RESULTS
Baseline Characteristics
At baseline (CARDIA enrollment, year 0), mean age was 25 ± 3.6 years, 46% were male, and 2% had a history of hypertension. Among the 1330 black participants included in our study, 176 (13%) had the APOL1 high-risk genotypes (Supplementary Table 1). When comparing by race, black individuals had higher mean systolic and mid-blood pressures, higher mean pulse pressure, higher cystatin-based estimated glomerular filtration rate (eGFR-cys), higher prevalence of albuminuria, and lower socioeconomic status compared to white individuals (p<0.01 for each; Table 1). When comparing by APOL1 risk status, black participants with the high-risk genotype were more likely to have albuminuria at year 10 compared to those with the low-risk genotype (0–1 risk alleles; p<0.01; Table 1). Similar conclusions were obtained when comparing by number of APOL1 risk alleles (Supplementary Table 2).
Table 1. Baseline characteristics of study participants, by race and APOL1 genotype status, in the Coronary Artery Disease in Young Adults Study (n=3030).
Values presented as mean ± standard deviation or number (percentage). BMI, body mass index; HDL, high-density lipoprotein; LDL, low-density lipoprotein; HS, high school; eGFRcys, cystatin c-based estimated glomerular filtration rate; ACR, urine albumin-to-creatinine ratio. Hypertension was defined as having a systolic blood pressure >140 mmHg, diastolic blood pressure >90 mmHg, or use of anti-hypertensive medications. Diabetes mellitus was defined as having an elevated fasting blood glucose level of ≥126 mg/dL and/or use of diabetes medications.
| Characteristic | Whites (n=1700) | Blacks | P value Comparing Black vs. White | P value Comparing APOL1 high-risk vs. low-risk | |
|---|---|---|---|---|---|
| APOL1 Low-risk (n=1154) | APOL1 High-risk (n=176) | ||||
| Age, years | 26 ± 3 | 25 ± 4 | 24 ± 4 | <0.01 | 0.01 |
| Male gender | 817 (48%) | 503 (44%) | 79 (45%) | 0.02 | 0.75 |
| Annual Income† | <0.01 | 0.84 | |||
| < $25,000 | 249 (15%) | 434 (38%) | 69 (39%) | ||
| $25,000 – $49,999 | 554 (33%) | 423 (37%) | 66 (38%) | ||
| ≥$50,000 | 895 (53%) | 291 (25%) | 41 (23%) | ||
| Employment | <0.01 | 0.21 | |||
| Employed | 1464 (89%) | 837 (74%) | 118 (68%) | ||
| Unemployed | 137 (8%) | 264 (23%) | 51 (29%) | ||
| Homemaker | 49 (3%) | 37 (3%) | 5 (3%) | ||
| Participant education | <0.01 | 0.30 | |||
| Less than HS | 76 (4%) | 139 (12%) | 17 (10%) | ||
| HS completed | 334 (20%) | 417 (36%) | 57 (32%) | ||
| College or more | 1290 (76%) | 598 (52%) | 102 (58%) | ||
| Caretaker education† | <0.01 | 0.85 | |||
| Less than HS | 153 (9%) | 221 (19%) | 33 (19%) | ||
| HS completed | 935 (55%) | 661 (57%) | 98 (56%) | ||
| College or more | 612 (36%) | 272 (24%) | 45 (26%) | ||
| Usual source of medical care† | 221 (13%) | 139 (12%) | 22 (13%) | 0.48 | 0.88 |
| Hypertension | 35 (2%) | 31 (3%) | 7 (4%) | 0.16 | 0.33 |
| Diabetes mellitus | 12 (1%) | 16 (1%) | 4 (2%) | 0.03 | 0.32 |
| Current smoker | 405 (24%) | 336 (29%) | 59 (34%) | <0.01 | 0.23 |
| Systolic blood pressure, mmHg | 109 ± 11 | 111 ± 11 | 113 ± 10 | <0.01 | 0.06 |
| Diastolic blood pressure, mmHg | 68 ± 9 | 69 ± 10 | 70 ± 11 | 0.21 | 0.15 |
| Mid-blood pressure, mmHg | 89 ± 9 | 90 ± 9 | 91 ± 9 | <0.01 | 0.08 |
| Pulse pressure, mmHg | 41 ± 9 | 43 ± 10 | 43 ± 10 | <0.01 | 0.54 |
| Anti-hypertensive medication use | 11 (1%) | 9 (1%) | 4 (2%) | 0.31 | 0.08 |
| BMI, kg/m2 | 24 ± 4 | 25 ± 6 | 25 ± 6 | <0.01 | 0.27 |
| Total cholesterol, mg/dL | 176 ± 32 | 178 ± 34 | 179 ± 35 | 0.07 | 0.59 |
| HDL, mg/dL | 52 ± 13 | 54 ± 13 | 55 ± 13 | <0.01 | 0.32 |
| LDL, mg/dL | 109 ± 30 | 111 ± 32 | 110 ± 33 | 0.05 | 0.92 |
| Triglycerides, mg/dL | 78 ± 56 | 65 ± 32 | 69 ± 43 | <0.01 | 0.38 |
| eGFRcys, ml/min/1.73 m2 † | 109 ± 12 | 113 ± 12 | 111 ± 15 | <0.01 | 0.18 |
| ACR ≥30 mg/g † | 41 (3%) | 57 (6%) | 22 (14%) | <0.01 | <0.01 |
Denotes measured at year 10.
