Abstract
BACKGROUND
The landscape of hereditary syndromes and clinicopathologic characteristics among US Latino/Hispanic individuals with colorectal cancer (CRC) remains poorly understood.
METHODS
A total of 265 patients with CRC who were enrolled in the Hispanic Colorectal Cancer Study were included in the current study. Information regarding CRC risk factors was elicited through interviews, and treatment and survival data were abstracted from clinical charts. Tumor studies and germline genetic testing results were collected from medical records or performed using standard molecular methods.
RESULTS
The mean age of the patients at the time of diagnosis was 53.7 years (standard deviation, 10.3 years), and 48.3% were female. Overall, 21.2% of patients reported a first‐degree or second‐degree relative with CRC; 3.4% met Amsterdam I/II criteria. With respect to Bethesda guidelines, 38.5% of patients met at least 1 criterion. Of the 161 individuals who had immunohistochemistry and/or microsatellite instability testing performed, 21 (13.0%) had mismatch repair (MMR)‐deficient (dMMR) tumors. dMMR tumors were associated with female sex (61.9%), earlier age at the time of diagnosis (50.4 ± 12.4 years), proximal location (61.9%), and first‐degree (23.8%) or second‐degree (9.5%) family history of CRC. Among individuals with dMMR tumors, 13 (61.9%) had a germline MMR mutation (MutL homolog 1 [MLH1] in 6 patients; MutS homolog 2 [MSH2] in 4 patients; MutS homolog 6 [MHS6] in 2 patients; and PMS1 homolog 2, mismatch repair system component [PMS2] in 1 patient). The authors identified 2 additional MLH1 mutation carriers by genetic testing who had not received immunohistochemistry/microsatellite instability testing. In total, 5.7% of the entire cohort were confirmed to have Lynch syndrome. In addition, 6 individuals (2.3%) had a polyposis phenotype.
CONCLUSIONS
The percentage of dMMR tumors noted among Latino individuals (13%) is similar to estimates in non‐Hispanic white individuals. In the current study, the majority of individuals with dMMR tumors were confirmed to have Lynch syndrome. Cancer 2017. © 2017 The Authors. Cancer published by Wiley Periodicals, Inc. on behalf of American Cancer Society. Cancer 2017;123:3732–3743. © 2017 American Cancer Society
Keywords: colorectal cancer, DNA mismatch repair, Hispanic, Latino, Lynch syndrome, microsatellite instability (MSI)
Short abstract
The landscape of hereditary syndromes and clinicopathologic characteristics among Latino/Hispanic individuals in the United States with colorectal cancer remains poorly understood. Using data from the Hispanic Colorectal Cancer Study, approximately 13% of cases in the current study appear to have mismatch repair‐deficient tumors, 61.9% of which will be confirmed to have Lynch syndrome.
INTRODUCTION
Colorectal cancer (CRC) is the second most common and lethal malignancy among Hispanic/Latino individuals (henceforth referred to as Latinos), who are the fastest growing minority in the United States.1, 2 Compared with non‐Hispanic white (NHW) individuals, Latino patients present with CRC at an earlier age, are 20% to 40% more likely to present with advanced disease, and have a 20% to 30% increased stage‐specific mortality.2, 3, 4, 5, 6, 7, 8 To the best of our knowledge, the reasons for such disparities are incompletely understood and may partly reflect different biology, treatment, and surveillance patterns.9, 10
Genomic instability results from a loss of DNA mismatch repair (MMR) activity. Approximately 10% to 15% of CRC tumors are associated with microsatellite instability (MSI),11, 12 whereby short repetitive DNA sequences undergo an increase or decrease in repeat length. Mechanistically, somatic events, such as MutL homolog 1 (MLH1) promoter hypermethylation, account for the majority of MSI‐H cancers, with a smaller percentage attributable to germline mutations in a DNA MMR gene (MLH1; MutS homolog 2 [MSH2]; MutS homolog 6 [MSH6]; or PMS1 homolog 2, mismatch repair system component [PMS2]).13, 14 Individuals with germline MMR mutations are classified as having Lynch syndrome,15, 16 which is the most common inherited form of CRC, accounting for 3% to 6% of cases.17, 18, 19
Two recent studies examined potential differences in MSI by ethnicity/race and reported no significant differences.20, 21 Both studies estimated the rate of MMR‐deficient (dMMR) tumors in Latino individuals to be approximately 12%.20, 21 Another study by De Jesus‐Monge et al found different results, reporting a rate of 4.3% for high MSI tumors in a cohort of 164 Puerto Rican patients with CRC.22 However, all these studies were limited by sample size and the lack of germline testing performed. A better understanding of tumor characteristics and the extent of CRC heterogeneity in Latino patients may help explain outcome disparities as well as inform screening and therapeutic decisions in this understudied population.
In the current study, we sought to better characterize the spectrum and prevalence of hereditary syndromes among patients enrolled in the ongoing Hispanic Colorectal Cancer Study (HCCS).
MATERIALS AND METHODS
Study Design and Population
The HCCS is a population‐based study of self‐identified Hispanic or Latino individuals with a diagnosis of CRC. Patients are identified through the California Cancer Registry or directly from local hospitals in the Los Angeles region. As of December 2015, a total of 1112 subjects have been enrolled into the HCCS. Men and women with an initial diagnosis of CRC (International Classification of Diseases for Oncology, 3rd Edition [ICD‐O‐3] codes C18‐C21) after January 1, 2008 were eligible for participation. The current study includes all patients recruited at 2 centers that are part of the HCCS and that report to the California Cancer Registry: the Los Angeles County (LAC) plus University of Southern California (USC) Medical Center (LAC) and USC Norris Comprehensive Cancer Center (Norris); hereafter, these patients will be referred to collectively as the USC subset (265 patients). All participants provided written informed consent. This protocol was approved by the USC Institutional Review Board and the California Institute for the Protection of Human Subjects.
Risk Factor Questionnaires
All participants completed a telephone‐based or face‐to‐face interview after study enrollment that included the collection of demographic information (age, sex, and country of birth) and lifestyle exposures during the 2 years before the diagnosis of CRC. Data were collected regarding personal and family histories of CRC, colon polyps, and other cancers. Medical diagnoses of diabetes, Crohn disease, ulcerative colitis, and familial adenomatous polyposis were self‐reported by patients and confirmed by review of the medical records when possible. Body mass index (BMI) was calculated as the individual's weight (in kg) 2 years before study recruitment divided by adult height in meters squared (m2). Several lifestyle risk factors were queried, including medication use, reproductive history, hormonal contraceptive use, physical activity, body height and weight, alcohol intake, and tobacco use. Ever‐use (yes vs no) of nonsteroidal anti‐inflammatory drugs was defined as use at least 2 times per week for >1 month during a participant's lifetime. Alcohol use was defined as the consumption of any alcoholic beverage (beer, wine, hard cider, sake, liquor, mixed drinks, or cocktails) at least once a week for ≥6 months during the most recent decade of life at the time of enrollment. Being an ever‐smoker was defined as ever smoking at least 1 cigarette per day for ≥3 months. Pack‐years of smoking were calculated based on the number of cigarettes smoked per day and the number of years smoked. An individual was considered to be physically active (yes vs no) if they reported >20 metabolic equivalent (MET) hours per week of physical activity during the most recent decade of life at the time of enrollment.
