Abstract
Aim
Selective dorsal rhizotomy (SDR) is a surgical treatment for spasticity in children with cerebral palsy (CP). Studies suggest long-lasting effects of SDR on spasticity; long-term effects on symptoms and function are not clear. This study tested whether adults with CP (average 22y after SDR) report less pain, fatigue, and functional decline than a retrospectively assessed non-surgical comparison group.
Method
This was a case–control study. Eighty-eight adults with CP (mean age 27y 0mo; SDR=38; non-surgical [comparison]=50) recruited from a tertiary care center and the community completed a battery of self-reported outcome measures. Regression models were used to test whether SDR status predicted pain, fatigue, functional change, and hours of assistance (controlling for Gross Motor Function Classification System level).
Results
SDR status did not significantly predict pain interference (p=0.965), pain intensity (p=0.512), or fatigue (p=0.404). SDR related to lower decline in gross motor functioning (p=0.010) and approximately 6 fewer hours of daily assistance than for those in the comparison group (p=0.001).
Interpretation
Adults with CP who had SDR in childhood reported less gross motor decline and fewer daily assistance needs than non-surgically treated peers, suggesting the functional impact of SDR persists long after surgery.
Cerebral palsy (CP) remains common in the USA, affecting as many as 3.1 in every 1000 children, and more children with CP are surviving to adulthood.1,2 As children with CP grow older, families may choose from a variety of options for managing spasticity and other symptoms that contribute to functional impairment. Of these options, selective dorsal rhizotomy (SDR) is among the most invasive. This neurosurgical procedure, which is often performed in the first decade of life, involves selective severing of lumbosacral sensory rootlets using electromyography guidance, with the goal of decreasing spasticity and improving motor function. After the procedure, the children participate in a rigorous program of physical and occupational therapy lasting several months, although protocols for postoperative rehabilitation vary between institutions. They may undergo further treatments such as oral medications, injected botulinum neurotoxin and phenol injections, and orthopedic surgery.3 Despite the variety of treatment options available to people with CP, functional declines with aging, particularly in gross motor function and gait, have been observed4–7 as early as adolescence or early adulthood.8 Currently, there are still limited data examining long-term outcomes of SDR, especially as these children reach adulthood.
Previous prospective studies in a single, small sample of people who have undergone SDR have demonstrated reductions in spasticity, pain intensity, and pain interference that are maintained even years after SDR.9,10 Although previous studies have demonstrated short- and long-term benefits of SDR in terms of reduced spasticity, prospective studies have differed over the benefit in functional outcomes.10–12 Furthermore, other than one study of 18 people that showed that SDR was related to relatively low pain and pain interference,10 we lack understanding of how SDR affects symptom experience as individuals age. This is important given that symptoms such as pain and fatigue are highly prevalent in adults with CP and have a detrimental effect on quality of life and functional ability.3,13–15
To address these limitations in our understanding of how SDR relates to symptoms and function in adulthood, in this case–control study we compared adults with CP who either did or did not undergo SDR as children in terms of self-reported pain (intensity and location of pain), pain interference, fatigue severity, and perceptions of change with aging in motor function and required daily assistance. We hypothesized that pain intensity, pain interference, prevalence of back and lower extremity pain, and fatigue severity would be lower in the SDR group than the comparison (non-surgical) group owing to reduced spasticity, improved biomechanics, and decreased energy expenditure with motor activities. Further, we hypothesized that SDR might delay gross motor decline through lasting effects on spasticity and thus contractures and other associated complications; we expected that those who underwent SDR compared with those who did not would require less daily assistance as a result of better gross motor functioning. We included a self-report functional measure to allow comparison of current function and assistance needs within Gross Motor Function Classification System (GMFCS) categories.
