Abstract
In the United States, ticks transmit the greatest diversity of arthropod-borne pathogens and are responsible for the most cases of all vector-borne diseases. In recent decades, the number of reported cases of notifiable tick-borne diseases has steadily increased, geographic distributions of many ticks and tick-borne diseases have expanded, and new tick-borne disease agents have been recognized. In this review, we (1) describe the known disease agents associated with the most commonly human-biting ixodid ticks, (2) review the natural histories of these ticks and their associated pathogens, (3) highlight spatial and temporal changes in vector tick distributions and tick-borne disease occurrence in recent decades, and (4) identify knowledge gaps and barriers to more effective prevention of tick-borne diseases. We describe 12 major tick-borne diseases caused by 15 distinct disease agents that are transmitted by the 8 most commonly human-biting ixodid ticks in the United States. Notably, 40% of these pathogens were described within the last two decades. Our assessment highlights the importance of animal studies to elucidate how tick-borne pathogens are maintained in nature, as well as advances in molecular detection of pathogens which has led to the discovery of several new tick-borne disease agents.
Keywords: Amblyomma, Anaplasma, Borrelia, Dermacentor, Ehrlichia, Francisella, Ixodes, Rhipicephalus, rickettsia, tick-borne virus
Introduction
Ticks, particularly ixodid (hard) ticks, are among the most medically important arthropods in the United States. They are unique among arthropods in the United States in the diversity of pathogens of humans and domestic animals they transmit, including protozoa, bacteria (including different species of rickettsiae and spirochetes), and viruses (Jongejan and Uilenberg 2004). Among the nearly 50,000 cases of autochthonous (locally acquired), nationally notifiable human vector-borne diseases reported annually to the Centers for Disease Control and Prevention (CDC) from states and the District of Columbia, approximately 95% are caused by pathogens vectored by ticks (Adams et al. 2016; Paddock et al. 2016). Moreover, in recent decades, the number of reported cases of notifiable tick-borne diseases has steadily increased, geographic distributions of many tick vectors and tick-borne diseases have expanded, and new tick-borne disease agents have been recognized (Paddock et al. 2016).
Of the 84 species of ticks known to occur in the United States, fewer than 10 species from 2 families (Ixodidae [hard ticks] and Argasidae [soft ticks]) commonly bite humans (Merten and Durden 2000). Soft ticks in the genus Ornithodoros are important vectors of relapsing fever Borrelia spirochetes. In the United States, tick-borne relapsing fever is endemic in the western United States; cases occur infrequently and are commonly associated with exposures in rustic settings (Dworkin et al. 2008). This review will focus only on common human-biting hard tick species, since they are responsible for the vast majority of tick-borne illnesses that occur in the United States. The most important ixodid tick vectors include: Amblyomma americanum (the lone star tick), A. maculatum (the Gulf Coast tick), Dermacentor andersoni (the Rocky Mountain wood tick), D. occidentalis (the Pacific coast tick), D. variabilis (the American dog tick), Ixodes pacificus (the western blacklegged tick), I. scapularis (the blacklegged tick or deer tick), and Rhipicephalus sanguineus sensu lato (the brown dog tick). These ticks take single blood meals from vertebrate hosts in all of their three active life stages: larva, nymph, and adult. The major human disease agents transmitted by each of these tick species and the most important natural vertebrate pathogen reservoirs are listed in Table 1.
Table 1.
Summary of major tick-borne diseases of humans in the United States and their etiological agents, primary vectors and vertebrate reservoirs
| Disease | Etiological agent(s) | Primary vector(s) | Vertebrate reservoir(s) |
|---|---|---|---|
| Anaplasmosis | Anaplasma phagocytophilum | Ixodes scapularis | Peromyscus leucopus, Sciurus spp., Neotoma spp., Tamias spp., Blarina brevicauda1 |
| Ixodes pacificus | |||
| Babesiosis | Babesia microti | Ixodes scapularis | Peromyscus leucopus2 |
| Borrelia miyamotoi disease | Borrelia miyamotoi | Ixodes scapularis, Ixodes pacificus | Peromyscus leucopus3/ not determined/ (transovarial transmission) |
| Colorado tick fever | Colorado tick fever virus (coltivirus) | Dermacentor andersoni | Spermophilus lateralis4 |
| Ehrlichiosis | Ehrlichia muris eauclairensis | Ixodes scapularis | Not determined |
| Ehrlichia chaffeensis | Amblyomma americanum | Odocoileus virginianus5 | |
| Ehrlichia ewingii | Amblyomma americanum | Odocoileus virginianus6 | |
| Heartland virus disease | Heartland virus (phlebovirus) | Amblyomma americanum | Not determined |
| Lyme disease | Borrelia burgdorferi | Ixodes scapularis, Ixodes pacificus | Peromyscus leucopus, Sciurus griseus Tamias spp.7 |
| Borrelia mayonii | Ixodes scapularis | Not determined | |
| Powassan encephalitis | Powassan virus (flavivirus) | Ixodes scapularis | Peromyscus leucopus8 |
| Rickettsia parkeri rickettsiosis | Rickettsia parkeri | Amblyomma maculatum | Not determined9 |
| Rocky Mountain spotted fever | Rickettsia rickettsii | Dermacentor variabilis, Dermacentor andersoni, Rhipicephalus sanguineus s. l. | (transovarial transmission), Microtus spp., Spermophilus spp., Sylvilagus Tamias spp.10 |
| Pacific Coast tick fever | Rickettsia philipii | Dermacentor occidentalis | Not determined11 |
| Tularemia | Francisella tularensis | Amblyomma americanum | Various terrestrial and aquatic mammals12 |
| Dermacentor variabilis | |||
| Dermacentor andersoni |
The objectives of this review are to (1) describe the known human disease agents associated with the most commonly human-biting ixodid ticks, (2) review the natural histories of these ticks and their associated pathogens, (3) highlight spatial and temporal changes in vector tick distributions and tick-borne disease occurrence in recent decades, and (4) identify knowledge gaps and barriers to more effective prevention of tick-borne diseases.
Blacklegged Ticks (Ixodes scapularis) and Western Blacklegged Ticks (Ixodes pacificus)
Associated Human Disease Agents
The blacklegged tick, I. scapularis, is the primary vector to humans in the eastern United States of a diverse array of pathogens including: Lyme disease spirochetes, Borrelia burgdorferi and Borrelia mayonii; a relapsing fever spirochete, Borrelia miyamotoi; other bacterial agents causing anaplasmosis (Anaplasma phagocytophilum) and ehrlichiosis (Ehrlichia muris euclairensis); a protozoan causing babesiosis (Babesia microti); and the deer tick virus lineage of Powassan encephalitis virus (Family: Flaviviridae) (Dolan et al. 1997, 2016; Ebel 2010; Johnson et al. 2015; Karpathy et al. 2016; Krause et al. 2015; Piesman and Eisen 2008; Pritt et al. 2011, 2016a, 2016b, 2016c; Teglas and Foley 2006). A closely related species, the western blacklegged tick, I. pacificus, is the primary vector to humans in the far western United States of Bo. burgdorferi and A. phagocytophilum, and most likely also of Bo. miyamotoi (Krause et al. 2015; Lane et al. 1994; Padgett et al. 2014; Teglas and Foley 2006).
Some Ixodes-borne pathogens were described long before they were recognized as etiological agents of disease in humans. Babesiosis (Texas fever) of cattle was the first disease of vertebrates that was experimentally shown to be caused by an agent (a protozoan originally named Pyrosoma bigeminum but now known as Babesia bigemina) transmitted by ticks (Smith and Kilborne 1893). Nearly a century later, Ba. microti was recognized as a tick-borne agent causing human babesiosis in the northeastern United States (Spielman 1994). Similarly, A. phagocytophilum was first described in sheep in the 1930s but not recognized to cause human disease until the 1990s (Dumler et al. 2005). Another case in point is Bo. miyamotoi, which was first described from Ixodes ticks in the United States in 2001, but not associated with human disease until more than a decade later (Krause et al. 2015).
By contrast, description of the etiological agents of other Ixodes-borne diseases lagged clinical recognition of illness. For example, although certainly present in the United States earlier, Lyme disease emerged along with babesiosis in the eastern United States during the 1970s (Spielman 1994); however, the etiological agents of Lyme disease in the United States were not described until 1982 (Bo. burgdorferi) and 2016 (Bo. mayonii) (Burgdorfer et al. 1982; Pritt et al. 2016b). The E. muris-like agent was described recently as a cause of ehrlichiosis, and subsequent to identification in human clinical samples, the organism was identified in I. scapularis ticks (Pritt et al. 2011). Powassan virus was first described as a human pathogen in the 1950s, but more recent effort has better defined the genetic diversity of closely-related genotypes that are linked to distinct enzootic transmission cycles (Ebel 2010).
Geographic Distribution of Tick Vectors
Although both ticks are generally found in dense brush and heavily forested woodlands (Lane et al. 1994), I. scapularis and I. pacificus have nonoverlapping geographic ranges (Figure 1). Owing largely to their need for high humidity, neither species is found in the arid Rocky Mountains or Inter-Mountain West (Colorado, Idaho, Montana, New Mexico, and Wyoming). Recently, I. scapularis has been reported from all states to the east and I. pacificus has been reported from all states west of this region (Eisen et al. 2016a). The current widespread distribution of I. scapularis likely represents a reemergence of the tick, which is believed to have been established across the eastern United States prior to the late 1800s. Because of the close association between I. scapularis, white-tailed deer (Odocoileus virginianus), and woodlands, deforestation and suppression of white-tailed deer populations in the late 1800s and early 1900s led to severe restrictions in the tick’s distribution and abundance. Reforestation and rebounding deer populations during the second half of the 20th century yielded ecological conditions that were conducive for the reemergence of I. scapularis (Dennis et al. 1998; Lane et al. 1991; Spielman et al. 1985). Over the past two decades, the geographic range of I. scapularis has continued to expand markedly, while the geographic range of I. pacificus remained unchanged (Eisen et al. 2016a). The number of counties in which I. scapularis is considered to be established more than doubled from 1996 through 2015. The greatest expansion in the tick’s range was observed in the north-central and northeastern states, while the distribution in the southeastern states remained relatively stable. Range expansion has been attributed to reforestation, the increasing abundance of white-tailed deer, and increasingly warmer temperatures (Lee et al. 2013; Ostfeld and Brunner 2015; Spielman 1994; Spielman et al. 1985).
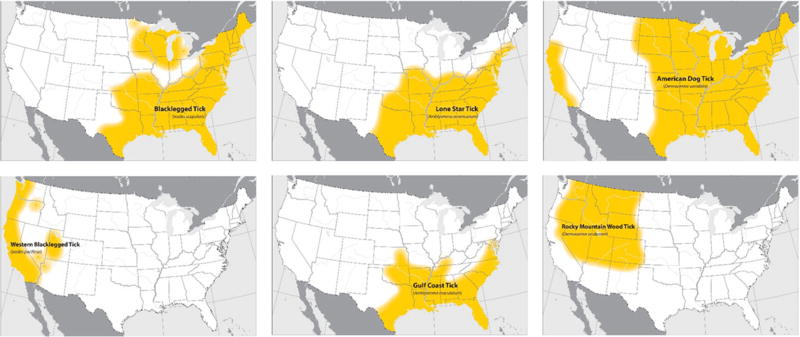
Figure 1.
Generalized distributions of key human biting ticks in the United States These maps provide general insight into the expected distribution of common human-biting ticks in the contiguous United States. Populations of these ticks may be found outside noted areas. The maps are not meant to represent risk for tick-borne diseases. The brown dog tick, Rhipicephalus sanguineus, is found throughout the continental United States and in Hawaii. The geographic distribution of the Pacific Coast tick, Dermacentor occidentalis, has not been assessed in recent years and is not shown.
Tick Life Cycles/Enzootic Pathogen Transmission
Both I. scapularis and I. pacificus are three-host ticks that host-seek openly (as opposed to nidicolous ticks, which remain in animal nests) and are considered host generalists. The human pathogens described above are acquired through blood feeding on reservoir hosts, or via co-feeding transmission from infected to noninfected ticks feeding in proximity to each other on reservoir or nonreservoir hosts (Costero and Grayson 1996; Dolan et al. 2016; Ebel 2010; Karpathy et al. 2016; Mather and Mather 1990; Piesman et al. 1987; Teglas and Foley 2006). However, Bo. miyamotoi and Powassan virus also can be transmitted transovarially from an infected female to her offspring (Costero and Grayson 1996; Rollend et al. 2013).
Although humans can be bitten by all life stages, bites by nymphs and adults are most commonly reported; humans serve as incidental tick hosts and do not contribute to pathogen transmission (Bishopp and Trembley 1945; Merten and Durden 2000). Larvae and nymphs feed on a wide range of hosts, including lizards, birds, insectivores, rodents, lagomorphs, and ungulates (Brinkerhoff et al. 2011; Eisen et al. 2004a; LoGiudice et al. 2003; Salkeld and Lane 2010). However, these blood meal hosts differ in their ability to serve as pathogen reservoirs by providing a source of infection for feeding ticks. For example, some lizard species are zooprophylactic (Lane and Quistad 1998), clearing Lyme disease spirochetes from feeding ticks, while others, such as the white-footed mouse (Peromyscus leucopus) or the western tree squirrel (Sciurus griseus), are highly competent reservoirs of Bo. burgdorferi and infect large numbers of feeding ticks (Mather et al. 1989; Salkeld and Lane 2010). Although adult ticks feed on a variety of medium- and large-sized mammals, I. scapularis and I. pacificus feed primarily on white-tailed deer and Columbian black-tailed deer (O. hemionus columbinanus), respectively (Furman and Loomis 1984; Piesman et al. 1979). Key vertebrate reservoirs of the pathogens listed above are shown in Table 1.
Tick Seasonality and Host-Seeking Behavior
As recently reviewed in more detail (Eisen et al. 2016b), depending on the geographic region and weather conditions, the ticks’ life cycles are completed in 2 to 4 years (Hamer et al. 2012; Padgett and Lane 2001; Yuval and Spielman 1990). In the northeastern and north-central United States during the warm months from spring through autumn, I. scapularis larvae and nymphs typically seek hosts from the top of leaf litter or twigs near the ground, whereas adults actively seek hosts by ascending vegetation (Hamer et al. 2012; Yuval and Spielman 1990). In the Southeast, the seasonality of host-seeking is poorly defined. Notably, in this region, I. scapularis nymphs are less likely than those in the North to ascend vegetation (leaf litter) to seek hosts, thus reducing the likelihood of humans encountering nymphal ticks in the South (Arsnoe et al. 2015; Diuk-Wasser et al. 2012; Stromdahl and Hickling 2012).