Longitudinal Blood Pressure by Race and APOL1 Risk Status
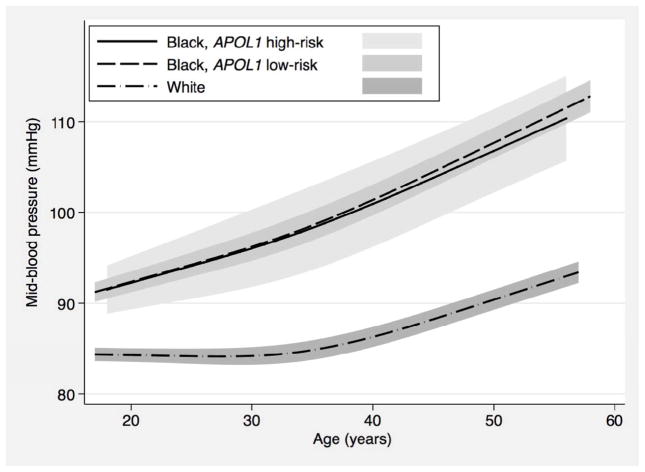
Over 8 possible CARDIA examinations (25 years of follow-up), the number of blood pressure readings available per participant ranged from a minimum of 3 to a maximum of 8, and was similar by race (mean of 7.5 measurements for black and 7.7 for white participants) and by APOL1 risk status (mean of 7.5 measurements for both high- and low-risk genotypes). Mean follow-up time was 23.8 years for black participants with the high-risk genotype, 23.7 years for black participants with the low-risk genotype, and 24.2 years for white participants. In our first approach, we used linear mixed-effects models to compare changes in mid-blood pressure by self-identified race. We found that black individuals experienced greater increases in mid-blood pressure from young adulthood to mid-life compared to white individuals. These racial differences in longitudinal mid-blood pressure persisted even after adjusting for demographic factors, socioeconomic factors, traditional risk factors for hypertension, use of anti-hypertensive medications, kidney function, and albuminuria. Figure 1 depicts the trajectories of the fitted mean mid-blood pressure by race and APOL1 risk status while Table 2 presents differences in annualized percent mid-blood pressure change at specific ages. In contrast to the observed race differences, analyses limited to black participants showed no difference in longitudinal mid-blood pressure change by APOL1 risk status (Figure 1 and Table 2). Similar findings were observed in analyses using systolic blood pressure, diastolic blood pressure, and pulse pressure as secondary outcomes (Supplementary Figure 1, Supplementary Tables 3, 4, and 5). Finally, in sensitivity analyses, we used an additive genetic model (0, 1, or 2 APOL1 risk alleles) and found no association between number of APOL1 risk alleles and each outcome (Supplementary Table 6).
Figure 1.
Longitudinal changes in mid-blood pressure over time, by race and APOL1 genotype status, in the Coronary Artery Disease in Young Adults Study (n=2760)
Table 2. Differences in annualized percentage change in mid-blood pressure for sample ages, by race and APOL1 genotype status, in the Coronary Artery Disease in Young Adults Study.
We examined the associations of race and APOL1 status with longitudinal mid-blood pressure using nested models. Model 1 adjusted for gender, global ancestry, and CARDIA center site. Model 2 additionally adjusted for body mass index†, smoking†, total cholesterol†, use of anti-hypertensive medications†, history of diabetes mellitus†, annual income, employment†, participant education†, caretaker education, and access to care. Model 3 additionally adjusted for estimated glomerular filtration rate (cystatin based)† and urine albumin-to-creatinine ratio ≥ 30 mg/g†. A positive percentage indicates numerically higher blood pressure percentage change in Blacks vs. Whites and in APOL1 high-risk vs. low-risk individuals.
| Age (years) | Comparing Black vs. White (overall) | Comparing APOL1 high-risk vs. low-risk (among Blacks only) | |||
|---|---|---|---|---|---|
| Model 1 (n=2760) | Model 2 (n=2753) | Model 3 (n=2744) | Model 1 (n=1160) | Model 2 (n=1155) | |
| Between group difference (%) 95% Confidence Interval |
Between group difference (%) 95% Confidence Interval |
Between group difference (%) 95% Confidence Interval |
Between group difference (%) 95% Confidence Interval |
Between group difference (%) 95% Confidence Interval |
|
| 30 | 0.36 ** (0.30, 0.43) | 0.22 ** (0.15, 0.29) | 0.12 (−0.20, 0.44) | −0.04 (−0.18, 0.10) | −0.03 (−0.17, 0.11) |
| 35 | 0.29 ** (0.25, 0.33) | 0.23 ** (0.19, 0.28) | 0.16 * (0.01, 0.31) | −0.03 (−0.12, 0.06) | −0.02 (−0.11, 0.07) |
| 40 | 0.23 ** (0.18, 0.28) | 0.24 ** (0.19, 0.30) | 0.19 ** (0.09, 0.30) | −0.03 (−0.15, 0.10) | −0.02 (−0.14, 0.11) |
| 45 | 0.20 ** (0.14, 0.26) | 0.25 ** (0.18, 0.32) | 0.21** (0.06, 0.37) | −0.03 (−0.19, 0.13) | −0.02 (−0.17, 0.14) |
| 50 | 0.20 ** (0.13, 0.26) | 0.25 ** (0.18, 0.32) | 0.21 * (0.05, 0.38) | −0.03 (−0.19, 0.14) | −0.02 (−0.18, 0.15) |
Denotes time-varying variable;
denotes p<0.05; and
denotes p<0.01.