Method
Participant recruitment
In this case–control study, we recruited adults with CP who had and had not undergone SDR in childhood. Selection for the surgery was not included in the study activities; participants were assigned to each group on the basis of retrospective self-report of their rhizotomy status. Local institutional review board approval was obtained before initiation of study activities. Participants were asked to complete an online self-reported survey battery and received US$15.00 for participation. A convenience sample was recruited from a tertiary care center (University of Michigan Hospital and Health Systems) and from the general community between January 2014 and May 2015. Recruitment methods included postal mail letters with the survey web address, face-to-face contact in an adult CP clinic, an institutional research recruitment website, and coordination with outside organizations such as United Cerebral Palsy. Informed consent was received through the initial survey access screen. Participants were allowed to complete the survey at their own pace in a location of their choosing. Those who wished to participate during their clinic visit were provided with an Apple iPad and a private location to use while completing the survey. Volunteers were eligible to participate if they had a diagnosis of CP, were between 18 and 35 years old, and had sufficient English fluency and cognitive ability to respond independently to survey questions (Appendix S1, online supporting information). Volunteers were ineligible if they underwent SDR at age 10 years or older or had any history of other spinal cord surgery. Only those whose SDR was performed before age 10 were included, to avoid confounding effects of age at time of surgery since older age at SDR has been linked to worse functional outcomes.16,17
Study procedures
Participants were asked to formulate their own response to each question, and were allowed to miss any question that they did not understand or feel comfortable answering. Participants could use a physical aide or another person to select each response if needed, as we anticipated that many of the potential participants would have physical barriers to completing the survey. Participants were not asked to report need for physical assistance with completing the survey.
Study measures
The survey included demographic information and information about motor function, communication abilities, medical treatments (including whether participants had ever had a baclofen pump, tendon lengthening surgery, or hip surgery), life satisfaction, health perception, and self-reported inputs of body mass index (self-reported weight and height). Demographic information included sex, living situation, educational level, and employment or school enrollment status. Current mobility was assessed using the self-report version of the GMFCS,18 which assigns a level ranging from I to V, from higher to lower independent mobility. It has been shown to be a valid self-report measure of mobility. Current fine motor functioning was assessed using the Manual Ability Classification System (MACS)19 and current communication ability was assessed using the Communication Function Classification System (CFCS).20 Similar to the GMFCS, both measures assign a level on an ordinal scale of I to V, ranging from better to worse functioning.
Pain outcomes were measured using the Patient Reported Outcome Measurement Information System (PROMIS) Pain Intensity – Short Form 3a and Pain Interference – Short Form 8a.21 PROMIS measures have previously not been widely used in CP research; however, these measures are widely accessible and address a multitude of relevant participant reported outcomes. The PROMIS Pain Intensity – Short Form asked respondents to rate their worst and average pain in the previous seven days as well as current pain. Items were rated on a 1 to 5 Likert scale and responses were summed for a possible total score of 3 to 15, with higher scores indicating higher pain intensity. The PROMIS Pain Interference – Short Form asked respondents to rate how much pain interfered with daily activities, housework, chores, participation in and enjoyment of social activities, enjoyment of life, recreation, and family life. Items were rated on a 1 to 5 Likert scale and responses were summed for a possible total score of 8 to 40, with higher scores indicating higher pain interference. Pain location was identified by multiple-choice options indicating various body areas: head, neck/upper back, lower back, upper extremities, lower extremities, chest/abdomen. Participants were asked to select all of the areas where they typically experienced pain. Fatigue was assessed using the Fatigue Severity Scale (FSS). The FSS has been used in many different patient populations, including adults with CP,13 and has been shown to be internally consistent, reliable, sensitive, and valid in multiple patient populations.22
Perceived change in functional status was assessed in a method similar to that of Opheim et al.5 Participants were asked to grade both their overall motor function and walking ability (if applicable) as having improved, stayed the same, or worsened with time. For those who noted worsening, they were asked to estimate when they began to notice that change in 5 year intervals. The Self-Reported Functional Measure (SRFM)23 was used as a secondary outcomes measure. The SRFM contains 13 questions about activities of daily living, for which the participant is asked to rate the amount of assistance they require on a 4-point scale, ranging from ‘no extra time or help’ to ‘total help or never do’. Possible scores for this measure ranged from 13 to 52. Additionally, the SRFM includes an item to report the number of hours of assistance (0–24; paid or unpaid) that they receive each day. Although this measure was developed for use in research into spinal cord injury, it contains questions about functional activities also relevant to people with CP.
Data analysis
Data analysis was performed using SPSS version 22 (IBM SPSS Statistics, IBM Inc., NY, USA). Descriptive statistics for all outcome variables were calculated and analyzed for normality. Student's t-tests and χ2 tests were used to examine group differences in demographic and clinical (e.g. GMFCS) variables. The primary research questions were examined using regression models. Linear regression was used to test whether SDR status predicted pain intensity, pain interference, and fatigue and functional (SRFM) scores. Multinomial linear regression was used to test whether SDR status predicted change in motor function (worse, no change, better). In some cases, mean results are presented to aid in interpreting findings. The fatigue measure (FSS) showed acceptable skew (0.12) and kurtosis (−1.18) values, but a slightly bimodal distribution; thus, a non-parametric test of group differences in fatigue (i.e. the Mann–Whitney U-test) was conducted to confirm/contrast with the parametric tests results. All regression analyses controlled for GMFCS level.