Clinical Chart Abstraction
A systematic review of each participant's medical record was performed at LAC and Norris. Information regarding the following tumor characteristics was retrieved: clinical stage of disease (AJCC 7th Ed., stage I‐IV); primary tumor location (rectal, distal, or proximal); MSI status (stable vs instable); KRAS exon 2/3 mutation status (mutant vs wild‐type); BRAF V600E mutation status (mutant vs wild‐type); and immunohistochemical (IHC) staining of the MMR protein products for hMLH1, hMSH2, hMSH6, and hPMS2 (absent vs present). These tumor studies were performed under standard clinical protocols at each facility. Records also were requested from diagnostic hospitals for those individuals with incomplete records regarding these tumor characteristics at LAC or Norris (32 patients; 12%). Not all patients were tested for MMR deficiency and/or MSI as part of routine clinical practice.
Results were reviewed for any participants who had germline genetic analyses performed under standard clinical protocols at Clinical Laboratory Improvement Amendments (CLIA)‐certified laboratories. The specific genetic test performed was indicated based on personal and family history and IHC results after a clinical cancer genetics evaluation. Participants either had genetic testing of ≥1 CRC risk genes (MLH1, MSH2, MSH6, PMS2, MUTYH, or adenomatous polyposis coli [APC]) or were tested using a broader multigene panel of 25 genes (APC, ATM, BARD1, bone morphogenetic protein receptor type 1A [BMPR1A], BRCA1, BRCA2, BRIP1, cadherin 1 [CDH1], CDK4, CDKN2A [p16INK4a and p14ARF], CHEK2, MLH1, MSH2, MSH6, MUTYH, NBN, PALB2, PMS2, phosphatase and tensin homolog [PTEN], RAD51C, RAD51D, SMAD family member 4 [SMAD4], serine/threonine kinase 11 [STK11], and tumor protein P53 [TP53]).
Medical records were reviewed and treatment records were abstracted, including neoadjuvant and adjuvant chemotherapy and radiotherapy, as well as all therapies received in the metastatic setting. The date of the initial diagnosis and date of death (if available) or last follow‐up also were recorded.
Family History
Data regarding family history of cancer were gathered from participant questionnaires and genetic counseling clinical notes. Having a first‐degree and/or second‐degree relative with CRC was recorded. If participants reported any family history of cancer, the details were reviewed to determine whether their history fulfilled Amsterdam I or II (AM‐I, AM‐II) clinical criteria for Lynch syndrome. AM‐I participants were those from families with ≥3 relatives with CRC, with 1 being a first‐degree relative of another, in 2 successive generations, and with at least 1 relative diagnosed at age <50 years.23 AM‐II uses the same 3‐2‐1 criteria, but allows for the inclusion of other Lynch syndrome‐associated cancers of the endometrium, small intestine, ureter, or renal pelvis, in addition to CRC.24 Each participant was classified as AM‐I, AMI‐II, or neither. In addition, each participant was classified with regard to whether they met ≥1 of the Bethesda guidelines (yes or no). The specific guideline(s) that the participant met was captured. An individual was categorized as “Bethesda 1” if diagnosed at age <50 years and “Bethesda 2” if they had an additional Lynch syndrome‐associated cancer diagnoses.25, 26 Individuals with ≥1 first‐degree relatives with a Lynch syndrome‐associated tumor, with 1 of the cancers being diagnosed before age 50 years, were categorized as “Bethesda 4.” Participants with ≥2 first‐degree or second‐degree relatives with Lynch syndrome‐associated tumors, regardless of age at the time of diagnosis, were categorized as “Bethesda 5.” The Bethesda guideline 3 pertaining to MSI histology (tumor‐infiltrating lymphocytes, Crohn‐like lymphocytic reaction, mucin/signet ring cell differentiation, medullary growth pattern) was not included in the current study because pathology reports did not uniformly capture these features.
Tumor Tissue Analysis
For individuals with dMMR tumors and no explanatory germline MMR gene mutation (5 patients) and 1 individual with absent staining for MLH1, MSH6, and PMS2 by IHC, tumor tissue and DNA were sent for tumor sequencing using ColoSeq at the University of Washington. This assay sequences all exons, nonrepeating intronic sequences, and select promoter regions of AKT1, APC, AXIN2, BMPR1A, CDH1, CTNNA1, EPCAM, GALNT12, GREM1, MLH1, MSH2, MSH6, MUTYH, PIK3CA, PMS2, POLE, POLD1, PTEN, RPS20, SMAD4, STK11, and TP53. A total of 445 kilobases were sequenced and the average depth of coverage ranged from 320 to >1000 sequencing reads per base pair (bp). Genomic regions were captured using biotinylated RNA oliognucleotides (SureSelect; Aligent Technologies, Santa Clara, Calif), prepared in paired‐end libraries with an approximately 200‐bp insert size, and sequenced on an Illumina HiSeq2000 instrument (Illumina Inc, San Diego, Calif) with 100‐bp read lengths, in a modification of a procedure described by Pritchard et al.27 Large deletions and duplications were detected using methods described by Walsh et al.28
Statistical Analysis
Descriptive statistics, including means with standard deviations (SDs) and frequency with percentage, were reported for continuous and categorical variables, respectively. Univariate analyses were performed using Student t tests and 1‐way analysis of variance for continuous variables, when appropriate. Pearson chi‐square or Fisher exact tests were conducted on categorical outcomes. Univariate multinomial logistic regression was applied to explore the relationship between clinicopathologic characteristics and tumor MMR status among patients in the USC subset (individuals with dMMR tumors, individuals with normal IHC/MSI tumors, and individuals with no testing performed). A Scheffe post hoc test was applied to examine the direction of the differences between groups for continuous risk factors. A macro, CHISQ_MC (SAS Institute Inc, Cary, NC), was implemented to perform an analogous Tukey‐type multiple comparison on a Pearson chi‐square test for categorical variables.29 All statistical tests were 2‐tailed, with an α level of .05. All analyses were performed using SAS statistical software (version 9.4; SAS Institute Inc) and STATA statistical software (version 14.2; StataCorp, College Station, Tex).
RESULTS
Patient and Tumor Characteristics of the USC Subset Versus the Population‐Based HCCS
Among the 265 participants in the USC subset (48.3% of whom were female and 51.7% of whom were male), the mean age at the time of diagnosis of CRC was 53.7 years (SD, 10.3 years), which was significantly younger than those patients in the population‐based HCCS (aged 57.2 ± 12.2 years; P<.01) (Table 1). The majority of the USC participants were foreign born (Mexico in 52.3% and other Latin countries in 28.6%), a rate that was statistically significantly higher than that of the HCCS cohort (45.1% of whom were born in Mexico and 16.3% in another Latin country; P<.01). Greater than 80% of the entire study population was either overweight or obese, and the mean BMI of the subset was 30.5 kg/m2 (SD, 6.6 kg/m2), but did not differ from the HCCS cohort. However, patients in the USC subset were less likely to have a prior history of colorectal polyps (P<.01) or to have taken hormone replacement therapy (P<.01) compared with the remaining HCCS cohort. Rates of physical activity, selected medications (nonsteroidal anti‐inflammatory drugs and oral contraceptives), alcohol use, tobacco use, and selected comorbidities (diabetes, Crohn disease, ulcerative colitis, and familial adenomatous polyposis) did not differ significantly between the USC subset and the entire cohort.
Table 1.
