Abstract
Background
Outcomes for children with acute myeloid leukemia (AML) have improved over the past 20 years even though the medication used for induction therapy have not changed.
Methods
We analyzed data from patients with AML enrolled on successive protocols (AML97 and AML02) to determine the contributors to the improved outcome of the latter clinical trial.
Results
There was a significant improvement in 5-year overall survival (48.9% vs 71.2%, P <0.0001) and event-free survival (43.5% vs 61.8%, P = .002) from AML97 to AML02. The 5-year cumulative incidence of early death/treatment related mortality was reduced for patients treated on AML02 (18.5% vs 7.9%, P = 0.007). While the overall incidence of refractory disease (6.5% vs 5.6%, P = 0.736) or relapse (29.3% vs 21.0%, P = 0.12) was not different between the two studies, patients with low-risk AML treated on AML02 had reduced incidence of relapse (27.3% vs 8.8%, P = .036).
Conclusions
Improved outcome on the AML02 trial resulted from improved disease control for low-risk patients and overall decreased early death/treatment-related mortality. These results emphasize the importance of supportive care measures throughout chemotherapy courses and HCT, and the value of treatment intensity for patients with low-risk AML, while underscoring the need for novel therapy, rather than increased therapy intensity, for children with high-risk AML.
Keywords: leukemia, pediatric, supportive care, outcomes
Introduction
Survival rates for children with acute myeloid leukemia (AML) have improved significantly over the past 40 years, reaching 70% on recent clinical trials.1 Almost all cooperative groups have reported improvements in event-free survival (EFS) and overall survival (OS) rates across consecutive trials.1 However, because most randomized trials have failed to demonstrate significant differences between treatment arms within each trial, it is difficult to ascertain which components of each trial contribute to the improved survival. As a result of this uncertainty, the improvements in outcome for children with AML have been variously attributed to refinement of supportive care, adaptation of therapy based on each patient's response to early therapy, intensification of chemotherapy, introduction of new agents, the selective use of hematopoietic cell transplantation (HCT), and improved salvage therapy. We previously reported EFS and OS rates of 44% and 50% for those treated on our AML97 trial compared to 63% and 71% for children treated on the St. Jude AML02 trial.2, 3 In the present report, we examine relapse rates, treatment-related mortality, and salvage rates after relapse to determine the contribution of each factor to the overall improvement in outcome.
Patients and Methods
Patients and therapy
From March 1997 to June 2002, 104 patients less than 22 years old with newly diagnosed AML, mixed-phenotype acute leukemia, or myelodysplastic syndrome were enrolled in the St. Jude AML97 trial.2 From October 2002 to June 2008, 232 such patients were enrolled in the AML02 trial.3 For the purpose of this retrospective analysis, we excluded patients with mixed phenotype acute leukemia or Down syndrome. After these exclusions, we analyzed 92 patients treated on AML97 and 216 on AML02. Both treatment protocols were approved by the institutional review boards, with signed informed consent obtained from the parents or guardians, and assent obtained from the patients, as appropriate.
AML97 was a single-institution trial that included an initial course of cladribine plus cytarabine given prior to standard therapy.2 In this trial, patients were randomly assigned to receive either short daily infusions of cytarabine (500 mg/m2/day on days 1-5) or a five-day continuous infusion of cytarabine (500 mg/m2/day on days 1-5) during the first course of therapy. Patients in both arms also received cladribine (9 mg/m2/day on days 2-6). The second and third courses were standard inductions courses consisting of daunorubicin (30 mg/m2/day by continuous infusion on days 1–3), cytarabine (250 mg/m2/day by continuous infusion on days 1–5) and etoposide (200 mg/m2/day by continuous infusion on days 4 and 5). Standard-risk patients with matched-sibling donors and all high-risk patients were eligible for allogeneic HCT, whereas all other patients received autologous HCT or consolidation chemotherapy. Within the AML97 HCT cohort, eight patients were treated with high dose chemotherapy with autologous stem cell rescue. These patients were conditioned with busulfan (1 mg/kg orally every 6 h for 16 doses on days 9, 8, 7 and 6) and cyclophosphamide (50 mg/kg per dose on days 5, 4, 3 and 2). Consolidation chemotherapy started with a course of cytarabine (3 g/m2/dose every 12 hours on days 1, 2, 8 and 9) and L-asparaginase (6000 units/m2/dose after the fourth and eighth doses of cytarabine), followed by a second course consisting of mitoxantrone (10 mg/m2/dose on days 1–5) and cytarabine (1 g/m2/dose every 12 hours on days 1–3).