Results
Eighty-eight adults with CP participated in this study. Characteristics of participants are described in Table I. The groups were not significantly different in age (t[86]=−0.46, p=0.646), sex (χ2[1,86]=2.60, p=0.128), education level (χ2[1,85]=3.45, p=0.631), baclofen pump use (χ2[1,85]=0.364, p=0.546), hip surgery (χ2[1,85]=1.862, p=0.172), tendon lengthening surgery (χ2[1,85]=0.810, p=0.368), CFCS level (χ2[1,86]=6.79, p=0.147), or MACS levels (χ2[1,88]=0.49, p=0.975). There was a significant difference in GMFCS levels between the two groups (χ2[4,88]=18.55, p=0.001), with the non-surgical (comparison) group having less motor involvement than the SDR group. The SDR group primarily comprised individuals who had undergone surgery at the University of Michigan Hospital and Health Systems between 1988 and 2002; 29 of the 38 surgeries were performed at University of Michigan Hospital and Health Systems.
Table I. Characteristics of participants.
| Parameter | Case | Comparison | Total | p |
|---|---|---|---|---|
| n | 38 | 50 | 88 | |
| Mean age, y:mo (SD) | 27:4 (4:6) | 26:10 (5:1) | 27:0 (4:10) | 0.646 |
| Time since SDR, y:mo (range) | 22:0 (11:7–28:4) | N/A | ||
| Sex, % male | 55.6 | 38.0 | 45.3 | 0.128 |
| Ever had baclofen pump, yes, n (% of group) | 5/35 (14.3) | 5/50 (10) | 11.8 | 0.546 |
| Ever had hip surgery, yes, n (% of group) | 14/35 (40) | 13/50 (26) | 31.8 | 0.172 |
| Ever had tendon lengthening surgery, yes, n (% of group) | 19/35 (54) | 32/50 (64) | 60 | 0.368 |
| Education level, n (% of group) | 0.631 | |||
| Less than high school diploma | 2 (5.7) | 5 (10.0) | 7 (8.2) | |
| High school diploma or GED | 13 (37.1) | 25 (50.0) | 38 (44.7) | |
| Vocational school/associates degree | 8 (22.9) | 8 (16.0) | 16 (18.2) | |
| Bachelor's degree | 9 (25.7) | 7 (14.0) | 16 (18.2) | |
| Master's degree | 2 (5.7) | 4 (8.0) | 6 (7.1) | |
| Doctorate | 1 (2.9) | 1 (2.0) | 2 (2.3) | |
| GMFCS level, n (% of group) | 0.001 | |||
| I | 2 (5.3) | 13 (26.0) | 15 (17.0) | |
| II | 7 (18.4) | 18 (36.0) | 25 (28.4) | |
| III | 11 (28.9) | 4 (8.0) | 15 (17.0) | |
| IV | 14 (36.8) | 7 (14.0) | 21 (23.9) | |
| V | 4 (10.5) | 8 (16.0) | 12 (13.6) | |
| CFCS level, n (% of group) | 0.147 | |||
| I | 28 (75.7) | 25 (51.0) | 53 (61.6) | |
| II | 4 (10.8) | 13 (26.5) | 17 (19.8) | |
| III | 4 (10.8) | 6 (12.2) | 10 (11.6) | |
| IV | 1 (2.7) | 3 (6.1) | 4 (4.7) | |
| V | 0 (0.0) | 2 (4.1) | 2 (2.3) | |
| MACS level, n (% of group) | 0.975 | |||
| I | 13 (34.2) | 16 (32.0) | 29 (33.0) | |
| II | 14 (36.8) | 20 (40.0) | 34 (38.6) | |
| III | 6 (15.8) | 7 (14.0) | 13 (14.8) | |
| IV | 3 (6.0) | 3 (6.0) | 6 (6.8) | |
| V | 2 (5.3) | 4 (8.0) | 6 (6.8) |
SDR, selective dorsal rhizotomy; N/A, not applicable; GED, general education development; GMFCS, current Gross Motor Function Classification System level; CFCS, current Communication Function Classification System level; MACS, current Manual Ability Classification System level.