In northern California, I. pacificus are active year-round with adults host-seeking in cooler months from late autumn through early spring and immature life stages host-seeking from early spring through summer or early autumn (Eisen et al. 2003, 2004b; Lane et al. 2007). In southern California, the tick’s life cycle is truncated compared with northern populations; adult host-seeking begins later and ends earlier and immature life stages generally terminate host-seeking behavior earlier in the season. Similar to I. scapularis in the Southeast, I. pacficus nymphs rarely ascend emergent vegetation and are seldom encountered using methods that aim to assess human risk of encounters with ticks, with the exception of leaf litter habitats without emergent vegetation (MacDonald and Briggs 2016).
Frequency, Seasonality, and Geographic Distribution of Ixodes-Borne Diseases
Collection and collation of data on occurrence of nationally notifiable conditions under the National Notifiable Diseases Surveillance System is the nation’s most comprehensive and systematic means of conducting public health surveillance (Centers for Disease Control and Prevention 2016c). In 1991, Lyme disease became the first Ixodes-associated illness to become a nationally notifiable condition. Since that time, anaplasmosis, Powassan virus disease, and babesiosis were added as notifiable conditions in 2000, 2002, and 2011, respectively. The incidence and geographic range of these infections have steadily increased since national reporting was initiated (Figure 2).
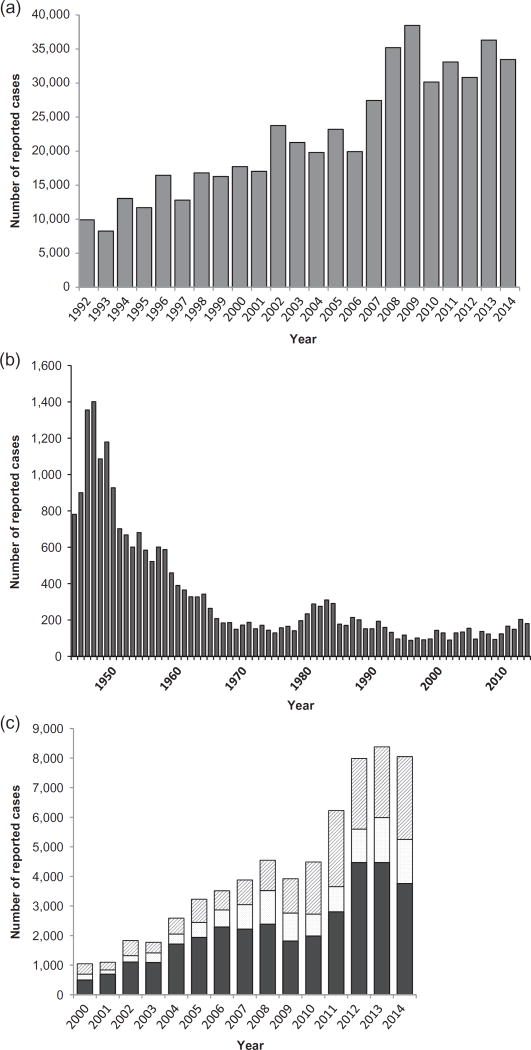
Figure 2.
Reported human cases in the United States of Lyme disease, 1992–2014 (A); tularemia, 1944–2014 (B); and anaplasmosis (diagonal lines), ehrlichiosis (stippled), and spotted fever group rickettsioses (black) 2000–2014 (C).
Lyme disease is the most commonly reported vector-borne disease in the United States. Although underreporting in public health surveillance is well documented, the annual number of human Lyme disease cases reported to the public health system has increased from approximately 10,000 to 30,000 cases between the early 1990s and mid-2010s (Figure 2) (Adams et al. 2016; Centers for Disease Control and Prevention 2016b). Nonetheless, the true magnitude of Lyme disease is considerably greater, with current estimates of 240,000 to 440,000 cases annually based on commercial laboratory test results. National numbers mask the highly focal nature of the illness, with 96% of all cases coming from just 14 states in the Northeast, mid-Atlantic, and upper Midwest (Figure 3) (Adams et al. 2016; Centers for Disease Control and Prevention 2016b; Hinckley et al. 2014). Human infection is sporadic in the far western United States. Importantly, Lyme disease cases have started occurring in new places as endemic foci have expanded over time. The number of counties with high Lyme disease incidence has increased by approximately 300% since the mid-1990s (Kugeler et al. 2015). The expansion of areas with high incidence of Lyme disease mirrors the geographic expansion of I. scapularis in the eastern United States, as described above (Eisen et al. 2016a, 2016b). In addition to the primary Lyme disease agent in the United States, Bo. burgdorferi, a second spirochete also belonging to the Bo. burgdorferi sensu lato complex, Bo. mayonii, was recently recognized as a cause of Lyme disease in the upper Midwest (Pritt et al. 2016b). This spirochete has yet to be detected from other parts of the eastern United States. Incidence of infection with Bo. mayonii is unknown, and Lyme disease cases may not be able to be differentiated by etiologic agent in national surveillance. Therefore, special studies are needed to better define the clinical course, geographic distribution, and frequency of human infection with Bo. mayonii.
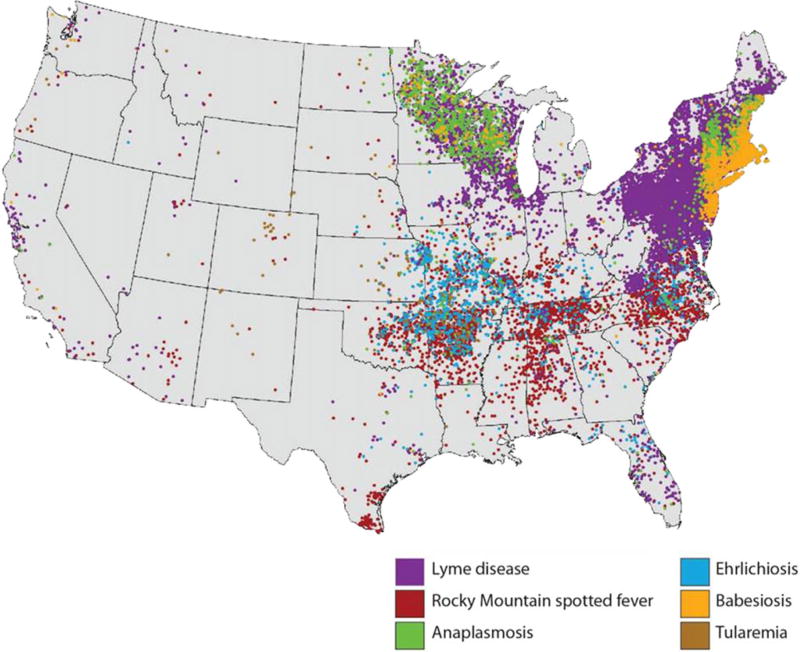
Figure 3.
Reported distribution of key tick-borne diseases in the United States, 2014 Each dot represents one case. Cases are reported from the infected person’s county of residence, not necessarily the county of exposure. During 2014, babesiosis was reportable in Alabama, California, Connecticut, Delaware, Illinois, Indiana, Louisiana, Maine, Maryland, Massachusetts, Michigan, Minnesota, Montana, Nebraska, New Hampshire, New Jersey, New York, North Dakota, Ohio, Oregon, Rhode Island, South Carolina, South Dakota, Tennessee, Texas, Utah, Vermont, Washington, West Virginia, Wisconsin, and Wyoming. CDC was not notified through the national surveillance system of cases in other states. In 2014, no cases of tick-borne illness were reported from Hawaii. In 2014, Alaska reported eight travel-related cases of Lyme disease.
The geographic distribution of anaplasmosis, Powassan virus disease, and babesiosis mirror that of Lyme disease, although frequency of human infection is several orders of magnitude lower (Figures 1 and 3). The number of reported anaplasmosis cases increased from 348 in 2000 to 2800 in 2014, with geographic expansion of areas reporting cases (Centers for Disease Control and Prevention 2016a; Dahlgren et al. 2015). Although Powassan virus disease became a notifiable condition in 2002, case reports were submitted to CDC and published in the literature before that time. These compiled sources indicate that cases of this rare illness have increased in frequency since the pathogen was first described, from an average of 0.7 cases recorded annually during 1958–1998 to an average of 7 cases per year since 2006 (Adams et al. 2016; Centers for Disease Control and Prevention 2016d; Hinten et al. 2008). Powassan virus infections in North America appear to be transmitted primarily by I. scapularis and occur in Northeast and upper Midwest states, where nymphal ticks more commonly bite humans compared with the southeastern United States (Stromdahl and Hickling 2012). Since babesiosis became a notifiable condition in 2011, between 900 and 1800 cases have been reported annually (Adams et al. 2016). A few cases of babesiosis caused by species other than Ba. microti have been documented from the western United States, although the reservoirs and vectors of these species have yet to be elucidated (Vannier et al. 2015).
Similar to Bo. mayonii, the E. muris-like agent was described recently from the north-central United States, and human infections appear to be limited to that geographic region (Johnson et al. 2015; Pritt et al. 2011, 2016b). The incidence of human infections is largely unknown, because they are not easily differentiated in national surveillance counts for ehrlichiosis and anaplasmosis (Dahlgren et al. 2016). Likewise, there is no standard surveillance for Bo. miyamotoi disease and frequency of human infection is unknown. In an assessment among banked samples from the northeastern United States, 3.9% of samples from healthy persons and 9.8% of those with suspected acute Lyme disease demonstrated reactivity to a Bo. miyamotoi-specific target (Krause et al. 2014). Additionally, acute coinfections with Bo. miyamotoi and Bo. burgdorferi were documented by seroconversion to antigens from both pathogens. Human illness has also been documented in the upper Midwest, and human infection along the Pacific Coast is likely given evidence that Bo. miyamotoi has been found in I. pacificus ticks in multiple locations in California (Cook et al. 2016; Jobe et al. 2016; Mun et al. 2006; Padgett et al. 2014). Nevertheless, the frequency of asymptomatic infection is unknown and the overall importance of Bo. miyamotoi as a human pathogen remains unclear.
Discordance between the broad distribution of I. scapularis and I. pacificus in the United States and the focal nature of associated human diseases is multifactorial (Figures 1 and 3). As described above, the host-seeking behaviors of immature I. scapularis and I. pacificus do not favor human exposure to the nymphal stages of these ticks in the southern United States. In addition, prevalence of B. burgdorferi infection is substantially lower in ticks collected from the South compared with the North (Lane et al. 2013; Stromdahl and Hickling 2012). While human tick-borne illnesses under surveillance are reported from most states, these cases are reported according to the patient’s location of residence rather than the probable location where the infected tick was encountered. Cases of Lyme disease reported from low-incidence areas may reflect exposure to focal areas of transmission in otherwise low-risk places; nonetheless, cases reported from these states often represent travel to highly endemic areas and possible misdiagnosis due to higher likelihood of false positive test results in these settings (Forrester et al. 2015; Lantos et al. 2015).
Nymphal stage I. scapularis are responsible for most human Lyme disease, babesiosis, and anaplasmosis, as deduced from the late spring through early summer peak in human disease occurrence, which coincides with peak nymphal host-seeking activity that is distinct from the peak host-seeking activity period for adult female ticks. Although adult ticks typically have higher rates of infection with Lyme disease, babesiosis, and anaplasmosis agents than nymphs, bites by female ticks account for a smaller fraction of human illness, presumably due to less frequent outdoor high-risk behaviors in the fall and early spring when the female ticks are most active, and adult ticks’ larger size compared with nymphs, which makes them easier to detect. Prompt detection and removal of ticks reduces risk of infection with A. phagocytophilum, Ba. microti, and Bo. burgdorferi owing to the documented time lag post-tick attachment before infectious organisms are transmitted from the tick to the host (Hodzic et al. 1998; Hojgaard et al. 2008; Piesman and Spielman 1980). Although data on human Bo. miyamotoi infections are still limited, transovarially infected larvae appear to play a role in transmission to humans, based on peak disease onset in July-August, which coincides with the peak period of host-seeking activity for larval stage I. scapularis (Molloy et al. 2015; Scoles et al. 2001). The roles of different tick life stages as vectors of Bo. mayonii, the E. muris-like agent, and Powassan virus to humans are not well understood.
Lone Star Tick (Amblyomma americanum) and Gulf Coast Tick (Amblyomma maculatum)
Associated Human Disease Agents
The lone star tick, A. americanum, was the first tick species described in the United States in 1754, yet, until the early 1990s it was considered primarily a nuisance species (Childs and Paddock 2003). It is now recognized as a primary vector of the etiological agents of tularemia (Francisella tularensis), ehrlichiosis (Ehrlichia chaffeensis and Ehrlichia ewingii), and Heartland virus (Family: Bunyaviridae) (Anziani et al. 1990; Ewing et al. 1995; Godsey et al. 2016; Hopla 1953, 1955). Southern tick-associated rash illness has been associated with the bite of A. americanum, but despite thorough investigation since its first description in the 1980s, an etiologic agent has not been linked to this condition, suggesting the possibility of a noninfectious cause (Masters et al. 1998; Wormser et al. 2005).
The disease now known as tularemia was first described in Japan in the 19th century. The causative agent, F. tularensis, was first identified among California ground squirrels in 1910 and linked to human illness in the United States shortly thereafter (Francis 1925; McCoy 1911). The highly infectious organism has been shown to infect hundreds of animal species and also to survive in water and soil; multiple tick species are likely involved in maintenance of F. tularensis in nature (Hopla 1974; Jellison 1974; Parker et al. 1951). Tick bites are one of several possible modes of transmission of F. tularensis to humans, and A. americanum nymphs and adults have been implicated as important vectors in the south-central United States (Brown et al. 2011; Eisen 2007a).
Beginning in the 1980s, additional A. americanum-borne human pathogens were increasingly recognized, particularly in Arkansas and Missouri. The first case of human monocytic ehrlichiosis was recognized in 1986 in a patient presumably exposed to A. americanum bites in Arkansas (Maeda et al. 1987). The etiological agent was later described as E. chaffeensis (Anderson et al. 1991; Dawson et al. 1991). In 1999, E. ewingii, initially described from four patients from Missouri, was recognized as another cause of ehrlichiosis in humans (Buller et al. 1999). In 2009, Heartland virus, the first pathogenic tick-borne phlebovirus in the United States, was described from two patients in northwestern Missouri and later detected in field collected A. americanum ticks (McMullan et al. 2012; Savage et al. 2013, 2016).