Discrete Blood Pressure Trajectories by Race and APOL1 Risk Status
In a second approach, we categorized participants into previously described discrete blood pressure trajectory groups defined by baseline levels and changes over time (low-stable, moderate-stable, elevated-stable, moderate-increasing, and elevated-increasing).6 We found that black persons were more likely to be assigned to higher and rising blood pressure trajectory categories compared to white persons, whereas there was no difference by APOL1 risk status (Table 3). In adjusted multinomial logistic regression models, black individuals were also more likely than white individuals to be assigned to one of the two classes with rising blood pressure. Adjustment only moderately attenuated these associations, which remained statistically significant. In analyses limited to black participants, we found no significant differences in trajectory assignment by APOL1 risk status (Table 4). Findings did not differ when using systolic or diastolic blood pressure separately as the outcome (Supplementary Tables 7 and 8).
Table 3. Distribution of five mid-blood pressure trajectories, by race and APOL1 genotype status, in the Coronary Artery Disease in Young Adults Study.
Discrete blood pressure trajectories were analyzed as previously described for this cohort by Allen et al.6 Differences in blood pressure trajectories were compared by Chi-squared test.
| Mid-Blood Pressure Trajectories | ||||||
|---|---|---|---|---|---|---|
| Low Stable | Moderate Stable | Elevated Stable | Moderate Increasing | Elevated Increasing | P-value | |
| Overall | ||||||
| White race | 513 (30.2%) | 761 (44.8%) | 282 (16.6%) | 114 (6.7%) | 30 (1.8%) | <0.001 |
| Black race | 159 (12.0%) | 559 (42.0%) | 291 (21.9%) | 224 (16.8%) | 97 (7.3%) | |
| Among Blacks only | ||||||
| APOL1 high-risk genotype | 20 (11.4%) | 73 (41.5%) | 38 (21.6%) | 28 (15.9%) | 17 (9.7%) | 0.78 |
| APOL1 low-risk genotype | 139 (12.1%) | 486 (42.1%) | 253 (21.9%) | 196 (17.0%) | 80 (6.9%) | |
Table 4. Relative risk ratios for five mid-blood pressure trajectories, by race and APOL1 genotype status, in the Coronary Artery Disease in Young Adults Study.
We examined the relative risk for being assigned to a particular category of mid-blood pressure change by race and APOL1 status using nested models. Model 1: adjusted for gender, global ancestry, and CARDIA center site. Model 2: additionally adjusted for body mass index, smoking, total cholesterol, use of anti-hypertensive medications, history of diabetes mellitus, annual income, employment, participant education, caretaker education, and access to care. RRR, relative risk ratio; Ref, reference group. Discrete blood pressure trajectories were determined in this cohort as previously described by Allen et al.6 Blacks and whites differed in blood pressure trajectories, but APOL1 high-risk and low-risk blacks were similar.
| Relative risk ratio comparing Black vs. White (overall) | |||||
|---|---|---|---|---|---|
| Low Stable | Moderate Stable | Elevated Stable | Moderate Increasing | Elevated Increasing | |
| RRR 95% Confidence Interval |
RR 95% Confidence Interval |
RR 95% Confidence Interval |
RR 95% Confidence Interval |
RR 95% Confidence Interval |
|
| Model 1 | Ref | 2.73** (2.16, 3.45) | 4.44** (3.36, 5.88) | 7.64** (5.57, 10.40) | 13.60** (8.35, 22.40) |
| Model 2 | Ref | 2.15** (1.66, 2.79) | 2.89** (2.10, 3.97) | 5.21** (3.66, 7.41) | 7.27** (4.17, 12.60) |
| Relative risk ratio comparing APOL1 high-risk vs. low-risk (among Blacks only) | |||||
| Low Stable | Moderate Stable | Elevated Stable | Moderate Increasing | Elevated Increasing | |
| RRR 95% Confidence Interval |
RRR 95% Confidence Interval |
RRR 95% Confidence Interval |
RRR 95% Confidence Interval |
RRR 95% Confidence Interval |
|
| Model 1 | Ref | 0.95 (0.52, 1.72) | 1.01 (0.52, 1.94) | 0.94 (0.48, 1.83) | 1.40 (0.64, 3.06) |
| Model 2 | Ref | 1.04 (0.56, 1.94) | 1.14 (0.57, 2.30) | 1.08 (0.53, 2.18) | 1.34 (0.57, 3.19) |
denotes p<0.01
DISCUSSION
In this large cohort study of healthy young adults, we found that self-reported black race is associated with blood pressures that are more likely to rise and become elevated from young adulthood to mid-life compared to white race. This racial difference in longitudinal blood pressure over 25 years of follow-up was not explained by traditional risk factors for hypertension, socioeconomic status, or access to care. Moreover, accounting for time-dependent values of eGFR-cys and albuminuria did not explain these observed differences. Among black participants, we found no association between APOL1 risk variants and longitudinal blood pressure. Taken together, our findings suggest that despite their strong association with kidney disease progression, APOL1 risk variants do not appear to play a role in longitudinal blood pressure changes among black individuals. Furthermore, the persistent racial differences in blood pressure rise after adjustment for traditional and socioeconomic risk factors indicate that additional, yet unknown environmental or genetic factors may account for the excess burden of hypertension among black Americans.