Pain intensity, interference, and location
In total, 65.9 per cent of participants had experienced some level of pain on average in the previous 7 days. Pain incidence was not significantly different between the case and comparison groups, at 65.8 per cent and 66.0 per cent respectively (χ2[1,88]=0.00, p=0.984). SDR status was not significantly associated with pain intensity (β[83]=−0.07, t=−0.66, p=0.512) or pain interference (β[83]=0.01, t=0.04, p=0.965). Pain location was also not significantly different between the two groups. Low back and lower extremities were the most commonly reported areas of pain for both groups, with no statistically significant difference between the two groups. Forty-two per cent of participants endorsed low back pain (i.e. 34.2% of cases and 48.0% of the comparison group; p=0.194). Over half (54.5%) of participants reported pain in their lower extremities (i.e. 52.6% [20 out of 38] of the SDR group and 56.0% [28 out of 50] of the comparison group; p=0.753).
Fatigue
SDR status was not significantly related to fatigue scores (β=−0.09; t=−0.79; p=0.404); non-parametric test results were similarly non-significant (U=796.50, p=0.582). Average FSS score for all participants was 3.9 (standard deviation [SD] 1.72). In the SDR group (n=35), the mean was 3.77 (SD 1.80). In the comparison group (n=49), the mean was 4.00 (SD 1.68). A mean FSS score of four or higher represents clinically significant fatigue.
Functional change
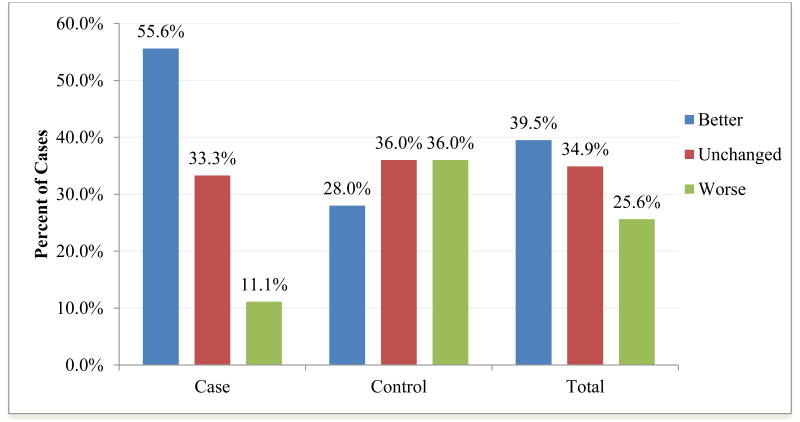
As depicted in Figure 1, in the SDR group, a larger proportion reported improvement in overall motor functioning and a smaller proportion reported motor decline than the non-surgical (comparison) group (χ2[2,86]=9.131, p=0.010). This finding was statistically significant even when accounting for GMFCS in multinomial logistic regression analysis (Wald test=10.21, p=0.001). However, when the 66 participants who reported being ambulatory at some point in their lives were asked about changes in walking ability specifically, there was no significant difference between the two groups (χ2[2,66]=1.625, p=0.444). For those who reported declines in motor functioning, changes were first noted between 1 and 5 years of age for three (13.6%), between 11 and 15 years for two (9.1%), between 16 and 20 years for four (18.2%), between 21 and 25 years for eight (36.4%), between 26 and 30 years for four (18.2%), and between 31 and 35 years for one (4.5%); notably, the age at which motor decline was first noted did not differ by SDR status (χ2[1,22]=1.553, p=0.907).
Figure 1.
Percent of participants in each group who reported better, unchanged, or worse overall motor functioning with time.