Rickettsia parkeri was first reported to cause spotted fever rickettsiosis in humans in 2004 (Paddock et al. 2004), but the disease agent had been described decades prior in association with the Gulf Coast tick, A. maculatum (Lackman et al. 1965, 1949). Rickettsia parkeri has now been detected in A. maculatum ticks from Alabama, Arizona, Delaware, Florida, Georgia, Kentucky, Louisiana, Maryland, Mississippi, North Carolina, Oklahoma, Tennessee, Texas, and Virginia, approximating the geographic distribution of human cases. Current estimates of infection rates of host-seeking adult ticks with R. parkeri range from 8% to 56%, remarkably higher than for any other recognized tick-borne rickettsial pathogen in the United States (Herrick et al. 2016; Paddock and Goddard 2015). Amblyomma maculatum ticks infected with R. parkeri have been collected from various species of wildlife, including feral pigs, white-tailed deer, cotton rats, and coyotes (Sumner et al. 2007; Trout and Steelman 2010), although a definitive vertebrate reservoir has not yet been identified.
Geographic Distribution of Tick Vectors
Similar to I. scapularis and I. pacificus, A. americanum is primarily a woodland-associated tick but also seeks hosts in fields and meadows. It is most common in second growth forests with dense understory vegetation (Hair and Howell 1970). The core of the lone star tick’s distribution is in the southern parts of the eastern United States, but it occurs along the Atlantic coastline as far north as Maine, and sporadically in the upper Midwest (Figure 1) (Childs and Paddock 2003; Springer et al. 2014). Review of historical records suggest a continuing northward range expansion of this tick (Childs and Paddock 2003; Paddock and Yabsley 2007; Springer et al. 2014). Range expansion and increasing abundance of A. americanum is largely attributed to the increasing range and abundance of white-tailed deer, the primary host for A. americanum (Childs and Paddock 2003; Paddock and Yabsley 2007).
Largely because of its ability to effectively regulate water balance, compared with the lone star tick, the Gulf Coast tick is associated with more xeric habitats including grasslands and scrub communities, upland and coastal prairies (Needham and Teel 1991; Teel et al. 2010). During the first half of the 20th century, A. maculatum was known to be distributed primarily within 100 to 150 miles inland from the Gulf Coast spanning from Texas to the Atlantic Coast as far north as South Carolina (Bishopp and Trembley 1945). The distribution of the Gulf Coast tick extends northward along the Atlantic coast to Delaware, as well as >250 miles inland from historically recognized habitats along the Gulf of Mexico and the Atlantic Ocean. Established populations of A. maculatum also now exist in several noncoastal states, including Arkansas, Kansas, Kentucky, and Oklahoma (Figure 1). Range expansion has been attributed to the expanding range of white-tailed deer and movement of infested cattle, feral swine, and migratory birds and is also possibly linked to restoration of inland grassland and savannah habitats (Paddock and Goddard 2015; Teel et al. 2010).
Tick Life Cycles/Enzootic Pathogen Transmission
Amblyomma americanum and A. maculatum are non-nidicolous, three-host ticks that are considered host generalists and, due to their very active host-seeking behavior, aggressive biters of humans (Childs and Paddock 2003; Merten and Durden 2000; Paddock and Goddard 2015; Paddock and Yabsley 2007; Stromdahl and Hickling 2012; Teel et al. 2010). Humans serve as incidental hosts of both ticks and do not contribute to transmission of their associated pathogens. All life stages of A. americanum commonly bite humans, whereas human encounters with A. maculatum are primarily with the adult life stage (Bishopp and Trembley 1945; Merten and Durden 2000).
Although A. americanum will feed on a wide range of hosts, they are highly dependent on large mammals to complete their life cycle. Adults feed primarily on white-tailed deer and other medium- and large-sized mammals (Bishopp and Trembley 1945; Childs and Paddock 2003; Kollars et al. 2000; Paddock and Yabsley 2007). Larval A. americanum are not known to transmit any human pathogens (Stromdahl and Hickling 2012) and are likely to acquire infections during blood feeding. Larvae and nymphs infest ground-feeding birds, medium- and large-sized mammals, and less frequently parasitize small mammals (Bishopp and Trembley 1945; Childs and Paddock 2003). White-tailed deer are naturally infected with E. chaffeensis and E. ewingii, and because all life stages of A. americanum feed commonly on this host, it is considered the primary reservoir of these pathogens (Table 1) (Childs and Paddock 2003; Lockhart et al. 1997a, 1997b; Yabsley et al. 2002). The potential role of other mammals as reservoirs of E. chaffeensis was reviewed previously by Childs and Paddock (2003).
Host feeding preferences of A. maculatum were recently reviewed by Teel et al. (2010). Adult ticks feed commonly on large-sized wild and domestic mammals, primarily ungulates and carnivores. By contrast, larvae and nymphs feed more commonly on birds and rodents. Although A. maculatum has been implicated as the vector of R. parkeri, little is known about how this pathogen is maintained in enzootic transmission cycles (Paddock et al. 2004).
Tick Seasonality and Host-Seeking Behavior
The host-seeking phenology of A. americanum is similar across the species range from New Jersey (Schulze et al. 1986) to Missouri (Kollars et al. 2000) and Georgia (Davidson et al. 1994). Adult ticks are host-seeking as early as March or April and reach peak abundance from March to July, with the peak occurring earlier in the South compared with the North. In each of the three locations, larval abundance peaks in September, but larvae are active as early as March and as late as October. Nymphs are active as early as March and as late as September, with the longest period of activity observed in Georgia. In Georgia, peak nymphal activity spans from March to May, whereas in Missouri and New Jersey, peak nymphal activity occurs in June.
Seasonal activity of A. maculatum differs by geographic region, with inland populations active from spring to summer and coastal populations from fall to spring. In coastal communities of Texas, adults are active primarily in September followed by peak activity of larvae and nymphs in January and February, respectively. By contrast, in inland communities in Oklahoma, with a more temperate climate, the peak of adult activity occurs during late April to early June, followed by peak activity of larvae and nymphs in July and August, respectively (Teel et al. 2010).
Frequency, Seasonality, and Geographic Distribution of Amblyomma-Borne Diseases
In the early to mid-20th century, human tularemia was a notifiable condition in many states and data were reported through systems predating current public health surveillance mechanisms. During that time, the disease occurred much more commonly than it does today, with >1000 cases reported annually during the 1940s (Jellison 1974). These numbers contrast with an average of 125 tularemia cases reported in recent years through National Notifiable Diseases Surveillance System (Figure 2) (Centers for Disease Control and Prevention 2002, 2013, 2016e). Human cases have been reported from all states other than Hawaii; however, frequency is often highest in the south-central states of Arkansas, Missouri, and Oklahoma (Figure 3). The decline in frequency of tularemia cases over time has been associated with decreased rabbit hunting, a food-gathering practice thought to account for a large proportion of human cases via direct transmission resulting from handling of infected rabbits (Eisen 2007a; Jellison 1974). The transition away from hunting exposures is also evident in the shifting seasonality of human cases over the decades. A once predominantly wintertime disease in the United States has transitioned to one that occurs primarily during the warmer months when ticks and other arthropod vectors of F. tularensis are most active (Centers for Disease Control and Prevention 2013; Centers for Disease Control and Prevention 2016e; Eisen 2007a). The proportion of human tularemia cases in the United States explicitly linked to tick bites remains substantial, particularly in south-central states (Eisen 2007a).
Ehrlichia chaffeensis and E. ewingii infections are nationally notifiable conditions in the United States. During 2008–2012, there were 4668 reported cases of ehrlichiosis and approximately 99% were attributable to infection with E. chaffeensis. The incidence of these infections in the United States during this period was 3.2 cases per 1 million persons, which represented a 4-fold increase since 2000 (Figure 2). States that consistently report the highest incidence include Arkansas, Missouri, Oklahoma, Tennessee, and Virginia (Figure 3) (Dahlgren et al. 2011; Heitman et al. 2016). Approximately two-thirds of all cases are reported during May to July. The geographical distribution of ehrlichiosis mirrors the distribution of the principal vector, the lone star tick (Figure 1), and increasing numbers of cases have been identified recently well beyond the historical range of A. americanum, to reflect the dynamic and expanding distribution of this medically important tick (Cortinas and Spomer 2013; Maegli et al. 2016).
Through 2015, approximately 40 cases of R. parkeri rickettsiosis have been identified from 10 states (Centers for Disease Control and Prevention 2016a; Herrick et al. 2016; Paddock et al. 2008). Confirmed infections have resulted from bites of nymphal and adult stage Gulf Coast ticks. Recognized infections have occurred from April through October with approximately 80% during July through September. Approximately 75% of the known case patients are men. In contrast to Rocky Mountain spotted fever (RMSF), there are no published descriptions of R. parkeri rickettsiosis in young children; indeed, most patients with this infection are considerably older and the median age from case reports is 53 years (range, 23–83 years) (Paddock and Goddard 2015).
American Dog Tick (Dermacentor variabilis), Rocky Mountain Wood Tick (Dermacentor andersoni), and Pacific Coast Tick (Dermacentor occidentalis)
Associated Human Disease Agents
The American dog tick, D. variabilis, has historically been implicated as a major vector to humans in the eastern United States of Rickettsia rickettsii, the etiological agent of RMSF (Burgdorfer 1975). However, more recent investigations have shown that D. variabilis is rarely infected with pathogenic R. rickettsii and instead more frequently harbors nonpathogenic spotted fever group rickettsia (Stromdahl et al. 2011). Although D. variabilis is capable of transmitting F. tularensis (Reese et al. 2011) and has been associated with local tularemia outbreaks, its overall importance as a vector to humans remains unclear (Petersen et al. 2009; Stromdahl and Hickling 2012).
In the Rocky Mountains and Inter-Mountain West, the Rocky Mountain wood tick, D. andersoni, has been implicated as a major vector to humans of R. rickettsii (Burgdorfer 1975). However, R. rickettsii has been shown experimentally to cause mortality in infected ticks, thus resulting in low prevalence of infection in ticks (Niebylski et al. 1999). This tick species is also a vector of F. tularensis; however, like D. variabilis, its importance as a vector to humans is poorly defined (Petersen et al. 2009). Finally, D. andersoni also serves as the primary vector to humans of Colorado tick fever virus (Family: Reoviridae) (Burgdorfer 1977).
In the Pacific Coast states, D. occidentalis, the Pacific Coast tick has long been associated with transmission of the agents of tularemia, RMSF, and Colorado tick fever virus (Brown et al. 2005). More recently, its significance in the transmission of the agent of Pacific Coast tick fever has been elucidated (Padgett et al. 2016). Originally described as “Rickettsia 364 D,” R. philipii, a spotted fever group rickettsia that is closely related to R. rickettsii, was first isolated from D. occidentalis in 1966 (Padgett et al. 2016; Lane et al. 1981b). In 2008, the first confirmed human infection with R. philipii was described from a patient in northwestern California (Shapiro et al. 2010).
Geographic Distribution of Tick Vectors
Dermacentor variabilis is found primarily in old fields and ecotones comprised of old fields and woodlands, and is rarely found in heavily forested areas (Bishopp and Trembley 1945; Campbell and MacKay 1979; Sonenshine and Stout 1968). It is broadly distributed east of the Rocky Mountains, with a limited distribution in Pacific Coast states, making it one of the most widely distributed ticks in the United States (Figure 1) (Bishopp and Trembley 1945; James et al. 2015). Because of the tick’s dependence on high humidity, D. variabilis is not found in the Rocky Mountains and Inter-Mountain West (James et al. 2015). During the last 70 years, the geographic range of the American dog tick in the United States has changed less than many other medically important tick species. However, more recent collection records of D. variabilis have been reported from Washington and Idaho where the tick had not been reported in early surveys (Bishopp and Trembley 1945; James et al. 2015).
In striking contrast, D. andersoni is found primarily in semi-arid and mountainous areas, favoring habitats comprised mostly of grasses and shrubs (Bishopp and Trembley 1945; Eisen 2008; James et al. 2006; Lane et al. 1981a). Its range is restricted to the Rocky Mountains and Inter-Mountain West and it is largely found in areas that are uninhabitable to D. variabilis (Figure 1) (Bishopp and Trembley 1945; James et al. 2006, 2015). The geographic range of D. andersoni appears to have remained relatively stable since 1932 (James et al. 2006).
Dermacentor occidentalis is most commonly encountered in woody and brushy habitats (Brown et al. 2005). Its range is restricted to Oregon, California, and northern Baja California in Mexico, but, based on abundance, its core distribution appears to be in the Coastal Ranges and Cascade Range in California and southern Oregon (Bishopp and Trembley 1945; Furman and Loomis 1984).
Tick Life Cycles/Enzootic Pathogen Transmission
All three Dermacentor vector species are three-host, non-nidicolous ticks that feed primarily on small mammals as larvae and nymphs and on a larger hosts as adults (Bishopp and Trembley 1945). While immature D. andersoni and D. occidentalis feed on a wide range of rodents and other small mammals, D. variabilis has a more narrow host range, feeding most commonly on voles (Microtus spp.) and white-footed mice (Peromyscus spp.) (Bishopp and Trembley 1945; Burgdorfer 1977). As adults, all three species feed on a wide range of large domestic and wild mammals; however, domestic dogs are common hosts for adult D. variabilis (Bishopp and Trembley 1945; Burgdorfer 1977). Similar to the other tick species described above, humans are incidental hosts of D. variabilis, D. andersoni, and D. occidentalis; the vast majority of human bites are by the adult life stage (Bishopp and Trembley 1945; Merten and Durden 2000).
Because of their high susceptibility to R. rickettsii infection and because they are commonly infested by D. andersoni and D. variabilis, small mammals including chipmunks (Tamias spp.), voles (Microtus spp.), ground squirrels (Spermophilus spp.), and cottontail rabbits (Sylvilagus spp.) are considered important amplifying hosts (Table 1) (Burgdorfer 1977; Niebylski et al. 1999). Rickettsia rickettsii also is maintained through transovarial transmission and through simultaneous feeding on a common host by infected and noninfected ticks. Despite multiple mechanisms of transmission that predict a high infection rate in ticks, prevalence of R. rickettsii in Dermacentor spp. ticks is typically <1% (Burgdorfer 1977; Stromdahl et al. 2011). Low infection prevalence may be explained in part by detrimental effects of infection on the survival and fecundity of the vector (Niebylski et al. 1999).