Characteristics of the Study Population
| Characteristics |
Subset N = 265 |
Other HCCS Participants N = 847 |
P a |
|---|---|---|---|
| Age at participation (mean ± SD), y | 56.0 (10.6) | 60.6 (12.3) | <.01 |
| Age at diagnosis (mean ± SD), y | 53.7 (10.3) | 57.2 (12.2) | <.01 |
| Sex | |||
| Male | 137 (51.7) | 465 (54.9) | .36 |
| Female | 128 (48.3) | 382 (45.1) | |
| Birth country | |||
| United States | 46 (19.1) | 318 (38.6) | <.01 |
| Mexico | 126 (52.3) | 372 (45.1) | |
| Other | 69 (28.6) | 134 (16.3) | |
| BMI (mean ± SD) | 30.5 ± 6.6 | 31.0 ± 14.2 | .40 |
| Physical activity | |||
| No | 76 (31.7) | 230 (28.0) | .26 |
| Yes | 164 (68.3) | 593 (72.0) | |
| Alcohol use | |||
| <1 per wk | 111 (45.9) | 337 (40.6) | .14 |
| ≥1 per wk | 131 (54.1) | 493 (59.4) | |
| Tobacco use | |||
| Never | 142 (59.2) | 451 (54.6) | .21 |
| Ever | 98 (40.8) | 375 (45.4) | |
| Mean pack per y ± SD | 20.5 ± 84.0 | 14.6 ± 25.5 | .51 |
| NSAID use | |||
| Never | 127 (52.7) | 434 (52.0) | .84 |
| Ever | 114 (47.3) | 401 (48.0) | |
| Hormone replacement therapy | |||
| Never | 109 (90.1) | 291 (78.2) | <.01 |
| Ever | 12 (9.9) | 81 (21.8) | |
| Contraceptive use | |||
| Never | 46 (38.3) | 160 (42.7) | .40 |
| Ever | 74 (61.7) | 215 (57.3) | |
| Diabetes | |||
| No | 184 (76.0) | 586 (69.9) | .08 |
| Yes | 58 (24.0) | 252 (30.1) | |
| Crohn disease | |||
| No | 238 (99.6) | 827 (99.3) | .51 |
| Yes | 1 (0.4) | 6 (0.7) | |
| Ulcerative colitis | |||
| No | 239 (97.6) | 797 (95.6) | .16 |
| Yes | 6 (2.4) | 37 (4.4) | |
| Familial adenomatous polyposis | |||
| No | 234 (98.7) | 804 (97.9) | .59 |
| Yes | 3 (1.3) | 17 (2.1) | |
| History of polyps | |||
| No | 140 (59.3) | 338 (41.1) | <.01 |
| Yes | 96 (40.7) | 484 (58.9) | |
| Family history | |||
| First‐degree relative with CRC | |||
| No | 231 (87.2) | 725 (88.3) | .62 |
| Yes | 34 (12.8) | 96 (11.7) | |
| Second‐degree relative with CRC | |||
| No | 250 (94.3) | 444 (92.5) | .34 |
| Yes | 15 (5.7) | 36 (7.5) | |
| Cancer localization | |||
| Localized | 46 (31.3) | 342 (44.7) | <.01 |
| Regional | 64 (43.5) | 366 (47.8) | |
| Metastatic | 37 (25.2) | 57 (7.5) | |
| Tumor location | |||
| Proximal colon | 69 (27.1) | 223 (26.6) | .28 |
| Distal colon | 72 (28.2) | 279 (33.3) | |
| Rectum | 114 (44.7) | 336 (40.1) | |
| Histologic differentiation | .28 | ||
| Well | 12 (9.0) | 74 (10.4) | |
| Moderate | 95 (71.4) | 539 (76.0) | |
| Poor | 25 (18.8) | 89 (12.6) | |
| Undifferentiated | 1 (0.8) | 7 (1.0) |
Abbreviations: BMI, body mass index; CRC, colorectal cancer; HCCS, Hispanic Colorectal Cancer Study; NSAID, nonsteroidal anti‐inflammatory drug; SD, standard deviation.
P values were derived from the Student t test for continuous variables and the chi‐square/Fisher exact test for categorical variables.
With respect to family history, 12.8% of participants in the USC subset reported a first‐degree relative, 5.7% reported a second‐degree relative, and 2.3% reported both a first‐degree and second‐degree relative with CRC, which was similar to estimates in the entire cohort.
Overall, 44.7% of patients in the USC subset had rectal cancer, compared with 40.1% in the HCCS cohort (P =.28). However, patients in the USC subset were more likely to have metastatic disease at the time of diagnosis (25.2% vs 7.5%; P<.01) compared with patients in the larger cohort.
In the USC subset, 30% of patients (85 patients) had KRAS testing performed, and 40.0% (34 patients) had KRAS‐mutant cancers. Among patients with localized colon cancer, 38% received fluoropyrimidine‐based adjuvant chemotherapy and 55% of patients with rectal cancer received neoadjuvant chemotherapy and radiotherapy. Overall, approximately 17% of patients participated in therapeutic clinical trials.
Bethesda Guidelines and Amsterdam Criteria
On initial review of the USC subset, 3.0% met AM‐I criteria and 0.4% met AM‐II criteria. With respect to Bethesda guidelines, 38.5% met at least 1 guideline, with diagnosis at age <50 years the most commonly fulfilled criterion. No differences were observed by sex or birth location, except for a marginally significant difference noted between the percentage of individuals meeting Bethesda guidelines who were born in the United States (54.3%) versus Mexico (41.3%) and other Latin countries (31.9%) (P =.05) (Table 2). Individuals aged <50 years were more likely to meet AM‐I and AM‐II criteria (P<.01).
Table 2.
Clinicopathological Characteristics of the Study Population by Age, Sex, and Birth Location
| Age at Diagnosis | Sex | Birth Location | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| <50 Years | ≥50 Years | Male | Female | United States | Mexico | Other | P a | ||||||||||
| No. | % | No. | % | P a | No. | % | No. | % | P a | No. | % | No. | % | No. | % | ||
| Bethesda guidelines | |||||||||||||||||
| No | 0 | 0.0 | 163 | 89.6 | NA | 79 | 57.7 | 83 | 64.8 | .23 | 21 | 45.7 | 74 | 58.7 | 49 | 68.1 | .05 |
| Yes | 83 | 100.0 | 19 | 10.4 | 58 | 42.3 | 45 | 35.2 | 25 | 54.3 | 52 | 41.3 | 23 | 31.9 | |||
| Criteria 1 | |||||||||||||||||
| No | 0 | 0.0 | 182 | 100.0 | NA | 89 | 65.0 | 93 | 72.7 | .19 | 27 | 58.7 | 84 | 66.7 | 53 | 73.6 | .24 |
| Yes | 83 | 100.0 | 0 | 0.0 | 48 | 35.0 | 35 | 27.3 | 19 | 41.3 | 42 | 33.3 | 19 | 26.4 | |||
| Criteria 2 | |||||||||||||||||
| No | 80 | 96.4 | 175 | 96.2 | 1.00 | 131 | 95.6 | 124 | 96.9 | .59 | 43 | 93.5 | 120 | 95.2 | 71 | 98.6 | .34 |
| Yes | 3 | 3.6 | 7 | 3.8 | 6 | 4.4 | 4 | 3.1 | 3 | 6.5 | 6 | 4.8 | 1 | 1.4 | |||
| Criteria 4 | |||||||||||||||||
| No | 73 | 88.0 | 172 | 94.5 | 0.06 | 127 | 92.7 | 118 | 92.2 | .87 | 42 | 91.3 | 116 | 92.1 | 66 | 91.7 | .99 |
| Yes | 10 | 12.0 | 10 | 5.5 | 10 | 7.3 | 10 | 7.8 | 4 | 8.7 | 10 | 7.9 | 6 | 8.3 | |||
| Criteria 5 | |||||||||||||||||
| No | 80 | 96.4 | 178 | 97.8 | 0.68 | 135 | 98.5 | 123 | 96.1 | .21 | 45 | 97.8 | 124 | 98.4 | 68 | 94.4 | .26 |
| Yes | 3 | 3.6 | 4 | 2.2 | 2 | 1.5 | 5 | 3.9 | 1 | 2.2 | 2 | 1.6 | 4 | 5.6 | |||
| Amsterdam criteria | |||||||||||||||||
| None | 76 | 91.6 | 181 | 98.9 | <.01 | 132 | 96.4 | 124 | 96.9 | .62 | 44 | 95.7 | 122 | 96.8 | 69 | 95.8 | .62 |
| AM I | 7 | 8.4 | 1 | 0.5 | 4 | 2.9 | 4 | 3.1 | 2 | 4.3 | 4 | 3.2 | 2 | 2.8 | |||
| AM II | 0 | 0.0 | 1 | 0.6 | 1 | 0.7 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 1.4 | |||
Abbreviation: NA, not applicable.