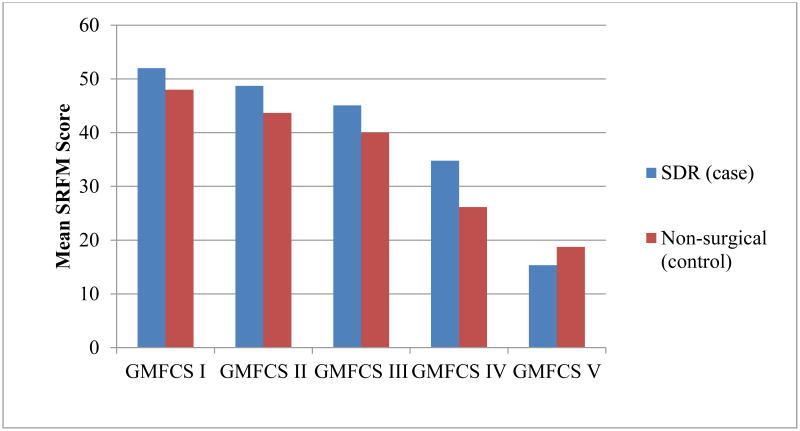
SRFM scores were also significantly different between the two groups, with the SDR group reporting higher scores (better function) when corrected for GMFCS. SDR status contributed to 5.6 per cent of variance in SRFM scores, with the SDR group achieving higher functional scores when controlling for GMFCS (β=0.24; t=3.04, p=0.003). As can be seen in Figure 2, SRFM scores were higher for the SDR group in all GMFCS levels except V.
Figure 2.
Mean Self-Reported Functional Measure (SRFM) scores for the selective dorsal rhizotomy (SDR) and non-surgical (comparison) groups within each Gross Motor Function Classification (GMFCS) category.
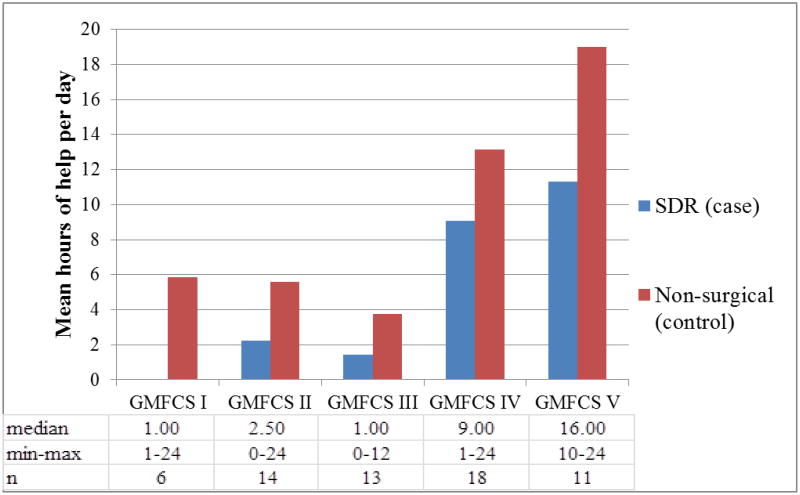
Results also showed that the SDR group reported significantly fewer total numbers of hours of help (paid or unpaid) each day (β=−0.36, t=−3.41, p=0.001) in regression models that controlled for GMFCS; these analyses indicate that the SDR group reported receiving 5.88 fewer hours of help per day than the comparison group. As can be seen in Figure 3, the number of hours of help per day was comparatively lower for the SDR group within each GMFCS category (no data were provided by the two individuals with SDR in GMFCS level I).
Figure 3.
Mean, median, and minimum/maximum hours of help (paid and unpaid) each day for the selective dorsal rhizotomy (SDR) and non-surgical (comparison) groups within each Gross Motor Function Classification (GMFCS) category.