The racial disparities that exist in blood pressure levels and hypertension are well established.1–3,6,25 Using data from the first four CARDIA examinations (years 0, 2, 5, and 7), Liu et al. reported that systolic and diastolic blood pressure were higher in black men and women compared to their white counterparts and more than 50% of the differences observed at Year 7 remained after controlling for obesity and other lifestyle factors.5 More recently, Allen et al. used data from all eight CARDIA examinations (years 0, 2, 5, 7, 10, 15, 20, and 25) and demonstrated that compared to white participants, black participants were more likely to have rising blood pressure trajectories.6 We extend upon these findings by: 1) employing an additional method (i.e. linear mixed-effects models) to model longitudinal blood pressure in this population, and 2) exploring potential explanatory variables and their changes over young adulthood. Our study shows that differences in blood pressure by race persisted even after adjustment for relevant demographic, clinical, and socioeconomic factors, and are consistent with past studies in middle aged and older individuals. We therefore propose that other factors likely contribute and still need to be identified.
Prior studies have suggested a role of the APOL1 risk variants in hypertension-attributed kidney disease and possibly also cardiovascular disease. Lipkowitz et al. and Parsa et al. reported that the APOL1 high-risk genotype was associated with CKD progression among black individuals with hypertension-attributed nephropathy.15,17 Some investigators have also described an increased risk of cardiovascular disease among individuals with two APOL1 high-risk alleles, though this finding has not been consistent across studies.26–29 In addition, Skorecki and Wasser illustrated that the geographic distribution of APOL1 risk variants correlates with the distribution of hypertension prevalence rates in West Africa.30 Finally, in APOL1 expression studies by Madhavan et al., de novo expression of APOL1 was found in arterial smooth muscle cells of diseased but not healthy kidneys.18 While Ma et al. also reported renal microvascular expression of APOL1 in normal kidneys,19 APOL1 may adversely affect the function of the renal microcirculation in ways that promote hypertension. Moreover, these pathways may not, at least initially, cause kidney damage that can be detected by routine clinical tests. The present study, however, did not find evidence for the predicted greater prevalence of hypertension among persons with APOL1 high-risk genotypes transitioning from young adulthood to middle age and with largely preserved kidney function. Histopathologic studies have also shown a lack of correlation between APOL1 risk variants and arteriosclerosis or arteriolar hyalinosis across a variety of kidney diseases.31–33 Perhaps, an association between APOL1 risk variants and high blood pressure exists but is much weaker than the variants’ associations with CKD or ESRD. Alternatively, APOL1 may contribute to hypertension through its effects on kidney function and that more severe kidney disease needs to be present before such associations are detectable. Overall, our results suggest that racial differences in longitudinal blood pressure are not explained by APOL1 risk variants in black Americans in the general population.
To further understand the association of race with longitudinal blood pressure in the context of kidney disease, we also studied whether this association persisted after adjusting for time-varying markers of kidney function. Peralta et al. previously demonstrated in CARDIA that black persons with the APOL1 high-risk genotype had onset of albuminuria at earlier ages and faster rates of eGFR-cys decline compared to white persons or black persons with the low-risk genotype.34 Other studies have also demonstrated increased serum cystatin C and albuminuria as independent predictors of incident hypertension.22,35–37 Our results suggest that in a population of healthy young adults without significant kidney disease, differences in kidney function do not significantly explain race differences in blood pressure changes over time. We further propose that the APOL1 risk variants’ associations with renal damage are unlikely to be mediated by preceding rising blood pressure.
Our study has several strengths. First, we utilized data from a large, well-characterized cohort of healthy young adults with relatively few comorbidities at baseline. Second, we assessed longitudinal blood pressure in two different ways, first using linear mixed-effects models and then with latent class models. Similar conclusions were obtained from both methods, thus suggesting that our findings are robust. Third, follow-up was long (up to 25 years) with high retention and few participants taking anti-hypertensive medications at baseline. This allowed us to comprehensively examine how blood pressure progresses from young adulthood to middle age. Limitations included the potential for residual or unknown sources of confounding, as we did not account for additional measures of socioeconomic status, dietary intake, or dose of anti-hypertensive therapy. Moreover, our results may not be generalizable to other populations, including older adults or individuals with established CKD.
In conclusion, we report that differences in longitudinal blood pressure exist by race but not by APOL1 risk variants among blacks. Additional studies are needed to replicate our findings in other cohorts of young individuals as well as to identify other genetic and/or environmental factors that might reduce the excess burden of hypertension among black persons.