P values were derived from the chi‐square or Fisher exact test.
A total of 161 participants in the USC subset (60.8%) had IHC and/or MSI testing performed, including 72.8% of individuals (75 individuals) who met Bethesda guidelines (103 individuals) (Fig. 1). Among those for whom tumor studies were performed, 13.0% (21 patients) had dMMR tumors. Of the individuals with dMMR tumors, greater than one‐third did not meet Bethesda guidelines, and only 1 met Amsterdam criteria. Thirty of the 104 patients who did not have IHC or MSI performed (28.8%) were diagnosed at outside hospitals and later transferred care to USC‐affiliated hospitals. Of these, 3 individuals were diagnosed outside of the United States, and none of the US‐based referring institutions reported ordering MSI or IHC. Two additional individuals did not undergo MSI or IHC testing, but underwent germline genetic testing based on clinical criteria (both met Bethesda guidelines and 1 patient met AM‐II) and were found to carry MLH1 mutations (patients22 and 23) (Table 3) (Fig. 1).
Figure 1.
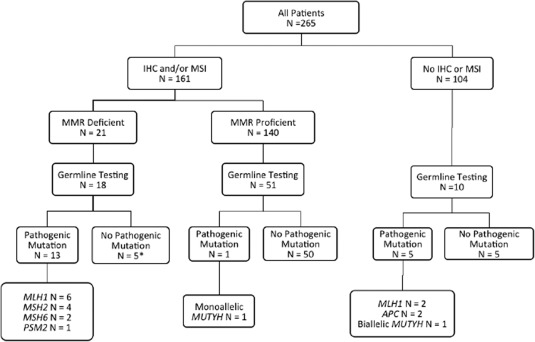
Tumor immunohistochemistry (IHC) of mismatch repair (MMR) proteins and germline testing for hereditary colorectal cancer syndromes. APC indicates adenomatous polyposis coli; MLH1, MutL homolog 1; MSH2, MutS homolog 2; MSH6, MutS homolog 6; MSI, microsatellite instability; PSM2, PMS1 homolog 2, mismatch repair system component.
Table 3.
Somatic and Germline Analysis of Patients With MMR‐Deficient Tumors and/or Lynch Syndrome
| Clinical History | Immunohistochemistry of MMR Proteins | Germline Analysis | Somatic Tumor Sequencing | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tumor Location/Age at Diagnosis, Years | Bethesda Guidelines | Amsterdam Criteria | MLH1 | MSH2 | MSH6 | PMS2 | MLH1 | MSH2 | MSH6 | PMS2 | ||
| 1 | Transverse colon/60 | No | No | Absent | Present | Present | Absent | c.2041G>A | NA | NA | NA | NA |
| 2 | Ascending colon/30 | Yes | No | Absent | Present | Present | Absent | c.1852_1854del | ‐ | ‐ | NA | |
| 3 | Ascending colon/28 | Yes | No | Absent | Present | Present | Absent | c.393_396del | ‐ | ‐ | ‐ | NA |
| 4 | Descending colon/51 | No | No | Absent | Present | Present | Absent | del exon 14 | ‐ | ‐ | ‐ | NA |
| 5 | Left‐sided colon/45; renal cell cancer/46 | Yes | No | Absent | Present | Present | Absent | del exons 2‐3 | ‐ | NA | NA | NA |
| 6 | Sigmoid colon/64 | Yes | Yes | Absent | Present | Present | Absent | c.2634+1G>T | NA | NA | NA | NA |
| 7 | Splenic flexure/51 | No | No | Absent | Present | Absent | Absent | ‐ | ‐ | del exon 6 | ‐ |
MLH1 c.350C>T MLH1 c.1975C>T MSH6 c.2079del Biallelic loss of MSH6 exon 6 |
| 8 | Transverse colon/58 | No | No | Present | Absent | Absent | Present | ‐ | c.1705_1706delAG | ‐ | NA | NA |
| 9 | Rectal/41 | Yes | No | Present | Absent | Absent | Present | ‐ | c.1786_1788del | ‐ | ‐ | NA |
| 10 | Ascending colon/41; transverse colon/41 | Yes | No | Present | Absent | Absent | Present | ‐ | c.1968C>G | NA | NA | NA |
| 11 | Rectum/43 | Yes | No | Present | Absent | Absent | Present | ‐ | c.1046C>G | ‐ | ‐ | NA |
| 12 | Transverse colon/50 | No | No | Present | Present | Absent | Present | NA | NA | c.3255_3256del2ins4 | NA | NA |
| 13 | Rectum/40 | Yes | No | Present | Present | Present | Absent | NA | NA | NA | c.538‐2A>G | NA |
| 14 | Ascending colon/60 | Yes | No | Absent | Present | Present | Absent | ‐ | ‐ | ‐ | ‐ | MLH1 c.1852_1854del with LOH |
| 15 | Sigmoid colon/50 | Yes | No | Absent | Present | Present | Absent | ‐ | ‐ | ‐ | ‐ |
No MMR mutations BRAF V600E negative MSI stable |
| 16 | Ascending colon/52 | No | No | Absent | Present | Present | Absent | ‐ | ‐ | ‐ | ‐ |
MLH1 c.–42C>T with LOH |
| 17 | Ascending colon/48 | Yes | No | Present | Absent | Absent | Present | ‐ | ‐ | ‐ | ‐ |
MSH2 c.1076+1G>T MSH2 c.14del MSH6 c.3261dup |
| 18 | Ascending colon/47 | Yes | No | Present | Absent | Absent | Present | NA | ‐ | ‐ | NA |
MSH2 c.1045C>G with LOH |
| 19 | Ascending colon/58 | No | No | Absent | Present | Present | Absent | NA | NA | NA | NA |
MLH1 c.1400del with LOH |
| 20 | Sigmoid colon/60 | No | No | Absent | Present | Present | Absent | NA | NA | NA | NA | NA |
| 21 | Ascending colon/85 | No | No | Absent | Present | Present | Absent | NA | NA | NA | NA | NA |
| 22 | Transverse colon/45 | Yes | No | NA | NA | NA | NA | c.1989 +5G>T | ‐ | ‐ | ‐ | NA |
| 23 | Appendix/40 | Yes | Yes | NA | NA | NA | NA | c.1852_1854del | ‐ | ‐ | ‐ | NA |
Abbreviations: —, the gene was tested and no mutations were identified; del, deletion; LOH, loss of heterozygosity; MLH1, MutL homolog 1; MMR, mismatch repair; MSH2, MutS homolog 2; MSH6, MutS homolog 6; MSI, microsatellite instability; NA, testing was not performed; PSM2, PMS1 homolog 2, mismatch repair system component.