Discussion
To our knowledge, this is the first study to compare adults with CP who underwent SDR as a child with those who did not have the surgery in terms of a broad range of clinically relevant outcomes, including pain intensity and interference, fatigue, changes in functional ability, and amount of daily assistance used. Notably, there were no significant differences in pain or fatigue outcomes between the SDR and the non-surgical comparison groups. While these data do not suggest a benefit of SDR in terms of pain and fatigue, they do suggest that SDR does not have a deleterious effect in terms of these symptoms as the children age and reach adulthood. Although previous research has shown that pain and fatigue are common in adults with CP, pain intensity, pain interference, and fatigue (FSS) scores were quite low in our study population, although in the same range as other long-term SDR studies.5,10,24
Research on outcomes for SDR has demonstrated good evidence for lasting reduction in spasticity and improved gait mechanics, with less support for significant improvement in function and participation.25 However, in the face of well-documented, multifactorial functional decline in adults with CP,4–8 it is pertinent to investigate whether childhood interventions have an effect on the rate of decline. For example, Tedroff et al.10 reported the best improvement in Gross Motor Function Measure scores 3 years after rhizotomy, followed by decline. Most of the decline that occurred was in participants who started with greater motoric impairment, whereas individuals in GMFCS levels I or II gained and plateaued in motor function. Ailon et al.11 followed 44 patients an average of 14 years 5 months after SDR, and found small improvements in motor function scores at long-term follow-up for those in GMFCS levels II or III, but not for those in GMFCS levels IV or V. These results must be considered within the context of expected functional trajectories for people with CP, and therefore may not represent improvement related to the surgery. Functional plateau and decline with age is expected in CP, and evidence suggests especially precipitous declines among those with greater motor involvement. For example, Opheim et al.5 found that individuals with bilateral involvement had deterioration of walking skills much sooner than those with unilateral involvement. We should ask, then, whether intervention affects the rate of this process across functional levels. In our study, adults who had SDR in childhood were less likely to self-report general motor decline, a finding that was supported by higher SRFM scores, and less reliance on daily assistance from others. Importantly, these findings held true even when controlling for GMFCS level. Interestingly, this result was not replicated for changes in walking function, perhaps because of the fewer participants for whom this question was applicable; only 25 participants in the SDR group and 41 in the comparison group reported ever walking, which suggests these analyses may be underpowered. Alternatively, it may suggest that our study population took a more global view of motor function, rather than focusing on walking alone.
Study strengths and limitations
Although we had a relatively modest sample size, this study compares well with other long-term SDR studies. We included an age-matched comparison group, which allowed interpretation of findings in the context of a similarly aged population with CP. Further, the study only included adults, leading to a longer follow-up time than many of the currently published SDR studies. The sole use of self-reported outcome measures in this study conveys both benefits and limitations; many health-related quality of life outcomes, especially those that are not easily observable (e.g. pain, fatigue), are best measured by self-report. However, a retrospective self-report of changes in function is likely to be less reliable than prospective measurement. A combination of self-reported measures, proxy-reported measures, and objective measures of functioning is recommended for future studies as a means of optimizing data reliability and validity. Responses to the question about the hours of unpaid and paid help received each day suggest that interpretation of the question may have differed somewhat across respondents; for instance, some individuals in GMFCS level I answered that they received 24 hours of care, whereas one person in GMFCS level IV answered that they received only 1 hour of care, suggesting that interpretation of ‘help’ may vary. Nonetheless, we have no reason to expect that differences in interpretation were systematically different across the SDR and comparison groups; in other words, any response bias is not expected to affect the group differences that were found. Future research should assess help or assistance further, with more specific questions and/or adjunctive reports from parents, caregivers, spouses, etc.
The SDR and comparison groups were significantly different in terms of GMFCS levels; however, we were able to control for this difference in multivariate statistical tests. The two groups were also likely to be different in terms of types of CP, since we included participants who reported any diagnosis of it. The SDR group probably primarily comprised participants with spastic diplegic or quadriplegic CP, whereas those in the comparison group might have been more likely to include other types of CP (dystonic, athetoid, hemiplegic, etc.). Therefore, this study has included a more global view of functional status via GMFCS, acknowledging that participants may not be able to accurately self-report the subtype of their CP diagnosis. Additionally, the SRFM, developed for use in participants with spinal cord injury, may be less valid in the CP population. Original questions included in the study may have been difficult for participants to interpret in the way the researchers intended; however, this potential for bias was present in both study groups.
Recruiting in clinics and collecting data via the Internet raises the potential of selecting participants who have regular medical follow-up and higher socioeconomic status. We also included only a few participants who had their SDR elsewhere than at the University of Michigan. This makes it more difficult to generalize these results to other medical centers that perform SDR, especially as selection criteria may vary between institutions. The high proportion of participants in the SDR group classified in GMFCS level IV probably reflects older selection criteria at the University of Michigan Hospital and Health Systems, as SDR is now more likely to be recommended in ambulatory patients. However, despite the greater motor impairment of those in the SDR group, they reported requiring less care than their GMFCS-matched peers.
Conclusion
Adults with CP who underwent SDR as children reported similar prevalence and impact of pain and fatigue as adults with CP who did not have the surgery. They reported higher levels of function, had fewer complaints of gross motor decline, and needed less assistance than their peers in the non-surgical group. More research is required to understand the nature of functional decline in adults with CP, and the impact of pediatric interventions. In addition, the lack of significant difference in pain prevalence, pain interference, and fatigue suggests that this particular spasticity treatment does not have a significant impact on these common symptoms, and more investigation is required to determine best practices to decrease pain and fatigue in adults with CP.