Colorado tick fever virus is maintained through horizontal transmission with infected D. andersoni nymphs maintaining the virus through winter, then infecting small mammal hosts in the spring, which then infect feeding larvae. Although Colorado tick fever virus has been isolated from numerous small mammal species, golden-mantled ground squirrels and chipmunks appear to be key hosts based on their ability to harbor high viral loads that are infectious to feeding ticks and due to their heavy infestation rates with immature D. andersoni (Table 1) (Burgdorfer 1977; Marfin and Campbell 2005). Local infection rates with Colorado tick fever virus in host-seeking D. andersoni can vary dramatically over short distances, but a recent study from Wyoming showed that 23% of females collected from Grand Teton National Park and Bridger-Teton National Forest carried this virus (Geissler et al. 2014).
Tick Seasonality and Host-Seeking Behavior
The host-seeking phenology of D. variabilis differs across its geographic range. For example, in Virginia, adults are actively host-seeking as early as April with peak abundance occurring in late June or early July and adult activity continues through August. Larval host-seeking commences in the early spring of the following year. Peak larval abundance typically is observed in April and declines rapidly after the peak, but larval activity persists at lower levels for an additional 2 to 3 months postpeak. Nymphs are typically active from spring through summer, with peak activity lagging behind the larval peak by 4 to 6 weeks (Sonenshine 1993). Further north in the tick’s range, larval host-seeking activity precedes nymphal activity as it does in the South; however, in contrast to the unimodal peaks observed in the South, in the North larval and nymphal host-seeking activities peak in the spring and their abundance often increases again in late summer. Adults are active from April through mid-August with peak activity in May or June (Garvie et al. 1978; Smart and Caccamise 1988). In contrast, in Oklahoma larval activity reportedly spans from June through September, with peak activity in June (Gage et al. 1992).
Although there are subtle regional differences in host-seeking behavior of D. andersoni, adults are generally active from February through November, with peak activity occurring between April and June. Larvae and nymphs are typically active from March through October, with peak host-seeking activity in May and June (Bishopp and Trembley 1945; Eads and Smith 1983; Easton et al. 1977; James et al. 2006; Sonenshine et al. 1976).
In California, adult D. occidentalis have been collected during all months of the year, but their activity peaks from March to May. Larvae and nymphs are active during the spring and summer, but peak activity is typically in July and August (Furman and Loomis 1984; Padgett et al. 2016).
Frequency, Seasonality, and Geographic Distribution of Dermacentor-Borne Diseases
RMSF is the most lethal tick-borne disease in the United States, and during the decade preceding discovery of effective antimicrobial therapy for RMSF, the aggregate mortality attributable to this disease was approximately 23%. Throughout most of the United States, RMSF is sporadic and uncommon relative to most other tick-transmitted infections. Cases of spotted fever have been reported from each of the 48 contiguous states and the District of Columbia; nonetheless, its distribution and frequency generally parallel the distribution and abundance of D. variabilis in central and eastern states and D. andersoni in western states (Biggs et al. 2016). In this context, Arkansas, Missouri, North Carolina, Oklahoma, and Tennessee demarcate a broad band of higher incidence states in the eastern and central United States, accounting for 63% of spotted fever cases during 2008–2012 (Figure 2). Montana and Wyoming report the highest state-wide incidence of RMSF in the western United States (Drexler et al. 2016). Approximately 70% of reported cases occur from May through August; however, sporadic cases of RMSF are reported during all months of the year (Lange et al. 1982). Endemic foci for RMSF have been documented to persist for decades within small regions or communities (Burgdorfer 1977; Hazard et al. 1969), and family clusters of RMSF involving as many as six persons have been described (Jones et al. 1999).
Through 2015, 14 cases of laboratory-confirmed Pacific Coast tick fever have been identified from 5 counties in California. Twelve (86%) of these cases occurred during July and August, corresponding to the peak host-seeking activity of nymphal stage D. occidentalis (Padgett et al. 2016). There are no known deaths attributable to this infection, but some patients had moderately severe illnesses and required hospitalization. The range of D. occidentalis also includes southern Oregon, but all known cases of Pacific Coast tick fever have been limited to California.
Colorado tick fever (CTF) occurs where humans come into contact with tick vectors of the causative virus, primarily D. andersoni (Emmons 1988). CTF has been reported from 11 western states, including California, Colorado, Idaho, Montana, Nevada, New Mexico, Oregon, South Dakota, Utah, Washington, and Wyoming, where it occurs predominantly in mountainous highland habitats from approximately 4000 to more than 10,000 feet elevation and where vegetation comprising sagebrush, juniper, and pine with moderate grass cover predominates. Recent studies suggest that the risk of acquiring CTF may be greatest at elevations above 7000 feet (Geissler et al. 2014). Approximately two-thirds of all cases of CTF reported during 1995–2012 occurred in male patients, and the highest age-specific incidence was in people ≥50 years of age (Brackney et al. 2010; Yendell et al. 2015). The majority of cases occur during May to July and reflect the peak of host-seeking adult D. andersoni ticks (Eisen 2007b; Spruance and Bailey 1973). Fatalities occur only rarely. Reported cases of CTF have declined dramatically since the early 1980s when more than 200 cases were reported each year (Emmons 1988). By comparison, a median of 55 cases of CTF were reported annually during 1987–2001, and the median annual number dropped to 5 during 2002–2012. These changes could be influenced by recent changes in reporting practices in some historically high-incidence states such as Colorado, which removed CTF from its list of reportable diseases in 1997 (Yendell et al. 2015).
Brown Dog Tick (Rhipicephalus sanguineus Sensu Lato)
Associated Disease Agents
Investigators in the 1930s determined that Rh. sanguineus s. l. was a competent experimental vector of R. rickettsii (Parker 1933); nonetheless, a conclusive role for this tick in the natural history of RMSF in the United States was not documented until 2005, when the brown dog tick was identified as the sole vector responsible for epidemic levels of RMSF in several tribal communities in Arizona (Demma et al. 2005; Nicholson et al. 2006a, 2006b). Environmental assessments of the affected communities revealed high rates of infection with R. rickettsii in adult ticks, including approximately 5% and 10% of the nonengorged specimens and engorged specimens, respectively (Eremeeva 2012). The taxonomic status of R. sanguineus sensu stricto is unresolved, and current data indicate that ticks identified collectively as R. sanguineus comprise a group of genetically distinct taxa that likely includes multiple sibling species. Because distinct populations of R. sanguineus s. l. often differ in vector competence with other infectious agents, it is possible that certain populations of R. sanguineus s. l ticks vary in efficiency as vectors of R. rickettsii (Dantas-Torres and Otranto 2015). The brown dog tick is also a vector of Rickettsia massiliae, a moderately pathogenic spotted fever group Rickettsia species, and Ehrlichia canis, an important bacterial pathogen of domestic dogs. Each of these agents is associated with disease in humans in other parts of the world and has been detected in Rh. sanguineus s. l. ticks and domestic dogs in several regions of the United States (Beall et al. 2012; Beeler et al. 2011; Eremeeva et al. 2006); nonetheless, there have been no confirmed cases of human illness caused by R. massiliae or E. canis in the United States.
Geographic Distribution
The brown dog tick is one of the most widely distributed hard tick species worldwide and is found approximately between the latitudes of 50°N and 30°S. In the United States collection records of Rh. sanguineus s. l. exist from all 50 states (Walker et al. 2000).
Tick Life Cycles/Enzootic Pathogen Transmission
Multiple biological and ecological features of Rh. sanguineus s. l. contribute to its role as a formidable vector of R. rickettsii. This three-host tick is predominantly monotropic and domesticated dogs serve as the principal hosts for all life stages. Because of its host preference, Rh. sanguineus s. l. is extremely well adapted to peridomestic and endophilic habitats and is also remarkably resistant to desiccation (Koch and Tuck 1986). Brown dog ticks spend most of their life span off of their hosts, concealed in cracks and crevices of walls in kennels, homes, and other peridomestic harborages. Because of the cryptic behavior and small size of larvae and nymphs, low-level peridomestic infestations can escape attention, allowing populations to increase rapidly (Eiden et al. 2015). The duration of the brown dog tick life cycle is temperature dependent, but under favorable conditions, Rh. sanguineus s. l. can complete 2 generations or more per year. In the United States, brown dog ticks are most active from late spring through early fall (Dantas-Torres 2008). The parasitism by Rh. sanguineus s. l. of hosts other than domestic dogs is generally uncommon but can occur in heavily infested environments, and all three feeding stages can bite humans (Dantas-Torres 2010). Nonetheless, ticks can acquire R. rickettsii bacteria from infected mammalian hosts at each feeding stage and rickettsiae are transmitted to subsequent stages as well as transovarially to the eggs of infected female ticks (Costa et al. 2011; Labruna et al. 2008; Parker 1933; Piranda et al. 2011). Although filial infection rates are typically <50%, female ticks can oviposit as many as 4000 eggs (Koch 1982), and survival rates among R. rickettsii-infected Rh. sanguineus s. l. ticks are generally greater than observed for other recognized vector species (Labruna et al. 2008; Piranda et al. 2011).
Frequency, Seasonality, and Geographic Distribution of Rh. sanguineus s. l.-Borne Rocky Mountain Spotted Fever
Among several American Indian reservations in eastern and southern Arizona, large populations of free-roaming dogs support enormous numbers of Rh. sanguineus s. l. that serve as efficient vectors for R. rickettsii and perpetuate extraordinarily high rates of RMSF (Demma et al. 2005; Nicholson et al. 2006b). During 2003–2013, approximately 300 cases of RMSF, including 20 deaths, were reported from tribal lands in Arizona compared with 3 RMSF cases reported from the entire state during the previous decade (Biggs et al. 2016). During 2009–2012, the average annual incidence of RMSF for the three most affected communities in Arizona was approximately 1360 cases per 1 million persons, more than 150 times the average for the United States (Drexler et al. 2014; Drexler et al. 2016).
Prevention Strategies
Human vaccines are currently lacking for all tick-borne pathogens that occur in the United States. Avoiding habitats where ticks occur is a first line of defense against tick-borne diseases; however, because some important tick vectors, such as I. scapularis, are abundant in peridomestic settings, this may be impractical and alternative strategies are required (Hayes and Piesman 2003). Prevention efforts focus on (i) minimizing the risk of tick bites through personal protective measures (e.g., using tick repellents on skin or clothing, wearing permethrin-treated clothing, and placing outdoor clothes into a dryer on high heat for 10 minutes to kill ticks lingering on the clothing), (ii) reducing the risk of pathogen transmission by prompt detection and removal of attached ticks (checking one’s body daily for attached ticks and showering or bathing within two hours after spending time in tick habitat), and (iii) environmentally based tick/pathogen control methods aimed at reducing the overall density of infected host-seeking ticks (e.g., landscape and vegetation management to reduce tick habitat and decrease the risk of tick bites on residential properties, suppression of host-seeking ticks with synthetic or natural product-based chemical acaricides or fungal biological agents, use of rodent reservoir-targeted topical acaricides to disrupt tick feeding and reduce enzootic pathogen transmission, and reduction of the availability of deer as tick hosts via deer culling or topical application of acaricide) (Connally et al. 2009; Eisen and Dolan 2016; Nelson et al. 2016).
Despite the availability of these various measures, prevention and control of tick-borne diseases have not, with the notable exception of RMSF associated with the brown dog tick (described below), met with great success in the United States. For example, no single personal protective measure or environmental tick/pathogen suppression method has consistently been shown to reduce Lyme disease cases, and no integrated strategy that combines two or more approaches has yet been evaluated with Lyme disease cases as an outcome measure (Eisen and Gray 2016; Hinckley et al. 2016). Indeed, there is an urgent need for strengthening the evidence base for the potential of existing personal protective measures and environmentally based control methods to reduce cases of tick-borne diseases, particularly when used in strategic combinations.
In addition to the lack of human vaccines, there are several other root causes that hinder the control and prevention of Amblyomma-, Dermacentor-, and Ixodes-transmitted infections. These vector ticks typically feed on a wide range of vertebrate hosts as immatures, often including multiple species that serve as pathogen reservoirs. Some of the vector ticks are largely dependent on deer as hosts for the adult stage, likely representing the weakest link in their life cycle, but deer populations have exploded in the eastern United States with limited potential for reducing deer densities to the very low levels required for substantial impact on tick populations (Eisen and Dolan 2016; Kugeler et al. 2016). Moreover, some of the most important tick vectors are ubiquitous both in natural environments and peridomestic settings, which makes these difficult to avoid. Although personal protective measures can be effective, they require extreme diligence and are highly inconvenient if the risk habitat includes your own backyard. Environmental suppression of ticks remains the responsibility of individual homeowners, leading to mosaics of treated and nontreated properties rather than larger tracts of treated area: this is a major difference between control of vector ticks and vector mosquitoes in the United States, and most likely part of the explanation for the greater success in controlling mosquito-borne diseases.
In contrast, efforts to curb outbreaks of RMSF associated with the brown dog tick have been largely successful, because all three feeding stages of Rh. sanguineus s. l. rely predominantly on one host species, the domesticated dog. The combination of an easily accessible tick host and clearly defined and readily accessible habitats for host-seeking brown dog ticks in and around homes set the stage for a successful campaign to control RMSF on American Indian reservations in Arizona. In year one of a two-year campaign known as the RMSF rodeo, all dogs in the community were outfitted with long-acting tick collars, acaricides were applied to yards monthly, and modifications to animal care practices (e.g., spaying and neutering and tethering of dogs) were encouraged. In year two, tick reduction efforts focused on the long-acting tick collar alone. Compared with control communities, tick infestations were significantly reduced in treated communities in years 1 and 2 of the study. Overall, incidence of RMSF decreased in treated and control communities. Although a more substantial decrease was observed in the treated community compared with the control, the significance and attribution of the decrease require further scrutiny (Drexler et al. 2014).
Conclusions
We described 12 major tick-borne diseases, caused by a total of 15 distinct disease agents that are transmitted by the 8 most commonly human-biting ixodid ticks in the United States (Table 1). Remarkably, only within the last two decades, 6 (40%) of these 15 pathogens (Bo. mayonii, E. muris euclairensis, Heartland virus, E. ewingii. R. parkeri, and R. philipii) have been recognized to cause illness in humans. This observed trend is attributable in part to improved diagnostic capabilities, astute clinical observations, and increased awareness of tick-borne pathogens by researchers and clinicians. Several of these etiological agents were described many years before recognition of confirmed infections of humans, while others are newly recognized agents of previously characterized diseases. Use of animal models has allowed for the rapid confirmation of tick vector competence for these newly discovered pathogens, and field studies have elucidated key tick species and vertebrate host species involved in the enzootic maintenance of the pathogens. Epidemiological surveillance has documented a marked increase in the incidence and geographic range of numerous tick-borne illnesses, particularly those associated with I. scapularis, A. americanum, and A. maculatum. Although vector surveillance is not standardized or routine in the United States, studies have revealed geographic expansion of several vectors in recent decades (notably, I. scapularis, A. americanum, and A. maculatum). Recognizing which reservoir hosts and tick vectors are critical to enzootic maintenance of these pathogens, which tick species and life stages serve as bridging vectors to humans, and where and when humans are at greatest risk for exposure are all critical in designing effective prevention measures.