Germline Genetic Testing and Somatic Tumor Studies
Of the 265 participants in the USC subset, 29.8% (79 patients) underwent germline genetic testing including 18 of the 21 individuals with dMMR tumors (Fig. 1). The majority of the analyses (64 patients; 81.0%) were performed using a 25‐gene panel and the remaining 15 individuals had genetic tests ordered for ≥1 MMR or polyposis genes (Table 3). Overall, 19.0% of the individuals (15 patients) who underwent genetic testing were confirmed to have Lynch syndrome, representing 5.7% of the USC subset. Six individuals (2.3%) had a colonic polyposis phenotype, and 3 underwent germline testing. Two of these patients were found to have an APC mutation and the third was revealed to be a biallelic MUTYH mutation carrier (see Supporting Information Table 1). Only 1 monoallellic MUTYH mutation carrier was identified in the tested group. No other pathogenic mutations were identified; however, 37 variants of uncertain clinical significance were identified among 27 individuals (see Supporting Information Table 2).
Among the 21 patients with dMMR tumors, 85.7% (18 patients) underwent clinical germline testing, with a pathogenic MMR mutation identified in 13 individuals (72.2%) (Fig. 1). Among the 9 individuals with dMMR tumors who either had uninformative germline genetic testing (5 patients) or who did not undergo clinical genetic testing (3 patients), 6 had tumor tissue available for ColoSeq analysis. Five of the 6 patients were found to have somatic mutations and/or evidence of loss of heterozygosity (LOH) that explained the dMMR nature of their tumors. In one individual (patient 15) (Table 3), no somatic or germline mutation was identified to explain the loss of expression of MLH1 and PMS2. In addition, the tumor from patient 7 demonstrated loss of MLH1, MSH6, and PMS2 staining and germline testing confirmed a deletion of exon 6 in MSH6. This deletion also was identified with somatic tumor sequencing along with 2 MLH1 mutations, most likely explaining the lack of MLH1 and PMS2 staining.
Among individuals with confirmed Lynch syndrome (15 patients), the majority of cancers occurred in the colon (80%) versus the rectum (20%) and among those occurring in the colon, 67% were located in the proximal colon. In addition, although the majority of patients with Lynch syndrome (10 patients; 66.7%) did meet Bethesda guidelines, only 2 (13.3%) met AM‐1 or AM‐II criteria. Overall, 5 of the patients with Lynch syndrome in the cohort (33.3%) would not have been identified by Bethesda guidelines or Amsterdam criteria alone.
Only 5.5% of all patients (15 patients) had BRAF testing performed, with 13.3% of the patients (2 patients) found to harbor tumors with BRAF V600E mutations. However, these 2 individuals were not part of the group with dMMR tumors; 1 had an MMR‐proficient tumor according to IHC results and the other individual had neither MSI nor IHC performed.
Clinicopathologic Characteristics Stratified by Tumor dMMR Status
The mean age at the time of CRC diagnosis of those with dMMR tumors was 50.4 years (SD, 12.4 years), which was similar to that of individuals with normal IHC/MSI MMR‐proficient tumors (51.4 ± 9.5 years; P =.91) (Table 4). Individuals diagnosed at older ages were significantly less likely to have had IHC or MSI performed, which is a reflection of clinical practice (57.5 ± 9.8 years; P<.01). The prevalence of dMMR tumors was higher in women compared with men, but this did not reach statistical significance after adjusting for multiple comparisons (61.9% vs 47.1%; P =.42). Individuals with a first‐degree (23.8% vs 10%) or second‐degree (9.5% vs 7.1%) family member diagnosed with CRC also were more likely to have dMMR tumors compared with MMR‐proficient tumors, but this finding did not reach statistical significance. However, dMMR tumors were statistically significantly more likely to be located in the proximal colon compared with the distal colon/rectum (P<.01).
Table 4.
Clinicopathologic Characteristics Stratified by Tumor Mismatch Repair Status
| Individuals With dMMR Tumors | Individuals With Proficient MMR Tumors | MSI/IHC Testing Not Performed | ||||
|---|---|---|---|---|---|---|
| No. (%) | No. (%) | No. (%) | ||||
| 21 (7.9) | 140 (52.8) | 104 (39.3) | P for dMMR Versus Proficient MMRa | P for Proficient Versus Untesteda | P a | |
| Mean age at the time of diagnosis (± SD), y | 50.4 ± 12.4 | 51.4 ± 9.5 | 57.5 ± 9.8 | .91 | <.01 | <.01 |
| Sex | ||||||
| Male | 8 (38.1) | 74 (52.9) | 55 (52.9) | .42 | .37 | .43 |
| Female | 13 (61.9) | 66 (47.1) | 49 (47.1) | |||
| Birth country | ||||||
| United States | 3 (15.0) | 22 (17.0) | 21 (22.1) | .92 | .13 | .82 |
| Mexico | 10 (50.0) | 70 (54.3) | 46 (48.4) | |||
| Other | 7 (35.0) | 37 (28.7) | 28 (29.5) | |||
| Cancer localization | ||||||
| Localized | 6 (60.0) | 17 (34.0) | 10 (29.4) | .43 | .88 | .58 |
| Regional | 3 (30.0) | 22 (44.0) | 17 (50.0) | |||
| Metastatic | 1 (10.0) | 11 (22.0) | 7 (20.6) | |||
| BMI | ||||||
| Mean ± SD, kg/m2 | 31.4 (9.0) | 30.4 (6.6) | 30.3 (6.1) | .84 | 1.00 | .82 |
| Diabetes | ||||||
| No | 15 (75.0) | 103 (80.5) | 66 (70.2) | .99 | .70 | .21 |
| Yes | 5 (25.0) | 25 (19.5) | 28 (29.8) | |||
| Alcohol use | ||||||
| <1 per wk | 12 (60.0) | 57 (44.5) | 42 (44.7) | .30 | .95 | .42 |
| ≥1 per wk | 8 (40.0) | 71 (55.5) | 52 (55.3) | |||
| Smoking | ||||||
| Never | 13 (65.0) | 76 (59.8) | 53 (57.0) | .95 | .95 | .78 |
| Ever | 7 (35.0) | 51 (40.2) | 40 (43.0) | |||
| Mean pack‐y ± SD | 6.51 ± 4.1 | 11.24 ± 17.9 | 34.43 ± 128.8 | .99 | .45 | .41 |
| NSAID Use | ||||||
| Never | 12 (60.0) | 69 (54.3) | 46 (48.9) | .96 | .68 | .58 |
| Ever | 8 (40.0) | 58 (45.7) | 48 (51.1) | |||
| Postmenopausal hormones | ||||||
| Never | 12 (100.0) | 56 (88.9) | 41 (87.2) | .56 | .83 | .44 |
| Ever | 0 (0.0) | 7 (11.1) | 6 (12.8) | |||
| Oral contraceptive use | ||||||
| Never | 6 (50.0) | 16 (25.8) | 25 (53.2) | .70 | .47 | .01 |
| Ever | 6 (50.0) | 46 (74.2) | 22 (46.8) | |||
| Family history | ||||||
| First‐degree relative with CRC | ||||||
| No | 16 (76.2) | 126 (90.0) | 89 (85.6) | .17 | .58 | .16 |
| Yes | 5 (23.8) | 14 (10.0) | 15 (14.4) | |||
| Second‐degree relative with CRC | ||||||
| No | 19 (90.5) | 130 (92.9) | 101 (97.1) | .91 | .23 | .17 |
| Yes | 2 (9.5) | 10 (7.1) | 3 (2.9) | |||
| Primary tumor location | ||||||
| Proximal colon | 13 (61.9) | 33 (24.4) | 23 (23.2) | <.01 | .79 | <.01 |
| Distal colon | 5 (23.8) | 42 (31.1) | 25 (25.3) | |||
| Rectum | 3 (14.3) | 60 (44.5) | 51 (51.5) |
Abbreviations: BMI, body mass index; CRC, colorectal cancer; dMMR, mismatch repair‐deficient; IHC, immunohistochemistry; MSI, microsatellite instability; NSAID, nonsteroidal anti‐inflammatory drug; SD, standard deviation.