METHODS
Study Population
Our study population consisted of 3030 participants from the CARDIA kidney function trajectories ancillary study as previously described.34,38 Among them, 1700 were white and 1330 were self-reported black Americans who had previously been genotyped for the APOL1 risk variants.34,38 Briefly, CARDIA is a prospective cohort study designed to study risk factors for cardiovascular disease among young adults. Beginning in 1985–1986, 5115 white and black individuals, aged 18–30 years, were recruited from one of four sites (Birmingham, Alabama; Chicago, Illinois; Minneapolis, Minnesota; and Oakland, California).25,34,38 The initial examination included the standardized collection of multiple risk factors relevant to the development of coronary artery disease.25 Subsequent examinations occurred at years 2, 5, 7, 10, 15, 20, and 25.25,34,38 Retention rates for these follow-up examinations were high at 90%, 86%, 81%, 77%, 74%, 72%, and 72%, respectively.6 The CARDIA study was approved by the institutional review boards of each participating institution.25,34,38
Measurement of Blood Pressure
At each examination, resting systolic and diastolic blood pressures were measured 3 times at 1-minute intervals by trained technicians using a random-zero sphygmomanometer (years 0–20) or an Omron sphygmomanometer (years 20–25). Blood pressure measurements for years 20 and 25 were calibrated, based on dual measurements in a subset of participants.39 The average of the second and third blood pressure measurements were subsequently used.6,25 Prior studies have suggested that in young adults, joint consideration of both systolic and diastolic blood pressure may be more predictive of cardiovascular disease than either alone.6,40–42 As such, our primary outcome in this study was change in mid-blood pressure (defined as the average of systolic and diastolic blood pressure or [SBP+DBP]/2) over the entire CARDIA follow-up period. We also considered systolic blood pressure, diastolic blood pressure, and pulse pressure (SBP-DBP) separately.
Genotyping
Genotyping for the APOL1 risk variants (G1 and G2) was performed using TaqMan assays (ABI, Foster City, CA) and DNA samples collected at year 10 or later.34 The G1 allele (rs73885319) is in near-absolute linkage equilibrium with a second G1 allele (rs60910145), both of which are missense mutations. The G2 allele (rs71785313) is defined by a 6 base pair deletion.12,16 In CARDIA, only black participants were genotyped for APOL1 because the frequency of the G1 and G2 risk variants is extremely low among individuals of European ancestry.34,43,44 Consistent with prior studies, we used a recessive genetic model in which the high-risk genotype was defined as having two APOL1 risk alleles and the low-risk genotype was defined as having one or no risk alleles.13,15,17,34 To estimate ancestry, we used Principal Components Analysis (PCA) as implemented in EIGENSTRAT on the QCed and LD-pruned Affymetrix 6.0 genotype data.34,45–47 To control for possible population stratification, we performed our analyses adjusting for the first four principal components.45–47
Covariates
Details regarding each participant’s age, gender, race, cigarette smoking status (years 0 and 10), and use of medications (years 0, 10, 15, and 20) were collected by questionnaires. Measured height and weight was used to calculate BMI (years 0, 10, 15, and 20). Blood specimens were also collected at each study visit and sent to the Northwest Lipid Research Clinic Laboratory at the University of Washington in Seattle for measurement of total cholesterol (years 0 and 10). History of diabetes mellitus (years 0, 10, 15, and 20) was defined as having an elevated fasting blood glucose level of ≥126 mg/dL, use of diabetes medications, or 2-hour glucose tolerance test ≥200 mg/dL (year 10 onwards). Socioeconomic factors including annual income (year 10), employment (years 0 and 10), participant education (years 0 and 10), caretaker education (year 10), and access to care (defined as having a usual source of medical care, year 10) were based on self-report. Urinary albumin and creatinine levels were available at years 10, 15, 20, and 25, with albuminuria being defined as a urine albumin-to-creatinine ratio (ACR) ≥30 mg/g.34 Kidney function was assessed by serum cystatin C, which was measured at years 10, 15, and 20 from stored samples that were processed using the N Latex cystatin C kit (Dade Behring, now Siemens, Munich, Germany) at a central laboratory.34 The following equation was used to estimate cystatin based GFR (GFR-cys): eGFR-cys=133*(min(cysC/0.8,1)**(−0.499))*(max(cysC/0.8, 1)**(−1.328))*(0.996**age)*(0.932**if female).34,48
Statistical Analyses
Baseline characteristics were compared using Kruskal-Wallis test for continuous variables and chi-squared or Fisher’s exact test for categorical variables. Change in blood pressure over time was analyzed using two different methods. First, we used linear mixed-effects models (LMMs) to examine differences in the rate of change in blood pressure between: 1) black vs. white persons (in the overall sample); and 2) APOL1 high- vs. low-risk status (among black persons only). We modeled within-group trends in log-transformed blood pressure using group-specific restricted cubic splines in age, with knots at 24, 33, and 48 years (corresponding to the 10th, 50th and 90th percentiles of the overall distribution, respectively). Within-subject correlation of the repeated responses was accounted for using random intercepts and slopes, with unstructured covariance. To determine whether differences in blood pressure trends by race or APOL1 status could be explained by other factors, we estimated a nested sequence of adjusted LMMs, adding covariates to the preceding model at each step. Model 1 adjusted for baseline demographic factors including gender, global ancestry, and CARDIA center site. Model 2 additionally adjusted for traditional risk factors for hypertension (BMI, smoking, total cholesterol, use of anti-hypertensive medications, and history of diabetes mellitus) and socioeconomic factors (annual income, employment, participant and caretaker education, and access to care). Model 3 additionally adjusted for markers of kidney function, including eGFR-cys and albuminuria. Model 3 was intended a priori to assess for potential mediators and used only if a main effect was detected. We therefore considered Model 2 to be our final model. In order to account for changes over the study period, BMI, smoking, total cholesterol, anti-hypertensive medication use, history of diabetes, employment, participant education, eGFR-cys, and albuminuria were updated at each visit in which they were measured, with the last value being carried forward for intervening visits. In a final-step, to quantify between-group differences, we estimated annualized percentage rates of change using the first derivatives (with respect to age) of the non-linear group-specific spline trajectories in log-transformed blood pressure, evaluated at ages 30, 35, 40, 45, and 50, and then back transformed using the formula 100*(exp(β)−1), where β is the between-group difference in age-specific derivatives. Standard errors, confidence intervals and p-values were calculated using the delta-method. In sensitivity analyses, we also considered an additive genetic model (0, 1, or 2 risk alleles).