Supplementary Material
What this paper adds.
After rhizotomy, adults report decreased motor decline and need for care, controlling for Gross Motor Function Classification System (GMFCS) level.
They also reported higher function.
They have similar prevalence of pain and fatigue as peers.
Pain and fatigue are prevalent in both groups, emphasizing the need to address other causes of these complaints in addition to spasticity.
Acknowledgments
This research was supported by the Foundation for PM&R Gabriella E. Molnar-Swafford Pediatric PM&R Research Grant. ALK was supported during manuscript preparation by a grant from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (1K01AR064275; principal investigator: ALK). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations
- FSS
Fatigue Severity Scale
- SDR
Selective dorsal rhizotomy
- SRFM
Self-Reported Functional Measure
Footnotes
The authors have stated that they had no interest that could be perceived as posing a conflict or bias.
Supplementary Information: The following additional materials may be found online:
Appendix S1: Motor function change questions.
References
- 1.Christensen D, Van Naarden Braun K, Doernberg NS, et al. Prevalence of cerebral palsy, co-occurring autism spectrum disorders, and motor functioning – Autism and Developmental Disabilities Monitoring Network, USA, 2008. Dev Med Child Neurol. 2014;56:59–65. doi: 10.1111/dmcn.12268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brooks JC, Strauss DJ, Shavelle RM, Tran LM, Rosenbloom L, Wu YW. Recent trends in cerebral palsy survival. Part I: period and cohort effects. Dev Med Child Neurol. 2014;56:1059–64. doi: 10.1111/dmcn.12520. [DOI] [PubMed] [Google Scholar]
- 3.Hurvitz EA, Marciniak CM, Daunter AK, et al. Functional outcomes of childhood dorsal rhizotomy in adults and adolescents with cerebral palsy. J Neurosurg Pediatr. 2013;11:380–8. doi: 10.3171/2013.1.PEDS12311. [DOI] [PubMed] [Google Scholar]
- 4.Peterson MD, Gordon PM, Hurvitz EA. Chronic disease risk among adults with cerebral palsy: the role of premature sarcopoenia, obesity and sedentary behaviour. Obes Rev. 2013;14:171–82. doi: 10.1111/j.1467-789X.2012.01052.x. [DOI] [PubMed] [Google Scholar]
- 5.Opheim A, Jahnsen R, Olsson E, Stanghelle JK. Walking function, pain, and fatigue in adults with cerebral palsy: a 7-year follow-up study. Dev Med Child Neurol. 2009;51:381–8. doi: 10.1111/j.1469-8749.2008.03250.x. [DOI] [PubMed] [Google Scholar]
- 6.Morgan P, McGinley J. Performance of adults with cerebral palsy related to falls, balance and function: a preliminary report. Dev Neurorehabil. 2013;16:113–20. doi: 10.3109/17518423.2012.725107. [DOI] [PubMed] [Google Scholar]
- 7.Johnson DC, Damiano DL, Abel MF. The evolution of gait in childhood and adolescent cerebral palsy. J Pediatr Orthop. 1997;17:392–6. [PubMed] [Google Scholar]
- 8.Day SM, Wu YW, Strauss DJ, Shavelle RM, Reynolds RJ. Change in ambulatory ability of adolescents and young adults with cerebral palsy. Dev Med Child Neurol. 2007;49:647–53. doi: 10.1111/j.1469-8749.2007.00647.x. [DOI] [PubMed] [Google Scholar]
- 9.Tedroff K, Löwing K, Jacobson DN, Åström E. Does loss of spasticity matter? A 10-year follow-up after selective dorsal rhizotomy in cerebral palsy. Dev Med Child Neurol. 2011;53:724–9. doi: 10.1111/j.1469-8749.2011.03969.x. [DOI] [PubMed] [Google Scholar]
- 10.Tedroff K, Lowing K, Astrom E. A prospective cohort study investigating gross motor function, pain, and health-related quality of life 17 years after selective dorsal rhizotomy in cerebral palsy. Dev Med Child Neurol. 2015;57:484–90. doi: 10.1111/dmcn.12665. [DOI] [PubMed] [Google Scholar]