Acknowledgments
We thank W.L. Nicholson and R.R. Lash for providing the tick species distribution maps.
Contributor Information
Rebecca J. Eisen, A Research Biologist in the Bacterial Diseases Branch, Division of Vector-Borne Diseases, Centers for Disease Control and Prevention in Fort Collins, Colorado..
Kiersten J. Kugeler, An Epidemiologist in the Bacterial Diseases Branch, Division of Vector-Borne Diseases, Centers for Disease Control and Prevention in Fort Collins, Colorado..
Lars Eisen, A Research Entomologist in the Bacterial Diseases Branch, Division of Vector-Borne Diseases, Centers for Disease Control and Prevention in Fort Collins, Colorado..
Charles B. Beard, A Branch Chief in the Bacterial Diseases Branch, Division of Vector-Borne Diseases, Centers for Disease Control and Prevention in Fort Collins, Colorado..
Christopher D. Paddock, A Medical Officer/Pathologist in the Rickettsial Zoonoses Branch, Division of Vector-Borne Diseases, Centers for Disease Control and Prevention in Atlanta, Georgia..
References
- Adams DA, Thomas KR, Jajosky R, Sharp P, Onweh D, Schley A, Anderson W, Faulkner A, Kugeler K. Summary of notifiable infectious disease conditions—United States, 2014. Morbid Mortal Wkly Rep. 2016;63:1–52. doi: 10.15585/mmwr.mm6253a1. [DOI] [PubMed] [Google Scholar]
- Anderson BE, Dawson JE, Jones DC, Wilson KH. Ehrlichia chaffeensis, a new species associated with human ehrlichiosis. J Clin Microbiol. 1991;29(12):2838–2842. doi: 10.1128/jcm.29.12.2838-2842.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anziani OS, Ewing SA, Barker RW. Experimental transmission of a granulocytic form of the tribe Ehrlichieae by Dermacentor variabilis and Amblyomma americanum to dogs. Am J Vet Res. 1990;51(6):929–931. [PubMed] [Google Scholar]
- Arsnoe IM, Hickling GJ, Ginsberg HS, McElreath R, Tsao JI. Different populations of blacklegged tick nymphs exhibit differences in questing behavior that have implications for human Lyme disease risk. PLoS One. 2015;10(5):e0127450. doi: 10.1371/journal.pone.0127450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour AG, Bunikis J, Travinsky B, Hoen AG, Diuk-Wasser MA, Fish D, Tsao JI. Niche partitioning of Borrelia burgdorferi and Borrelia miyamotoi in the same tick vector and mammalian reservoir species. Am J Trop Med Hyg. 2009;81(6):1120–1131. doi: 10.4269/ajtmh.2009.09-0208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beall MJ, Alleman AR, Breitschwerdt EB, Cohn LA, Couto CG, Dryden MW, Guptill LC, Iazbik C, Kania SA, Lathan P, et al. Seroprevalence of Ehrlichia canis, Ehrlichia chaffeensis and Ehrlichia ewingii in dogs in North America. Parasit Vectors. 2012;5:29. doi: 10.1186/1756-3305-5-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeler E, Abramowicz KF, Zambrano ML, Sturgeon MM, Khalaf N, Hu R, Dasch GA, Eremeeva ME. A focus of dogs and Rickettsia massiliae-infected Rhipicephalus sanguineus in California. Am J Trop Med Hyg. 2011;84(2):244–249. doi: 10.4269/ajtmh.2011.10-0355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggs HM, Behravesh CB, Bradley KK, Dahlgren FS, Drexler NA, Dumler JS, Folk SM, Kato CY, Lash RR, Levin ML, et al. Diagnosis and management of tickborne rickettsial diseases: Rocky Mountain Spotted Fever and other spotted fever group rickettsioses, ehrlichioses, and anaplasmosis - United States. MMWR Recomm Rep. 2016;65(2):1–44. doi: 10.15585/mmwr.rr6502a1. [DOI] [PubMed] [Google Scholar]
- Bishopp FC, Trembley HL. Distribution and hosts of certain North American ticks. J Parasitol. 1945;31:1–54. [Google Scholar]
- Brackney MM, Marfin AA, Staples JE, Stallones L, Keefe T, Black WC, Campbell GL. Epidemiology of Colorado tick fever in Montana, Utah, and Wyoming, 1995–2003. Vector Borne Zoonotic Dis. 2010;10(4):381–385. doi: 10.1089/vbz.2009.0065. [DOI] [PubMed] [Google Scholar]
- Brinkerhoff RJ, Folsom-O’Keefe CM, Streby HM, Bent SJ, Tsao K, Diuk-Wasser MA. Regional variation in immature Ixodes scapularis parasitism on North American songbirds: implications for transmission of the Lyme pathogen, Borrelia burgdorferi . J Med Entomol. 2011;48(2):422–428. doi: 10.1603/me10060. [DOI] [PubMed] [Google Scholar]
- Brown HE, Yates KF, Dietrich G, MacMillan K, Graham CB, Reese SM, Helterbrand WS, Nicholson WL, Blount K, Mead PS, et al. An acarologic survey and Amblyomma americanum distribution map with implications for tularemia risk in Missouri. Am J Trop Med Hyg. 2011;84(3):411–419. doi: 10.4269/ajtmh.2011.10-0593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RN, Lane RS, Dennis DT. Geographic distributions of tick-borne diseases and their vectors. In: Goodman JL, Dennis DT, Sonenshine DE, editors. Tick-Borne Diseases of Humans. Washington DC: American Society of Microbiology Press; 2005. pp. 363–391. [Google Scholar]
- Buller RS, Arens M, Hmiel SP, Paddock CD, Sumner JW, Rikhisa Y, Unver A, Gaudreault-Keener M, Manian FA, Liddell AM, et al. Ehrlichia ewingii, a newly recognized agent of human ehrlichiosis. N Engl J Med. 1999;341(3):148–155. doi: 10.1056/NEJM199907153410303. [DOI] [PubMed] [Google Scholar]
- Burgdorfer W. A review of Rocky Mountain spotted fever (tick-borne typhus), its agent, and its tick vectors in the United States. J Med Entomol. 1975;12(3):269–278. doi: 10.1093/jmedent/12.3.269. [DOI] [PubMed] [Google Scholar]
- Burgdorfer W. Tick-borne diseases in the United States: Rocky Mountain spotted fever and Colorado tick fever. A review. Acta Trop. 1977;34(2):103–126. [PubMed] [Google Scholar]
- Burgdorfer W, Barbour AG, Hayes SF, Benach JL, Grunwaldt E, Davis JP. Lyme disease-a tick-borne spirochetosis? Science. 1982;216(4552):1317–1319. doi: 10.1126/science.7043737. [DOI] [PubMed] [Google Scholar]
- Campbell A, MacKay PR. Distribution of the American dog tick, Dermacentor variabilis (Say), and its small-mammal hosts in relation to vegetation types in a study area in Nova Scotia. Can J Zool. 1979;57:1950–1959. doi: 10.1139/z79-258. [DOI] [PubMed] [Google Scholar]
- Castillo CG, Eremeeva ME, Paskewitz SM, Sloan LM, Lee X, Irwin WE, Tonsberg S, Pritt BS. Detection of human pathogenic Ehrlichia muris-like agent in Peromyscus leucopus . Ticks Tick Borne Dis. 2015;6(2):155–157. doi: 10.1016/j.ttbdis.2014.11.006. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Tularemia— United States, 1990–2000. MMWR Morb Mortal Wkly Rep. 2002;51(9):181–184. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Tularemia -United States, 2001–2010. MMWR Morb Mortal Wkly Rep. 2013;62(47):963–966. [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. [accessed on November 12, 2016];Anaplasmosis statistics and epidemiology [Internet] 2016a Available online ( http://www.cdc.gov/anaplasmosis/stats/index.html)
- Centers for Disease Control and Prevention. [accessed on November 12, 2016];Lyme disease data and statistics [Internet] 2016b Available online ( http://www.cdc.gov/lyme/stats/index.html)
- Centers for Disease Control and Prevention. [accessed on November 12, 2016];National Notifiable Diseases Surveillance System (NNDSS) [Internet] 2016c Available online ( http://wwwn.cdc.gov/nndss/default.aspx)
- Centers for Disease Control and Prevention. [accessed on November 12, 2016];Powassan virus statistics and maps [Internet] 2016d Available online ( http://www.cdc.gov/powassan/statistics.html)
- Centers for Disease Control and Prevention. [accessed on November 12, 2016];Tularemia statistics [Internet] 2016e Available online ( http://www.cdc.gov/tularemia/statistics/index.html)
- Childs JE, Paddock CD. The ascendancy of Amblyomma americanum as a vector of pathogens affecting humans in the United States. Annu Rev Entomol. 2003;48:307–337. doi: 10.1146/annurev.ento.48.091801.112728. [DOI] [PubMed] [Google Scholar]
- Connally NP, Durante AJ, Yousey-Hindes KM, Meek JI, Nelson RS, Heimer R. Peridomestic Lyme disease prevention: results of a population-based case-control study. Am J Prev Med. 2009;37(3):201–206. doi: 10.1016/j.amepre.2009.04.026. [DOI] [PubMed] [Google Scholar]
- Cook VJ, Fedorova N, Macdonald WP, Lane RS, Barbour AG. Unique strain of Borrelia miyamotoi in Ixodes pacificus ticks, California, USA. Emerg Infect Dis. 2016;22:2205–2207. doi: 10.3201/eid2212.152046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortinas R, Spomer S. Lone star tick (Acari: Ixodidae) occurrence in Nebraska: Historical and current perspectives. J Med Entomol. 2013;50(2):244–251. doi: 10.1603/me12207. [DOI] [PubMed] [Google Scholar]
- Costa LFD, Nunes PH, Soares JF, Labruna MB, Camargo-Mathias MI. Distribution of Rickettsia rickettsii in ovary cells of Rhipicephalus sanguineus (Latreille 1806) (Acari: Ixodidae) Parasit Vectors. 2011:222. doi: 10.1186/1756-3305-4-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costero A, Grayson MA. Experimental transmission of Powassan virus (Flaviviridae) by Ixodes scapularis ticks (Acari: Ixodidae) Am J Trop Med Hyg. 1996;55(5):536–546. doi: 10.4269/ajtmh.1996.55.536. [DOI] [PubMed] [Google Scholar]
- Dahlgren FS, Heitman KN, Behravesh CB. Undetermined human ehrlichiosis and anaplasmosis in the United States, 2008–2012: A catch-all for passive surveillance. Am J Trop Med Hyg. 2016;94(2):299–301. doi: 10.4269/ajtmh.15-0691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlgren FS, Heitman KN, Drexler NA, Massung RF, Behravesh CB. Human granulocytic anaplasmosis in the United States from 2008 to 2012: a summary of national surveillance data. Am J Trop Med Hyg. 2015;93(1):66–72. doi: 10.4269/ajtmh.15-0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlgren FS, Mandel EJ, Krebs JW, Massung RF, McQuiston JH. Increasing incidence of Ehrlichia chaffeensis and Anaplasma phagocytophilum in the United States, 2000–2007. Am J Trop Med Hyg. 2011;85(1):124–131. doi: 10.4269/ajtmh.2011.10-0613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantas-Torres F. The brown dog tick, Rhipicephalus sanguineus (Latreille, 1806) (Acari: Ixodidae): from taxonomy to control. Vet Parasitol. 2008;152(3–4):173–185. doi: 10.1016/j.vetpar.2007.12.030. [DOI] [PubMed] [Google Scholar]
- Dantas-Torres F. Biology and ecology of the brown dog tick, Rhipicephalus sanguineus . Parasit Vectors. 2010;3:26. doi: 10.1186/1756-3305-3-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantas-Torres F, Otranto D. Further thoughts on the taxonomy and vector role of Rhipicephalus group ticks. Vet Parasitol. 2015;208:9–13. doi: 10.1016/j.vetpar.2014.12.014. [DOI] [PubMed] [Google Scholar]
- Davidson WR, Siefken DA, Creekmore LH. Seasonal and annual abundance of Amblyomma americanum (Acari: Ixodidae) in central Georgia. J Med Entomol. 1994;31(1):67–71. doi: 10.1093/jmedent/31.1.67. [DOI] [PubMed] [Google Scholar]
- Dawson JE, Anderson BE, Fishbein DB, Sanchez JL, Goldsmith CS, Wilson KH, Duntley CW. Isolation and characterization of an Ehrlichia sp. from a patient diagnosed with human ehrlichiosis. J Clin Microbiol. 1991;29(12):2741–2745. doi: 10.1128/jcm.29.12.2741-2745.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demma LJ, Traeger MS, Nicholson WL, Paddock CD, Blau DM, Eremeeva ME, Dasch GA, Levin ML, Singleton J, Jr, Zaki SR, et al. Rocky Mountain spotted fever from an unexpected tick vector in Arizona. N Engl J Med. 2005;353(6):587–594. doi: 10.1056/NEJMoa050043. [DOI] [PubMed] [Google Scholar]
- Dennis DT, Nekomoto TS, Victor JC, Paul WS, Piesman J. Reported distribution of Ixodes scapularis and Ixodes pacificus (Acari: Ixodidae) in the United States. J Med Entomol. 1998;35(5):629–638. doi: 10.1093/jmedent/35.5.629. [DOI] [PubMed] [Google Scholar]
- Diuk-Wasser MA, Hoen AG, Cislo P, Brinkerhoff R, Hamer SA, Rowland M, Cortinas R, Vourc’h G, Melton F, Hickling GJ, et al. Human risk of infection with Borrelia burgdorferi, the Lyme disease agent, in eastern United States. Am J Trop Med Hyg. 2012;86(2):320–327. doi: 10.4269/ajtmh.2012.11-0395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan MC, Hojgaard A, Hoxmeier JC, Replogle AJ, Respicio-Kingry LB, Sexton C, Williams MA, Pritt BS, Schriefer ME, Eisen L. Vector competence of the blacklegged tick, Ixodes scapularis, for the recently recognized Lyme borreliosis spirochete Candidatus Borrelia mayonii . Ticks Tick Borne Dis. 2016;7(5):665–669. doi: 10.1016/j.ttbdis.2016.02.012. [DOI] [PubMed] [Google Scholar]