The multi-institutional AML02 study, featuring a randomized comparison of high-dose versus low-dose cytarabine-based induction therapy, was the first study to use minimal residual disease (MRD) levels to guide therapy.3 In AML02, patients were stratified by cytogenetic or morphologic subtype and randomized to receive either high-dose cytarabine (3 g/m2 every 12 hours on day 1, 3, and 5) or low-dose cytarabine (100 mg/m2/dose every 12 hours on days 1–10) in combination with daunorubicin (50 mg/m2/dose on days 2, 4, and 6) and etoposide (100 mg/m2/dose on days 2–6) plus during the first course of induction. For the second course of induction, all patients received daunorubicin (50 mg/m2/dose on days 1, 3, and 5), etoposide (100 mg/m2/dose on days 1-5) and cytarabine (100 mg/m2/dose every 12 hours on days 1-8) with or without gemtuzumab ozogamicin (3 mg/m2 on day 1) based upon response to first course of induction. Standard-risk patients with matched sibling donors and high-risk patients were eligible for allogeneic HCT, whereas all other patients received three courses of consolidation chemotherapy. Consolidation one consisted of cytarabine (500 mg/m2/day by continuous infusion for 5 days) and cladribine (9 mg/m2/dose on days 1–5) for patients with t(9;11) and inv(16); cytarabine (3 g/m2/dose every 12 hours on days 1–3) and etoposide (125 mg/m2/dose on days 2–5) for patients with M4 or M5 AML without t(9;11) or inv(16); and cytarabine (3 g/m2/dose every 12 hours on days 1–3) and mitoxantrone (10 mg/m2/dose on days 3–4) for all other patients. Consolidation two consisted of cytarabine (3 g/m2/dose every 12 hours on days 1, 2, 8, 9) and L-asparaginase (6000 Units/m2/dose 3 hours after fourth and eighth doses of cytarabine). Consolidation three included mitoxantrone (10 mg/m2/dose on days 1–3) and cytarabine (1 g/m2/dose every 12 hours on days 1–3). Further details of risk designations and therapy decisions in each protocol have been previously published.2, 3
Definitions
Complete remission (CR) was defined as trilineage hematopoietic recovery with less than 5% blasts in the marrow, platelet count greater than 30 × 109 per liter and absolute neutrophil count (ANC) greater than 0.3 × 109 per liter. Early death (ED) is defined as death in induction prior to CR. Treatment related mortality (TRM) is defined as any death in first CR. Refractory disease represents cases that failed to achieve CR after two courses of induction therapy, and relapse denotes disease recurrence after initial CR. Other events were study withdrawal or secondary malignancy. Risk categorization was defined similarly in each trial. Low risk patients had presence of t(8;21)/AML1-ETO, inv(16)/CBFB-MYH11, or t(9;11)/MLL-AF9. High risk patients had presence of one of the following: monosomy 7, t(6;9), FAB M6 or M7 morphology, treatment-related AML, AML arising from prior MDS, FLT3 ITD, or RAEB-T. Standard-risk patients had absence of low-risk or high-risk features; or the presence of both a high-risk and a low-risk feature.
Statistical Analysis
Overall survival was defined as the time elapsed from study enrollment to death with surviving patients censored at last follow-up. Event-free survival was defined as the time from study enrollment to refractory disease, relapse, death, withdrawal, or secondary malignancy, with those living and event-free at the last follow-up considered censored. Throughout this paper, we present the five-year EFS and OS estimates and associated statistical comparisons. EFS and OS were estimated by the method of Kaplan and Meier4, with standard errors and confidence intervals calculated by the method of Link using the log-survival function.5 The cumulative incidence of failure due to specific causes (early death, treatment related mortality, refractory disease, and relapse) was estimated by the Aalen's method6; the cumulative incidence curves were compared using Gray's test.7 For the analyses of relapse, all other first events (refractory disease, death as first event, second neoplasms, and withdrawal) were considered competing events in the estimation of cumulative incidence of relapse. All analyses were performed using R software Windows version 3.2.4 and the cmprsk and survival packages.
Results
Baseline characteristics of patients on the trials are presented in Table 1. There was no difference in age, sex, presenting WBC count, FAB subtypes, or risk category between the two clinical trials. The OS of patients improved significantly between AML97 and AML02 trials (48.9% vs 71.2%, P <0.001). Similarly, there was an 18% improvement in EFS (43.5% vs 61.8%, P = .002) between the AML97 and AML02 trials (Figure 1a). We sought to elucidate the reasons for these improvements by analyzing first events and survival among patients who relapsed or had refractory leukemia (Figure 1b).
Table 1.
Presenting features of patients on AML97 and AML902.
| AML97 (n=92) | AML02 (n=216) | p value | |
|---|---|---|---|
|
| |||
| Age (years) | 9.0 (0.0 - 20.0) | 8.1 (0.0 - 21.0) | |
| Sex | |||
| Male | 49% (45) | 56% (121) | 0.454 |
| Female | 51% (47) | 44% (95) | |
| WBC Count | |||
| < 50,000 | 76% (70) | 74% (160) | 0.776 |
| ≥ 50,000 | 24% (22) | 26% (56) | |
| FAB | |||
| M0 | 2% (2) | 1% (2) | 0.586 |
| M1 | 17% (16) | 11% (24) | 0.141 |
| M2 | 24% (22) | 18% (38) | 0.211 |
| M4 | 21% (19) | 25% (55) | 0.387 |
| M5 | 17% (16) | 25% (54) | 0.181 |
| M6 | 0% (0) | 1% (2) | 0.999 |
| M7 | 14% (13) | 12% (25) | 0.571 |
| Unknown | 4% (4) | 7% (16) | 0.45 |
| Initial Risk | |||
| Low | 24% (22) | 32% (68) | 0.218 |
| Standard | 42% (39) | 36% (77) | 0.304 |
| High | 34% (31) | 33% (71) | 0.937 |
Figure 1.