- 11.Ailon T, Beauchamp R, Miller S, et al. Long-term outcome after selective dorsal rhizotomy in children with spastic cerebral palsy. Child Nerv Syst. 2015;31:415–23. doi: 10.1007/s00381-015-2614-9. [DOI] [PubMed] [Google Scholar]
- 12.Langerak NG, Lamberts RP, Fieggen AG, et al. A prospective gait analysis study in patients with diplegic cerebral palsy 20 years after selective dorsal rhizotomy. J Neurosurg Pediatr. 2008;1:180–6. doi: 10.3171/PED/2008/1/3/180. [DOI] [PubMed] [Google Scholar]
- 13.van der Slot W. PhD thesis. Rotterdam: Erasmus University; 2012. Health issues and participation in adults with cerebral palsy. [Google Scholar]
- 14.Engel JM, Jensen MP, Hoffman AJ, Kartin D. Pain in persons with cerebral palsy: extension and cross validation. Arch Phys Med Rehabil. 2003;84:1125–8. doi: 10.1016/s0003-9993(03)00263-6. [DOI] [PubMed] [Google Scholar]
- 15.Jahnsen R, Villien L, Stanghelle JK, Holm I. Fatigue in adults with cerebral palsy in Norway compared with the general population. Dev Med Child Neurol. 2003;45:296–303. doi: 10.1017/s0012162203000562. [DOI] [PubMed] [Google Scholar]
- 16.MacWilliams BA, Johnson BA, Shuckra AL, D'Astous JL. Functional decline in children undergoing selective dorsal rhizotomy after age 10. Dev Med Child Neurol. 2011;53:717–23. doi: 10.1111/j.1469-8749.2011.04010.x. [DOI] [PubMed] [Google Scholar]
- 17.Funk JF, Panthen A, Bakir MS, et al. Predictors for the benefit of selective dorsal rhizotomy. Res Dev Disabil. 2015;37:127–34. doi: 10.1016/j.ridd.2014.11.012. [DOI] [PubMed] [Google Scholar]
- 18.Palisano RJ, Rosenbaum P, Bartlett D, Livingston MH. Content validity of the expanded and revised Gross Motor Function Classification System. Dev Med Child Neurol. 2008;50:744–50. doi: 10.1111/j.1469-8749.2008.03089.x. [DOI] [PubMed] [Google Scholar]
- 19.Eliasson AC, Krumlinde-Sundholm L, Rosblad B, et al. The Manual Ability Classification System (MACS) for children with cerebral palsy: scale development and evidence of validity and reliability. Dev Med Child Neurol. 2006;48:549–54. doi: 10.1017/S0012162206001162. [DOI] [PubMed] [Google Scholar]
- 20.Hidecker MJ, Paneth N, Rosenbaum PL, et al. Developing and validating the Communication Function Classification System for individuals with cerebral palsy. Dev Med Child Neurol. 2011;53:704–10. doi: 10.1111/j.1469-8749.2011.03996.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Amtmann D, Cook KF, Jensen MP, et al. Development of a PROMIS item bank to measure pain interference. Pain. 2010;150:173–82. doi: 10.1016/j.pain.2010.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krupp LB, LaRocca NG, Muir-Nash J, Steinberg AD. The fatigue severity scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol. 1989;46:1121–3. doi: 10.1001/archneur.1989.00520460115022. [DOI] [PubMed] [Google Scholar]
- 23.Hoenig H, McIntyre L, Sloane R, Branch LG, Truncali A, Horner RD. The reliability of a self-reported measure of disease, impairment, and function in persons with spinal cord dysfunction. Arch Phys Med Rehabil. 1998;79:378–87. doi: 10.1016/s0003-9993(98)90137-x. [DOI] [PubMed] [Google Scholar]
- 24.Van Der Slot WM, Nieuwenhuijsen C, Van Den Berg-Emons RJ, et al. Chronic pain, fatigue, and depressive symptoms in adults with spastic bilateral cerebral palsy. Dev Med Child Neurol. 2012;54:836–42. doi: 10.1111/j.1469-8749.2012.04371.x. [DOI] [PubMed] [Google Scholar]
- 25.Novak I, Mcintyre S, Morgan C, et al. A systematic review of interventions for children with cerebral palsy: state of the evidence. Dev Med Child Neurol. 2013;55:885–910. doi: 10.1111/dmcn.12246. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.