In additional analysis, we also assessed the associations of race/ethnicity and APOL1 status with previously identified categories of blood pressure trajectory of each participant. Briefly, a mixture model was used to identify latent classes characterized by distinct blood pressure trajectories, and to assign each person to one of the classes. Based on the Bayesian Information Criterion (BIC), models with 5 latent classes were selected: low-stable, moderate-stable, elevated-stable, moderate-increasing, and elevated-increasing.6 We then used multinomial logistic regression models to estimate the associations of race and APOL1 risk status with latent class assignment. As in our primary analyses, we estimated a nested sequence of models adjusting for demographic factors, traditional risk factors for hypertension, and socioeconomic factors (Models 1 and 2).
Data were analyzed using Stata Version 14.1 (StataCorp, College Station, TX). Blood pressure trajectory groups had previously been determined using Proc Traj in SAS Version 9.3 (SAS Institute Inc).6 P values less than 0.05 were considered statistically significant.
Supplementary Material
Acknowledgments
TKC is funded by the Extramural Grant Program (EGP) by Satellite Healthcare, a not-for-profit renal care provider. CAP is funded by NIH/NIDDK grant R03DK095877. We would like to thank the participants of the CARDIA Study. This project has been funded in part with federal funds from the National Cancer Institute, National Institutes of Health, under contract HHSN26120080001E. The Coronary Artery Risk Development in Young Adults Study (CARDIA) is conducted and supported by the National Heart, Lung, and Blood Institute (NHLBI) in collaboration with the University of Alabama at Birmingham (HHSN268201300025C & HHSN268201300026C), Northwestern University (HHSN268201300027C), University of Minnesota (HHSN268201300028C), Kaiser Foundation Research Institute (HHSN268201300029C), and Johns Hopkins University School of Medicine (HHSN268200900041C). CARDIA is also partially supported by the Intramural Research Program of the National Institute on Aging (NIA) and an intra-agency agreement between NIA and NHLBI (AG0005). This manuscript has been reviewed by CARDIA for scientific content. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government. This research was supported in part by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research, and National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD.
Footnotes
DISCLOSURES
TKC previously owned stock in Pfizer Pharmaceuticals. CAP owns stock in and is a consultant for Cricket Health, Inc. and previously was a consultant for Vital Labs, Inc. The other authors have nothing to disclose.