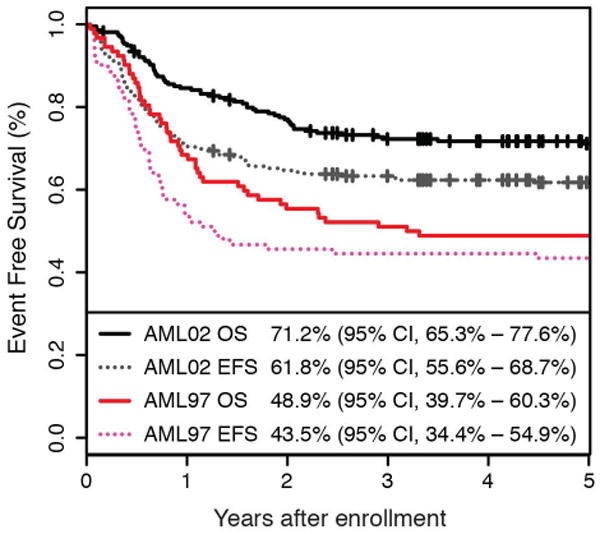
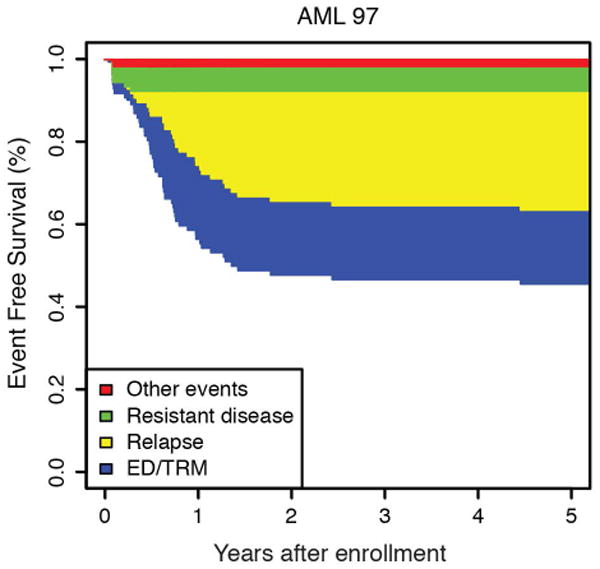
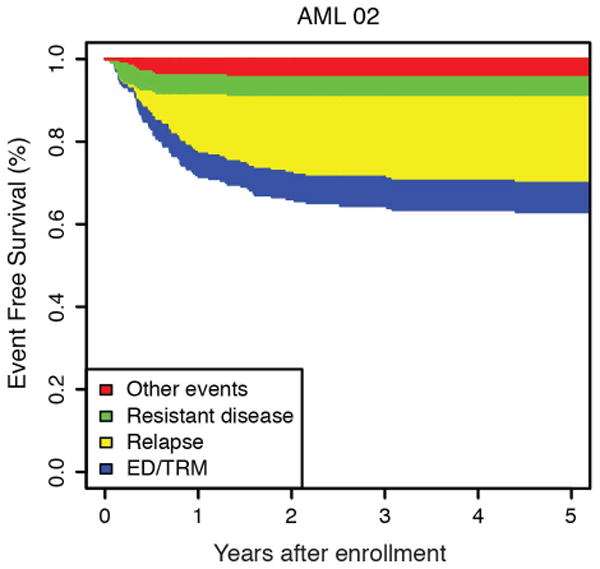
(a) Overall and event free survival and AML97 and AML02. (b) Event free survival of AML97 and (c) AML02 showing the relative contribution of first events to the failure rate. First events shown are ED/TRM (early death / treatment related mortality), relapsed disease, refractory disease, and other events (withdrawal or secondary malignancy).
Withdrawal or secondary malignancy
Two patients (2%) withdrew from AML97 and ten patients (5%) withdrew from AML02. One secondary malignancy occurred in each trial: a case of malignant mesothelioma after AML97 therapy and one case of diffuse large B-cell lymphoma after AML02 therapy.
Decreased Early Death and Treatment-Related Mortality in First Complete Remission
A lower rate of ED/TRM was the largest contributor to the improvements in OS and EFS between AML97 and AML02. The 5-year cumulative incidence of ED/TRM was significantly improved for patients treated on AML02 (18.5% vs 7.9%, P = 0.007). (Figure 2) There were two ED in AML97 and one ED in AML02. The causes of ED and TRM for each trial are listed in Table 2. We next examined whether the improved cumulative incidence of TRM occurred in patients treated with chemotherapy / chemotherapy with autologous stem cell rescue only, or for patients treated with allogeneic HCT. The improvement in TRM after allogeneic HCT (35.5% vs 15.1%, P = 0.022) was the primary contributing factor to the improved TRM seen in AML02. There was no difference between the two studies for patients treated with chemotherapy, including chemotherapy with autologous stem cell rescue, (6.5% vs 4.2%, P = 0.371).
Figure 2.