P values were derived from analysis of variance for continuous variables with Scheffe adjustment for multiple comparisons and from the chi‐square/Fisher exact test for categorical variables with Tukey adjustment for multiple comparisons.
b P values were derived from analysis of variance for continuous variables and the chi‐square/Fisher exact test for categorical variables.
DISCUSSION
To our knowledge, the current study represents one of the largest cohorts of Latino patients diagnosed with CRC with reported germline genetic and somatic tumor testing in the United States performed to date. The findings suggest that the rate of dMMR tumors in Latino individuals is 13.0%, which is similar to previous estimates.20, 30 In contrast with other studies, we were able to perform more in‐depth molecular analysis to confirm that the majority of the dMMR tumors (61.9%) were indeed attributable to germline MMR gene mutations. In the current study sample, we also observed a younger age at the time of onset, a higher percentage of rectal cancers, and advanced disease in Latino patients, which is consistent with observations in other studies.6, 8
The incidence of dMMR tumors in Latino patients has been examined in various Latino populations in the United States as well as in South America and the Caribbean.31, 32, 33, 34, 35 In the United States, 2 small studies reported MSI in 16.9%36 and 19.0%37 of Latinos, respectively. In Puerto Rico, a larger retrospective study investigated an unselected group of Latino patients with CRC, among whom IHC staining of only 2 MMR proteins (MLH1 and MSH2) was performed.22 Among 164 individuals, only 8 demonstrated any loss of protein expression by IHC (7 patients with absent MSH2 and 1 patient with absent MLH1) and were presumed to have Lynch syndrome. Overall, those studies concluded that the rate of dMMR tumors (4.3%) was lower than that reported in other populations, with the majority of cases attributable to Lynch syndrome. A hospital‐based study in Texas30 conducted a retrospective review of tumor registry data in Latino patients and performed MSI and IHC on all 4 MMR proteins (MLH1, MSH2, MSH6, and PMS2). In the 111 patients with CRC they studied, 9.8% had tumors demonstrating MSI and 14.6% had abnormal IHC expression. The authors concluded that the rate of dMMR tumors was similar to that of other populations, and that a greater percentage of tumors were attributable to Lynch syndrome, although these authors also were unable to perform genetic testing for confirmation. A third hospital‐based study of 103 surgically resected CRC specimens from Latino patients in Miami found that 12.6% of tumors demonstrated abnormal IHC, but again the authors were unable to confirm cases of Lynch syndrome with germline testing.21 Lastly, a meta‐analysis combining data in 3 of these studies reported that 12% of tumors (range, 7%‐16%) diagnosed among Latino patients were dMMR.20 The results of the current study similarly suggest that the rate of dMMR tumors in Latino individuals is approximately 13%.
Berera et al compared rates among NHW, Latino, and African American patients and observed no differences in the rate of dMMR tumors by ethnicity/race21; similar results also were observed in a larger meta‐analysis.20 However, several studies have hypothesized that Lynch syndrome may explain a high percentage of Latino patients with CRC who have dMMR tumors. After conducting additional germline and somatic tumor testing, which had been lacking in the previously reported studies, we observed that 13 of 21 patients (61.9%) had a germline mutation in a MMR gene, confirming that these individuals have Lynch syndrome. The current study findings, although suggestive, should be interpreted with caution because the entire cohort did not have IHC or MSI performed. Furthermore, the apparent lower prevalence of sporadic MSI CRC among Latino individuals is not entirely understood and may be due to chance. Some have hypothesized that this finding may reflect differences in environmental and lifestyle factors between Latino individuals and other populations. For example, MSI‐H CRC has been associated with tobacco use,36, 38 which is less prevalent in Latinos compared with other populations,39 and a high BMI, which is more common in Latino individuals, is associated with microsatellite stable CRC tumors.40 Further studies are needed to investigate these hypotheses.
Furthermore, recent studies investigating the underlying etiology of dMMR41, 42, 43 have demonstrated that 52% to 69% of unexplained dMMR cases are attributable to multiple somatic MMR mutations or LOH. This is clinically significant because individuals with unexplained dMMR tumors, especially loss of MSH2 and MSH6, often are managed as having Lynch syndrome, despite the lack of a detectable germline mutation. Among individuals in the current study with dMMR tumors, there were 5 individuals in whom no germline mutations were identified as well as 1 individual who did not undergo germline genetic testing who had tumor tissue available for further studies. Sequencing was performed and 5 of the 6 patients (83.3%) were found to have multiple somatic MMR mutations and/or evidence of LOH, which potentially explains the dMMR. The findings of the current study add to the growing body of literature demonstrating the contribution of double somatic mutations to dMMR CRC tumors.
Although to our knowledge only a few studies have been conducted to date, the mutational spectrum of Lynch syndrome may vary by Latino subgroup. Latinos are the result of >500 years of admixture of European, Amerindian, and African individuals, with varying degrees across Latin America.44 Moreover, US Latinos include recent immigrants who make similar lifestyle and dietary choices as those in their countries of origin, as well as second‐generation or higher immigrants born in the United States who are partially or fully assimilated to the US lifestyle. Both genetic ancestry and lifestyle factors may be associated with tumor characteristics and help to explain differences in the mutation spectrum as observed in different studies. For example, Berera et al observed that MSH2‐deficient tumors were overrepresented in Latino individuals from Miami21 (who are disproportionately of Cuban origin). Similarly, a case series study among Caribbean Hispanics from Puerto Rico and the Dominican Republic demonstrated that the mutation spectrum was largely composed of MSH2 (66.7%) mutations followed by MLH1 (25%) and MSH6 (8.3%) mutations.35 In comparison, the current California‐based study identified MLH1 mutations in 53.3% of individuals, MSH2 mutations in 26.7% of individuals, MSH6 mutations in 13.3% of individuals, and PMS2 mutations in 6.7% of individuals. Latinos from California are largely of Mexican origin, with a higher percentage of Amerindian ancestry compared with those Latino individuals from Florida or the Caribbean, who are more likely to have a higher percentage of African ancestry.45 We were unable to fully investigate differences in the mutational spectrum by ancestry and further studies are needed on the subject.
It is interesting to note that approximately one‐half of the current study cohort had rectal cancer, which is higher than that reported in the general population (approximately one‐third of patients with CRC). This may reflect the disproportionately younger age of the patients in the current study. Rectal cancer has been steadily rising in incidence among individuals aged <50 years,46, 47, 48 and younger patients are more likely to present with poorly differentiated and late‐stage cancers. Previous studies also have suggested that Latino individuals (in particular Mexicans)8 have high rates of rectal cancer,9, 47 although the reasons remain unclear.