Portions of this work were presented at the 2016 American Society of Hypertension Annual Scientific Meeting in New York, New York (May 13–17, 2016).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Egan BM, Zhao Y, Axon RN. US trends in prevalence, awareness, treatment, and control of hypertension, 1988–2008. JAMA. 2010;303:2043–2050. doi: 10.1001/jama.2010.650. [DOI] [PubMed] [Google Scholar]
- 2.Ayanian JZ, Landon BE, Newhouse JP, et al. Racial and ethnic disparities among enrollees in Medicare Advantage plans. N Engl J Med. 2014;371:2288–2297. doi: 10.1056/NEJMsa1407273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kramer H, Han C, Post W, et al. Racial/ethnic differences in hypertension and hypertension treatment and control in the multi-ethnic study of atherosclerosis (MESA) Am J Hypertens. 2004;17:963–970. doi: 10.1016/j.amjhyper.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 4.Levine DA, Lewis CE, Williams OD, et al. Geographic and demographic variability in 20-year hypertension incidence: the CARDIA study. Hypertension. 2011;57:39–47. doi: 10.1161/HYPERTENSIONAHA.110.160341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu K, Ruth KJ, Flack JM, et al. Blood pressure in young blacks and whites: relevance of obesity and lifestyle factors in determining differences. The CARDIA Study. Coronary Artery Risk Development in Young Adults. Circulation. 1996;93:60–66. doi: 10.1161/01.cir.93.1.60. [DOI] [PubMed] [Google Scholar]
- 6.Allen NB, Siddique J, Wilkins JT, et al. Blood pressure trajectories in early adulthood and subclinical atherosclerosis in middle age. JAMA. 2014;311:490–497. doi: 10.1001/jama.2013.285122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hebert LA, Kusek JW, Greene T, et al. Effects of blood pressure control on progressive renal disease in blacks and whites. Modification of Diet in Renal Disease Study Group. Hypertension. 1997;30:428–435. doi: 10.1161/01.hyp.30.3.428. [DOI] [PubMed] [Google Scholar]
- 8.Pergola PE, White CL, Szychowski JM, et al. Achieved blood pressures in the secondary prevention of small subcortical strokes (SPS3) study: challenges and lessons learned. Am J Hypertens. 2014;27:1052–1060. doi: 10.1093/ajh/hpu027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu X, Cooper RS. Admixture mapping provides evidence of association of the VNN1 gene with hypertension. PLoS One. 2007;2:e1244. doi: 10.1371/journal.pone.0001244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhu X, Luke A, Cooper RS, et al. Admixture mapping for hypertension loci with genome-scan markers. Nat Genet. 2005;37:177–181. doi: 10.1038/ng1510. [DOI] [PubMed] [Google Scholar]
- 11.Simino J, Rao DC, Freedman BI. Novel findings and future directions on the genetics of hypertension. Curr Opin Nephrol Hypertens. 2012;21:500–507. doi: 10.1097/MNH.0b013e328354e78f. [DOI] [PubMed] [Google Scholar]
- 12.Genovese G, Friedman DJ, Ross MD, et al. Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science. 2010;329:841–845. doi: 10.1126/science.1193032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kopp JB, Nelson GW, Sampath K, et al. APOL1 genetic variants in focal segmental glomerulosclerosis and HIV-associated nephropathy. J Am Soc Nephrol. 2011;22:2129–2137. doi: 10.1681/ASN.2011040388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kopp JB. Rethinking hypertensive kidney disease: arterionephrosclerosis as a genetic, metabolic, and inflammatory disorder. Curr Opin Nephrol Hypertens. 2013;22:266–272. doi: 10.1097/MNH.0b013e3283600f8c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parsa A, Kao WH, Xie D, et al. APOL1 risk variants, race, and progression of chronic kidney disease. N Engl J Med. 2013;369:2183–2196. doi: 10.1056/NEJMoa1310345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tzur S, Rosset S, Shemer R, et al. Missense mutations in the APOL1 gene are highly associated with end stage kidney disease risk previously attributed to the MYH9 gene. Hum Genet. 2010;128:345–350. doi: 10.1007/s00439-010-0861-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lipkowitz MS, Freedman BI, Langefeld CD, et al. Apolipoprotein L1 gene variants associate with hypertension-attributed nephropathy and the rate of kidney function decline in African Americans. Kidney Int. 2013;83:114–120. doi: 10.1038/ki.2012.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Madhavan SM, O’Toole JF, Konieczkowski M, et al. APOL1 localization in normal kidney and nondiabetic kidney disease. J Am Soc Nephrol. 2011;22:2119–2128. doi: 10.1681/ASN.2011010069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ma L, Shelness GS, Snipes JA, et al. Localization of APOL1 protein and mRNA in the human kidney: nondiseased tissue, primary cells, and immortalized cell lines. J Am Soc Nephrol. 2015;26:339–348. doi: 10.1681/ASN.2013091017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bruggeman LA, Wu Z, Luo L, et al. APOL1-G0 or APOL1-G2 Transgenic Models Develop Preeclampsia but Not Kidney Disease. J Am Soc Nephrol. 2016 doi: 10.1681/ASN.2015111220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wen Q, Liu LY, Yang T, et al. Peptidomic Identification of Serum Peptides Diagnosing Preeclampsia. PLoS One. 2013;8:e65571. doi: 10.1371/journal.pone.0065571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kestenbaum B, Rudser KD, de Boer IH, et al. Differences in kidney function and incident hypertension: the multi-ethnic study of atherosclerosis. Ann Intern Med. 2008;148:501–508. doi: 10.7326/0003-4819-148-7-200804010-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klag MJ, Whelton PK, Randall BL, et al. End-stage renal disease in African-American and white men. 16-year MRFIT findings. JAMA. 1997;277:1293–1298. [PubMed] [Google Scholar]