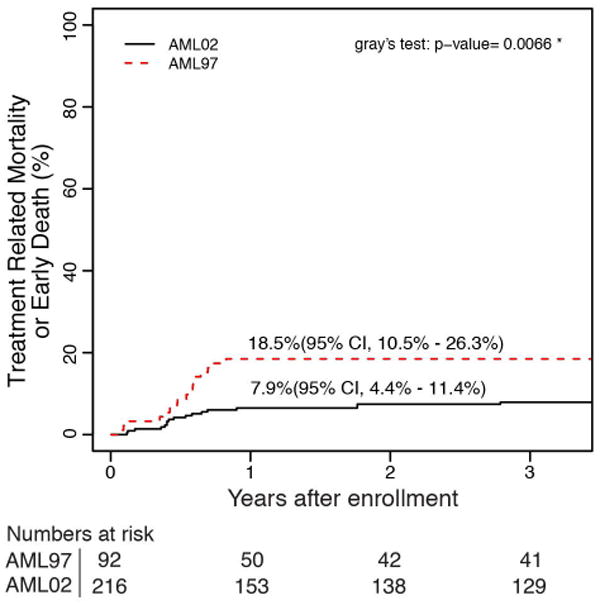
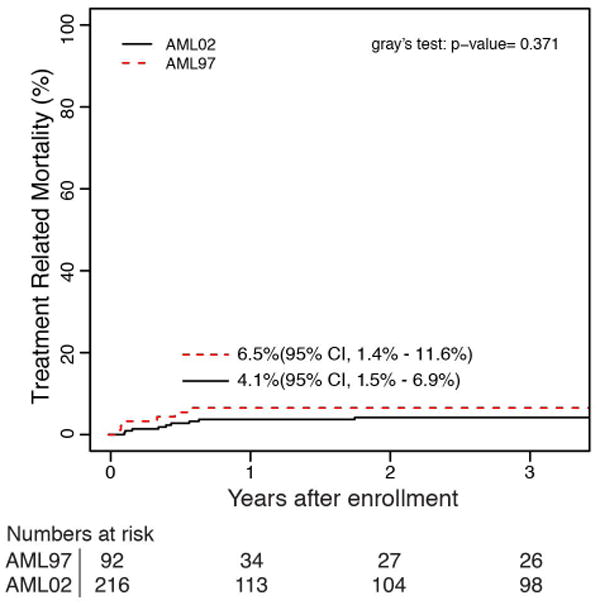
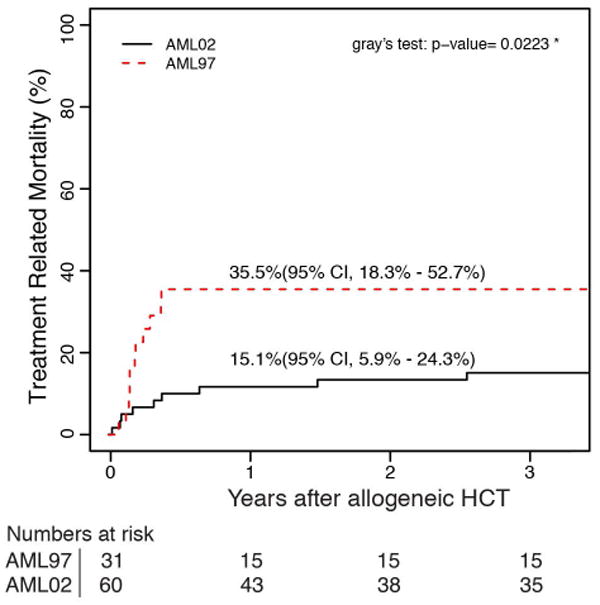
Cumulative incidence of (a) ED/TRM as first event for all patients on AML97 and AML02, (b) TRM as first event for patient in receiving chemotherapy or autologous HCT and (c) TRM as first event for patients receiving allogeneic HCT.
Table 2.
Cause of early death (ED) and treatment related mortality (TRM) in the AML97 and AML02 trials. Early death (ED) is defined as death in induction prior to CR. Treatment related mortality (TRM) is defined as any death in first CR.
| AML97 (n=92) | AML02 (n=216) | |||||
|---|---|---|---|---|---|---|
| Cause of death | ED | TRM | ED | TRM | ||
| Chemotherapy alone | Allogeneic HCT (n=39) | Chemotherapy alone | Allogneic HCT (n=60) | |||
| Infection | 2 | 4 | 10 | 0 | 7 | 4 |
| Bleeding | 0 | 0 | 0 | 1 | 0 | 1 |
| Cardiovascular failure | 0 | 0 | 1 | 0 | 1 | 0 |
| Graft versus host | 0 | 0 | 0 | 0 | 0 | 1 |
| Other | 0 | 0 | 0 | 0 | 0 | 3 |
| Total - N (5 year CI) | 2 | 4 (6.5%) | 11 (35.5%) | 1 | 8 (4.1%) | 9 (15.1%) |
Leukemia Eradication
The cumulative incidence of refractory disease was the same on AML97 and AML02 (6.5% vs 5.6%, P = 0.736). No patients with low-risk AML on either trial had refractory disease. The difference we observed in the cumulative incidence of relapse between AML97 and AML02, did not achieve statistical significance (29.3% vs 21.0%, P = 0.12). To further explore any potential difference in relapse incidence between protocols, we analyzed risk groups separately. We compared incidence of relapse between trials in high, standard, and low–risk patients (Figure 3). Neither the high-risk nor standard-risk patients showed differences in relapse/refractory leukemia rates between AML97 and AML02 (high risk: 22.6% vs 28.4%, P = .613; standard risk: 35.9% vs 24.7%, P = .211). However, patients with low-risk AML, defined as those with core-binding factor leukemia, had a significantly lower incidence of relapse disease on AML02 (27.3% vs 8.8%, P = .036). We analyzed the cases with t(8;21) and inv(16) independently to assess the possible contributions of each subtype to the improvement observed in the low-risk group on AML02. Patients with both cytogenetic abnormalities (t(8;21): 45.5% vs 0%, P < 0.01; inv(16): 28.6% vs 7.7%, P = 0.15) appear to have lower rates of relapse on AML02, though the group with inv(16) did not achieve statistical significance, likely due to low number of patients. (Supplemental Fig. 1). For the overall cohort, we also compared results based on MRD at the end of induction I. There was no significant difference in EFS between AML97 and AML02 among patients with positive MRD using cutoffs of either ≥ 0.1% (31.6% vs 39.0%, P = .575) or ≥ 1% (31.3% vs 30.0%, P = .720), again emphasizing that patients with disease that has poor initial response did not see appreciable improvements on the more recent AML02 protocol.