- Dolan MC, Maupin GO, Panella NA, Golde WT, Piesman J. Vector competence of Ixodes scapularis, I. spinipalpis, and Dermacentor andersoni (Acari:Ixodidae) in transmitting Borrelia burgdorferi, the etiologic agent of Lyme disease. J Med Entomol. 1997;34(2):128–135. doi: 10.1093/jmedent/34.2.128. [DOI] [PubMed] [Google Scholar]
- Dworkin MS, Schwan TG, Anderson DE, Borchardt SM. Tick-borne relapsing fever. Infect Dis Clin N Am. 2008;22:449–468. doi: 10.1016/j.idc.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drexler N, Miller M, Gerding J, Todd S, Adams L, Dahlgren FS, Bryant N, Weis E, Herrick K, Francies J, et al. Community-based control of the brown dog tick in a region with high rates of Rocky Mountain spotted fever, 2012–2013. PLoS One. 2014;9(12):pe112368. doi: 10.1371/journal.pone.0112368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drexler NA, Dahlgren FS, Heitman KN, Massung RF, Paddock CD, Behravesh CB. National Surveillance of Spotted Fever Group Rickettsioses in the United States, 2008–2012. Am J Trop Med Hyg. 2016;94(1):26–34. doi: 10.4269/ajtmh.15-0472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumler JS, Choi KS, Garcia-Garcia JC, Barat NS, Scorpio DG, Garyu JW, Grab DJ, Bakken JS. Human granulocytic anaplasmosis and Anaplasma phagocytophilum . Emerg Infect Dis. 2005;11(12):1828–1834. doi: 10.3201/eid1112.050898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eads RB, Smith GC. Seasonal activity and Colorado tick fever virus infection rates in Rocky Mountain wood ticks, Dermacentor andersoni (acari: Ixodidae), in north-central Colorado, USA. J Med Entomol. 1983;20(1):49–55. doi: 10.1093/jmedent/20.1.49. [DOI] [PubMed] [Google Scholar]
- Easton ER, Keirans JE, Gresbrink RA, Clifford CM. The distribution in Oregon of Ixodes pacificus, Dermacentor andersoni, and Dermacentor occidentalis with a note on Dermacentor variabilis (Acarina: Ixodidae) J Med Entomol. 1977;13(4–5):501–506. doi: 10.1093/jmedent/13.4-5.501. [DOI] [PubMed] [Google Scholar]
- Ebel G. Update on Powassan virus: emergence of a North American tick-borne flavivirus. Ann Rev Entomol. 2010;55:95–110. doi: 10.1146/annurev-ento-112408-085446. [DOI] [PubMed] [Google Scholar]
- Eiden AL, Kaufman PE, Oi FM, Allan SA, Miller RJ. Detection of permethrin resistance and fipronil tolerance in Rhipicephalus sanguineus (Acari: Ixodidae) in the United States. J Med Entomol. 2015;52(3):429–436. doi: 10.1093/jme/tjv005. [DOI] [PubMed] [Google Scholar]
- Eisen L. A call for renewed research on tick-borne Francisella tularensis in the Arkansas-Missouri primary national focus of tularemia in humans. J Med Entomol. 2007a;44(3):389–397. doi: 10.1603/0022-2585(2007)44[389:acfrro]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Eisen L. Seasonal pattern of host-seeking activity by the human-biting adult life stage of Dermacentor andersoni (Acari: Ixodidae) J Med Entomol. 2007b;44(2):359–366. doi: 10.1603/0022-2585(2007)44[359:spohab]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Eisen L. Climate change and tick-borne diseases: a research field in need of long-term empirical field studies. Int J Med Microbiol. 2008;298:12–18. [Google Scholar]
- Eisen L, Dolan MC. Evidence for personal protective measures to reduce human contact with blacklegged ticks and for environmentally based control methods to suppress host-seeking blacklegged ticks and reduce infection with Lyme disease spirochetes in tick vectors and rodent reservoirs. J Med Entomol. 2016 doi: 10.1093/jme/tjw103. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen L, Eisen RJ, Lane RS. The roles of birds, lizards, and rodents as hosts for the western black-legged tick Ixodes pacificus . J Vector Ecol. 2004a;29(2):295–308. [PubMed] [Google Scholar]
- Eisen L, Gray JS. Lyme borreliosis prevention strategies: United States versus Europe. In: Braks MAH, Van Wierer SE, Takken W, Sprong H, editors. Ecology and Prevention of Lyme borreliosis. Wageningen, The Netherlands: Wageningen Academic Publishers; 2016. [Google Scholar]
- Eisen RJ, Eisen L, Beard CB. County-scale distribution of Ixodes scapularis and Ixodes pacificus (Acari: Ixodidae) in the Continental United States. J Med Entomol. 2016a;53(2):349–386. doi: 10.1093/jme/tjv237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen RJ, Eisen L, Castro MB, Lane RS. Environmentally related variability in risk of exposure to Lyme disease spirochetes in Northern California: Effect of climatic conditions on habitat type. Environ Entomol. 2003;32:1010–1018. [Google Scholar]
- Eisen RJ, Eisen L, Ogden NH, Beard CB. Linkages of weather and climate with Ixodes scapularis and Ixodes pacificus (Acari: Ixodidae), enzootic transmission of Borrelia burgdorferi, and Lyme Disease in North America. J Med Entomol. 2016b;53(2):250–261. doi: 10.1093/jme/tjv199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen RJ, Mun J, Eisen L, Lane RS. Life stage-related differences in density of questing ticks and infection with Borrelia burgdorferi sensu lato within a single cohort of Ixodes pacificus (Acari: Ixodidae) J Med Entomol. 2004b;41(4):768–773. doi: 10.1603/0022-2585-41.4.768. [DOI] [PubMed] [Google Scholar]
- Emmons RW. Ecology of Colorado tick fever. Annu Rev Microbiol. 1988;42:49–64. doi: 10.1146/annurev.mi.42.100188.000405. [DOI] [PubMed] [Google Scholar]
- Eremeeva ME. Molecular epidemiology of rickettsial diseases in North America. Ticks Tick Borne Dis. 2012;3(5–6):332–337. doi: 10.1016/j.ttbdis.2012.10.022. [DOI] [PubMed] [Google Scholar]
- Eremeeva ME, Bosserman EA, Demma LJ, Zambrano ML, Blau DM, Dasch GA. Isolation and identification of Rickettsia massiliae from Rhipicephalus sanguineus ticks collected in Arizona. Appl Environ Microbiol. 2006;72(8):5569–5577. doi: 10.1128/AEM.00122-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewing SA, Dawson JE, Kocan AA, Barker RW, Warner CK, Panciera RJ, Fox JC, Kocan KM, Blouin EF. Experimental transmission of Ehrlichia chaffeensis (Rickettsiales: Ehrlichieae) among white-tailed deer by Amblyomma americanum (Acari: Ixodidae) J Med Entomol. 1995;32(3):368–374. doi: 10.1093/jmedent/32.3.368. [DOI] [PubMed] [Google Scholar]
- Foley JE, Nieto NC, Adjemian J, Dabritz H, Brown RN. Anaplasma phagocytophilum infection in small mammal hosts of Ixodes ticks, western United States. Emerg Infect Dis. 2008;14(7):1147–1150. doi: 10.3201/eid1407.071599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrester JD, Brett M, Matthias J, Stanek D, Springs CB, Marsden-Haug N, Oltean H, Baker JS, Kugeler KJ, Mead PS, et al. Epidemiology of Lyme disease in low-incidence states. Ticks Tick Borne Dis. 2015;6(6):721–723. doi: 10.1016/j.ttbdis.2015.06.005. [DOI] [PubMed] [Google Scholar]
- Francis E. Tularemia. JAMA. 1925;84:1243–1250. doi: 10.1001/jama.250.23.3216. [DOI] [PubMed] [Google Scholar]
- Furman DP, Loomis EC. The ticks of California (Acari: Ixodidae) Bull Calif Insect Surv. 1984;25:1–239. [Google Scholar]
- Gage KL, Hopla CE, Schwan TG. Cotton rats and other small mammals as hosts for immature Dermacentor variabilis (Acari: Ixodidae) in central Oklahoma. J Med Entomol. 1992;29(5):832–842. doi: 10.1093/jmedent/29.5.832. [DOI] [PubMed] [Google Scholar]
- Garvie MB, McKiel JA, Sonenshine DE, Campbell A. Seasonal dynamics of American dog tick, Dermacentor variabilis (Say), populations in southwestern Nova Scotia. Can J Zool. 1978;56(1):28–39. doi: 10.1139/z78-004. [DOI] [PubMed] [Google Scholar]
- Geissler AL, Thorp E, Van Houten C, Lanciotti RS, Panella N, Cadwell BL, Murphy T, Staples JE. Infection with Colorado tick fever virus among humans and ticks in a national park and forest, Wyoming, 2010. Vector Borne Zoonotic Dis. 2014;14(9):675–680. doi: 10.1089/vbz.2013.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godsey MS, Jr, Savage HM, Burkhalter KL, Bosco-Lauth AM, Delorey MJ. Transmission of Heartland Virus (Bunyaviridae: Phlebovirus) by experimentally infected Amblyomma americanum (Acari: Ixodidae) J Med Entomol. 2016 doi: 10.1093/jme/tjw080. in press. [DOI] [PubMed] [Google Scholar]
- Hair JA, Howell DE. Lone star ticks: Their biology and control in Ozark recreation areas. Oklahoma Agric Exp Stn Bull. 1970:679. [Google Scholar]
- Hamer SA, Hickling GJ, Sidge JL, Walker ED, Tsao JI. Synchronous phenology of juvenile Ixodes scapularis, vertebrate host relationships, and associated patterns of Borrelia burgdorferi ribotypes in the midwestern United States. Ticks Tick Borne Dis. 2012;3(2):65–74. doi: 10.1016/j.ttbdis.2011.11.004. [DOI] [PubMed] [Google Scholar]
- Hayes EB, Piesman J. How can we prevent Lyme disease? N Engl J Med. 2003;348(24):2424–2430. doi: 10.1056/NEJMra021397. [DOI] [PubMed] [Google Scholar]
- Hazard GW, Ganz RN, Nevin RW, Nauss AH, Curtis E, Bell DW, Murray ES. Rocky Mountain spotted fever in the eastern United States. N Engl J Med. 1969;280(2):57–62. doi: 10.1056/NEJM196901092800201. [DOI] [PubMed] [Google Scholar]
- Heitman KN, Dahlgren FS, Drexler NA, et al. Increasing incidence of ehrlichiosis in the United States: a summary of national surveillance of Ehrlichia chaffeensis and Ehrlichia ewingii infections in the United States, 2008–2012. Am J Trop Med Hyg. 2016;94:52–60. doi: 10.4269/ajtmh.15-0540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrick KL, Pena SA, Yaglom HD, Layton BJ, Moors A, Loftis AD, Condit ME, Singleton J, Kato CY, Denison AM, et al. Rickettsia parkeri rickettsiosis, Arizona, USA. Emerg Infect Dis. 2016;22(5):780–785. doi: 10.3201/eid2205.151824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinckley AF, Connally NP, Meek JI, Johnson BJ, Kemperman MM, Feldman KA, White JL, Mead PS. Lyme disease testing by large commercial laboratories in the United States. Clin Infect Dis. 2014;59(5):676–681. doi: 10.1093/cid/ciu397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinckley AF, Meek JI, Ray JA, Niesobecki SA, Connally NP, Feldman KA, Jones EH, Backenson PB, White JL, Lukacik G, et al. Effectiveness of residential acaricides to prevent Lyme and other tick-borne diseases in humans. J Infect Dis. 2016;214(2):182–188. doi: 10.1093/infdis/jiv775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinten SR, Beckett GA, Gensheimer KF, Pritchard E, Courtney TM, Sears SD, Woytowicz JM, Preston DG, Smith RP, Jr, Rand PW, et al. Increased recognition of Powassan encephalitis in the United States, 1999–2005. Vector Borne Zoonotic Dis. 2008;8(6):733–740. doi: 10.1089/vbz.2008.0022. [DOI] [PubMed] [Google Scholar]
- Hodzic E, Fish D, Maretzki CM, De Silva AM, Feng S, Barthold SW. Acquisition and transmission of the agent of human granulocytic ehrlichiosis by Ixodes scapularis ticks. J Clin Microbiol. 1998;36(12):3574–3578. doi: 10.1128/jcm.36.12.3574-3578.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hojgaard A, Eisen RJ, Piesman J. Transmission dynamics of Borrelia burgdorferi s.s. during the key third day of feeding by nymphal Ixodes scapularis (Acari: Ixodidae) J Med Entomol. 2008;45(4):732–736. doi: 10.1603/0022-2585(2008)45[732:TDOBBS]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Hopla CE. Experimental studies on tick transmission of tularemia organisms. Am J Hyg. 1953;58(1):101–118. doi: 10.1093/oxfordjournals.aje.a119585. [DOI] [PubMed] [Google Scholar]
- Hopla CE. The multiplication of tularemia organisms in the lone star tick. Am J Hyg. 1955;61(3):371–380. doi: 10.1093/oxfordjournals.aje.a119761. [DOI] [PubMed] [Google Scholar]
- Hopla CE. The ecology of tularemia. Adv Vet Sci Comp Med. 1974;18(0):25–53. [PubMed] [Google Scholar]
- James AM, Burdett C, McCool MJ, Fox A, Riggs P. The geographic distribution and ecological preferences of the American dog tick, Dermacentor variabilis (Say), in the U.S.A. Med Vet Entomol. 2015;29(2):178–188. doi: 10.1111/mve.12099. [DOI] [PubMed] [Google Scholar]
- James AM, Freier JE, Keirans JE, Durden LA, Mertins JW, Schlater JL. Distribution, seasonality, and hosts of the Rocky Mountain wood tick in the United States. J Med Entomol. 2006;43(1):17–24. [PubMed] [Google Scholar]
- Jellison WL. Tularemia in North America, 1930–1974. Missoula: University of Montana; 1974. [Google Scholar]