Certain limitations of the current study should be acknowledged. The study population herein was recruited from Los Angeles, an area where the majority of Latino individuals are of Mexican origin compared with other Latin American countries,45 which may limit the generalizability of the current study findings to all Latino groups. Furthermore, we were unable to analyze the data by ancestry or nativity due to the limited sample size. Another limitation is that approximately 40% of the current study cohort did not have MSI and/or IHC performed. The subgroup without tumor studies was similar to the group with MMR‐proficient tumors with regard to the lower frequency of proximal tumors and the lack of affected relatives. These individuals also were older (mean age, 57.5 years). Therefore, the untested cohort is less likely to have dMMR tumors and if such tumors were present, the etiology is more likely to be sporadic or somatic rather than germline in nature. This limitation could lead to an underrepresentation of sporadic dMMR tumors over the cohort and could explain, in part, the large percentage of dMMR tumors explained by Lynch syndrome.
The current study had many strengths. Among them, all 4 MMR proteins were studied, which is in contrast to previous reports, and nearly 30% of the cohort in the current study underwent germline genetic testing, including the majority of individuals with dMMR tumor studies (18 of 21 individuals; 85.7%), thereby allowing for a more comprehensive assessment of the underlying mechanism behind the dMMR tumors. In addition, somatic tumor testing provided greater insight into the etiology of the unexplained dMMR tumors. Cases were identified using a population‐based cancer registry, which contributes to the generalizability of the current study findings. Although our characterization was restricted to 2 specific centers, we were able to compare the subset of the data used in the current study from USC‐affiliated hospitals with the characteristics of a population‐based sample in California, which provides additional information regarding the generalizability of the findings presented herein.
The results of the current study suggest that Latino individuals have similar rates of dMMR tumors compared with NHW individuals. We confirmed that the majority of these cancers are attributable to Lynch syndrome. Further research is needed to understand whether there is a lower percentage of tumors with high MSI in the Latino population.
FUNDING SUPPORT
Supported by National Institutes of Health awards R01CA155101 (to Jane C. Figueiredo) and P30CA014089 (to Charité N. Ricker). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
CONFLICT OF INTEREST DISCLOSURES
The authors made no disclosures.
AUTHOR CONTRIBUTIONS
Charité N. Ricker: Project conceptualization, methodology, investigation, resources, and article writing. Diana L. Hanna: Project conceptualization, methodology, investigation, resources, and article writing. Cheng Peng: Statistical analysis, data curation, supportive writing, and project administration. Nathalie T. Nguyen: Statistical analysis, data curation, supportive writing, and project administration. Mariana C. Stern: Statistical analysis and supportive writing. Stephanie L. Schmit: Statistical analysis and supportive writing. Greg E. Idos: Statistical analysis and supportive writing. Ravi Patel: Data curation and supportive writing. Steven Tsai: Data curation and supportive writing. Veronica Ramirez: Data curation and supportive writing. Sonia Lin: Data curation and supportive writing. Vinay Shamasunadara: Data curation and supportive writing. Afsaneh Barzi: Data curation and supportive writing. Heinz‐Josef Lenz: Project supervision, resource allocation, and article writing support. Jane C. Figueiredo: Project conceptualization, methodology, investigation, resources, data visualization, supervision, funding acquisition, and article writing.
Supporting information
Additional supporting information may be found in the online version of this article.
Supporting Information Tables.
We are indebted to the individuals who participated in the current study. We thank the following individuals for their assistance with logistical support and management, interviewing patients, and data entry: Julissa Ramirez, Yaquelin Perez, Alicia Rivera, Lauren Gerstmann, and the student intern staff.
REFERENCES
- 1. Robbins AS, Siegel RL, Jemal A. Racial disparities in stage‐specific colorectal cancer mortality rates from 1985 to 2008. J Clin Oncol. 2012;30:401‐405. [DOI] [PubMed] [Google Scholar]
- 2. Chien C, Morimoto LM, Tom J, Li CI. Differences in colorectal carcinoma stage and survival by race and ethnicity. Cancer. 2005;104:629‐639. [DOI] [PubMed] [Google Scholar]
- 3. Stefanidis D, Pollock BH, Miranda J, et al. Colorectal cancer in Hispanics: a population at risk for earlier onset, advanced disease, and decreased survival. Am J Clin Oncol. 2006;29:123‐126. [DOI] [PubMed] [Google Scholar]
- 4. Jafri NS, Gould M, El‐Serag HB, Duan Z, Davila JA. Incidence and survival of colorectal cancer among Hispanics in the United States: a population‐based study. Dig Dis Sci. 2013;58:2052‐2060. [DOI] [PubMed] [Google Scholar]
- 5. Soto‐Salgado M, Suarez E, Calo W, Cruz‐Correa M, Figueroa‐Valles NR, Ortiz AP. Incidence and mortality rates for colorectal cancer in Puerto Rico and among Hispanics, non‐Hispanic whites, and non‐Hispanic blacks in the United States, 1998‐2002. Cancer. 2009;115:3016‐3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cruz‐Correa M. Increasing colorectal cancer burden among young US Hispanics: is it time to change current screening guidelines? Dig Dis Sci. 2013;58:1816‐1818. PMID: 23722565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lathroum L, Ramos‐Mercado F, Hernandez‐Marrero J, Villafana M, Cruz‐Correa M. Ethnic and sex disparities in colorectal neoplasia among Hispanic patients undergoing screening colonoscopy. Clin Gastroenterol Hepatol. 2012;10:997‐1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Stern MC, Zhang J, Lee E, Deapen D, Liu L. Disparities in colorectal cancer incidence among Latino subpopulations in California defined by country of origin. Cancer Causes Control. 2016;27:147‐155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ollberding NJ, Nomura AM, Wilkens LR, Henderson BE, Kolonel LN. Racial/ethnic differences in colorectal cancer risk: the multiethnic cohort study. Int J Cancer. 2011;129:1899‐1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Du XL, Meyer TE, Franzini L. Meta‐analysis of racial disparities in survival in association with socioeconomic status among men and women with colon cancer. Cancer. 2007;109:2161‐2170. [DOI] [PubMed] [Google Scholar]
- 11. Peltomaki P. Role of DNA mismatch repair defects in the pathogenesis of human cancer. J Clin Oncol. 2003;21:1174‐1179. PMID: 12637487. [DOI] [PubMed] [Google Scholar]
- 12. Boland CR, Goel A. Microsatellite instability in colorectal cancer. Gastroenterology. 2010;138:2073‐2087.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Markowitz SD, Bertagnolli MM. Molecular origins of cancer: molecular basis of colorectal cancer. N Engl J Med. 2009;361:2449‐2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vilar E, Gruber SB. Microsatellite instability in colorectal cancer‐the stable evidence. Nat Rev Clin Oncol. 2010;7:153‐162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jasperson KW, Tuohy TM, Neklason DW, Burt RW. Hereditary and familial colon cancer. Gastroenterology. 2010;138:2044‐2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lynch HT, de la Chapelle A. Hereditary colorectal cancer. N Engl J Med. 2003;348:919‐932. [DOI] [PubMed] [Google Scholar]