Figure 3.
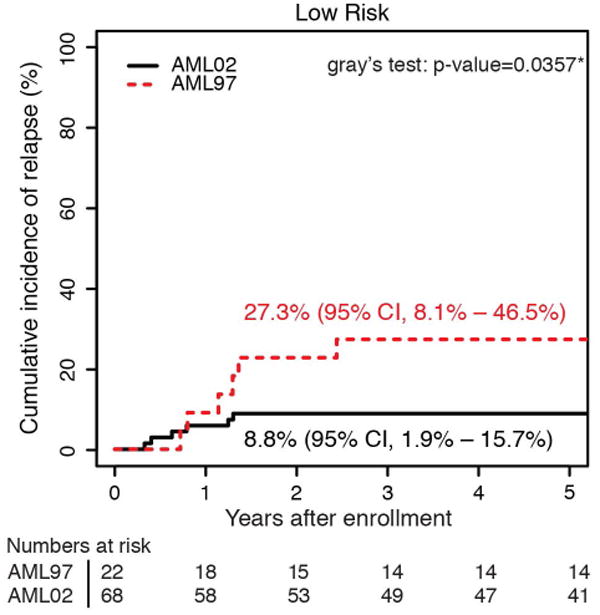
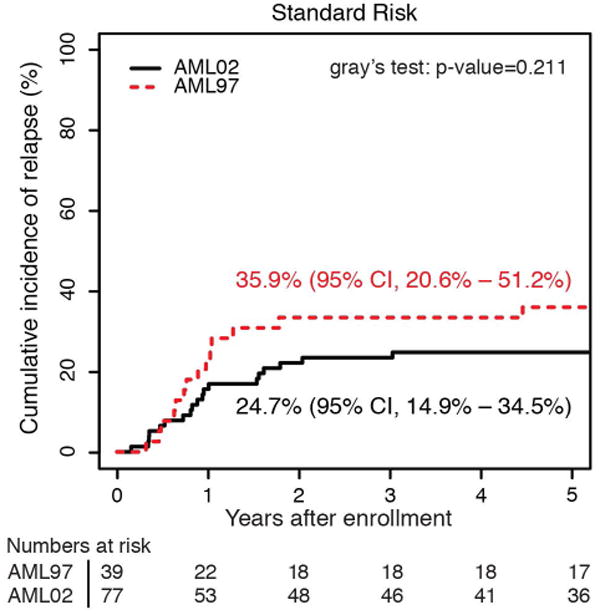
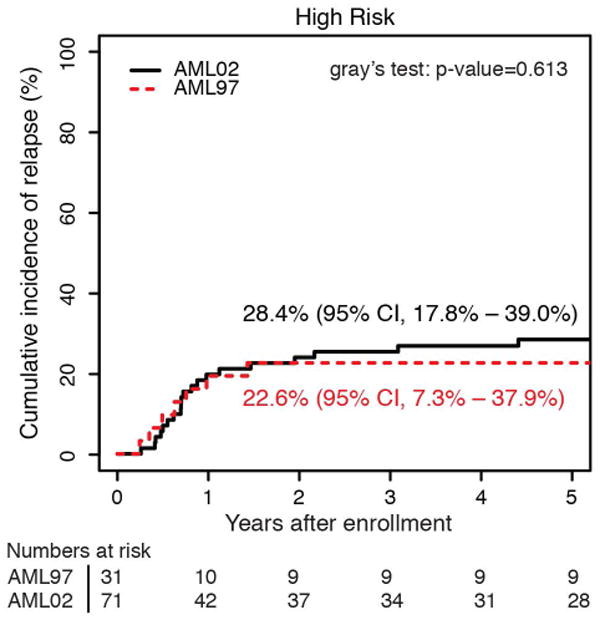
Cumulative incidence of relapsed disease for patients AML97 and AML02, based upon initial risk classification as (a) low risk, (b) standard risk, and (c) high risk.
Retrieval Therapy
To examine whether new treatment options or improvements in HCT may led to higher survival rates after relapse or refractory disease, we analyzed the overall survival for patients enrolled on AML97 compared to AML02. The OS for patients with relapsed or refractory disease was higher for patients initially treated on AML02 (12.1% vs 27.5%), albeit the difference did not reach statistical significance (P = .110). (Figure 4)
Figure 4.