- 24.Group SR, Wright JT, Jr, Williamson JD, et al. A Randomized Trial of Intensive versus Standard Blood-Pressure Control. N Engl J Med. 2015;373:2103–2116. doi: 10.1056/NEJMoa1511939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Friedman GD, Cutter GR, Donahue RP, et al. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol. 1988;41:1105–1116. doi: 10.1016/0895-4356(88)90080-7. [DOI] [PubMed] [Google Scholar]
- 26.Ito K, Bick AG, Flannick J, et al. Increased burden of cardiovascular disease in carriers of APOL1 genetic variants. Circ Res. 2014;114:845–850. doi: 10.1161/CIRCRESAHA.114.302347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mukamal KJ, Tremaglio J, Friedman DJ, et al. APOL1 Genotype, Kidney and Cardiovascular Disease, and Death in Older Adults. Arterioscler Thromb Vasc Biol. 2016;36:398–403. doi: 10.1161/ATVBAHA.115.305970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Langefeld CD, Divers J, Pajewski NM, et al. Apolipoprotein L1 gene variants associate with prevalent kidney but not prevalent cardiovascular disease in the Systolic Blood Pressure Intervention Trial. Kidney Int. 2015;87:169–175. doi: 10.1038/ki.2014.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grams ME, Rebholz CM, Chen Y, et al. Race, APOL1 Risk, and eGFR Decline in the General Population. J Am Soc Nephrol. 2016;27:2842–2850. doi: 10.1681/ASN.2015070763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Skorecki KL, Wasser WG. Hypertension-misattributed kidney disease in African Americans. Kidney Int. 2013;83:6–9. doi: 10.1038/ki.2012.369. [DOI] [PubMed] [Google Scholar]
- 31.Larsen CP, Beggs ML, Saeed M, et al. Histopathologic findings associated with APOL1 risk variants in chronic kidney disease. Mod Pathol. 2015;28:95–102. doi: 10.1038/modpathol.2014.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Atta MG, Estrella MM, Kuperman M, et al. HIV-associated nephropathy patients with and without apolipoprotein L1 gene variants have similar clinical and pathological characteristics. Kidney Int. 2012;82:338–343. doi: 10.1038/ki.2012.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kopp JB, Winkler CA, Zhao X, et al. Clinical Features and Histology of Apolipoprotein L1-Associated Nephropathy in the FSGS Clinical Trial. J Am Soc Nephrol. 2015;26:1443–1448. doi: 10.1681/ASN.2013111242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peralta CA, Bibbins-Domingo K, Vittinghoff E, et al. APOL1 Genotype and Race Differences in Incident Albuminuria and Renal Function Decline. J Am Soc Nephrol. 2016;27:887–893. doi: 10.1681/ASN.2015020124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang TJ, Evans JC, Meigs JB, et al. Low-grade albuminuria and the risks of hypertension and blood pressure progression. Circulation. 2005;111:1370–1376. doi: 10.1161/01.CIR.0000158434.69180.2D. [DOI] [PubMed] [Google Scholar]
- 36.Forman JP, Fisher ND, Schopick EL, et al. Higher levels of albuminuria within the normal range predict incident hypertension. J Am Soc Nephrol. 2008;19:1983–1988. doi: 10.1681/ASN.2008010038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang M, Matsushita K, Sang Y, et al. Association of kidney function and albuminuria with prevalent and incident hypertension: the Atherosclerosis Risk in Communities (ARIC) study. Am J Kidney Dis. 2015;65:58–66. doi: 10.1053/j.ajkd.2014.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peralta CA, Vittinghoff E, Bansal N, et al. Trajectories of kidney function decline in young black and white adults with preserved GFR: results from the Coronary Artery Risk Development in Young Adults (CARDIA) study. Am J Kidney Dis. 2013;62:261–266. doi: 10.1053/j.ajkd.2013.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jacobs DR, Jr, Yatsuya H, Hearst MO, et al. Rate of decline of forced vital capacity predicts future arterial hypertension: the Coronary Artery Risk Development in Young Adults Study. Hypertension. 2012;59:219–225. doi: 10.1161/HYPERTENSIONAHA.111.184101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Franklin SS. The importance of diastolic blood pressure in predicting cardiovascular risk. J Am Soc Hypertens. 2007;1:82–93. doi: 10.1016/j.jash.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 41.Franklin SS, Larson MG, Khan SA, et al. Does the relation of blood pressure to coronary heart disease risk change with aging? The Framingham Heart Study. Circulation. 2001;103:1245–1249. doi: 10.1161/01.cir.103.9.1245. [DOI] [PubMed] [Google Scholar]
- 42.Tverdal A. Systolic and diastolic blood pressures as predictors of coronary heart disease in middle aged Norwegian men. Br Med J (Clin Res Ed) 1987;294:671–673. doi: 10.1136/bmj.294.6573.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Limou S, Nelson GW, Kopp JB, et al. APOL1 kidney risk alleles: population genetics and disease associations. Adv Chronic Kidney Dis. 2014;21:426–433. doi: 10.1053/j.ackd.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.O’Seaghdha CM, Parekh RS, Hwang SJ, et al. The MYH9/APOL1 region and chronic kidney disease in European-Americans. Hum Mol Genet. 2011;20:2450–2456. doi: 10.1093/hmg/ddr118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kumar R, Seibold MA, Aldrich MC, et al. Genetic ancestry in lung-function predictions. N Engl J Med. 2010;363:321–330. doi: 10.1056/NEJMoa0907897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Price AL, Patterson NJ, Plenge RM, et al. Principal components analysis corrects for stratification in genome-wide association studies. Nat Genet. 2006;38:904–909. doi: 10.1038/ng1847. [DOI] [PubMed] [Google Scholar]
- 47.Patterson N, Price AL, Reich D. Population structure and eigenanalysis. PLoS Genet. 2006;2:e190. doi: 10.1371/journal.pgen.0020190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Inker LA, Schmid CH, Tighiouart H, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367:20–29. doi: 10.1056/NEJMoa1114248. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.