- 17. Lynch HT, Lanspa S, Smyrk T, Boman B, Watson P, Lynch J. Hereditary nonpolyposis colorectal cancer (Lynch syndromes I & II). Genetics, pathology, natural history, and cancer control, Part I. Cancer Genet Cytogenet. 1991;53:143‐160. [DOI] [PubMed] [Google Scholar]
- 18. Hampel H, Frankel WL, Martin E, et al. Feasibility of screening for Lynch syndrome among patients with colorectal cancer. J Clin Oncol. 2008;26:5783‐5788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hampel H, Frankel WL, Martin E, et al. Screening for the Lynch syndrome (hereditary nonpolyposis colorectal cancer). N Engl J Med. 2005;352:1851‐1860. [DOI] [PubMed] [Google Scholar]
- 20. Ashktorab H, Ahuja S, Kannan L, et al. A meta‐analysis of MSI frequency and race in colorectal cancer. Oncotarget. 2016;7:34546‐34557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Berera S, Koru‐Sengul T, Miao F, et al. Colorectal tumors from different racial and ethnic minorities have similar rates of mismatch repair deficiency. Clin Gastroenterol Hepatol. 2016;14:1163‐1171. [DOI] [PubMed] [Google Scholar]
- 22. De Jesus‐Monge WE, Gonzalez‐Keelan C, Zhao R, Hamilton SR, Rodriguez‐Bigas M, Cruz‐Correa M. Mismatch repair protein expression and colorectal cancer in Hispanics from Puerto Rico. Fam Cancer. 2010;9:155‐166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Vasen HF, Mecklin JP, Khan PM, Lynch HT. The International Collaborative Group on Hereditary Non‐Polyposis Colorectal Cancer (ICG‐HNPCC). Dis Colon Rectum. 1991;34:424‐425. [DOI] [PubMed] [Google Scholar]
- 24. Vasen HF, Watson P, Mecklin JP, Lynch HT. New clinical criteria for hereditary nonpolyposis colorectal cancer (HNPCC, Lynch Syndrome) proposed by the International Collaborative Group on HNPCC. Gastroenterology. 1999;116:1453‐1456. [DOI] [PubMed] [Google Scholar]
- 25. Rodriguez‐Bigas MA, Boland CR, Hamilton SR, et al. A National Cancer Institute Workshop on Hereditary Nonpolyposis Colorectal Cancer Syndrome: meeting highlights and Bethesda guidelines. J Natl Cancer Inst. 1997;89:1758‐1762. [DOI] [PubMed] [Google Scholar]
- 26. Umar A, Boland CR, Terdiman JP, et al. Revised Bethesda Guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. J Natl Cancer Inst. 2004;96:261‐268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pritchard CC, Smith C, Salipante SJ, et al. ColoSeq provides comprehensive Lynch and polyposis syndrome mutational analysis using massively parallel sequencing. J Mol Diagn. 2012;14:357‐366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Walsh T, Casadei S, Lee MK, et al. Mutations in 12 genes for inherited ovarian, fallopian tube, and peritoneal carcinoma identified by massively parallel sequencing. Proc Natl Acad Sci U S A. 2011;108:18032‐18037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jin M, Wang B. Implementing Multiple Comparisons on Pearson Chi‐square Test for an R×C Contingency Table in SAS®. In SAS Global Forum Proceedings. 2014;1544:1‐6. http://support.sas.com/resources/papers/proceedings14/1544-2014.pdf. [Google Scholar]
- 30. Gupta S, Ashfaq R, Kapur P, et al. Microsatellite instability among individuals of Hispanic origin with colorectal cancer. Cancer. 2010;116:4965‐4972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dominguez‐Valentin M, Nilbert M, Wernhoff P, et al. Mutation spectrum in South American Lynch syndrome families. Hered Cancer Clin Pract. 2013;11:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Alvarez K, Hurtado C, Hevia MA, et al. Spectrum of MLH1 and MSH2 mutations in Chilean families with suspected Lynch syndrome. Dis Colon Rectum. 2010;53:450‐459. [DOI] [PubMed] [Google Scholar]
- 33. da Silva FC, de Oliveira LP, Santos EM, et al. Frequency of extracolonic tumors in Brazilian families with Lynch syndrome: analysis of a hereditary colorectal cancer institutional registry. Fam Cancer. 2010;9:563‐570. [DOI] [PubMed] [Google Scholar]
- 34. Giraldo A, Gomez A, Salguero G, et al. MLH1 and MSH2 mutations in Colombian families with hereditary nonpolyposis colorectal cancer (Lynch syndrome)–description of four novel mutations. Fam Cancer. 2005;4:285‐290. [DOI] [PubMed] [Google Scholar]
- 35. Cruz‐Correa M, Diaz‐Algorri Y, Perez‐Mayoral J, et al. Clinical characterization and mutation spectrum in Caribbean Hispanic families with Lynch syndrome. Fam Cancer. 2015;14:415‐425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Slattery ML, Curtin K, Anderson K, et al. Associations between cigarette smoking, lifestyle factors, and microsatellite instability in colon tumors. J Natl Cancer Inst. 2000;92:1831‐1836. [DOI] [PubMed] [Google Scholar]
- 37. Carethers JM, Smith EJ, Behling CA, et al. Use of 5‐fluorouracil and survival in patients with microsatellite‐unstable colorectal cancer. Gastroenterology. 2004;126:394‐401. [DOI] [PubMed] [Google Scholar]
- 38. Poynter JN, Haile RW, Siegmund KD, et al; Colon Cancer Family Registry . Associations between smoking, alcohol consumption, and colorectal cancer, overall and by tumor microsatellite instability status. Cancer Epidemiol Biomarkers Prev. 2009;18:2745‐2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. King DE, Mainous AG 3rd, Carnemolla M, Everett CJ. Adherence to healthy lifestyle habits in US adults, 1988‐2006. Am J Med. 2009;122:528‐534. [DOI] [PubMed] [Google Scholar]
- 40. Campbell PT, Jacobs ET, Ulrich CM, et al; Colon Cancer Family Registry . Case‐control study of overweight, obesity, and colorectal cancer risk, overall and by tumor microsatellite instability status. J Natl Cancer Inst. 2010;102:391‐400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Haraldsdottir S, Hampel H, Tomsic J, et al. Colon and endometrial cancers with mismatch repair deficiency can arise from somatic, rather than germline, mutations. Gastroenterology. 2014;147:1308‐1316.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mensenkamp AR, Vogelaar IP, van Zelst‐Stams WA, et al. Somatic mutations in MLH1 and MSH2 are a frequent cause of mismatch‐repair deficiency in Lynch syndrome‐like tumors. Gastroenterology. 2014;146:643‐646.e8. PMID: 24333619. [DOI] [PubMed] [Google Scholar]
- 43. Geurts‐Giele WR, Leenen CH, Dubbink HJ, et al. Somatic aberrations of mismatch repair genes as a cause of microsatellite‐unstable cancers. J Pathol. 2014;234:548‐559. [DOI] [PubMed] [Google Scholar]
- 44. Salari K, Choudhry S, Tang H, et al. Genetic admixture and asthma‐related phenotypes in Mexican American and Puerto Rican asthmatics. Genet Epidemiol. 2005;29:76‐86. [DOI] [PubMed] [Google Scholar]
- 45. Pew Research Center . Hispanic population and origin in select U.S. metropolitan areas, 2014. http://www.pewhispanic.org/interactives/hispanic‐population‐in‐select‐u‐s‐metropolitan‐areas/. Accessed December 21, 2016.
- 46. Meyer JE, Narang T, Schnoll‐Sussman FH, Pochapin MB, Christos PJ, Sherr DL. Increasing incidence of rectal cancer in patients aged younger than 40 years: an analysis of the Surveillance, Epidemiology, and End Results database. Cancer. 2010;116:4354‐4359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Berger MD, Yang D, Sunakawa Y, et al. Impact of sex, age, and ethnicity/race on the survival of patients with rectal cancer in the United States from 1988 to 2012. Oncotarget. 2016;7:53668‐53678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bailey CE, Hu CY, You YN, et al. Increasing disparities in the age‐related incidences of colon and rectal cancers in the United States, 1975‐2010. JAMA Surg. 2015;150:17‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional supporting information may be found in the online version of this article.
Supporting Information Tables.