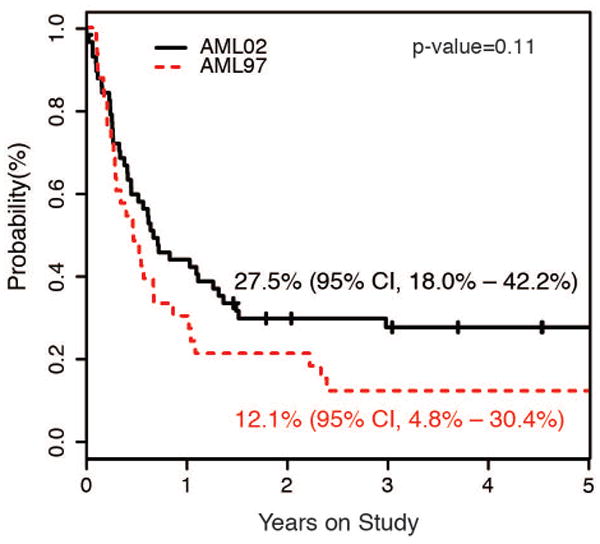
Overall Survival for patients with relapsed or refractory AML according to frontline treatment protocol.
Discussion
The outcome for children with AML has improved over the last three decades across a range of collaborative group studies. This is in spite of the fact that most randomized trials have shown no difference in randomized treatment arms, and the backbone therapy has not changed significantly.1 Nevertheless, there could be improvement in disease eradication because of adjustments to chemotherapy dosing or timing, intensification of therapy, effectiveness of HCT for disease eradication, and improved salvage therapy. Alternatively, the improvement in outcomes for children with AML could be the result of improved understanding and identification of therapy-related complications and improved supportive care measures as well as risk-directed treatment to avoid over-treatment of low-risk patients or under-treatment of those with high-risk leukemia.
We did not see a statistically significant difference in the overall cumulative incidence of relapsed or refractory disease between AML97 and AML02. However, patients classified as low risk showed a decreased cumulative incidence of relapse on AML02. This improvement likely reflects the differences in treatment intensity between protocols. During the two standard induction courses, patients on AML97 received a lower dose of both daunorubicin (180 mg/m2 vs 300 mg/m2) and cytarabine (2.5g/m2 vs 4 or 18 g/m2). (Figure 5) Studies conducted by the Medical Research Council and the Berlin-Frankfurt-Münster Study Group (BFM) have also shown that children with AML and t(8;21) benefit from higher intensity therapy, based on either higher dose anthracycline or high dose cytarabine.8-10 In contrast, increasing the intensity of therapy failed to decrease the risk of relapsed or refractory disease for high-risk patients on our AML02 protocol, emphasizing the futility of this approach for this subgroups of patients. Therefore, addition of novel agents, possibly with decreased intensity of conventional AML chemotherapy, may be appropriate for high-risk AML. Patients with standard risk AML may have had some improvement in relapse rates between the two trials, but our numbers limit definitive conclusions for this group of patients.
Figure 5.
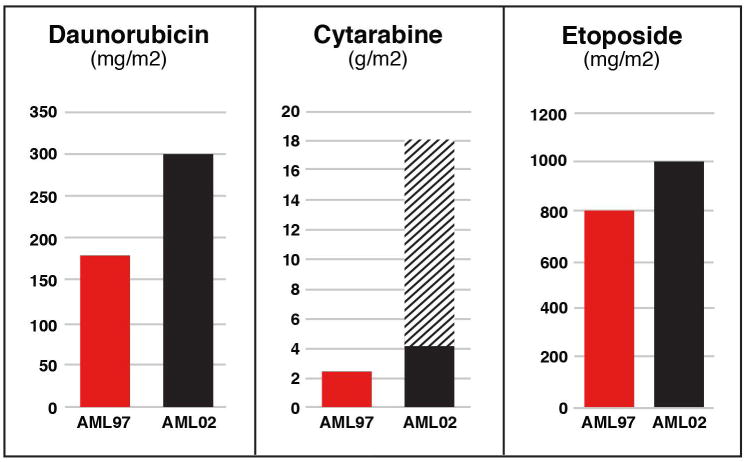
Cumulative dosing of chemotherapy received during the two standard induction courses on AML97 and AML02. Patients on AML02 were randomized between high (hatched bar) and low dose cytarabine (black bar) during the first induction course.
A recent analysis by the AML-BFM group shows improved efficacy of salvage therapy after relapsed or refractory disease is an important contributor to improved outcome of pediatric AML.11 Likewise, the survival rate of our patients with relapsed or refractory AML also trended towards improvement over the treatment era.