- Jobe DA, Lovrich SD, Oldenburg DG, Kowalski TJ, Callister SM. Borrelia miyamotoi infection in patients from Upper Midwestern United States, 2014–2015. Emerg Infect Dis. 2016;22(8):1471–1473. doi: 10.3201/eid2208.151878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DK, Schiffman EK, Davis JP, Neitzel DF, Sloan LM, Nicholson WL, Fritsche TR, Steward CR, Ray JA, Miller TK, et al. Human infection with Ehrlichia muris-like pathogen, United States, 2007–2013(1) Emerg Infect Dis. 2015;21(10):1794–1799. doi: 10.3201/eid2110.150143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones TF, Craig AS, Paddock CD, McKechnie DB, Childs JE, Zaki SR, Schaffner W. Family cluster of Rocky Mountain spotted fever. Clin Infect Dis. 1999;28(4):853–859. doi: 10.1086/515213. [DOI] [PubMed] [Google Scholar]
- Jongejan F, Uilenberg G. The global importance of ticks. Parasitology. 2004;(129):S3–14. doi: 10.1017/s0031182004005967. [DOI] [PubMed] [Google Scholar]
- Karpathy SE, Allerdice ME, Sheth M, Dasch GA, Levin ML. Co-feeding transmission of the Ehrlichia muris-like agent to mice (Mus musculus) Vector Borne Zoonotic Dis. 2016;16(3):145–150. doi: 10.1089/vbz.2015.1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keesing F, Hersh MH, Tibbetts M, McHenry DJ, Duerr S, Brunner J, Killilea M, LoGiudice K, Schmidt KA, Ostfeld RS. Reservoir competence of vertebrate hosts for Anaplasma phagocytophilum . Emerg Infect Dis. 2012;18(12):2013–2016. doi: 10.3201/eid1812.120919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch HG. Oviposition of the brown dog tick (Acari: Ixodidae) in the laboratory. Ann Entomol Soc Am. 1982;75:583–586. [Google Scholar]
- Koch HG, Tuck MD. Moulting and survival of the brown dog tick (Acari: Ixodidae) under different temperatures and humidities. Ann Entomol Soc Am. 1986;79:11–14. [Google Scholar]
- Kollars TM, Jr, Oliver JH, Jr, Durden LA, Kollars PG. Host association and seasonal activity of Amblyomma americanum (Acari: Ixodidae) in Missouri. J Parasitol. 2000;86(5):1156–1159. doi: 10.1645/0022-3395(2000)086[1156:HAASAO]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Krause PJ, Fish D, Narasimhan S, Barbour AG. Borrelia miyamotoi infection in nature and in humans. Clin Microbiol Infect. 2015;21:631–639. doi: 10.1016/j.cmi.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause PJ, Narasimhan S, Wormser GP, Barbour AG, Platonov AE, Brancato J, Lepore T, Dardick K, Mamula M, Rollend L, et al. Borrelia miyamotoi sensu lato seroreactivity and seroprevalence in the northeastern United States. Emerg Infect Dis. 2014;20(7):1183–1190. doi: 10.3201/eid2007.131587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kugeler KJ, Farley GM, Forrester JD, Mead PS. Geographic distribution and expansion of human Lyme disease, United States. Emerg Infect Dis. 2015;21(8):1455–1457. doi: 10.3201/eid2108.141878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kugeler KJ, Jordan RA, Schulze TL, Griffith KS, Mead PS. Will culling white-tailed deer prevent Lyme disease? Zoonoses Public Health. 2016;63(5):337–345. doi: 10.1111/zph.12245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labruna MB, Ogrzewalska M, Martins TF, Pinter A, Horta MC. Comparative susceptibility of larval stages of Amblyomma aureolatum, Amblyomma cajennense, and Rhipicephalus sanguineus to infection by Rickettsia rickettsii . J Med Entomol. 2008;45(6):1156–1159. doi: 10.1603/0022-2585(2008)45[1156:csolso]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Lackman DB, Bell EJ, Stoenner HG, Pickens EG. The Rocky Mountain spotted fever group of Rickettsias. Health Lab Sci. 1965;2:135–141. [PubMed] [Google Scholar]
- Lackman DB, Parker RR, Gerloff RK. Serological characteristics of a pathogenic rickettsia occurring in Amblyomma maculatum . Public Health Rep. 1949;64(43):1342–1349. [PubMed] [Google Scholar]
- Lane RS, Brown RN, Piesman J, Peavey CA. Vector competence of Ixodes pacificus and Dermacentor occidentalis (Acari: Ixodidae) for various isolates of Lyme disease spirochetes. J Med Entomol. 1994;31:417–424. doi: 10.1093/jmedent/31.3.417. [DOI] [PubMed] [Google Scholar]
- Lane RS, Emmons RW, Dondero DV, Nelson BC. Ecology of tick-borne agents in California. I. Spotted fever group rickettsiae. Am J Trop Med Hyg. 1981a;30(1):239–252. doi: 10.4269/ajtmh.1981.30.239. [DOI] [PubMed] [Google Scholar]
- Lane RS, Fedorova N, Kleinjan JE, Maxwell M. Eco-epidemiological factors contributing to the low risk of human exposure to ixodid tick-borne borreliae in southern California, USA. Ticks Tick Borne Dis. 2013;4(5):377–385. doi: 10.1016/j.ttbdis.2013.02.005. [DOI] [PubMed] [Google Scholar]
- Lane RS, Mun J, Eisen RJ, Eisen L. Western gray squirrel (Rodentia: Sciuridae): a primary reservoir host of Borrelia burgdorferi in Californian oak woodlands? J Med Entomol. 2005;42(3):388–396. doi: 10.1093/jmedent/42.3.388. [DOI] [PubMed] [Google Scholar]
- Lane RS, Mun J, Peribanez MA, Stubbs HA. Host-seeking behavior of Ixodes pacificus (Acari: Ixodidae) nymphs in relation to environmental parameters in dense-woodland and woodland-grass habitats. J Vector Ecol. 2007;32(2):342–357. doi: 10.3376/1081-1710(2007)32[342:hboipa]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Lane RS, Phillip RN, Casper EA. II. Further observations on rickettsiae. In: Burgdorfer W, Anacker RL, editors. Rickettsiae and Rickettsial Diseases. NY: Academic Press; 1981b. pp. 575–583. [Google Scholar]
- Lane RS, Piesman J, Burgdorfer W. Lyme borreliosis: relation of its causative agent to its vectors and hosts in North America and Europe. Annu Rev Entomol. 1991;36:587–609. doi: 10.1146/annurev.en.36.010191.003103. [DOI] [PubMed] [Google Scholar]
- Lane RS, Quistad GB. Borreliacidal factor in the blood of the western fence lizard (Sceloporus occidentalis) J Parasitol. 1998;84(1):29–34. [PubMed] [Google Scholar]
- Lange JV, Walker DH, Wester TB. Documented Rocky Mountain spotted fever in wintertime. JAMA. 1982;247(17):2403–2404. [PubMed] [Google Scholar]
- Lantos PM, Branda JA, Boggan JC, Chudgar SM, Wilson EA, Ruffin F, Fowler V, Auwaerter PG, Nigrovic LE. Poor positive predictive value of Lyme disease serologic testing in an area of low disease incidence. Clin Infect Dis. 2015;61(9):1374–1380. doi: 10.1093/cid/civ584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee X, Hardy K, Johnson DH, Paskewitz SM. Hunter-killed deer surveillance to assess changes in the prevalence and distribution of Ixodes scapularis (Acari: Ixodidae) in Wisconsin. J Med Entomol. 2013;50(3):632–639. doi: 10.1603/me12234. [DOI] [PubMed] [Google Scholar]
- Lockhart JM, Davidson WR, Stallknecht DE, Dawson JE, Howerth EW. Isolation of Ehrlichia chaffeensis from wild white-tailed deer (Odocoileus virginianus) confirms their role as natural reservoir hosts. J Clin Microbiol. 1997a;35(7):1681–1686. doi: 10.1128/jcm.35.7.1681-1686.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockhart JM, Davidson WR, Stallknecht DE, Dawson JE, Little SE. Natural history of Ehrlichia chaffeensis (Rickettsials: Ehrlichieae) in the piedmont physiographic province of Georgia. J Parasitol. 1997b;83(5):887–894. [PubMed] [Google Scholar]
- LoGiudice K, Ostfeld RS, Schmidt KA, Keesing F. The ecology of infectious disease: Effects of host diversity and community composition on Lyme disease risk. Proc Natl Acad Sci USA. 2003;100(2):567–571. doi: 10.1073/pnas.0233733100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald AJ, Briggs CJ. Truncated seasonal activity patterns of the western blacklegged tick (Ixodes pacificus) in central and southern California. Ticks Tick Borne Dis. 2016;7(1):234–242. doi: 10.1016/j.ttbdis.2015.10.016. [DOI] [PubMed] [Google Scholar]
- Maeda K, Markowitz N, Hawley RC, Ristic M, Cox D, McDade JE. Human infection with Ehrlichia canis, a leukocytic rickettsia. N Engl J Med. 1987;316(14):853–856. doi: 10.1056/NEJM198704023161406. [DOI] [PubMed] [Google Scholar]
- Maegli A, Loy JD, Cortinas R. Note on Ehrlichia chaffeensis, Ehrlichia ewingii, and “Borrelia lonestari” infection in lone star ticks (Acari: Ixodidae), Nebraska, USA. Ticks Tick Borne Dis. 2016;7(1):154–158. doi: 10.1016/j.ttbdis.2015.10.008. [DOI] [PubMed] [Google Scholar]
- Marfin AA, Campbell GL. Colorado tick fever and related Coltivirus infections. In: Goodman JL, Dennis DT, Sonenshine DE, editors. Tick-Borne Diseases of Humans. Washington, D. C: American Society of Microbiology Press; 2005. pp. 143–149. [Google Scholar]
- Masters E, Granter S, Duray P, Cordes P. Physician-diagnosed erythema migrans and erythema migrans-like rashes following Lone Star tick bites. Arch Dermatol. 1998;134(8):955–960. doi: 10.1001/archderm.134.8.955. [DOI] [PubMed] [Google Scholar]
- Mather TN, Mather ME. Intrinsic competence of three ixodid ticks (Acari) as vectors of the Lyme disease spirochete. J Med Entomol. 1990;27(4):646–650. doi: 10.1093/jmedent/27.4.646. [DOI] [PubMed] [Google Scholar]
- Mather TN, Wilson ML, Moore SI, Ribeiro JM, Spielman A. Comparing the relative potential of rodents as reservoirs of the Lyme disease spirochete (Borrelia burgdorferi) Am J Epidemiol. 1989;130(1):143–150. doi: 10.1093/oxfordjournals.aje.a115306. [DOI] [PubMed] [Google Scholar]
- McCoy G. A plague-like disease in rodents. Public Health Bulletin. 1911;43:53–71. [Google Scholar]
- McMullan LK, Folk SM, Kelly AJ, MacNeil A, Goldsmith CS, Metcalfe MG, Batten BC, Albarino CG, Zaki SR, Rollin PE, et al. A new phlebovirus associated with severe febrile illness in Missouri. N Engl J Med. 2012;367(9):834–841. doi: 10.1056/NEJMoa1203378. [DOI] [PubMed] [Google Scholar]
- Merten HA, Durden LA. A state-by-state survey of ticks recorded from humans in the United States. J Vector Ecol. 2000;25(1):102–113. [PubMed] [Google Scholar]
- Molloy PJ, Telford SR, 3rd, Chowdri HR, Lepore TJ, Gugliotta JL, Weeks KE, Hewins ME, Goethert HK, Berardi VP. Borrelia miyamotoi disease in the Northeastern United States: A case series. Ann Intern Med. 2015;163(2):91–98. doi: 10.7326/M15-0333. [DOI] [PubMed] [Google Scholar]
- Mun J, Eisen RJ, Eisen L, Lane RS. Detection of a Borrelia miyamotoi sensu lato relapsing-fever group spirochete from Ixodes pacificus in California. J Med Entomol. 2006;43(1):120–123. doi: 10.1093/jmedent/43.1.120. [DOI] [PubMed] [Google Scholar]
- Needham GR, Teel PD. Off-host physiological ecology of ixodid ticks. Annu Rev Entomol. 1991;36:659–681. doi: 10.1146/annurev.en.36.010191.003303. [DOI] [PubMed] [Google Scholar]
- Nelson CA, Hayes CM, Markowitz MA, Flynn JJ, Graham AC, Delorey MJ, Mead PS, Dolan MC. The heat is on: killing blacklegged ticks in residential washers and dryers to prevent tickborne diseases. Ticks Tick Borne Dis. 2016;7(5):958–963. doi: 10.1016/j.ttbdis.2016.04.016. [DOI] [PubMed] [Google Scholar]
- Nicholson WL, Gordon R, Demma LJ. Spotted fever group rickettsial infection in dogs from eastern Arizona: how long has it been there? Ann N Y Acad Sci. 2006a;1078:519–522. doi: 10.1196/annals.1374.102. [DOI] [PubMed] [Google Scholar]
- Nicholson WL, Paddock CD, Demma L, Traeger M, Johnson B, Dickson J, McQuiston J, Swerdlow D. Rocky Mountain spotted fever in Arizona: documentation of heavy environmental infestations of Rhipicephalus sanguineus at an endemic site. Ann N Y Acad Sci. 2006b;1078:338–341. doi: 10.1196/annals.1374.065. [DOI] [PubMed] [Google Scholar]
- Niebylski ML, Peacock MG, Schwan TG. Lethal effect of Rickettsia rickettsii on its tick vector (Dermacentor andersoni) Appl Environ Microbiol. 1999;65(2):773–778. doi: 10.1128/aem.65.2.773-778.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieto NC, Foley JE. Reservoir competence of the redwood chipmunk (Tamias ochrogenys) for Anaplasma phagocytophilum . Vector Borne Zoonotic Dis. 2009;9(6):573–577. doi: 10.1089/vbz.2008.0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostfeld RS, Brunner JL. Climate change and Ixodes tick-borne diseases of humans. Philos Trans R Soc Lond B Biol Sci. 2015;370(1665) doi: 10.1098/rstb.2014.0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paddock CD, Finley RW, Wright CS, Robinson HN, Schrodt BJ, Lane CC, Ekenna O, Blass MA, Tamminga CL, Ohl CA, et al. Rickettsia parkeri rickettsiosis and its clinical distinction from Rocky Mountain spotted fever. Clin Infect Dis. 2008;47(9):1188–1196. doi: 10.1086/592254. [DOI] [PubMed] [Google Scholar]
- Paddock CD, Goddard J. The evolving medical and veterinary importance of the Gulf Coast tick (Acari: Ixodidae) J Med Entomol. 2015;52(2):230–252. doi: 10.1093/jme/tju022. [DOI] [PubMed] [Google Scholar]
- Paddock CD, Lane RS, Staples JE, Labruna MB. Changing paradigms for tick-borne diseases in the Americas. In: Mack A, editor. Global health impacts of vector-borne diseases: workshop summary. National Academies Press; 2016. pp. 221–257. [PubMed] [Google Scholar]