Several studies have demonstrated that children with AML who have residual MRD after induction therapy have worse prognosis compared to those who are MRD negative.3, 12 Therefore, another component of treatment that could contribute to improved disease eradication is the intensification of therapy for patients with persistent MRD. AML02 was the first AML clinical trial to prospectively evaluate MRD and to adjust therapy based upon the results. We did not see a decrease in the incidence of relapse among patients with residual disease after the first course of therapy in AML02 , using cutoffs of 0.1% or 1%, though a direct comparison of patients in MRD positive categories is confounded by differences in the first course between the two trials. This may be related to the limited treatment options for AML patients with persistent MRD. In contrast to patients with ALL and positive MRD early in therapy, patients with AML and persistent MRD may have limited benefit from intensification with currently available treatments. Patients with persistent MRD, like patients with other high-risk features, may be stratified to receive allogeneic HCT, but overall benefit for such patients remains an area of active investigation.14-16 Previous analysis of our AML02 trial suggested that gemtuzumab ozogamicin may be beneficial for patients with persistent MRD, but the benefits of prospective MRD also remains to be demonstrated.13
Given the lack of significant alterations to induction regimens for pediatric AML, it is intuitive that reduction in TRM contributes to the improved EFS rates seen in cooperative group trials over the past 30 years.1 Studies conducted by the Nordic Society of Paediatric Haematology and Oncology and the Dutch Children's Oncology Group showed that ED/TRM in AML did not improve over successive clinical trials in the 1980s and 1990s.19, 20 However, a study from the AML-BFM group showed improvement of ED/TRM, which is consistent with our results.21 Similarly, we observed a significant improvement in ED/TRM between the AML97 and AML02 trials. This improvement was seen despite the increased intensity of chemotherapy administered in AML02. Standard supportive care guidelines for metabolic derangements, PJP prophylaxis, transfusion support, and management of febrile neutropenia were similar for both studies. The only significant differences in protocol specific supportive care guidelines was the recommendation to provide prophylactic antifungal therapy on AML02 and the requirement starting midway through AML02 to administer prophylactic antibiotics to prevent viridans streptococcus bacteremia.17, 18 While many factors could have contributed to the overall decrease in ED/TRM seen in our AML02 clinical trial, including improved critical care support, improved management of active infections, and prophylactic antimicrobials, the primary improvement we observed was specifically following allogeneic HCT. Recent studies have also confirmed improvement in transplant related mortality for children over similar treatment eras.22, 23
Our study has several limitations resulting from performing a retrospective analysis. The assessment of the potential benefit of prospective MRD assessment used on AML02 was limited by small numbers and by the different chemotherapy schedules used on the two trials. Our analysis of ED/TRM is confounded by supportive care measures that evolved over time outside of protocol specification. Finally, allogeneic HCT and management of refractory and relapsed patients occurred after these patients were removed from frontline protocols and was largely influenced by treatment era rather than initial therapy.
In conclusion, EFS and OS improved from AML97 to AML02 because of decreased ED/TRM, likely due to a combination of improved supportive care measures, and improved disease eradication in patients with low risk AML. Despite our efforts to increase the intensity of therapy by incorporating high-dose cytarabine during induction and adding gemtuzumab ozogamicin for patients with high or persistent MRD, the outcome of patients with high risk AML did not improve. These results support the importance of treatment intensity for patients with low-risk AML, while emphasizing the need for novel therapy, rather than increased therapy intensity with current chemotherapy, for children with high-risk AML.
Supplementary Material
Acknowledgments
This work was supported in part by Cancer Center Support (CORE) grant P30 CA021765-30 from the National Institutes of Health and by the American Lebanese Syrian Associated Charities (ALSAC). Ching-Hon Pui is an American Cancer Society Professor.
Footnotes
Author contributions: RR, BT, SP, RR, CHP, JR conceptualized the overall study. TA, LW, HI, BT, SP, RR, CHP, JR development the design of methodology. LW, SP completed data management and statistical analysis. TA, LW, SP performed the formal analysis. TA, HI, BT, RR, JR performed the research and investigation process. TA, LW, HI, BT, SP, RR, CHP, JR all contributed resources. LW, SP performed data curation. TA, JR wrote the original draft. TA, LW, HI, BT, SP, RR, CHP, JR reviewed and edited the final manuscript. TA, LW, SP, JR helped prepare the visualization of the manuscript. HI, BT, SP, RR, CHP, JR assisted with supervision. TA, LW, HI, BT, SP, RR, CHP, JR contributed to administration of the project. RR, CHP, JR were responsible for ensuring financial support.
There are no conflicts of interest and no financial disclosures.
References
- 1.Zwaan CM, Kolb EA, Reinhardt D, et al. Collaborative Efforts Driving Progress in Pediatric Acute Myeloid Leukemia. J Clin Oncol. 2015;33:2949–2962. doi: 10.1200/JCO.2015.62.8289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rubnitz JE, Crews KR, Pounds S, et al. Combination of cladribine and cytarabine is effective for childhood acute myeloid leukemia: results of the St Jude AML97 trial. Leukemia. 2009;23:1410–1416. doi: 10.1038/leu.2009.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rubnitz JE, Inaba H, Dahl G, et al. Minimal residual disease-directed therapy for childhood acute myeloid leukaemia: results of the AML02 multicentre trial. Lancet Oncol. 2010;11:543–552. doi: 10.1016/S1470-2045(10)70090-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaplan E, Meier P. Non-parametric estimation for incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 5.Link CL. Confidence intervals for the survival function using Cox's proportional-hazard model with covariates. Biometrics. 1984;40:601–609. [PubMed] [Google Scholar]
- 6.Aalen O. Nonparametric Estimation of Partial Transition-Probabilities in Multiple Decrement Models. Annals of Statistics. 1978;6:534–545. [Google Scholar]
- 7.Gray R. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16 [Google Scholar]
- 8.Creutzig U, Zimmermann M, Bourquin JP, et al. Second induction with high-dose cytarabine and mitoxantrone: different impact on pediatric AML patients with t(8;21) and with inv(16) Blood. 2011;118:5409–5415. doi: 10.1182/blood-2011-07-364661. [DOI] [PubMed] [Google Scholar]
- 9.Harrison CJ, Hills RK, Moorman AV, et al. Cytogenetics of childhood acute myeloid leukemia: United Kingdom Medical Research Council Treatment trials AML 10 and 12. J Clin Oncol. 2010;28:2674–2681. doi: 10.1200/JCO.2009.24.8997. [DOI] [PubMed] [Google Scholar]
- 10.Klein K, Kaspers G, Harrison CJ, et al. Clinical Impact of Additional Cytogenetic Aberrations, cKIT and RAS Mutations, and Treatment Elements in Pediatric t(8;21)-AML: Results From an International Retrospective Study by the International Berlin-Frankfurt-Munster Study Group. J Clin Oncol. 2015;33:4247–4258. doi: 10.1200/JCO.2015.61.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rasche M, Zimmermann M, Kellerstrass L, Creutzig U, Klusmann J, Reinhardt D. Successes and Challenges of Pediatric AML: A Report on Survival, Salvage Therapy and Causes of Deaths in the AML-BFM Study Group from 1987-2012. American Society of Hematology Annual Conference; San Diego, California. 2016. [Google Scholar]
- 12.van der Velden VH, van der Sluijs-Geling A, Gibson BE, et al. Clinical significance of flowcytometric minimal residual disease detection in pediatric acute myeloid leukemia patients treated according to the DCOG ANLL97/MRC AML12 protocol. Leukemia. 2010;24:1599–1606. doi: 10.1038/leu.2010.153. [DOI] [PubMed] [Google Scholar]
- 13.O'Hear C, Inaba H, Pounds S, et al. Gemtuzumab ozogamicin can reduce minimal residual disease in patients with childhood acute myeloid leukemia. Cancer. 2013;119:4036–4043. doi: 10.1002/cncr.28334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kelly MJ, Horan JT, Alonzo TA, et al. Comparable survival for pediatric acute myeloid leukemia with poor-risk cytogenetics following chemotherapy, matched related donor, or unrelated donor transplantation. Pediatr Blood Cancer. 2014;61:269–275. doi: 10.1002/pbc.24739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klingebiel T, Reinhardt D, Bader P, Party EPDW Place of HSCT in treatment of childhood AML. Bone Marrow Transplant. 2008;42(Suppl 2):S7–9. doi: 10.1038/bmt.2008.276. [DOI] [PubMed] [Google Scholar]
- 16.Niewerth D, Creutzig U, Bierings MB, Kaspers GJ. A review on allogeneic stem cell transplantation for newly diagnosed pediatric acute myeloid leukemia. Blood. 2010;116:2205–2214. doi: 10.1182/blood-2010-01-261800. [DOI] [PubMed] [Google Scholar]
- 17.Inaba H, Gaur AH, Cao X, et al. Feasibility, efficacy, and adverse effects of outpatient antibacterial prophylaxis in children with acute myeloid leukemia. Cancer. 2014;120:1985–1992. doi: 10.1002/cncr.28688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kurt B, Flynn P, Shenep JL, et al. Prophylactic antibiotics reduce morbidity due to septicemia during intensive treatment for pediatric acute myeloid leukemia. Cancer. 2008;113:376–382. doi: 10.1002/cncr.23563. [DOI] [PubMed] [Google Scholar]
- 19.Molgaard-Hansen L, Mottonen M, Glosli H, et al. Early and treatment-related deaths in childhood acute myeloid leukaemia in the Nordic countries: 1984-2003. Br J Haematol. 2010;151:447–459. doi: 10.1111/j.1365-2141.2010.08389.x. [DOI] [PubMed] [Google Scholar]
- 20.Slats AM, Egeler RM, van der Does-van den Berg A, et al. Causes of death--other than progressive leukemia--in childhood acute lymphoblastic (ALL) and myeloid leukemia (AML): the Dutch Childhood Oncology Group experience. Leukemia. 2005;19:537–544. doi: 10.1038/sj.leu.2403665. [DOI] [PubMed] [Google Scholar]
- 21.Creutzig U, Zimmermann M, Reinhardt D, Dworzak M, Stary J, Lehrnbecher T. Early deaths and treatment-related mortality in children undergoing therapy for acute myeloid leukemia: analysis of the multicenter clinical trials AML-BFM 93 and AML-BFM 98. J Clin Oncol. 2004;22:4384–4393. doi: 10.1200/JCO.2004.01.191. [DOI] [PubMed] [Google Scholar]
- 22.Majhail NS, Chitphakdithai P, Logan B, et al. Significant improvement in survival after unrelated donor hematopoietic cell transplantation in the recent era. Biol Blood Marrow Transplant. 2015;21:142–150. doi: 10.1016/j.bbmt.2014.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wood WA, Lee SJ, Brazauskas R, et al. Survival improvements in adolescents and young adults after myeloablative allogeneic transplantation for acute lymphoblastic leukemia. Biol Blood Marrow Transplant. 2014;20:829–836. doi: 10.1016/j.bbmt.2014.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