- Paddock CD, Sumner JW, Comer JA, Zaki SR, Goldsmith CS, Goddard J, McLellan SL, Tamminga CL, Ohl CA. Rickettsia parkeri: a newly recognized cause of spotted fever rickettsiosis in the United States. Clin Infect Dis. 2004;38(6):805–811. doi: 10.1086/381894. [DOI] [PubMed] [Google Scholar]
- Paddock CD, Yabsley MJ. Ecological havoc, the rise of white-tailed deer, and the emergence of Amblyomma americanum-associated zoonoses in the United States. Curr Top Microbiol Immunol. 2007;315:289–324. doi: 10.1007/978-3-540-70962-6_12. [DOI] [PubMed] [Google Scholar]
- Padgett K, Bonilla D, Kjemtrup A, Vilcins IM, Yoshimizu MH, Hui L, Sola M, Quintana M, Kramer V. Large scale spatial risk and comparative prevalence of Borrelia miyamotoi and Borrelia burgdorferi sensu lato in Ixodes pacificus . PLoS One. 2014;9(10):e110853. doi: 10.1371/journal.pone.0110853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padgett KA, Bonilla D, Eremeeva ME, Glaser C, Lane RS, Porse CC, Castro MB, Messenger S, Espinosa A, Hacker J, et al. The eco-epidemiology of Pacific Coast Tick Fever in California. PLoS Negl Trop Dis. 2016;10(10):e0005020. doi: 10.1371/journal.pntd.0005020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padgett KA, Lane RS. Life cycle of Ixodes pacificus (Acari: Ixodidae): timing of developmental processes under field and laboratory conditions. J Med Entomol. 2001;38(5):684–693. doi: 10.1603/0022-2585-38.5.684. [DOI] [PubMed] [Google Scholar]
- Parker RR. Potentialities of tick transmission in relation to geographical occurrence in the United States. Am J Trop Med Hyg. 1933;13:341–379. [Google Scholar]
- Parker RR, Steinhaus EA, Kohls GM, Jellison WL. Contamination of natural waters and mud with Pasteurella tularensis and tularemia in beavers and muskrats in the northwestern United States. Bull Natl Inst Health. 1951;193:1–161. [PubMed] [Google Scholar]
- Petersen JM, Mead PS, Schriefer ME. Francisella tularensis: an arthropod-borne pathogen. Vet Res. 2009;40(2):7. doi: 10.1051/vetres:2008045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piesman J, Eisen L. Prevention of tick-borne diseases. Ann Rev Entomol. 2008:323–343. doi: 10.1146/annurev.ento.53.103106.093429. [DOI] [PubMed] [Google Scholar]
- Piesman J, Hicks TC, Sinsky RJ, Obiri G. Simultaneous transmission of Borrelia burgdorferi and Babesia microti by individual nymphal Ixodes dammini ticks. J Clin Microbiol. 1987;25(10):2012–2013. doi: 10.1128/jcm.25.10.2012-2013.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piesman J, Spielman A. Human babesiosis on Nantucket Island: prevalence of Babesia microti in ticks. Am J Trop Med Hyg. 1980;29(5):742–746. doi: 10.4269/ajtmh.1980.29.742. [DOI] [PubMed] [Google Scholar]
- Piesman J, Spielman A, Etkind P, Ruebush TK2nd, Juranek DD. Role of deer in the epizootiology of Babesia microti in Massachusetts, USA. J Med Entomol. 1979;15(5–6):537–540. doi: 10.1093/jmedent/15.5-6.537. [DOI] [PubMed] [Google Scholar]
- Piranda EM, Faccini JL, Pinter A, Pacheco RC, Cancado PH, Labruna MB. Experimental infection of Rhipicephalus sanguineus ticks with the bacterium Rickettsia rickettsii, using experimentally infected dogs. Vector Borne Zoonotic Dis. 2011;11(1):29–36. doi: 10.1089/vbz.2009.0250. [DOI] [PubMed] [Google Scholar]
- Pritt BS, Allerdice MEJ, Sloan LM, et al. Proposal to rename Ehrlichia muris as Ehrlichia muris subsp. muris subsp. nov. and description of Ehrlichia muris subsp. eauclairensis subsp. nov., a newly recognized tick-borne pathogen of humans in the upper midwestern United States. Int J Sys Evol Bacteriol. 2016a in press. [Google Scholar]
- Pritt BS, Mead PS, Johnson DK, Neitzel DF, Respicio-Kingry LB, Davis JP, Schiffman E, Sloan LM, Schriefer ME, Replogle AJ, et al. Identification of a novel pathogenic Borrelia species causing Lyme borreliosis with unusually high spirochaetaemia: a descriptive study. Lancet Infect Dis. 2016b doi: 10.1016/S1473-3099(15)00464-8. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritt BS, Respicio-Kingry LB, Sloan LM, Schriefer ME, Replogle AJ, Bjork J, Liu G, Kingry LC, Mead PS, Neitzel DF, et al. Borrelia mayonii sp. nov., a member of the Borrelia burgdorferi sensu lato complex, detected in patients and ticks in the upper midwestern United States. Int J Syst Evol Microbiol. 2016c doi: 10.1099/ijsem.0.001445. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritt BS, Sloan LM, Johnson DK, Munderloh UG, Paskewitz SM, McElroy KM, McFadden JD, Binnicker MJ, Neitzel DF, Liu G, et al. Emergence of a new pathogenic Ehrlichia species, Wisconsin and Minnesota, 2009. N Engl J Med. 2011;365(5):422–429. doi: 10.1056/NEJMoa1010493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reese SM, Petersen JM, Sheldon SW, Dolan MC, Dietrich G, Piesman J, Eisen RJ. Transmission efficiency of Francisella tularensis by adult american dog ticks (Acari: Ixodidae) J Med Entomol. 2011;48(4):884–890. doi: 10.1603/me11005. [DOI] [PubMed] [Google Scholar]
- Rollend L, Fish D, Childs JE. Transovarial transmission of Borrelia spirochetes by Ixodes scapularis: a summary of the literature and recent observations. Ticks Tick Borne Dis. 2013;4(1–2):46–51. doi: 10.1016/j.ttbdis.2012.06.008. [DOI] [PubMed] [Google Scholar]
- Salkeld DJ, Lane RS. Community ecology and disease risk: lizards, squirrels, and the Lyme disease spirochete in California, USA. Ecology. 2010;91(1):293–298. doi: 10.1890/08-2106.1. [DOI] [PubMed] [Google Scholar]
- Savage HM, Godsey MS, Jr, Lambert A, Panella NA, Burkhalter KL, Harmon JR, Lash RR, Ashley DC, Nicholson WL. First detection of heartland virus (Bunyaviridae: Phlebovirus) from field collected arthropods. Am J Trop Med Hyg. 2013;89(3):445–452. doi: 10.4269/ajtmh.13-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage HM, Godsey MS, Jr, Panella NA, Burkhalter KL, Ashley DC, Lash RR, Ramsay B, Patterson T, Nicholson WL. Surveillance for Heartland Virus (Bunyaviridae: Phlebovirus) in Missouri during 2013: First detection of virus in adults of Amblyomma americanum (Acari: Ixodidae) J Med Entomol. 2016 doi: 10.1093/jme/tjw028. [DOI] [PubMed] [Google Scholar]
- Schulze TL, Bowen GS, Lakat MF, Parkin WE, Shisler JK. Seasonal abundance and hosts of Ixodes dammini (Acari: Ixodidae) and other ixodid ticks from an endemic Lyme disease focus in New Jersey, USA. J Med Entomol. 1986;23(1):105–109. doi: 10.1093/jmedent/23.1.105. [DOI] [PubMed] [Google Scholar]
- Scoles GA, Papero M, Beati L, Fish D. A relapsing fever group spirochete transmitted by Ixodes scapularis ticks. Vector Borne Zoonotic Dis. 2001;1(1):21–34. doi: 10.1089/153036601750137624. [DOI] [PubMed] [Google Scholar]
- Shapiro MR, Fritz CL, Tait K, Paddock CD, Nicholson WL, Abramowicz KF, Karpathy SE, Dasch GA, Sumner JW, Adem PV, et al. Rickettsia 364D: a newly recognized cause of eschar-associated illness in California. Clin Infect Dis. 2010;50(4):541–548. doi: 10.1086/649926. [DOI] [PubMed] [Google Scholar]
- Smart DL, Caccamise DF. Population dynamics of the American dog tick (Acari: Ixodidae) in relation to small mammal hosts. J Med Entomol. 1988;25(6):515–522. doi: 10.1093/jmedent/25.6.515. [DOI] [PubMed] [Google Scholar]
- Smith T, Kilborne FL. Investigations into the nature, causation, and prevention of southern cattle fever. US Dept Agric Bur Anim Ind Bull. 1893;1:301. [Google Scholar]
- Sonenshine DE. Chapter 23. Ecology of non-nidicolous ticks. Biology of Ticks. 1993:3–65. [Google Scholar]
- Sonenshine DE, Stout IJ. Use of old-field habitats by the American dog tick, Dermacentor variabilis . Ann Entomol Soc Am. 1968;61(3):679–686. doi: 10.1093/aesa/61.3.679. [DOI] [PubMed] [Google Scholar]
- Sonenshine DE, Yunker CE, Clifford CM, Clark GM, Rudbach JA. Contributions to the ecology of Colorado tick fever virus. 2. Population dynamics and host utilization of immature stages of the Rocky Mountain wood tick, Dermacentor andersoni. J Med Entomol. 1976;12(6):651–656. doi: 10.1093/jmedent/12.6.651. [DOI] [PubMed] [Google Scholar]
- Spielman A. The emergence of Lyme disease and human babesiosis in a changing environment. Ann N Y Acad Sci. 1994;740:146–156. doi: 10.1111/j.1749-6632.1994.tb19865.x. [DOI] [PubMed] [Google Scholar]
- Spielman A, Wilson ML, Levine JF, Piesman J. Ecology of Ixodes dammini-borne human babesiosis and Lyme disease. Annu Rev Entomol. 1985;30:439–460. doi: 10.1146/annurev.en.30.010185.002255. [DOI] [PubMed] [Google Scholar]
- Springer YP, Eisen L, Beati L, James AM, Eisen RJ. Spatial distribution of counties in the continental United States with records of occurrence of Amblyomma americanum (Ixodida: Ixodidae) J Med Entomol. 2014;51(2):342–351. doi: 10.1603/me13115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spruance SL, Bailey A. Colorado Tick Fever. A review of 115 laboratory confirmed cases. Arch Intern Med. 1973;131(2):288–293. [PubMed] [Google Scholar]
- Stromdahl EY, Hickling GJ. Beyond Lyme: aetiology of tick-borne human diseases with emphasis on the southeastern United States. Zoonoses Public Health. 2012;59(Suppl 2):48–64. doi: 10.1111/j.1863-2378.2012.01475.x. [DOI] [PubMed] [Google Scholar]
- Stromdahl EY, Jiang J, Vince M, Richards AL. Infrequency of Rickettsia rickettsii in Dermacentor variabilis removed from humans, with comments on the role of other human-biting ticks associated with spotted fever group Rickettsiae in the United States. Vector Borne Zoonotic Dis. 2011;11(7):969–977. doi: 10.1089/vbz.2010.0099. [DOI] [PubMed] [Google Scholar]
- Sumner JW, Durden LA, Goddard J, Stromdahl EY, Clark KL, Reeves WK, Paddock CD. Gulf Coast ticks (Amblyomma maculatum) and Rickettsia parkeri, United States. Emerg Infect Dis. 2007;13(5):751–753. doi: 10.3201/eid1305.061468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teel PD, Ketchum HR, Mock DE, Wright RE, Strey OF. The Gulf Coast tick: a review of the life history, ecology, distribution, and emergence as an arthropod of medical and veterinary importance. J Med Entomol. 2010;47(5):707–722. doi: 10.1603/me10029. [DOI] [PubMed] [Google Scholar]
- Teglas MB, Foley E. Differences in the transmissibility of two Anaplasma phagocytophilum strains by the North American ticks vector species, Ixodes pacificus and Ixodes scapularis (Acari: Ixodidae). Exp. Appl. Acarol. 2006;38:47–58. doi: 10.1007/s10493-005-5293-5. [DOI] [PubMed] [Google Scholar]
- Telford SR3rd, Dawson JE, Katavolos P, Warner CK, Kolbert CP, Persing DH. Perpetuation of the agent of human granulocytic ehrlichiosis in a deer tick-rodent cycle. Proc Natl Acad Sci U S A. 1996;93(12):6209–6214. doi: 10.1073/pnas.93.12.6209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telford SR3rd, Spielman A. Reservoir competence of white-footed mice for Babesia microti . J Med Entomol. 1993;30(1):223–227. doi: 10.1093/jmedent/30.1.223. [DOI] [PubMed] [Google Scholar]
- Trout RT, Steelman CD. Ticks (Acari: Ixodidae) parasitizing canines and deer in Arkansas. J Entomol. Sci. 2010;45:140–149. [Google Scholar]
- Vannier EG, Diuk-Wasser MA, Ben Mamoun C, Krause PJ. Babesiosis. Infect Dis Clin North Am. 2015;29(2):357–370. doi: 10.1016/j.idc.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker JB, Keirans JE, Horak IG. The genus Rhipicephalus (Acari: Ixodidae): A guide to brown ticks of the world. Cambridge University Press; 2000. [Google Scholar]
- WHO. WHO Guidelines on Tularaemia. World Health Organization; 2007. p. 115. [Google Scholar]
- Wormser GP, Masters E, Nowakowski J, McKenna D, Holmgren D, Ma K, Ihde L, Cavaliere LF, Nadelman RB. Prospective clinical evaluation of patients from Missouri and New York with erythema migrans-like skin lesions. Clin Infect Dis. 2005;41(7):958–965. doi: 10.1086/432935. [DOI] [PubMed] [Google Scholar]
- Yabsley MJ, Varela AS, Tate CM, Dugan VG, Stallknecht DE, Little SE, Davidson WR. Ehrlichia ewingii infection in white-tailed deer (Odocoileus virginianus) Emerg Infect Dis. 2002;8(7):668–671. doi: 10.3201/eid0807.020018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yendell SJ, Fischer M, Staples JE. Colorado tick fever in the United States, 2002–2012. Vector Borne Zoonotic Dis. 2015;15(5):311–316. doi: 10.1089/vbz.2014.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuval B, Spielman A. Duration and regulation of the developmental cycle of Ixodes dammini (Acari: Ixodidae) J Med Entomol. 1990;27(2):196–201. doi: 10.1093/jmedent/27.2.196. [DOI] [PubMed] [Google Scholar]